Abstract
Simple Summary
Salmonella is an important foodborne pathogen that represents a very critical threat to poultry industry worldwide. This study concerns an important aspect of human food and health problem by treating a common zoonotic bacterial disease in poultry industry. Owing to the increased resistance to antibiotics among Salmonella enterica serotypes, we aimed to explore the beneficial effects of different probiotics strains as alternative sources of protection against infection in broiler chickens. Three probiotic strains Lactobacillus (Lacticaseibacillus) casei ATTC334, Bifidobacterium breve JCM1192 and Bifidobacterium infantis BL2416) improved body weight gain and prevented the deleterious effects and mortality induced by Salmonella infection in chicks through different mechanisms, including competitive exclusion and the promotion of cytokines’ release.
Abstract
Chicken Salmonella enterica serovars are enteric bacteria associated with massive public health risks and economic losses. There is a widespread antimicrobial resistance among S. enterica serotypes, and innovative solutions to antibiotic resistance are needed. We aimed to use probiotics to reduce antibiotic resistance and identify the major probiotic players that modify the early interactions between S. enterica and host cells. One-day-old cobb broiler chicks were challenged with S. typhimurium after oral inoculation with different probiotic strains for 3 days. The adherence of different probiotic strains to Caco-2 intestinal epithelial cells was studied in vitro. Lactobacillus (Lacticaseibacillus) casei ATTC334 and Bifidobacterium breve JCM1192 strains attached to Caco-2 cells stronger than B. infantis BL2416. L. casei ATTC334 and B. breve JCM1192 reduced S. typhimurium recovery from the cecal tonsils by competitive exclusion mechanism. Although B. infantis BL2416 bound poorly to Caco-2 epithelial cells, it reduced S. typhimurium recovery and increased IFN-γ and TNF-α production. L. casei ATTC334, B. breve JCM1192 and B. infantis BL2416 improved body weight gain and the food conversion rate in S. typhimurium-infected broilers. B. longum Ncc2785 neither attached to epithelial cells nor induced IFN-γ and TNF-α release and consequently did not prevent S. typhimurium colonization in broiler chickens. In conclusion, probiotics prevented the intestinal colonization of S. typhimurium in infected chickens by competitive exclusion or cytokine production mechanisms.
Keywords: Salmonella typhimurium, Bifidobacteria, Lactobacilli, probiotics, antibiotic alternatives, broiler chickens
1. Introduction
Salmonella is one of most important pathogenic bacteria implicated in foodborne bacterial outbreak throughout the world. In the last two decades, Salmonella represented the major causative agent of foodborne gastroenteritis [1,2]. Salmonella threaten poultry welfare and exert a substantial economic burden owing to their ability to migrate to systemic sites, causing fowl typhoid and paratyphoid. S. typhimurium has been documented to be the major cause of avian salmonellosis in broiler chickens [3]. The levels of Salmonella infection in poultry in Egypt are amongst the highest in the world [4]. The outbreaks of human salmonellosis are originating from key food production animals. This food associated zoonosis is linked with a wide variety of foods of animal origin; however, the main source of human infection is poultry meat and eggs [5,6].
Host-bacterial interactions have a profound influence on health and disease. The chicken gastrointestinal tract (GIT) is inhabited by multiple commensal bacteria that aid in the digestion of food and production of vitamins and short-chain fatty acids that play vital roles in poultry physiology and disease [7,8,9]. The GIT pathogens such as S. enterica measure carbon sources as an important signal to regulate the expression of its virulence genes. Salmonella senses whether it is in a gluconeogenic versus a glycolytic environment, as well as fluctuations in sugar levels to fine tuned regulation of its virulence repertoire [10]. A number of studies have defined that the use of probiotics was associated with resistance to a very wide range of infections [11,12], in particular, systemic salmonellosis in poultry [13,14]. These probiotics oppose Salmonella colonization by reducing the pH by production of short-chain fatty acids to a level that is not suitable for Salmonella survival [7]. Furthermore, probiotic bacteria can interfere with the gene expression pathways of pathogenic bacteria, rendering the pathogen unable to colonize and cause disease [15]. These probiotics also manipulate rapid immune mechanisms by intestinal cells to produce antimicrobial peptides that lead to more effective control strategies to reduce the impact of Salmonella infection [16]. The antimicrobial peptides kill bacteria by forming pores in the membrane; however, these peptides play a key role in immune response through the recruitment of neutrophils to sites of infection [17]. In addition, the presence of these probiotics as feed supplements for poultry can promote immunomodulatory activities, mitigate lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α factor (LITAF), interleukin (IL)-1β, IL-6 and IL-12, and increase the IL-10 during a challenge with Salmonella. This innate immune response links to and directs the adaptive immune system, and thus translates into more efficacious immunomodulatory mechanisms that reduce the colonization of Salmonella [13,18].
Antibiotic resistance is a major problem in the treatment of bacterial infections. Salmonella is becoming resistant to several antibiotics that lead to treatment failure [19]. In addition to improvements in biosecurity and husbandry practices, using probiotic bacteria as an immune-modulator and competitor with pathogenic bacteria for colonization sites is another important procedure to reduce the prevalence of foodborne pathogens, like Salmonella, in the reservoir species especially broiler chicken [20], thereby limiting their entry to the human food chain. In this regard, we aimed to develop innovative interventions that could serve as effective antibiotic alternatives, especially for treatment of salmonellosis in chicken farms. We studied the adherence of different Lacticaseibacillus (Lactobacillus) and Bifidobacterium strains to Caco-2 intestinal epithelial cells and evaluated their beneficial effects in S. typhimurium-infected broiler chickens.
2. Materials and Methods
2.1. S. typhimurium Culture and Determination of Colony Forming Unit (CFU)
A primary poultry isolate of S. typhimurium was used for these experiments as described previously [4]. S. typhimurium was incubated at 37 °C for 24 h and passed every 12 h. The bacterial cells were washed three times in sterile saline by centrifugation at 4000 rpm. The culture was subjected to 10-fold serial dilution in phosphate-buffered saline (PBS) and concentrations of S. typhimurium were determined by spread-plating each dilution on XLD agar (Oxoid, Basingstoke, UK).
2.2. Probiotics Culture
Lacticaseibacillus casei ATTC334, Bifidobacterium breve JCM1192, B. longum Ncc2785 and B. infantis BL2416 were provided by Professor Tohru Suzuki (Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan). The probiotic isolates were cultured in MRS broth medium and then diluted in reconstituted powdered skimmed milk to an expected concentration of 108 CFU/mL. Actual CFUs administered per chick from each experiment were determined retrospectively by serially diluting the overnight culture of the probiotic strains, and plating onto MRS agar (BD Diagnostics, Franklin Lakes, NJ, USA) to determine the number of bacteria in culture as CFU.
2.3. Adhesion of Probiotic Strains to Caco-2 Cells
Caco-2 intestinal epithelial cells (American Type Culture Collection) were seeded for 2 weeks on minimum essential medium (MEM; Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 1% glutamine and 1% penicillin/streptomycin (100 U/mL) at 37 °C in 5% CO2 and moisture to allow cellular differentiation. Caco-2 cells were then seeded onto 20 mm coverslip coated with mouse collagen IV (Trevigen, Gaithersburg, MA, USA) in 12-well plate at 105 cells per well 24 h before being challenged with the bacteria. The adhesion of probiotics strains to Caco-2 cells was evaluated as previously described [21]. Briefly, the stationary phase lactic acid bacteria (LAB) strains grown in MRS were diluted in MEM to an optical density of 0.9 at 600 nm. An aliquot of this culture was used to challenge a confluent Caco-2 intestinal epithelial cells at a multiplicity of infection of ~100 bacteria to epithelial cell in triplicate. The binding studies were performed at 37 °C, 5% CO2 and 80% humidity for 1 h. For direct visualization of adherent bacteria, the cells were fixed in 99.8% methanol for 10 min at room temperature then stained with Giemsa stain (Muto Pure Chemicals, Tokyo, Japan) at 1:10 dilution for 20 min. A total of 100 cells were examined under the light microscope and the number of adhered bacteria to each cell was counted in 20 randomly selected fields.
2.4. Birds and Housing
One hundred and twenty apparently healthy, one-day-old Cobb broiler chicks were enrolled in the experiment. The chicks were housed in six separate pens with sawdust bedded floors. Each pen was equipped with a feeder, drinker and brooder. The house was equipped with fans, air suction and a thermometer to control temperature and humidity. The experiment was conducted at the Poultry Research Unit and approved by the Animal Care and Use Committee of Faculty of Veterinary Medicine, Kafrelsheikh University (Egypt).
The birds were fed ad libitum and feed intake was measured daily at 08:00 h in triplicates as feed offered refusals. The birds were blocked by body weight, with each block assigned randomly to one of the six treatments. Starter, grower and finisher diets were formulated according to nutrients specification of Cobb broiler requirements (Table 1). Birds were fed starter diet from 1–21 days, grower diet from 22–28 days, and finisher diet from 29–35 days.
Table 1.
Ingredients, composition and nutrient content of starter, grower and finisher diets.
| Ingredients (%) | Starter | Grower | Finisher |
|---|---|---|---|
| Corn grains | 56.9 | 61.6 | 66.4 |
| Soybean meal 48% | 34.31 | 29.71 | 24.6 |
| Corn gluten meal 60% | 3.5 | 3 | 3 |
| Soybean oil | 1.5 | 2 | 2.71 |
| Dicalcium phosphate | 1.6 | 1.37 | 1.27 |
| Limestone | 1.05 | 1.11 | 1 |
| Salt | 0.22 | 0.24 | 0.25 |
| Sodium bicarbonate | 0.32 | 0.37 | 0.24 |
| Lysine hydrochloride | 0.2 | 0.2 | 0.15 |
| D.L Methionine | 0.1 | 0.1 | 0.08 |
| Premix | 0.3 | 0.3 | 0.3 |
| Nutrients content | |||
| Metabolizable energy (K Cal/kg) | 3050 | 3120 | 3150 |
| Crude protein % | 23.12 | 21.02 | 19.01 |
| Crude fat % | 4.01 | 4.8 | 5.5 |
| Ash | 6.1 | 5.5 | 5.0 |
| Acid detergent fiber % | 4.51 | 4.34 | 4.3 |
| Calcium % | 0.97 | 0.92 | 0.86 |
| Available phosphorus % | 0.45 | 0.4 | 0.38 |
Premix supplies vitamin A 12,000 Iu, vitamin D 5000 Iu, vitamin E 50 mg, vitamin K3 3 mg, vitamin B1 3 mg, vitamin B2 8 mg, nicotinic acid 30 mg, pantothenic acid 15 mg, vitamin B6 4 mg, vitamin B12 0.016 mg, folic acid 2 mg, biotin 0.2 mg, manganese 120 mg, zinc 100 mg, iron 40 mg, copper 16 mg, iodine 1.25 mg, selenium 0.3 mg) per 1 kg diet. The diet composition was calculated according to the National Research Council (NRC) 1994 feed composition table [22].
2.5. Experimental Design
The experiment was designed according to the method described by Akbari et al. [18], with some modifications. One-day-old broiler chicks were obtained from a local Salmonella-free hatchery. Immediately after arrival, the chicks and their boxes were sampled for the presence of Salmonella. The birds were divided randomly into six groups (n = 20) as described in Table 2. Half of the birds of each group were scarified the next day of S. typhimurium challenge and the others continued to be treated with probiotics every day until the end of the experiment to check the effect of probiotics on body weight gain and food conversion ratio (FCR). The birds were inoculated orally with 1 mL containing 2 × 109 CFU from each candidate of probiotic bacteria via oral gavage for 3 days and then challenged with 1 mL containing 108 CFU wild-type strain of S. typhimurium via oral gavage. Chicks were scarified 24 h after challenge with S. typhimurium and cecal tonsils were aseptically removed and placed into sterile tubes containing selenite-F broth. The samples were incubated overnight at 37 °C and streaked on XLD agar, incubated for 16 h at 37 °C and the presence or absence of S. typhimurium colonies was documented. The cecum from each chicken was removed aseptically and the contents were homogenized and diluted with sterile PBS (10% w/v) and the spread plated onto XLD plates to enumerate S. typhimurium. The plates were incubated at 37 °C for 16 h, and the CFU of S. typhimurium per cecal pair were determined.
Table 2.
The group of chicks treated S. typhimurium and different probiotic strains.
| Groups | Treatment |
|---|---|
| Control | Inoculated orally with saline. |
| S. typhimurium | Inoculated orally with S. typhimurium. |
| B. breve JCM1192 + S. typhimurium | Inoculated orally with B. breve JCM1192 and S. typhimurium. |
| L. casei ATTC334 + S. typhimurium | Inoculated orally with L. casei ATTC334 and S. typhimurium. |
| B. longum Ncc2785 + S. typhimurium | Inoculated orally with B. longum Ncc2785 and S. typhimurium. |
| B infantis BL2416 + S. typhimurium | Inoculated orally with B infantis BL2416 and S. typhimurium. |
To investigate the clinical signs and lesions, the birds were observed daily from the start of challenge to the end of the experiment.
2.6. Determination of Serum IFN-γ and TNF-α
Blood samples were collected, and serum was separated for the assay of IFN-γ and TNF-α levels using specific ELISA kits supplied by MyBioSource (San Diego, CA, USA).
2.7. Statistical Analysis
The results were presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA followed by Tukey’s test on GraphPad Prism 7 and differences were indicated to be statistically significant at p < 0.05.
3. Results
3.1. LAB-Epithelial Adherence
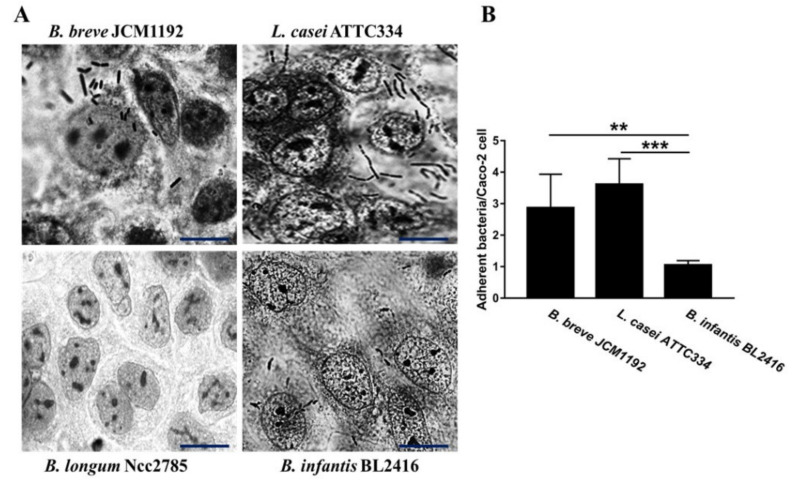
To examine whether there are differences in the interaction of LAB with Caco-2 epithelial cells, a panel of Bifidobacteria spp. and L. casei ATTC334 strains were used in the adherence assays. Using previously established criteria to define adherence phenotypes [21], L. casei ATTC334, B. breve JCM1192 and B. infantis BL2416 exhibited significant adherence, whereas B. longum Ncc2785 did not attach to Caco-2 cells (Figure 1). Quantitative analysis of the bacteria adherent demonstrated that L. casei ATTC334 and B. breve JCM1192 strains adheres significantly to Caco-2 cells relative to B. infantis BL2416 (Figure 1).
Figure 1.
Comparison of different probiotics interactions with Caco-2. (A) Images acquired using a phase contrast microscopy showing the adherence of bacterial strains to Caco-2 cells. Confluent monolayers of the Caco-2 cells were challenged with B. breve JCM1192, L. casei ATTC334, B. longum Ncc2785 or B. infantis BL2416 strains at a multiplicity of infection of ~100 (bacteria to epithelial cell) for 60 min. The cells were washed 3 times with PBS, fixed with methanol and stained with Giemsa stain. [Scale bar = 20 µm] (B) A total of 100 cells were examined under the light microscope and the number of bacteria adhered to each cell were counted in 20 randomly selected fields. The number of attached B. breve JCM1192 B. L. casei ATTC334, infantis BL2416 cells per Caco-2 cell were 2.9 ± 1.1, 3.6 ± 0.8 and 1.05 ± 0.1 (nucleus ± SD) respectively; but no adherent bacteria were observed in B. longum Ncc2785. Data are represented as mean ± SD. ** p < 0.01 and *** p < 0.001. The experiment was repeated three times (n = 3).
To investigate whether the binding of the probiotic strains to epithelial cells in vitro could affect Salmonella infection in vivo through their competition for binding sites and interaction with host immune cells, chicks were inoculated orally with the four different strains of probiotic bacteria for 3 days before challenge with wild type S. typhimurium. The results indicated that each probiotic strain which has the ability to bind to Caco-2 epithelial cells in vitro was sufficient to cause a significant reduction in S. typhimurium colonization to cecal tonsils with L. casei ATTC334 and B. breve JCM1192 strains being the most effective (Table 3). Treatment of the broiler chicks with B. breve JCM1192, L. casei ATTC334 or B. infantis BL2416 before S. typhimurium challenge significantly reduced cecal tonsils colonization by 20%, 10% and 30%, respectively. In addition, B. breve JCM1192, L. casei ATTC334 or B. infantis BL2416 resulted in a significant reduction in S. typhimurium-cecal recovery. The results showed 26.4 ± 8.067 (×104), 17.18 ± 3.45 (×104) and 22.61 ± 6.65 (×104) S. typhimurium-cecal recovery in chicks treated with B. breve JCM1192, L. casei ATTC334 or B. infantis BL2416, respectively, when compared with the control (230.0 ± 4.14 (×104)) and B. longum Ncc2785-treated (179.03 ± 7.81 (×104)) groups as outlined in Table 3.
Table 3.
S. typhimurium recovered from the cecal tonsils and cecal contents of chicks treated with different probiotic strains.
| Group | S. typhimurium-Positive Cecal Tonsils/Total Cecal Tonsils (%) | S. typhimurium-Cecal Recovery (×104) |
|---|---|---|
| S. typhimurium | 10/10 (100%) | 230.0 ± 4.14 |
| B. breve JCM1192 + S. typhimurium | 2/10 (20%) * | 26.4 ± 8.067 *** |
| L. casei ATTC334 + S. typhimurium | 1/10 (10%) * | 17.18 ± 3.45 *** |
| B. longum Ncc2785 + S. typhimurium | 9/10 (90%) | 179.03 ± 7.81 |
| B infantis BL2416 + S. typhimurium | 3/10 (30%) | 22.61 ± 6.65 *** |
Data are mean ± SD. * p < 0.05 and *** p < 0.001 versus S. typhimurium.
3.2. Clinical Signs and Postmortem (PM) Lesions
Birds infected with S. typhimurium alone or with B. longum Ncc2785 showed variable clinical signs including inappetence, depression, ruffled feathers and pasty diarrhea started at the 3rd day post-challenge. The mortality rate was 30% in the control positive group and 20% in the group treated with B. longum Ncc2785. In the PM lesions in the birds in the above two groups, the liver was enlarged and friable with observed necrotic foci and the gall bladder was distended. There were also lesion of pericarditis and splenomegaly. All the deaths occur from day 3 to 7 after challenge with S. typhimurium. Birds treated with B. breve JCM1192, L. casei ATTC334 or B. infantis BL2416 then infected with S. typhimurium did nt exhibit clinical signs or mortality.
3.3. Probiotics Improve Growth Performance in S. typhimurium-Infected Broiler Chickens
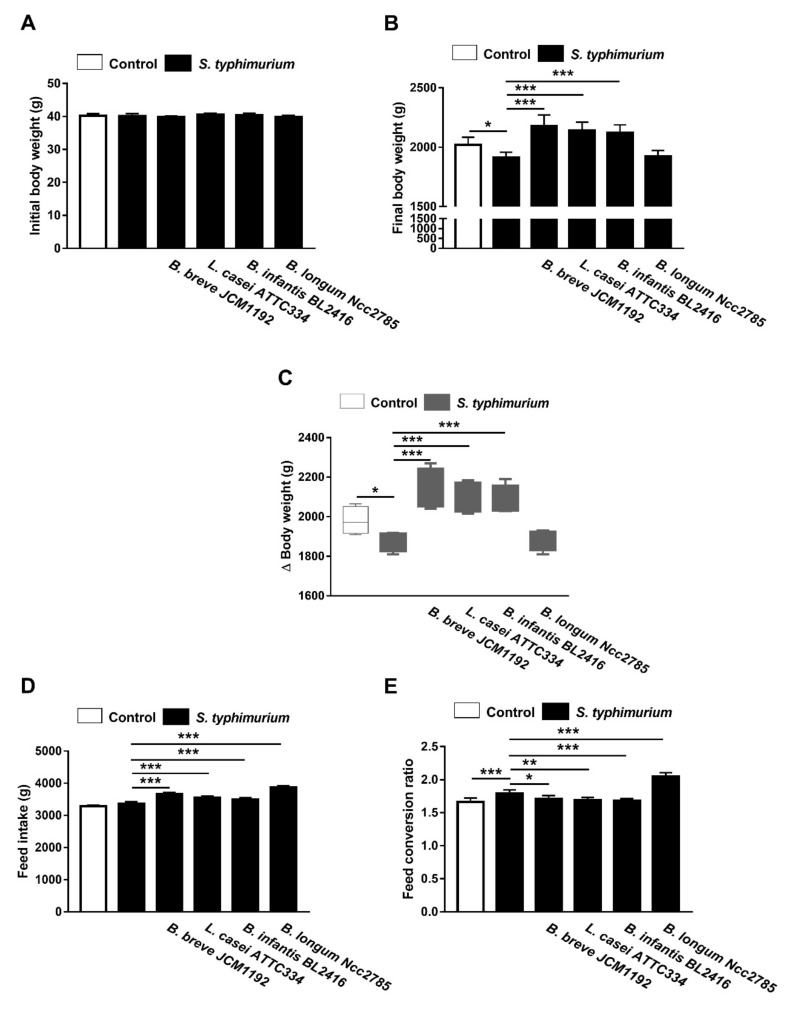
The results of growth performance are shown in Figure 2. Chicks infected with S. typhimurium showed a decrease in final body weight at day 35 (5.2%) and body weight gain (BWG; 5.3%) as compared to the control. Oral supplementation of B. longum Ncc2785 failed to improve the growth rate of the control chicks and was nearly similar to the infected non-treated chicks. Chicks treated with L. casei ATTC334, B. breve JCM1192 or B. infantis BL2416 had a significantly higher growth rate than the control chicks (Figure 2).
Figure 2.
Effect of Bifidobacteria and Lacticaseibacillus probiotics on growth performance of broiler chickens challenged with S. typhimurium. Probiotics improved body weight gain (A–C), feed intake (D) and feed conservation ratio (E). Data are mean ± SD, (n = 10). * p < 0.05, ** p < 0.01 and *** p < 0.001.
S. typhimurium showed that infected chicks had poor FCR (7.83%) compared to the control chicks, although there was non-significant difference in feed intake. Treatment with B. longum Ncc2785 offended FCR (23.49%) compared to the control chicks. On the other hand, treatment with L. casei ATTC334, B. breve JCM1192 or B. infantis BL2416 achieved similar FCR when compared to the control chicks (Figure 2).
3.4. Effect of Probiotics on Serum TNF-α and IFN-γ Levels in and S. typhimurium-Infected Broiler Chickens
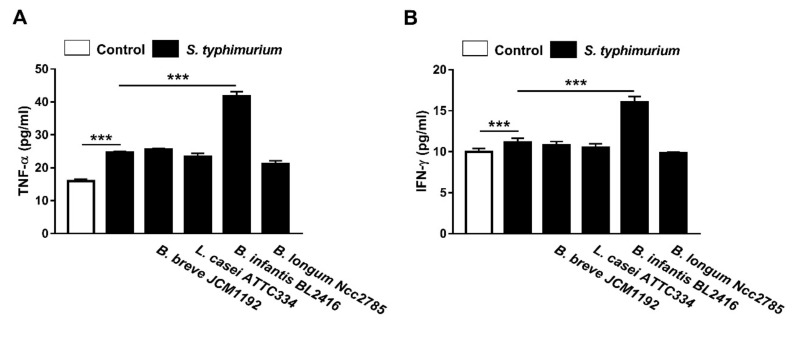
We investigated whether the exposure to probiotics and S. typhimurium triggers cytokines secretions in the peripheral blood of challenge broiler chickens. Serum TNF-α (Figure 3A) and IFN-γ (Figure 3B) levels were investigated by ELISA. TNF-α was increased significantly in the serum of S. typhimurium-infected chicks when compared with the control group. Although B. breve JCM1192, L. casei ATTC334 and B. longum Ncc2785 strains produced non-significant changes, B. infantis BL2416 significantly increased serum TNF-α in S. typhimurium-infected chicks (Figure 3A).
Figure 3.
Effect of oral administration of different probiotics on (A) TNF-α and (B) IFN-γ levels in the peripheral blood of broiler chickens. Data are mean ± SD, (n = 10). *** p < 0.001.
Similarly, serum IFN-γ was significantly increased in S. typhimurium-infected chicks received B. infantis BL2416 (Figure 3B), whereas other strains did not exert significant effects.
4. Discussion
The ability of bacteria to bind to intestinal epithelial cells has been considered as one of the most important selection properties for probiotic strains [23,24]. It is apparent that probiotics bacterial adhesions to the intestinal epithelial cells facilitates their attachments during intestinal colonization by avoiding bacterial removal by peristalsis and providing a competitive exclusion advantage, supporting its role in the ecosystem as an anti-infection strategy [25,26]. Our results indicated that L. casei ATTC334 and B. breve JCM1192 strains exhibited significant adherence when compared with B. infantis BL2416 and B. longum Ncc2785. Therefore, these strains may be associated with a greater capacity to be used as a probiotic. The attachment of probiotic to epithelial cells is a process that involves manipulation of the pathogenic bacterial infection [27]. Our results suggest that a reduction in S. typhimurium colonization to cecal tonsils with L. casei ATTC334 and B. breve JCM1192 strains were due to their ability to attach to epithelial cells, as shown by the in vitro study. B. longum Ncc2785 strain was not able to attach to Caco-2 cells in vitro, which could be explained in terms of the composition of surface polysaccharide that can prevent adhesion by inhibiting the expression of some bacterial factors, such as fimbriae that aid bacterial adherence [21].
Previous studies have shown that cytokines are implicated in the clearance of Salmonella from the gut [28,29]. IFN-γ is a major factor that has been implicated in many of the intestinal and systemic immunological consequences [30,31]. Its induced increase in gut motility during an infection is likely to enhance the clearing and fecal shedding of pathogens [32]. Given that enhanced phagocytosis could arise through increased production of IFN-γ [33], we examined the effect of all probiotic strains used in this study on serum IFN-γ. The results indicated that only B. infantis BL2416 was able to increase IFN-γ secretion in chicks. This probiotic strain reduced the recovery rate of S. typhimurium from cecal tonsils that may occur as a result of synergy between the probiotics and host macrophages. These interactions and the colonization of probiotics to the host epithelial cells caused a trend towards further reduction rates of Salmonella due to competition for the colonization sites. Similar results have been reported in previous studies [34,35,36,37].
Moreover, B. infantis BL2416 was the only probiotic strain that increased serum TNF-α in S. typhimurium-induced chicks. TNF-α is a major factor of inflammation in birds and is produced by monocytes, tissue macrophages, enterocytes, and other cells [32]. Owing to its ability to recruit and activate immune cells, TNF-α is decisive for early inflammatory responses that help in clearing infection [38]. Additionally, synergy between the activities of IFN-γ and TNF-α promotes clearing of Salmonella from broilers gut [13,38]. Although B. infantis BL2416 attached to host cells in lower rate than L. casei ATTC334 and B. breve JCM1192 strains, it promoted IFN-γ and TNF-α production which may contribute to the clearance of infection. Our study demonstrated that the probiotic strain that did not completely clear S. typhimurium infection still appeared to significantly lower its counts in the cecal contents. The ability of B. infantis BL2416 to increase the immune factors might be mediated via toll-like receptor-regulated signaling pathways that play a role in early events of the innate immunity and modulate pathogen-induced inflammation [39]. In this context, B. infantis has been reported to increase the production of Th1-related cytokines, including IFN-γ in mice [40]. However, the exact mechanism needs further studies to be experimentally addressed.
The clinical signs (inappetence, depression, ruffled feathers, and pasty diarrhea) and mortalities observed in birds infected with S. typhimurium alone those treated B. longum Ncc2785 were consistent with the findings of previous studies [3,4]. All the deaths occurred from day 3 to 7 post challenge with S. typhimurium. Birds treated with B. breve JCM1192, L. casei ATTC334 or B. infantis BL2416 then infected with S. typhimurium did not exhibit clinical signs or mortality due to the competitive exclusion by B. breve JCM1192 and L. casei ATTC334 [41] or the increased secretion of IFN-γ and TNFα by B. infantis BL2416 [38]. Accordingly, previous studies have shown that treatment with probiotics in case of Salmonella infection has improved broiler chickens’ body weight gain and FCR [14,42]. Our results also indicated that the treatment of S. typhimurium-infected birds with L. casei ATTC334, B. breve JCM1192 or B. infantis BL2416, respectively, improved body weight gain and FCR when compared with B. longum Ncc2785 or the non-treated infected birds.
5. Conclusions
Our results identified the set of probiotic bacteria that are likely to contribute to the poultry health and participate in modifying the key immune response in birds. Pre-treatment of the chicks with B. breve JCM1192, L. casei ATTC334 and B. infantis BL2416 prevented the deleterious effects of acquired Salmonella infection. These three probiotics showed an ability to bind to intestinal cells in vitro, and reduced S. typhimurium recovery from the cecal tonsils in vivo. In addition, probiotics improved body weight gain and FCR, and prevented all clinical signs and mortality in S. typhimurium-infected chicks. The results suggest that competitive exclusion is the mechanism underlying the protective effect of B. breve and L. casei and the reduced recovery of S. typhimurium from cecal tonsils, whereas B. infantis BL2416 exerted its protective effects via promoting IFN-γ and TNF-α production.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding this research through the Fast-track Research Funding Program.
Author Contributions
Conceptualization, A.T. and H.E.-S., T.S.; methodology H.E.-S., A.T., E.N., A.M.R., M.S. and A.M.M.; software, H.E.-S., A.T., A.M.M. and W.E.; validation, H.E.-S., A.T. and A.M.M.; formal analysis, H.E.-S., A.T., M.B.-J., W.E. and A.M.M.; investigation, A.T., H.E.-S., T.S. and A.M.M.; resources, A.T., A.M.M., M.O.G. and M.B.-J.; funding acquisition; M.B.-J.; data curation, H.E.-S.; writing—original draft preparation, H.E.-S., A.T.; writing—review and editing, H.E.-S., A.T., M.O.G., A.M.M., F.F., A.M.R., T.S.; visualization, H.E.-S., A.T., A.M.M.; supervision, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fantasia M., Filetici E. Salmonella enteritidis in Italy. Int. J. Food Microbiol. 1994;21:7–13. doi: 10.1016/0168-1605(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 2.Omwandho C.O.A., Kubota T. Salmonella enterica serovar enteritidis: A mini-review of contamination routes and limitations to effective control. Jpn. Agric. Res. Q. JARQ. 2010;44:7–16. doi: 10.6090/jarq.44.7. [DOI] [Google Scholar]
- 3.Padron M. Salmonella typhimurium outbreak in broiler chicken flocks in Mexico. Avian Dis. 1990;34:221–223. [PubMed] [Google Scholar]
- 4.El-Sharkawy H., Tahoun A., El-Gohary A.E.-G.A., El-Abasy M., El-Khayat F., Gillespie T., Kitade Y., Hafez H.M., Neubauer H., El-Adawy H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9:8. doi: 10.1186/s13099-017-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sean F.A., Nathan B., Amy C., Robert D., Alecia N., Wayne S., Robert U., Patricia W. Salmonella enteritidis in broiler chickens, United States, 2000–2005. Emerg. Infect. Dis. J. 2006;12:1848. doi: 10.3201/eid1212.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coble D.J., Sandford E.E., Ji T., Abernathy J., Fleming D., Zhao H., Lamont S.J. Impacts of Salmonella enteritidis infection on liver transcriptome in broilers. Genesis. 2013;51:357–364. doi: 10.1002/dvg.22351. [DOI] [PubMed] [Google Scholar]
- 7.Casas I.A., Dobrogosz W.J. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb. Ecol. Health Dis. 2000;12:247–285. [Google Scholar]
- 8.Spinler J.K., Taweechotipatr M., Rognerud C.L., Ou C.N., Tumwasorn S., Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008;14:166–171. doi: 10.1016/j.anaerobe.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talarico T.L., Dobrogosz W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob Agents Chemother. 1989;33:674–679. doi: 10.1128/AAC.33.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacheco A.R., Sperandio V. Enteric pathogens exploit the microbiota-generated nutritional environment of the gut. Microbiol. Spectr. 2015;3:279–296. doi: 10.1128/microbiolspec.MBP-0001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rostami F.M., Mousavi H., Mousavi M.R.N., Shahsafi M. Efficacy of probiotics in prevention and treatment of infectious diseases. Clin. Microbiol. Newsl. 2018;40:97–103. doi: 10.1016/j.clinmicnews.2018.06.001. [DOI] [Google Scholar]
- 12.Lebeer S., Bron P.A., Marco M.L., Van Pijkeren J.-P., O’Connell Motherway M., Hill C., Pot B., Roos S., Klaenhammer T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018;49:217–223. doi: 10.1016/j.copbio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Feng J., Wang L., Zhou L., Yang X., Zhao X. Using in vitro immunomodulatory properties of lactic acid bacteria for selection of probiotics against Salmonella infection in broiler chicks. PLoS ONE. 2016;11:e0147630. doi: 10.1371/journal.pone.0147630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Shall N.A., Awad A.M., El-Hack M.E.A., Naiel M.A.E., Othman S.I., Allam A.A., Sedeik M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals. 2019;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., Yuan Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:217. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazorla S.I., Maldonado-Galdeano C., Weill R., De Paula J., Perdigón G.D.V. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front. Microbiol. 2018;9:736. doi: 10.3389/fmicb.2018.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond G., Beckloff N., Weinberg A., Kisich K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari M.R., Haghighi H.R., Chambers J.R., Brisbin J., Read L.R., Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with salmonella enterica serovar typhimurium. Clin. Vaccine Immunol. CVI. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma M., Wang H., Yu Y., Zhang D., Liu S. Detection of antimicrobial resistance genes of pathogenic Salmonella from swine with DNA microarray. J. Vet. Diagn. Investig. 2007;19:161–167. doi: 10.1177/104063870701900204. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Higgins S.E., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Vicente J.L., Hargis B.M., Tellez G. Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella enteritidis in neonatal broilers. Poult. Sci. 2010;89:243–247. doi: 10.3382/ps.2009-00436. [DOI] [PubMed] [Google Scholar]
- 21.Tahoun A., Masutani H., El-Sharkawy H., Gillespie T., Honda R.P., Kuwata K., Inagaki M., Yabe T., Nomura I., Suzuki T. Capsular polysaccharide inhibits adhesion of Bifidobacterium longum 105-a to enterocyte-like caco-2 cells and phagocytosis by macrophages. Gut Pathog. 2017;9:27. doi: 10.1186/s13099-017-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Research Council . Nutrient Requirements of Poultry: Ninth Revised Edition. The National Academies Press; Washington, DC, USA: 1994. p. 176. [Google Scholar]
- 23.Salminen S., Laine M., Vonwright A., Vuopio-Varkila J., Korhonen T., Mattila-Sandholm T. Development of selection criteria for probiotic strains to assess their potential in functional foods: A Nordic and European approach. Biosci. Microflora. 1996;15:61–67. doi: 10.12938/bifidus1996.15.61. [DOI] [Google Scholar]
- 24.Jacobsen C.N., Rosenfeldt Nielsen V., Hayford A.E., Møller P.L., Michaelsen K.F., Paerregaard A., Sandström B., Tvede M., Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999;65:4949–4956. doi: 10.1128/AEM.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crociani J., Grill J.P., Huppert M., Ballongue J. Adhesion of different Bifidobacteria strains to human enterocyte-like caco-2 cells and comparison with in vivo study. Lett. Appl. Microbiol. 1995;21:146–148. doi: 10.1111/j.1472-765X.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 26.Alander M., Korpela R., Saxelin M., Vilpponen-Salmela T., Mattila-Sandholm T., Von Wright A. Recovery of Lactobacillus rhamnosus gg from human colonic biopsies. Lett. Appl. Microbiol. 1997;24:361–364. doi: 10.1046/j.1472-765X.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 27.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins. 2018;10:11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S., Beagley K.W., France M.P., Shen J., Husband A.J. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology. 2000;99:464–472. doi: 10.1046/j.1365-2567.2000.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raupach B., Kaufmann S.H. Bacterial virulence, proinflammatory cytokines and host immunity: How to choose the appropriate salmonella vaccine strain? Microbes Infect. 2001;3:1261–1269. doi: 10.1016/S1286-4579(01)01486-1. [DOI] [PubMed] [Google Scholar]
- 30.Held T.K., Weihua X., Yuan L., Kalvakolanu D.V., Cross A.S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 1999;67:206–212. doi: 10.1128/IAI.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibuki M., Kovacs-Nolan J., Fukui K., Kanatani H., Mine Y. Β 1-4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 2011;139:289–295. doi: 10.1016/j.vetimm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Fasina Y.O., Holt P.S., Moran E.T., Moore R.W., Conner D.E., McKee S.R. Intestinal cytokine response of commercial source broiler chicks to Salmonella typhimurium infection. Poult. Sci. 2008;87:1335–1346. doi: 10.3382/ps.2007-00526. [DOI] [PubMed] [Google Scholar]
- 33.Foster N., Hulme S.D., Barrow P.A. Induction of antimicrobial pathways during early-phase immune response to Salmonella spp. In murine macrophages: Gamma interferon (ifn-gamma) and upregulation of ifn-gamma receptor alpha expression are required for nadph phagocytic oxidase gp91-stimulated oxidative burst and control of virulent salmonella spp. Infect. Immun. 2003;71:4733–4741. doi: 10.1128/IAI.71.8.4733-4741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rantala M., Nurmi E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- 35.Blankenship L.C., Bailey J.S., Cox N.A., Stern N.J., Brewer R., Williams O. Two-step mucosal competitive exclusion flora treatment to diminish salmonellae in commercial broiler chickens. Poult. Sci. 1993;72:1667–1672. doi: 10.3382/ps.0721667. [DOI] [PubMed] [Google Scholar]
- 36.Corrier D.E., Hinton A., Jr., Ziprin R.L., Beier R.C., DeLoach J.R. Effect of dietary lactose on cecal ph, bacteriostatic volatile fatty acids, and Salmonella typhimurium colonization of broiler chicks. Avian Dis. 1990;34:617–625. doi: 10.2307/1591254. [DOI] [PubMed] [Google Scholar]
- 37.Schneitz C. Competitive exclusion in poultry––30 years of research. Food Control. 2005;16:657–667. doi: 10.1016/j.foodcont.2004.06.002. [DOI] [Google Scholar]
- 38.Eckmann L., Kagnoff M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–1200. doi: 10.1016/S1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- 39.Vanderpool C., Yan F., Polk D.B. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008;14:1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 40.Zuo L., Yuan K.T., Yu L., Meng Q.H., Chung P.C., Yang D.H. Bifidobacterium infantis attenuates colitis by regulating t cell subset responses. World J. Gastroenterol. 2014;20:18316–18329. doi: 10.3748/wjg.v20.i48.18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revolledo L., Ferreira C.S., Ferreira A.J. Prevention of Salmonella typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- 42.Abd El-Ghany A.W., El-Shafii S.A.S., Hatem M.E., Dawood E. A trial to prevent Salmonella enteritidis infection in broiler chickens using autogenous bacterin compared with probiotic preparation. J. Agric. Sci. 2012;4:91–108. doi: 10.5539/jas.v4n5p91. [DOI] [Google Scholar]