Abstract
Simple Summary
The Bardigiano horse is a native Italian breed bred for living in rural areas, traditionally used in agriculture. The breed counts about 3000 horses, and it is nowadays mainly used for recreational purposes. The relatively small size and the closed status of the breed raise the issue of monitoring genetic diversity. We therefore characterized the breed’s genetic diversity based on molecular data. We showed a critical reduction of genetic variability mainly driven by past bottlenecks. We also highlighted homozygous genomic regions that might be the outcome of directional selection in recent years, in line with the conversion of Bardigiano horses from agricultural to riding purposes.
Abstract
Horses are nowadays mainly used for sport and leisure activities, and several local breeds, traditionally used in agriculture, have been exposed to a dramatic loss in population size and genetic diversity. The loss of genetic diversity negatively impacts individual fitness and reduces the potential long-term survivability of a breed. Recent advances in molecular biology and bioinformatics have allowed researchers to explore biodiversity one step further. This study aimed to evaluate the loss of genetic variability and identify genomic regions under selection pressure in the Bardigiano breed based on GGP Equine70k SNP data. The effective population size based on Linkage Disequilibrium (Ne) was equal to 39 horses, and it showed a decline over time. The average inbreeding based on runs of homozygosity (ROH) was equal to 0.17 (SD = 0.03). The majority of the ROH were relatively short (91% were ≤2 Mbp long), highlighting the occurrence of older inbreeding, rather than a more recent occurrence. A total of eight ROH islands, shared among more than 70% of the Bardigiano horses, were found. Four of them mapped to known quantitative trait loci related to morphological traits (e.g., body size and coat color) and disease susceptibility. This study provided the first genome-wide scan of genetic diversity and selection signatures in an Italian native horse breed.
Keywords: conservation, selection scan, autochthonous, genomics, coat color, LCORL, IBH, ROH, MC1R, horse
1. Introduction
The Bardigiano is an Italian autochthonous horse breed whose origin can be traced back to the age of the Roman Empire in the Belgian Gaul province [1]. The breed consists of a homogeneous population characterized by typical and distinct traits: (a) the height ranges between 140 and 149 cm for males, and 135 and 147 cm for females; (b) the only admitted coat color is bay, with a preference for dark bay, while chestnuts and extremely light bays are not allowed; (c) limited white markings on the legs and face are allowed, although not preferred. Conformation related characteristics include a small head with a straight or concave profile, low withers, a slightly straight back, deep girth and an overall muscular appearance [2]. The rustic and docile Bardigiano horse was traditionally used as a working horse in mountain areas, and for meat production. After the Second World War, the number of Bardigiano horses dramatically decreased, numbering only five stallions and 150 mares [3]. The decrease in population size led to the creation of the Bardigiano studbook in 1977, and since then the Bardigiano has been considered a purebred breed due to the closure policy applied by the studbook. Nowadays, the Bardigiano breed counts roughly 3000 live horses, mainly used for riding and light draft purposes. It is classified as a small native population, possibly at risk of extinction [4]. However, the relatively small size and the closed status of the current population raises the issue of monitoring genetic diversity. A recent study exploring genetic diversity in the Bardigiano breed at pedigree level highlighted alarming levels of inbreeding and a loss of genetic diversity [5]. In this study, we present an extension of the above-mentioned work by using genomic data to analyze the genetic diversity in the breed.
Recent techniques in genomics permit present researchers to gain more insights into genetic diversity, population history and selection signatures compared to pedigree-based methods [6,7]. Several genome-wide population structure and genetic diversity studies have been performed in livestock species [8,9,10,11]. The first comprehensive insight into equine genetic diversity among a large breed cohort was published by Petersen et al. in 2013 [12]. This study used fixation index (Fst) statistics, one of the most popular methods for the capture of between-breed divergence [9,13,14,15]. Runs of homozygosity (ROH) are likewise a well-established method, widely used to detect the within-breed loss of genetic diversity [16]. Runs of homozygosity are long consecutive homozygous segments distributed across the genome. Among other evolutionary forces, they also arise from identical-by-descendent haplotypes which came from common ancestors [17]. Therefore, ROHs have been used nowadays as a valuable source of information for the description of genomic inbreeding (FROH) [18,19,20,21]. Compared to pedigree-based inbreeding, FROH is able to capture variation due to Mendelian sampling and linkage during gamete formation [7]. In addition, FROH does not rely on pedigree quality, which may be a limiting factor in inbreeding estimation based on genealogical data [22,23]. Overlapping homozygous regions, highly shared among individuals belonging to the same population, are called ROH islands. Since directional artificial selection reduces genomic variability, ROH islands are thought to be potential signs of selection around a target locus [16,24,25]. Several recent examples of ROH and population structure analyses applied to European horse breeds show key aspects of history and selection pressure [25,26,27,28,29,30,31,32,33]. However, few studies were conducted in the framework of European small native horse breeds [29,31,32,34] and, to the best of our knowledge, none of them specifically analyzed Italian autochthonous horse breeds. Therefore, based on a medium-density single nucleotide polymorphism (SNP) genotype panel, we aimed to investigate genomic diversity in the Bardigiano breed as an example of the reservoir of Italian native breeds.
The detailed aims of this study were to (i) investigate genomic effective population size throughout generations, (ii) calculate within-breed genomic diversity based on FROH, and (iii) evaluate the presence of potential signs of selection based on ROH islands.
2. Materials and Methods
2.1. Sample Collection and Genotyping
The study included genotype data from 89 Bardigiano horses (38 males and 51 females). The horses submitted to genotyping were carefully selected to represent the most of genetic variability from the pedigree information [5]. The following criteria were applied in the selection procedure: (i) verification of pedigree depth, excluding animals with an equivalent complete generation (defined as the sum for (1/2)n, where n is the number of generations separating the individual from each of its known ancestors [35]) lower than two; (ii) horses with available conformation measurements and linear and traditional evaluations were preferred [2]; (iii) no more than three animals descending from the same stallion were allowed; (iv) animals had to belong to the last two generations, considering an average generation interval of 8.74 years [5]. All samples were genotyped with the GGP Equine70k® (Illumina, San Diego, CA, USA) SNP chip, containing 65,157 SNPs separated on average by 40 kb. The SNP physical positions were remapped from the former reference genome EquCab2 to EquCab3 [36], as described in [37]. Only SNPs located on the 31 Equus caballus autosome (ECA) chromosomes were retrieved and used in this study. The quality control (QC) was performed in PLINK v1.9 [38] by removing SNPs with a call rate lower than 0.90, a Hardy–Weinberg equilibrium (HWE) deviation with p < 10−6 and minor allele frequencies (MAF) <0.01. Horses with a call rate lower than 0.90 were excluded.
2.2. Effective Population Size
Past and present effective population sizes (Ne) were estimated with the SNeP v.1.1 software [39] using only horses in the last birth year cohort (2011–2019). SNeP v.1.1 estimates the trends of the historical effective population size trajectories from SNP data based on linkage disequilibrium (LD). The recombination rate was calculated using the Sved and Feldman’s mutation rate modifier [40], and sample size correction was made for unphased genotypes. The cM unit in this study was set to 1.24 Mbp [41]. The minimum and maximum distance between pairs of SNPs was set to 0.5 Mbp and 26 Mbp, respectively. We also performed an Ne slope analysis (NeS) [42] to look into the rate and directionality of Ne changes occurring throughout generations. The NeS analysis identifies subtle changes in the inferred Ne curve not visually explicit in the Ne plot. The slope of each segment linking pairs of neighboring Ne estimates was first calculated and then normalized using the median of the two most proximal past Ne slope values.
2.3. Runs of Homozygosity
The ROH segments were detected using the DetectRUNS [43] package in R [44], and defined as follows: (i) at least 15 SNPs in a run, (ii) a minimum length of a run equal to 500 kb, (iii) a maximum distance between consecutive SNPs in a window 1000 kb, (iv) a lower density limit of 1 SNP per 100 kb [27] and (v) allowing for a maximum of one missing and one heterozygous SNP in a run [25]. The ROH segments were divided into the following five length classes: 0.5–1 Mbp, 1–2 Mbp, 2–4 Mbp, 4–8 Mbp and >8 Mbp. The total number of ROHs (NROH), average length of ROHs (LROH) and the average ROH number (SROH) were summarized according to each ROH length category. The genomic inbreeding coefficients (FROH) were calculated following the method described in [18]:
| (1) |
with LROH being the length of ROHs in each individual and LAUTO being the length of the autosomal genome, which was set to 2276 Gb, based on the genome length covered by SNPs. Based on the hypothesis that the length of ROH reflects the chronological time points when inbreeding happened [20], the genomic inbreeding was expressed separately for five length ROH categories (0.5–1 Mbp, 1–2 Mbp, 2–4 Mbp, 4–8 Mbp, >8 Mbp). By using the formula 1/2 g Morgan, with g being equal to generation [20,45], and the relationship between Centimorgan and Mb in horses [41], we estimated the timepoint of the inbreeding event based on ROH length. The inbreeding was also calculated per chromosome, and the type of distribution was evaluated based on skewness and kurtosis.
Putative ROH islands were determined based on overlapping homozygous regions within more than 70% of the horses. The EqCab3 genomic coordinates of these regions were used to retrieve candidate gene lists and annotations from the Biomart web interface in Ensembl [46] and from the UCSC genome browser platform [47]. Putative ROH islands were compared with quantitative trait loci (QTL) regions previously identified and reported in the Horse QTL database [48]. Functional analyses were performed on the genes potentially under selection, located in ROH shared in more than 85% of the animals [25] or overlapping with known QTLs.
3. Results
3.1. Genotyping Quality Control
All horses except one passed the genotype data quality control (37 males, 51 females), and 58,047 SNPs were retrieved. The average genotype call rate was 0.99 and the average pedigree depth based on complete generation equivalent of the selected horses was 6.28 (SD = 0.79). The horses descended from 54 stallions with an average of 1.65 offspring each (SD = 0.82) and 76 mares with 1–2 offspring each. In total, 80% of the horses were born between 2011 and 2019, whereas 18 horses were born between 2001 and 2010.
3.2. Effective Population Size
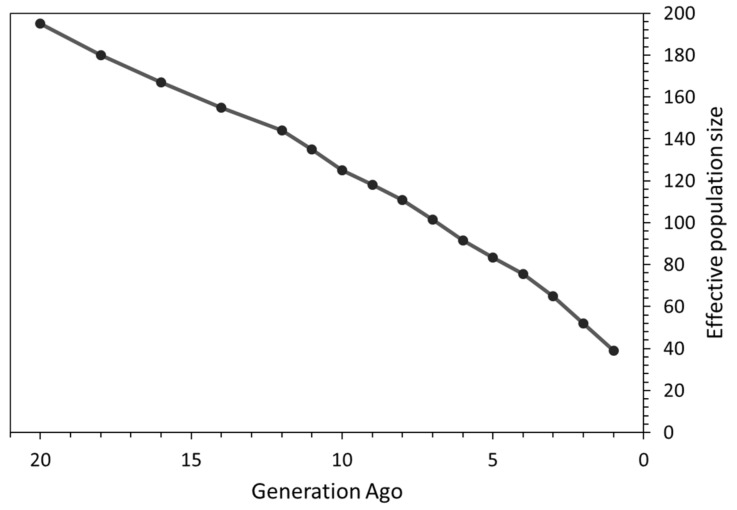
For the Ne calculation, we only included animals born between 2011 and 2019, which numbered 70 horses. The Ne declined over time, and was estimated to be ~39 horses one generation ago. Instead, the estimated Ne 20 generations ago was around 195 horses (Figure 1).
Figure 1.
Plot of effective population size change between 20 generations ago and the last generation in the Bardigiano horse breed.
To investigate the change in slope of the inferred Ne obtained from LD-based estimation, we used the NeS method, which offers more detailed information about population changes 1–20 generations ago. A constant rate of change is shown as a flat line at 0 in the Y-axis, whereas deviations above and below 0 represent relative increases and reductions, respectively, of the Ne change in slope compared to the previous two generations. This analysis highlighted two sharp reductions in Ne in the Bardigiano breed, being approximately ten and six generations ago (Figure 2).
Figure 2.
Ne Slope analysis (NeS) between 20 generations ago and the last generation in the Bardigiano horse breed. A constant rate of change in the Ne is shown as a flat line at 0 in the Y-axis.
3.3. Runs of Homozygosity
A total of 28,423 ROHs were found among the 88 Bardigiano horses analyzed in this study. An average of 323 ROHs were found per horse, with a maximum number equal to 365 and minimum of 285 ROHs. The average length of ROHs per individual was equal to 1215.8 Kb ± 173.7 Kb (SD). The majority of ROHs were shorter than 2 Mbp, with 69.5% being shorter than 1 Mbp and 21.4% being between 1 and 2 Mbp length (Table 1). The proportion of ROHs longer than 8 Mbp was equal to 1.2%, with an average length of 13.4 Mbp. A total of 84 horses exhibited ROHs in the >8 Mbp length class, with an average of 4.2 ROHs per horse in this class and a minimum and maximum equal to 1 and 13 ROHs, respectively.
Table 1.
Descriptive statistics of runs of homozygosity (ROH).
| ROH | |||||
|---|---|---|---|---|---|
| Length Class (Mbp) | N. 1 | NROH 2 | Percentage 3 | SROH 4 | LROH 5 |
| 0.5–1 | 88 | 19,746 | 69.5% | 224.4 | 0.70 |
| 1–2 | 88 | 6073 | 21.4% | 69.0 | 1.33 |
| 2–4 | 88 | 1587 | 5.6% | 18.0 | 2.74 |
| 4–8 | 88 | 664 | 2.3% | 7.5 | 5.42 |
| >8 | 84 | 353 | 1.2% | 4.2 | 13.40 |
1 Number of animals, 2 total number of ROH, 3 relative percentage, 4 average ROH number per animal, 5 average length of ROH.
The number of ROHs and length of ROH segments varied across chromosomes, as shown in Figure 3. The highest number of ROHs was identified on ECA1 (2386), while the lowest was identified on ECA31, with 273 ROHs detected. Nevertheless, when normalizing for the chromosome length, the highest portion was found on ECA29 and ECA28. The highest chromosome length covered by ROH was detected on ECA10, with 1.49 Mbp covered by ROHs, followed by ECA5 (1.46 Mbp), ECA1 (1.43 Mbp) and ECA3 (1.40 Mbp).
Figure 3.
Distribution and average length of runs of homozygosity (ROH) in Mbp detected across the autosomal genome in the Bardigiano horses. The bar plots show the ROH counts per chromosome and the orange line shows the average ROH size (Mbp) per chromosome.
3.3.1. ROH as a Measure of Inbreeding
The inbreeding, measured as the proportion of the genome covered by ROH, resulted in an average FROH equal to 0.17 ± 0.03 (SD). To differentiate ancient and recent inbreeding, we calculated FROH based upon five ROH length classes, as presented in Table 2. Inbreeding events occurring over ~31 generations ago were presented by FROH calculated per length below or equal to 2 Mbp. For the ROH length class above 8 Mbp, four horses did not have ROHs and thus their inbreeding resulted in being equal to 0. The degree of inbreeding based on the longest ROH class showed an average inbreeding equal to 0.02, reflecting inbreeding events which happened <8 generations ago.
Table 2.
Descriptive statistics of inbreeding based on runs of homozygosity (FROH) within each ROH length class for the 88 Bardigiano horses that passed the QC.
| Inbreeding Based on ROH (FROH) | ||||
|---|---|---|---|---|
| Length Class (Mbp) | Mean | Min. 1 | Max. 2 | SD 3 |
| 0.5–1 | 0.07 | 0.06 | 0.08 | 0.004 |
| 1–2 | 0.04 | 0.03 | 0.05 | 0.005 |
| 2–4 | 0.02 | 0.01 | 0.04 | 0.006 |
| 4–8 | 0.02 | 0.002 | 0.04 | 0.008 |
| >8 | 0.02 | 0.00 | 0.09 | 0.02 |
| Total | 0.17 | 0.12 | 0.26 | 0.03 |
1 Minimum, 2 maximum, 3 standard deviation.
The inbreeding calculated per chromosome showed large variation among chromosomes both in terms of average values and the distribution of inbreeding level per individual (Figure 4). The highest average inbreeding was found in ECA 1 (mean = 0.21 ± 0.09 SD) and the lowest on ECA 30 (0.12 ± 0.09 SD). For all chromosomes except ECA 3, ECA 15 and ECA 17, a highly skewed distribution towards higher values was found (i.e., a distribution skewness above one). A kurtosis value above three was used to investigate the presence of outlier individuals per each chromosome. In total, 21 out of the 31 chromosomes showed a kurtosis higher than three, highlighting the presence of some horses exhibiting an excess of homozygosity.
Figure 4.
Violin plot of the inbreeding based on runs of homozygosity (FROH) calculated per chromosome in the Bardigiano horse breed.
3.3.2. ROH as a Measure to Detect Signatures of Selection
A total of five chromosomes (ECA3, ECA10, ECA11, ECA15 and ECA19) contained ROH islands that were shared by more than 70% of individuals in the total sample. Three ROH islands were detected on ECA3, two on ECA10, and the remainder were equally distributed among ECA11, ECA15 and ECA19 (Figure 5). The three ROH islands located and ECA3 were shared in more than 85% of the animals, and in particular the ROH island located on ECA3 (ECA3: 35.48–36.01 Mbp) was shared in 93% of the animals (82 out of the 88).
Figure 5.
ROH islands on ECA3, ECA10, ECA11, ECA15 and ECA19 in the Bardigiano horse breed. The x-axis represents the chromosome position in Mbp and the y-axis represents the number of horses showing an ROH in each chromosome position.
Table 3 reports the genomic coordinates of the ROH islands and the annotated genes. A total of 115 annotated genes, two miRNAs and three snoRNA were located within the eight ROH islands. The two ROH islands on ECA3, located between positions 35,477,778 and 37,590,699 bp, overlapped with four known QTL regions; two related to insect bite hypersensitivity (IBH) [49,50], one to guttural pouch tympany [51] and one to hair pigmentation [52]. The ROH island on ECA3: 106,769,095: 107,709,841 bp coincided with a known QTL for body height at the withers [53]. The ROH island on ECA11 coincided with four QTL regions related to insect bite hypersensitivity [49], hair density [54], overall body size [55] and height at the withers [56]. The ROH islands on ECA10, ECA15 and ECA19 did not overlap with any known QTLs.
Table 3.
Runs of homozygosity (ROH) islands shared in over 70% of the Bardigiano horses, with genomic coordinates and a list of annotated genes located within each ROH island.
| ECA 1 | Start (bp) 2 | End (bp) 2 | Length (Kb) | Annotated Genes | % of Horses |
|---|---|---|---|---|---|
| 3 | 35,477,778 | 36,012,699 | 535 | ZNF469, ZFPM1,ZC3H18, IL17C, CYBA, MVD, SNAI3, RNF166, CTU2, PIEZO1 | 93% |
| 3 | 36,131,080 | 37,590,699 | 1460 |
CBFA2T3, ACSF3, CDH15, SLC22A31, ANKRD11, SPG7, RPL13, CPNE7, DPEP1, CHMP1A, SPATA33, CDK10, SPATA2L, VPS9D1, ZNF276, FANCA, SPIRE2, TCF25,
MC1R,TUBB3, DEF8, DBNDD1, GAS8, PRDM7, CENPE, BDH2, SLC9B2 |
92% |
| 3 | 106,769,095 | 107,709,841 | 941 | LCORL, NCAPG, DCAF16, FAM184B, | 92% |
| 10 | 24,965,648 | 25,501,456 | 536 | IL11, TMEM190, TMEM238, RPL28, UBE2S, SHISA7, ISOC2, C19orf85, ZNF628, NAT14, SSC5D, SBK2, SBK3, ZNF579, FIZ1, ZNF524, ZNF865, ZNF784, ZNF580, ZNF581, CCDC106, ZNF835, U2AF2, EPN1, RFPL4A, EQUCABV1R902, EQUCABV1R903, NLRP4, NLRP13, NLRP5 | 76% |
| 10 | 25,636,022 | 26,106,403 | 470 | EDDM13, ZNF667, ZNF583, ZNF582, SMIM17, ZNF471, ZFP28, ZNF470, ZNF71 | 76% |
| 11 | 22,957,922 | 24,177,108 | 1219 | CDK12, MED1, STAC2, CACNB1, ARL5C, PLXDC1, FBXO47, LINC00672, LASP1, RPL23, C17orf98, CWC25, PIP4K2B, PSMB3, MLLT6, PCGF2, CISD3, EPOP, SRCIN1, ARHGAP23, SOCS7, GPR179, MRPL45, NPEPPS, KPNB1, TBKBP1, TBX21, OSBPL7, MRPL10, LRRC46, SCRN2, SP6, SP2, PNPO | 73% |
| 15 | 67,045,471 | 67,539,463 | 494 | LCLAT1, LBH, YPEL5 | 73% |
| 19 | 55,113,101 | 55,782,316 | 669 | // | 77% |
1 ECA: Equine chromosome, 2 position in base pairs (bp) on EquCab3.
4. Discussion
The evaluation of genetic diversity with traditional methods based on pedigree analyses have been widely used to manage animals’ genetic resources. In small livestock populations, where financial support is generally a limiting factor, pedigree-based methods are still commonly adopted due to their cost–effectiveness ratio [5,57,58,59,60]. However, with novel molecular and bioinformatics approaches, genetic variability can be more proficiently evaluated by using genomic information, and could lead to more precise and effective conservation programs. This study can be considered as the first step in deepening our knowledge about Bardigiano genomic diversity.
Effective population size (Ne), computed using genealogical data, could be biased if pedigree depth and quality are low [61]. To this end, molecular markers could be a valuable alternative form of information for the characterization of Ne in livestock populations [62]. The value of Ne in the Bardigiano horses based on LD information was approximately 39 horses in the last generation. This value of Ne is lower than that calculated in the Persian Arabian Horses (Ne = 113) [63], in the Saxon Thuringa Coldblood (Ne = 48.1) and in the Rhenish German Draught Horse (Ne = 46.1) [64]. Interestingly, the Ne estimate based on pedigree data (calculated as individual increases in inbreeding [65,66] in the live Bardigiano population) was lower than (Ne = 30.7), yet comparable to, that found by molecular data analyses [5]. This evidence highlights that the Ne estimate obtained from pedigree can be considered a proper approximation of molecular based Ne in the case of Bardigiano horses. The estimation of Ne in the last 20 generations showed a steady decrease in effective population size, in line with the known history of the breed. The NeS analysis highlighted two sharp reductions in Ne in the Bardigiano breed, approximately ten and six generations ago. Since the average generation interval in this breed is 8.74 years [5], we hypothesized that those two reductions occurred, roughly, around 1933–1942 and 1968–1977, respectively. During World War II, the number of Bardigiano horses decreased dramatically, with only five stallions and 150 mares surviving [3]. This evidence may justify the sharp reduction in the Ne about 10 generations ago. The Bardigiano studbook was founded in 1977 with the aim to recover the breed, just after the second sharp reduction in Ne found in this study. This second sharp reduction in Ne might highlight the loss of genetic diversity due to the selection of breeding candidates during the initial steps of the breed recovery. In contrast, the general increase and stabilization of the Ne from the 4th generation onwards might reflect the breeding policies applied by the Bardigiano studbook to conserve the breed.
The extent and frequency of ROH have been widely used to infer ancestry at the individual and breed level [16]. Long ROH are generally considered to be a sign of recent inbreeding, whereas short ROH can capture ancient inbreeding which derived from older ancestors and can capture population bottlenecks. Under the assumption that 1 cM equals 1.24 Mbp, ROH can be separated in length classes to express different points in time when inbreeding occurred [19]. In the Bardigiano horse, the majority of ROH belonged to the shortest length class, being equal to 0.5–1 Mbp (69.5%). Similar results were observed in local breeds, as in the Bosnian Mountain Horse (61.7%) [32] and in the Noriker horse breed, where the majority (60–85%) of ROH segments were shorter than 4 Mbp [34]. This result might mainly originate from the small population size of the Bardigiano breed and the strong founder effect due to the experienced bottlenecks. In contrast, the lowest percentage of ROH (1.2%) resided into the longest class (ROH > 8Mbp), which highlights a rather small reduction in genetic variability occurred in the last generations. This result is coherent with the breeding strategies applied by the studbook to reduce matings between highly related animals. Likewise, the FROH ranged from 17.0%, considering all ROH regardless of the size, to 2.0%, considering only the longest class (ROH > 8Mbp). This last result highlights the occurrence of older inbreeding, rather than inbreeding which happened in the last few generations. Nevertheless, the average FROH in the Bardigiano was higher compared to other small-sized horse populations. For example, in the Croatian Posavje horse breed, the FROH was equal to 8.59%; in the Bosnian mountain horse (originating from Bosnia and Herzegovina) this value reached a mean of 13.21%; and in the Austrian Noriker horse it ranged between 8.00% and 13.00%, depending on coat color strains [31,34]. Similar levels of FROH to those in the Bardigiano horses were found in the Polish Koniks breed (mean = 16.00%), which is a primitive breed closely related to the extinct wild Tarpan [67], and in Purebred Arabian horses (mean =1 7.70%), despite the larger population size [32]. In contrast, a higher level of inbreeding was found in Friesian horses (mean = 22.30%) compared to the Bardigiano [68]. As expected from previous studies [7,69,70], the inbreeding based on molecular data in the Bardigiano tended to be higher than that previously found at the pedigree level [5].
In general, the ROHs caused by inbreeding tend to be distributed unevenly over the genome, as we have found in the Bardigiano horse, with a different distribution of numbers and sizes of ROH for each chromosome [71]. However, ROH that are located in specific genomic regions and shared among several individuals are thought to be potential signs of selection [24,25,27,71]. The reasons behind this theory are the driving forces of directional selection, which increase homozygosity around a target locus (ROH island). The breeding objective of the Bardigiano studbook can be divided into three main goals: (a) ensure long-term survival of the breed; (b) preserve the Bardigiano’s distinctive morphological features; and (c) increase the use of this breed in riding and draft activities [2]. For the latter aspect, in the ’90s, the Bardigiano studbook introduced a breeding value for the height at the withers to help the conversion of taller horses, which are thus more suitable for leisure. It is noteworthy that, in this study, we found ROH islands in two of the four loci previously found to explain 83% of the size variation in horses [55]. Specifically, one ROH was located on ECA3 and shared among 93% of the Bardigiano horses, which overlapped with the ligand dependent nuclear receptor corepressor-like (LCORL) gene [72]. The LCORL gene is a transcription factor that has been associated with human height [73,74,75]. In cattle, LCORL was identified in a screen for loci under selection [76], and the immediately adjacent gene, NCAPG, has been implicated in prenatal growth [77] as well as in the body size in Franches–Montagnes horses [77]. The other ROH that overlapped with a locus among the four explaining 83% of the size variation in horses was on ECA11, where the LIM and SH3 protein 1 (LASP1) gene is located. The LASP1 gene mediates cell migration and survival, and its expression is induced by insulin-like growth factor (IGF1) [78]. However, the ROH on ECA11 is located within a gene-dense region counting thirty-four genes; therefore, we cannot exclude that selection in this region might target other genes. Based on the overlap with known QTLs for body size, we hypothesize that the ongoing selection toward higher horses might have led to a reduction in variability in those two regions.
In addition, this latter region of ECA11, together with two ROH islands on ECA3 between 35.48 and 37.59 Mbp, overlapped with known QTLs for equine insect bite hypersensitivity (IBH). IBH is a pruritic skin allergy caused primarily by biting midges, Culicoides spp [49]. This skin disease has polygenic inheritance and occurs at high frequencies in several horse breeds worldwide, thus causing increased costs and reduced horse welfare. To the best of our knowledge, so far, no cases of IBH have been reported in the Bardigiano breed, even if the animals are mostly farmed outside, and pasture in the mountain area is commonly practiced in the breed. The summer pasture exposure and the surrounding vegetation have been identified as risk factors associated with IBH in warmblood horses [79]. We therefore hypothesize that long and extensive exposure to insect bites might have shaped the genome of Bardigiano horses towards more resistant animals. Nevertheless, those three ROH islands located on ECA3 and ECA11 also overlapped with known QTLs for hair pigmentation [52] and hair density [54]. Interestingly, some of the distinctive features of the Bardigiano horse are actually related to hair type and pigmentation. Indeed, the only coat color accepted by the breeding association is bay, with a slight preference for dark bay, whereas chestnuts and extremely light bays are not allowed in the breed. In addition, only limited white markings on the legs and face are allowed and, in general, if present, they are scored as a defect in the horse evaluation [2]. In the study by Haase et al. 2013 [52], seven QTLs were significantly associated with the white marking phenotype, which explained ~54% of the total genetic variance [52,80]. The ROH island identified on ECA3 (36.13–37.59 Mbp) in the Bardigiano horses overlapped with the melanocortin receptor gene (MC1R). The MC1R encoded by the Extension (E) locus controls, together with the peptide antagonist agouti-signaling-protein (ASIP), the amounts of melanin pigments in mammals [81]. We, therefore, cannot rule out the hypothesis that this region might be under selection pressure because of morphological requirements in terms of coat color in the Bardigiano breed.
5. Conclusions
The genetic diversity of the Bardigiano breed was analyzed via Ne based on LD and ROHs detected in this study. We identified a decrease in the LD-based Ne, when calculated over the last 20 generations, in line with the known history of the breed. The ROH analysis provided additional information to further optimize the breeding and management decisions needed to ensure the long term survival of the breed. Inbreeding levels, especially those based on short ROH segments, were moderate to high, suggesting the presence of past bottlenecks in the genome of Bardigiano horses. Conversely, the lowest percentage of ROH resided in the longest ROH classes, showing a small reduction in genetic variability during the last generations. Eight ROH islands were found in the breed; these islands were shared among more than 70% of the Bardigiano horses analyzed in this study. Four of them overlapped with known QTLs associated with conformation traits and disease susceptibility. Genes related with conformation traits, coat color and disease susceptibility appear to be targets of directional selection in the Bardigiano breed. This study has outlined the first genome-wide map of selection signatures in an Italian native horse breed, and provides material for further functional studies on the biological mechanisms of complex traits in horses.
Author Contributions
Conceptualization, M.A. and A.S.; methodology, M.A., S.E., S.M.; formal analysis M.A.; resources, M.V., A.S.; data curation, M.A, M.V.; writing–original draft preparation, M.A.; writing–review and editing, S.M., S.E., C.D., A.S.; project administration, A.S.; supervision, S.M., S.E., A.S.; funding, A.S., M.V. All authors have read and agreed to the published version of the manuscript.
Funding
The presented work received financial support from the PSRN 2016–2019 ‘Biodiversità—Sottomisura 10.2—Progetto Equinbio—Innovazione e Biodiversità per gli equidi’, which is an Italian National Operational Program intended to preserve genetic diversity in autochthonous Italian horse breeds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hendricks L.B. International Encyclopedia of Horse Breeds. University of Oklahoma Press; Norman, OK, USA: 1995. [Google Scholar]
- 2.Ablondi M., Summer A., Vasini M., Simoni M., Sabbioni A. Genetic parameters estimation in an Italian horse native breed to support the conversion from agricultural uses to riding purposes. J. Anim. Breed. Genet. 2020;137:200–210. doi: 10.1111/jbg.12425. [DOI] [PubMed] [Google Scholar]
- 3.Di Stasio L., Perrotta G., Blasi M., Lisa C. Genetic characterization of the Bardigiano horse using microsatellite markers. Ital. J. Anim. Sci. 2008;7:243–250. doi: 10.4081/ijas.2008.243. [DOI] [Google Scholar]
- 4.Sherf B. World Watch List for Domestic Animal Diversity. 3rd ed. FAO; Rome, Italy: 2000. [Google Scholar]
- 5.Ablondi M., Vasini M., Beretti V., Superchi P., Sabbioni A. Exploring genetic diversity in an Italian horse native breed to develop strategies for preservation and management. J. Anim. Breed. Genet. 2018;135:1–10. doi: 10.1111/jbg.12357. [DOI] [PubMed] [Google Scholar]
- 6.Kim E.S., Cole J.B., Huson H., Wiggans G.R., Van Tassel C.P., Crooker B.A., Liu G., Da Y., Sonstegard T.S. Effect of artificial selection on runs of homozygosity in U.S. Holstein cattle. PLoS ONE. 2013;8:1–14. doi: 10.1371/journal.pone.0080813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard J.T., Pryce J.E., Baes C., Maltecca C. Invited review: Inbreeding in the genomics era: Inbreeding, inbreeding depression, and management of genomic variability. J. Dairy Sci. 2017;100:6009–6024. doi: 10.3168/jds.2017-12787. [DOI] [PubMed] [Google Scholar]
- 8.Purfield D.C., Berry D.P., McParland S., Bradley D.G. Runs of homozygosity and population history in cattle. BMC Genet. 2012;13:70. doi: 10.1186/1471-2156-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz M., Bozzi R., García-Casco J., Núñez Y., Ribani A., Franci O., García F., Škrlep M., Schiavo G., Bovo S., et al. Genomic diversity, linkage disequilibrium and selection signatures in European local pig breeds assessed with a high density SNP chip. Sci. Rep. 2019;9:13546. doi: 10.1038/s41598-019-49830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talenti A., Bertolini F., Pagnacco G., Pilla F., Ajmone-Marsan P., Rothschild M.F., Crepaldi P. The Italian goat consortium the valdostana goat: A genome-wide investigation of the distinctiveness of its selective sweep regions. Mamm. Genome. 2017;28:129. doi: 10.1007/s00335-017-9685-8. [DOI] [PubMed] [Google Scholar]
- 11.Kijas J.W., Lenstra J.A., Hayes B., Boitard S., Neto L.R., Cristobal M.S., Servin B., McCulloch R., Whan V., Gietzen K., et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012:10. doi: 10.1371/journal.pbio.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen J.L., Mickelson J.R., Cothran E.G., Andersson L.S., Axelsson J., Bailey E., Bannasch D., Binns M.M., Borges A.S., Brama P., et al. Genetic diversity in the modern horse illustrated from genome-wide SNP data. PLoS ONE. 2013:8. doi: 10.1371/journal.pone.0054997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiorano A.M., Lourenco D.L., Tsuruta S., Toro Ospina A.M., Stafuzza N.B., Masuda Y., Filho A.E.V., Dos Santos-Goncalves C.J.N., Curi R.A., De Vasconcelos-Silva J.A. Assessing genetic architecture and signatures of selection of dual purpose Gir cattle populations using genomic information. PLoS ONE. 2018;13:1–24. doi: 10.1371/journal.pone.0200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khayatzadeh N., Mészáros G., Utsunomiya Y.T., Garcia J.F., Schnyder U., Gredler B., Curik I., Sölkner J. Locus-specific ancestry to detect recent response to selection in admixed Swiss Fleckvieh cattle. Anim. Genet. 2016;47:637–646. doi: 10.1111/age.12470. [DOI] [PubMed] [Google Scholar]
- 15.Msalya G., Kim E.S., Laisser E.L.K., Kipanyula M.J., Karimuribo E.D., Kusiluka L.J.M., Chenyambuga S.W., Rothschild M.F. Determination of genetic structure and signatures of selection in three strains of Tanzania shorthorn Zebu, Boran and Friesian cattle by genome-wide SNP analyses. PLoS ONE. 2017;12:1–18. doi: 10.1371/journal.pone.0171088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peripolli E., Munari D.P., Silva M.V.G.B., Lima A.L.F., Irgang R., Baldi F. Runs of homozygosity: Current knowledge and applications in livestock. Anim. Genet. 2017;48:255–271. doi: 10.1111/age.12526. [DOI] [PubMed] [Google Scholar]
- 17.Ceballos F.C., Joshi P.K., Clark D.W., Ramsay M., Wilson J.F. Runs of homozygosity: Windows into population history and trait architecture. Nat. Rev. Genet. 2018;19:220–234. doi: 10.1038/nrg.2017.109. [DOI] [PubMed] [Google Scholar]
- 18.McQuillan R., Leutenegger A.L., Abdel-Rahman R., Franklin C.S., Pericic M., Barac-Lauc L., Smolej-Narancic N., Janicijevic B., Polasek O., Tenesa A., et al. Runs of homozygosity in European populations. Am. J. Hum. Genet. 2008;83:359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curik I., Ferenčaković M., Sölkner J. Inbreeding and runs of homozygosity: A possible solution to an old problem. Livest. Sci. 2014;166:26–34. doi: 10.1016/j.livsci.2014.05.034. [DOI] [Google Scholar]
- 20.Ferenčaković M., Hamzić E., Gredler B., Solberg T.R., Klemetsdal G., Curik I., Sölkner J. Estimates of autozygosity derived from runs of homozygosity: Empirical evidence from selected cattle populations. J. Anim. Breed. Genet. 2013;130:286–293. doi: 10.1111/jbg.12012. [DOI] [PubMed] [Google Scholar]
- 21.Marras G., Gaspa G., Sorbolini S., Dimauro C., Ajmone-Marsan P., Valentini A., Williams J.L., Macciotta N.P.P. Analysis of runs of homozygosity and their relationship with inbreeding in five cattle breeds farmed in Italy. Anim. Genet. 2015;46:110–121. doi: 10.1111/age.12259. [DOI] [PubMed] [Google Scholar]
- 22.Schurink A., Arts D.J.G., Ducro B.J. Genetic diversity in the Dutch harness horse population using pedigree analysis. Livest. Sci. 2012;143:270–277. doi: 10.1016/j.livsci.2011.10.005. [DOI] [Google Scholar]
- 23.Sørensen M.K., Sørensen A.C., Baumung R., Borchersen S., Berg P. Optimal genetic contribution selection in Danish holstein depends on pedigree quality. Livest. Sci. 2008;118:212–222. doi: 10.1016/j.livsci.2008.01.027. [DOI] [Google Scholar]
- 24.Metzger J., Karwath M., Tonda R., Beltran S., Águeda L., Gut M., Gut I.G., Distl O. Runs of homozygosity reveal signatures of positive selection for reproduction traits in breed and non-breed horses. BMC Genom. 2015;16:764. doi: 10.1186/s12864-015-1977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ablondi M., Viklund Å., Lindgren G., Eriksson S., Mikko S. Signatures of selection in the genome of Swedish warmblood horses selected for sport performance. BMC Genom. 2019;20:717. doi: 10.1186/s12864-019-6079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grilz-Seger G., Neuditschko M., Ricard A., Velie B., Lindgren G., Mesarič M., Cotman M., Horna M., Dobretsberger M., Brem G., et al. Genome-wide homozygosity patterns and evidence for selection in a set of European and near eastern horse breeds. Genes. 2019;10:491. doi: 10.3390/genes10070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolte W., Thaller G., Kuehn C. Selection signatures in four German warmblood horse breeds: Tracing breeding history in the modern sport horse. PLoS ONE. 2019;14:1–25. doi: 10.1371/journal.pone.0215913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grilz-Seger G., Druml T., Neuditschko M., Dobretsberger M., Horna M., Brem G. High-resolution population structure and runs of homozygosity reveal the genetic architecture of complex traits in the Lipizzan horse. BMC Genom. 2019;20:1–17. doi: 10.1186/s12864-019-5564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moravčíková N., Kasarda R., Kadlečík O., Trakovická A., Halo M., Candrák J. Runs of homozygosity as footprints of selection in the norik of muran horse genome. Acta Univ. Agric. Silv. Mendel. Brun. 2019;67:1165–1170. doi: 10.11118/actaun201967051165. [DOI] [Google Scholar]
- 30.Ablondi M., Eriksson S., Tetu S., Sabbioni A., Viklund A., Mikko S. Genomic divergence in swedish warmblood horses selected for equestrian disciplines. Genes. 2019;10:976. doi: 10.3390/genes10120976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grilz-Seger G., Mesarič M., Cotman M., Neuditschko M., Druml T., Brem G. Runs of homozygosity and population history of three horse breeds with small population size. J. Equine Vet. Sci. 2018;71:27–34. doi: 10.1016/j.jevs.2018.09.004. [DOI] [Google Scholar]
- 32.Druml T., Neuditschko M., Grilz-Seger G., Horna M., Ricard A., Mesarič M., Cotman M., Pausch H., Brem G. Population networks associated with runs of homozygosity reveal new insights into the breeding history of the haflinger horse. J. Hered. 2018;109:384–392. doi: 10.1093/jhered/esx114. [DOI] [PubMed] [Google Scholar]
- 33.Ardestani S.S., Aminafshar M., Zandi-Baghche M.M.B., Banabazi M.H., Sargolzaei M., Miar Y. Whole-genome signatures of selection in sport horses revealed selection footprints related to musculoskeletal system development processes. Animals. 2019;10:53. doi: 10.3390/ani10010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grilz-Seger G., Druml T., Neuditschko M., Mesarič M., Cotman M., Brem G. Analysis of ROH patterns in the Noriker horse breed reveals signatures of selection for coat color and body size. Anim. Genet. 2019;50:334–346. doi: 10.1111/age.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maignel L., Boichard D., Verrier E. Genetic variability of French dairy breeds estimated from pedigree information. Interbull Bull. 1996;14:49–53. [Google Scholar]
- 36.Kalbfleisch T.S., Rice E.S., DePriest M.S., Walenz B.P., Hestand M.S., Vermeesch J.R., O′Connell B.L., Fiddes I.T., Vershinina A.O., Saremi N.F., et al. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun. Biol. 2018;1:1–8. doi: 10.1038/s42003-018-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beeson S.K., Schaefer R.J., Mason V.C., McCue M.E. Robust remapping of equine SNP array coordinates to EquCab3. Anim. Genet. 2019;50:114–115. doi: 10.1111/age.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbato M., Orozco-ter W.P., Tapio M., Bruford M.W. SNeP: A tool to estimate trends in recent effective population size trajectories using genome-wide SNP data. Front. Genet. 2015;6:109. doi: 10.3389/fgene.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sved J.A., Feldman M.W. Correlation and probability methods for one and two loci. Popul. Biol. 1973;4:129–132. doi: 10.1016/0040-5809(73)90008-7. [DOI] [PubMed] [Google Scholar]
- 41.Beeson S.K., Mickelson J.R., McCue M.E. Exploration of fine-scale recombination rate variation in the domestic horse. Genome Res. 2019;29:1744–1752. doi: 10.1101/gr.243311.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitt D., Bruford M.W., Barbato M., Orozco-ter W.P., Martínez R., Sevane N. Demography and rapid local adaptation shape creole cattle genome diversity in the tropics. Evol. Appl. 2019;12:105–122. doi: 10.1111/eva.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biscarini F., Cozzi P., Gaspa G., Marras G. detectRUNS: An R Package to Detect Runs of Homozygosity and Heterozygosity in Diploid Genomes. University of Sassari; Sassari, Italy: 2019. [Google Scholar]
- 44.R Development Core Team . R: A Language and Environment for Statistical Computing. R Development Core Team; Vienna, Austria: 2011. [Google Scholar]
- 45.De Souza-Fonseca P.A., dos Santos F.C., Rosse I.C., Ventura R.V., Brunelli F.Â.T., Penna V.M., da Silva Verneque R., Machado M.A., da Silva M.V.G.B., Carvalho M.R.S., et al. Retelling the recent evolution of genetic diversity for Guzerá: Inferences from LD decay, runs of homozygosity and Ne over the generations. Livest. Sci. 2016;193:110–117. doi: 10.1016/j.livsci.2016.10.006. [DOI] [Google Scholar]
- 46.Aken B.L., Ayling S., Barrell D., Clarke L., Curwen V., Fairley S., Fernandez Banet J., Billis K., García Girón C., Hourlier T., et al. The Ensembl gene annotation system. Database. 2016;2016:93. doi: 10.1093/database/baw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karolchik D. The UCSC table browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Z.-L., Park C.A., Reecy J.M. Building a livestock genetic and genomic information knowledgebase through integrative developments of animal QTLdb and CorrDB. Nucleic Acids Res. 2019;47:D701–D710. doi: 10.1093/nar/gky1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schurink A., Wolc A., Ducro B.J., Frankena K., Garrick D.J., Dekkers J.C.M., Van Arendonk J.A.M. Genome-wide association study of insect bite hypersensitivity in two horse populations in The Netherlands. Genet. Sel. Evol. 2012;44:1. doi: 10.1186/1297-9686-44-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shrestha M., Solé M., Ducro B.J., Sundquist M., Thomas R., Schurink A., Eriksson S., Lindgren G. Genome-wide association study for insect bite hypersensitivity susceptibility in horses revealed novel associated loci on chromosome 1. J. Anim. Breed. Genet. 2020;137:223–233. doi: 10.1111/jbg.12436. [DOI] [PubMed] [Google Scholar]
- 51.Metzger J., Ohnesorge B., Distl O. Genome-wide linkage and association analysis identifies major gene loci for guttural pouch tympany in Arabian and German warmblood horses. PLoS ONE. 2012;7:e41640. doi: 10.1371/journal.pone.0041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haase B., Signer-Hasler H., Binns M.M., Obexer-Ruff G., Hauswirth R., Bellone R.R., Burger D., Rieder S., Wade C.M., Leeb T. Accumulating mutations in series of haplotypes at the KIT and MITF loci are major determinants of white markings in franches-montagnes horses. PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0075071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tetens J., Widmann P., Kühn C., Thaller G. A genome-wide association study indicates LCORL/NCAPG as a candidate locus for withers height in german warmblood horses. Anim. Genet. 2013;44:467–471. doi: 10.1111/age.12031. [DOI] [PubMed] [Google Scholar]
- 54.Thomer A., Gottschalk M., Christmann A., Naccache F., Jung K., Hewicker-Trautwein M., Distl O., Metzger J. An epistatic effect of KRT25 on SP6 is involved in curly coat in horses. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-24865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makvandi-Nejad S., Hoffman G.E., Allen J.J., Chu E., Gu E., Chandler A.M., Loredo A.I., Bellone R.R., Mezey J.G., Brooks S.A., et al. Four loci explain 83% of size variation in the horse. PLoS ONE. 2012;7:1–6. doi: 10.1371/journal.pone.0039929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skujina I., Winton C.L., Hegarty M.J., McMahon R., Nash D.M., Davies Morel M.C.G., McEwan N.R. Detecting genetic regions associated with height in the native ponies of the British Isles by using high density SNP genotyping. Genome. 2018;61:767–770. doi: 10.1139/gen-2018-0006. [DOI] [PubMed] [Google Scholar]
- 57.Vostrá-Vydrová H., Vostrý L., Hofmanová B., Krupa E., Zavadilová L. Pedigree analysis of the endangered old kladruber horse population. Livest. Sci. 2016;185:17–23. doi: 10.1016/j.livsci.2016.01.001. [DOI] [Google Scholar]
- 58.Fabbri M.C., de Rezende M.P.G., Dadousis C., Biffani S., Negrini R., Carneiro P.L.S., Bozzi R. Population structure and genetic diversity of Italian beef breeds as a tool for planning conservation and selection strategies. Animals. 2019;9:880. doi: 10.3390/ani9110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mariani E., Summer A., Ablondi M., Sabbioni A. Genetic variability and management in nero di parma swine breed to preserve local diversity. Animals. 2020;10:538. doi: 10.3390/ani10030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giontella A., Pieramati C., Silvestrelli M., Sarti F.M. Analysis of founders and performance test effects on an autochthonous horse population through pedigree analysis: Structure, genetic variability and inbreeding. Animal. 2018;13:1–10. doi: 10.1017/S1751731118001180. [DOI] [PubMed] [Google Scholar]
- 61.Flury C., Tapio M., Sonstegard T., Drögemüller C., Leeb T., Simianer H., Hanotte O., Rieder S. Effective population size of an indigenous Swiss cattle breed estimated from linkage disequilibrium. J. Anim. Breed. Genet. 2010;127:339–347. doi: 10.1111/j.1439-0388.2010.00862.x. [DOI] [PubMed] [Google Scholar]
- 62.Goyache F., Álvarez I., Fernández I., Pérez-Pardal L., Royo L.J., Lorenzo L. Usefulness of molecular-based methods for estimating effective population size in livestock assessed using data from the endangered black-coated Asturcón pony. J. Anim. Sci. 2011;89:1251–1259. doi: 10.2527/jas.2010-3620. [DOI] [PubMed] [Google Scholar]
- 63.Sadeghi R., Moradi-Shahrbabak M., Ashtiani S.R.M., Schlamp F., Cosgrove E.J., Antczak D.F. Genetic diversity of Persian arabian horses and their relationship to other native iranian horse breeds. J. Hered. 2019;110:173–182. doi: 10.1093/jhered/esy061. [DOI] [PubMed] [Google Scholar]
- 64.Druml T., Curik I., Baumung R., Aberle K., Distl O., Sölkner J. Individual-based assessment of population structure and admixture in Austrian, Croatian and German draught horses. Heredity. 2007;98:114–122. doi: 10.1038/sj.hdy.6800910. [DOI] [PubMed] [Google Scholar]
- 65.Gutiérrez J.P., Cervantes I., Molina A., Valera M., Goyache F. Individual increase in inbreeding allows estimating effective sizes from pedigrees. Genet. Sel. Evol. 2008;40:359–378. doi: 10.1186/1297-9686-40-4-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutiérrez J.P., Cervantes I., Goyache F. Improving the estimation of realized effective population sizes in farm animals. J. Anim. Breed. Genet. 2009;126:327–332. doi: 10.1111/j.1439-0388.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 67.Kamiński S., Hering D.M., Jaworski Z., Zabolewicz T., Ruść A. Assessment of genomic inbreeding in Polish konik horses. Pol. J. Vet. Sci. 2017;20:603–605. doi: 10.1515/pjvs-2017-0074. [DOI] [PubMed] [Google Scholar]
- 68.Schurink A., Shrestha M., Eriksson S., Bosse M., Bovenhuis H., Back W., Johansson A.M., Ducro B.J. The genomic makeup of nine horse populations sampled in The Netherlands. Genes. 2019;10:480. doi: 10.3390/genes10060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Velie B.D., Solé M., Fegraeus K.J., Rosengren M.K., Røed K.H., Ihler C.F., Strand E., Lindgren G. Genomic measures of inbreeding in the Norwegian-Swedish coldblooded trotter and their associations with known QTL for reproduction and health traits. Genet. Sel. Evol. 2019;51:1–10. doi: 10.1186/s12711-019-0465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solé M., Gori A.S., Faux P., Bertrand A., Farnir F., Gautier M., Druet T. Age-based partitioning of individual genomic inbreeding levels in Belgian blue cattle. Genet. Sel. Evol. 2017;49:1–18. doi: 10.1186/s12711-017-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pemberton T.J., Absher D., Feldman M.W., Myers R.M., Rosenberg N.A., Li J.Z. Genomic patterns of homozygosity in worldwide human populations. Am. J. Hum. Genet. 2012;91:275–292. doi: 10.1016/j.ajhg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takasuga A. PLAG1 and NCAPG-LCORL in livestock. Anim. Sci. J. 2016;87:159–167. doi: 10.1111/asj.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen L.H., Estrada K., Lettre G., Berndt S.I., Weedon M.N., Rivadeneira F., Willer C.J., Jackson A.U., Vedantam S., Raychaudhuri S., et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gudbjartsson D.F., Walters G.B., Thorleifsson G., Stefansson H., Halldorsson B.V., Zusmanovich P., Sulem P., Thorlacius S., Gylfason A., Steinberg S., et al. Many sequence variants affecting diversity of adult human height. Nat. Genet. 2008;40:609–615. doi: 10.1038/ng.122. [DOI] [PubMed] [Google Scholar]
- 75.Weedon M.N., Lango H., Lindgren C.M., Wallace C., Evans D.M., Mangino M., Freathy R.M., Perry J.R.B., Stevens S., Hall A.S., et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat. Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flori L., Fritz S., Jaffrézic F., Boussaha M., Gut I., Heath S., Foulley J.-L., Gautier M. The genome response to artificial selection: A case study in dairy cattle. PLoS ONE. 2009;4:e6595. doi: 10.1371/journal.pone.0006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberlein A., Takasuga A., Setoguchi K., Pfuhl R., Flisikowski K., Fries R., Klopp N., Fürbass R., Weikard R., Kühn C. Dissection of genetic factors modulating fetal growth in cattle indicates a substantial role of the non-SMC condensin I complex, subunit G (NCAPG) gene. Genetics. 2009;183:951–964. doi: 10.1534/genetics.109.106476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loughran G., Huigsloot M., Kiely P.A., Smith L.M., Floyd S., Ayllon V., O’Connor R. Gene expression profiles in cells transformed by overexpression of the IGF-I receptor. Oncogene. 2005;24:6185–6193. doi: 10.1038/sj.onc.1208772. [DOI] [PubMed] [Google Scholar]
- 79.Peeters L.M., Janssens S., Coussé A., Buys N. Insect bite hypersensitivity in Belgian warmblood horses: Prevalence and risk factors. Vlaams Diergeneeskd. Tijdschr. 2014;83:240–249. [Google Scholar]
- 80.Hauswirth R., Haase B., Blatter M., Brooks S.A., Burger D., Drögemüller C., Gerber V., Henke D., Janda J., Jude R., et al. Mutations in MITF and PAX3 cause “splashed white” and other white spotting phenotypes in horses. PLoS Genet. 2012;8:e1002653. doi: 10.1371/journal.pgen.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rieder S., Taourit S., Mariat D., Langlois B., Guérin G. Mutations in the agouti (ASIP), the extension (MCIR), and the brown (TYRP1) loci and their association to coat color phenotypes in horses (Equus caballus) Mamm. Genome. 2001;12:450–455. doi: 10.1007/s003350020017. [DOI] [PubMed] [Google Scholar]