Abstract
PURPOSE:
To assess the relationship between depressive symptoms and self-perceived severity of autonomic dysfunction in patients with multiple system atrophy (MSA).
METHODS:
Cross-sectional evaluation of patients with MSA who underwent autonomic testing, Unified MSA Rating Scale (UMSARS)-1 and −2, rating of the presence and severity of depressive symptoms (Zung scale), quality of life (SF36), body vigilance, anxiety (Spielberg’s anxiety scale), severity of autonomic dysfunction with the Composite Autonomic Symptoms Score (COMPASS-31) and of orthostatic hypotension (OH) symptoms with the Orthostatic Hypotension Questionnaire (OHQ).
RESULTS:
Fifty-eight patients (32 women) with probable MSA (aged 61.8±8.6 years; disease duration: 4.3±2.1 years) were studied. Forty patients (69%) had symptoms of depression in the Zung scale. Age, disease duration, and motor disability were similar in those with and without symptoms of depression. Despite a similar orthostatic blood pressure fall, the severity of orthostatic symptoms was higher in patients with symptoms of depression (P=0.004). Depression scores were associated with higher burden of autonomic symptoms (R=0.401, P=0.02), specifically with the COMPASS-31 items related to orthostatic intolerance (R=0.337, P=0.045), and with the OHQ (R=0.529; P<0.001). A multivariable regression model including age, sex, UMSARS and drop in systolic blood pressure upon head-up tilt as covariates, showed that the burden of depressive symptoms was independently associated with the OHQ score : for every 1-unit increase in the Zung depression score, there was a 1.181-increase in the total OHQ score.
CONCLUSIONS:
In patients with MSA, depressive symptoms worsen the perceived severity of autonomic symptoms in general and orthostatic hypotension in particular. Our findings have implications for clinical trial design.
Keywords: Multiple system atrophy, Depression, Orthostatic hypotension, Non-motor symptoms, Symptomatic burden
INTRODUCTION
Multiple system atrophy (MSA) is a fatal, neurodegenerative synucleinopathy that progressively impairs motor and autonomic function. Patients are wheelchair-bound within 4 years of diagnosis and usually survive 8 years or fewer [5]. The prevalence of depression in patients with MSA is estimated as high as 80% [1, 2, 6, 8, 15, 17, 19] and is associated with worse overall health status and poorer quality of life [2, 8, 17]. Depression being so frequent, its symptoms could impact the self-perceived severity of autonomic symptoms, particularly orthostatic hypotension. Thus, we conducted a cross-sectional study to assess whether symptoms of depression had any relationship with the patients’ self-perceived severity of autonomic symptoms in a well-characterized cohort of patients with MSA.
We hypothesized that the affective, psychological, and somatic symptoms associated with depression would result in higher symptomatic burden of autonomic deficits, specifically of symptoms of orthostatic hypotension. This is an important consideration when using patient-reported outcomes in clinical trials.
METHODS
We recruited patients who fulfilled current consensus criteria for probable MSA [9] evaluated at the New York University (NYU) Dysautonomia Center between 2014 and 2017. All patients signed informed consent and the NYU School of Medicine Institutional Review Board approved the study. Assessments were performed at baseline on entry into the study.
Symptomatic assessment:
Overall clinical severity was assessed using the validated disease-specific Unified Multiple System Atrophy Rating Scale (UMSARS) administered by a neurologist trained in movement disorders. The first sub-score (UMSARS-1) measures activities of daily living and the second sub-score (UMSARS-2) measures motor impairment [20]. The presence and severity of depressive symptoms was assessed using the the Zung Self-Rating Depression Scale [3] and the prevalence of anxiety with the Spielberger’s State-Trait Anxiety Inventory, which evaluates both the state and trait characteristics of anxiety [14]. The Body Vigilance Scale was used to determine how much a focus the patients placed on their bodily sensations [16]. The Short Form Healthy Survey (SF36) was administered as a measure of quality of life, with domains to evaluate physical function, bodily pain, limitations due to physical health problems, limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions [4]. Symptoms of autonomic dysfunction were assessed using the Composite Autonomic Symptoms Score (COMPASS-31) [18]. To specifically assess the self-percieved symptomatic burden of orthostatic hypotension we used the Orthostatic Hypotension Questionnaire (OHQ) [12]. We used both sub-scales of the OHQ: the 6-item symptom assessment (OHSA) scale and the 4-item scale to assess the burden of OH symptoms on daily activities (OHDAS) [12]. Each individual item is rated on an 11-point scale, from 0 to 10, with 10 indicating the highest possible symptom burden or limitation. Thus, the maximum score is 60 in OHSA, 40 in OHDAS, and 100 in the total OHQ.
Autonomic function test:
All patients underwent baseline autonomic function tests including a passive 60-degree head-up-tilt test with continuous blood pressure (BP) and heart rate (HR) monitoring. Orthostatic hypotension was defined as a sustained drop in systolic blood pressure of at least 20 mmHg or a drop in diastolic blood pressure of at least 10 mmHg within 3 minutes of head-up tilt [7]. Sympathetic and parasympathetic cardiovascular reflexes were further assessed using a standardized Valsalva maneuver and deep-paced breathing, as described [10].
Statistical analysis:
Normality of the data was assessed using the Kolmogorov-Smirnov test. Patients were dichotomized into those with high and low burden of depressive symptoms , based on their Zung scale scores. A score of 50 or below was defined as low burden, whereas a score of 51 or above was defined as high burden of depressive symptoms. T-tests were applied between these two groups for continuous normally distributed variables. Simple linear regressions were used to evaluate the relationship between depression scores and OHQ scores, COMPASS-31, quality of life domains, autonomic testing scores, and UMSARS scores. A multiple linear regression model was used to determine if the total OHQ scores (i.e., the burden of symptoms of orthostatic hypotension) depends on the Zung depression symptoms core, including age, sex, UMSARS-1, UMSARS-2 and the reduction in systolic blood pressure after 3-min of head-up tilt as covariates. Statistical analyses were performed with SPSS version 18.0 (SPSS Inc. Chicago IL USA). Significance was set at P<0.05. Data are expressed in mean ± standard deviation unless otherwise specified.
RESULTS
Patient characteristics
Fifty-eight patients with probable MSA were included. There were 26 men, 32 women; mean age 61.8±8.6 years; mean disease duration 4.3±2.1 years; mean age at disease onset 54.6±13.4 years. Sixty percent (35 patients, 11 men and 24 women) were classified as having the cerebellar-predominant phenotype (MSA-C) and 40% (23 patients 15 men and 8 women) the parkinsonian-predominant phenotype (MSA-P). Patients with both phenotypes had similar quality of life, motor disability, autonomic symptoms, and autonomic function test results (Table 1).
Table 1.
Baseline characteristics of patients with multiple system atrophy according to their predominant motor phenotype (MSA-C vs. MSA-P)
| Variable | MSA-C (n=35) | MSA-P (n=23) | P-value |
|---|---|---|---|
| Age, years | 61.7±7.7 | 61.9±10.1 | 0.95 |
| Sex | 7M/17F | 13M/8F | 0.027* |
| Disease duration, years | 4.3±2.0 | 4.6±2.3 | 0.58 |
| Age at disease onset, years | 52.3±15.6 | 57.3±10.1 | 0.32 |
| UMSARS-1 (activities of daily living) | 18.9±7.8 | 20.0±9.7 | 0.65 |
| UMSARS-2 (neurological exam) | 20.8±9.25 | 22.3±9.7 | 0.57 |
| Zung depression score | 57.1±9.6 | 55.2±9.7 | 0.46 |
| Anxiety score (state) | 43.6±11.3 | 43.3±13.8 | 0.94 |
| Anxiety score (trait) | 43.7±11.9 | 40.2±11.5 | 0.35 |
| Body vigilance score | 18.6±8.3 | 18.1±9.3 | 0.85 |
| Orthostatic Hypotension Questionnaire (OHQ) | 40.7±21.4 | 45.1±19.6 | 0.51 |
| COMPASS-31 | 56.4±37.6 | 70.7±13.5 | 0.15 |
| Systolic blood pressure supine, mmHg | 149.9±27.3 | 159.5±27.7 | 0.21 |
| Diastolic blood pressure supine, mmHg | 83.0±15.5 | 84.2±13.7 | 0.76 |
| Heart rate supine, bpm | 78.9±10.7 | 77.7±14.5 | 0.72 |
| Systolic blood pressure 3-min head-up tilt, mmHg | 121.3±26.9 | 119.0±19.4 | 0.74 |
| Diastolic blood pressure 3-min head-up tilt, mmHg | 68.9±17.3 | 63.3±12.9 | 0.22 |
| Heart rate head-up after 3-min head-up tilt, bpm | 87.3±16.0 | 83.8±15.9 | 0.45 |
| Drop (Δ) in systolic blood pressure at 3-min head-up tilt, mmHg | 30±23 | 38±29 | 0.23 |
| Valsalva ratio | 1.20±0.18 | 1.20±0.15 | 0.59 |
| E:I ratio | 1.07±0.05 | 1.07±0.05 | 0.53 |
Asterisks denote statistical significance.
Symptoms of depression
Forty patients (69%, 27 with MSA-C and 13 with MSA-P) scored above 50 on the Zung scale, indicating depressive symptoms. Of these, 48% (19) had mild, 40% (16) had moderate and 12% (5) had severe depressive symptoms. Symptoms of depression occurred more often in patients with MSA-C (77%; 27 of 35) than in those with MSA-P (57%; 13 of 23), but this was not significant (χ2=2.76; P=0.10). Men and women had similar prevalence and severity distribution of depressive symptoms. Patients with higher depression scores had lower physical (R=−0.610, P=0.02) and social functioning (R=−0.861, P=0.001) as measured on the SF-36 quality-of-life questionnaire. The magnitude of self-perceived depression (Zung score) was not associated with the duration of disease (R=0.40; P=0.37).
At the time of assessment, 33% of the patients (19 of 58) were on antidepressant medication, including selective serotonin reuptake inhibitors in 12, serotonin and norepinephrine reuptake inhibitors in 3, bupropion in 3, and amitriptyline in 1. Despite treatment, 13 of these 19 patients still scored as having depressive symptoms on the Zung scale. Sixty seven percent of the patients (27 of 40) who scored as being depressed were not being treated for their depression at the time of assessments.
Patients with and without symptoms of depression
The age, disease duration, age at disease onset and severity of motor impairment (UMSARS-2) were similar in those with symptoms of depression vs. those without (Table 2). Patients with symptoms of depression had higher body vigilance scores, suggesting they were more focused on their symptoms (20.0±8.3 vs. non-depressed patients 14.2±8.3; P=0.029). Anxiety scores (both state and trait) were also significantly higher in patients with symptoms of depression.
Table 2.
Patients’ characteristics according to their depression status
| High burden of depressive symptoms (n=40) |
Low budern of depressive symptoms (n=18) |
P-value | |
|---|---|---|---|
| Age, years | 62.4±8.6 | 60.6±8.8 | 0.48 |
| Sex | 23M/17F | 9M/9F | 0.56 |
| Disease duration, years | 4.6±2.2 | 4.1±2.1 | 0.45 |
| Age at disease onset, years | 57.6±8.4 | 49.5±18.5 | 0.11 |
| UMSARS-1 (activities of daily living) | 21.0±8.8 | 15.5±6.2 | 0.012* |
| UMSARS-2 (neurological exam) | 21.8±10.0 | 20.1±7.9 | 0.55 |
| Zung depression score | 61.0±7.6 | 46.1±3.9 | 0.001* |
| Anxiety score (State) | 46.8±12.4 | 37±9.5 | 0.014* |
| Anxiety score (Trait) | 46.1±11.7 | 34.4±7.1 | 0.001* |
| Body vigilance score | 20.0±8.3 | 14.2±8.3 | 0.029* |
| OHQ total score | 47.2±17.3 | 26.5±23.4 | 0.004* |
| OHSA sub-score | 25.5±12.9 | 12.0±11.8 | 0.003* |
| COMPASS-31 | 68.3±28.5 | 52.5±28.5 | 0.088 |
| Systolic blood pressure supine, mmHg | 157.6±28.1 | 145.5±24.9 | 0.13 |
| Diastolic blood pressure supine, mmHg | 86.6±14.9 | 76.8±12.2 | 0.051 |
| Heart rate supine, bpm | 79.2±12.4 | 76.7±12.2 | 0.50 |
| Systolic blood pressure 3-min head-up tilt, mmHg | 121.3 ±27.1 | 118.3±16.1 | 0.61 |
| Diastolic blood pressure 3-min head-up tilt, mmHg | 67.7±17.3 | 64.7±12.4 | 0.53 |
| Heart rate 3-min head-up tilt, bpm | 87.0±16.8 | 83.4±13.8 | 0.45 |
| Drop (Δ) in systolic blood pressure at 3-min head-up tilt, mmHg | 27.6±32 | 37.2±23 | 0.23 |
| Valsalva ratio | 1.20±0.03 | 1.24±0.06 | 0.13 |
| E:I ratio | 1.08±0.02 | 1.08±0.01 | 0.87 |
| Plasma norepinephrine, pg/ml | 339±166# | 266±202 | 0.29 |
Asterisks denote statistical significance.
n=38 (two outliers with plasma norepinephrine > 1000 pg/ml were excluded).
Systolic blood pressures supine and standing were similar in both groups. The COMPASS-31 was numerically higher in patients with depressive symptoms, but the difference was not significant (P=0.08).
However, the UMSARS-1, which measures activities of daily living, and the OHQ, which measures the self-perceived symptomatic burden of the blood pressure fall, were both significantly higher in patients with depressive symptoms (P=0.004, Figure 1A).
Figure 1. Self-perceived severity of depressive symptoms and symptoms of orthostatic hypotension in multiple system atrophy.
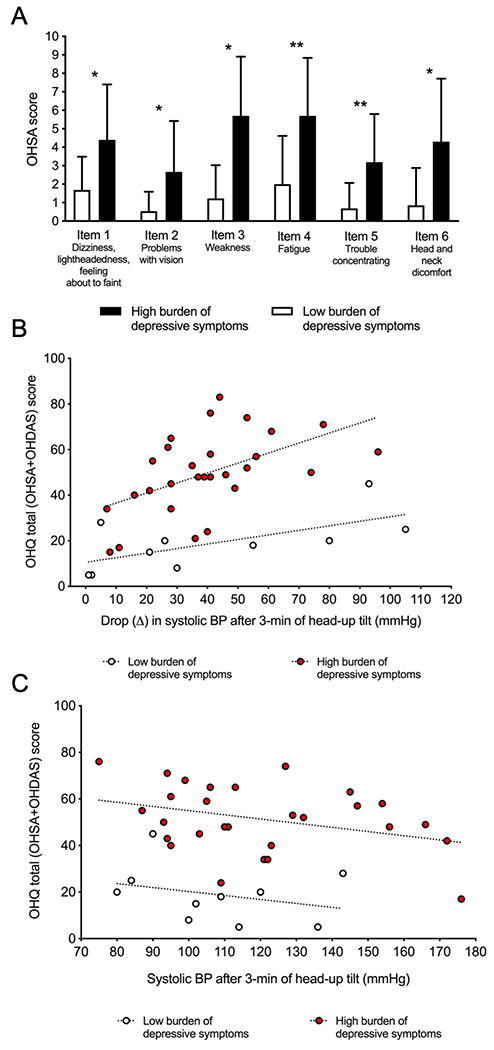
A. Orthostatic Hypotension Symptoms Assessment (OHSA) scores in depressed and non-depressed patients. Depressed patients had significantly more burden of orthostatic hypotension symptoms in each of the items of the OHSA. * P<0.05; **P<0.01. B. Symptomatic burden of orthostatic hypotension according to the reduction in systolic blood pressure after 3-minutes of head-up tilt. Even though the drop in systolic blood pressure was similar in both groups, the burden of orthostatic symptoms, as measured by the orthostatic hypotension questionnaire (OHQ) was higher in patients with depressive symptoms (red dots) compared to patients without depressive symptoms (white dots) C. Symptomatic burden of orthostatic hypotension according to systolic blood pressure after 3-mininutes of head-up tilt. Even though the systolic blood pressure after 3-minutes of head-up tilt was similar in both groups, the burden of orthostatic symptoms, as measured by the orthostatic hypotension questionnaire (OHQ) was higher in patients with depressive symptoms (red dots) compared to patients without depressive symptoms (white dots).
Depressive symptoms and orthostatic hypotension
The severity of depressive symptoms was correlated with the OHSA score, as well as with each individual item of this scale as well as with activities that require standing and walking for a long time on the OHDAS, and with the OHQ total score, i.e., the sum of both sub-scales (Figure 2 and Supplementary Table 1). Depressive symptoms scores were also associated with higher burden of autonomic symptoms as measured by the COMPASS-31 scale (R=0.401, P=0.02). When each of the specific domains of the COMPASS-31 were analyzed, only the items related to symptoms of orthostatic hypotension were significantly associated with depressive symptoms (R=0.337, P=0.045), with the items related to bladder dysfunction being close to significance (R=0.321, P=0.057).
Figure 2. Association between depressive symptoms and symptoms of orthostatic hypotension in multiple system atrophy.
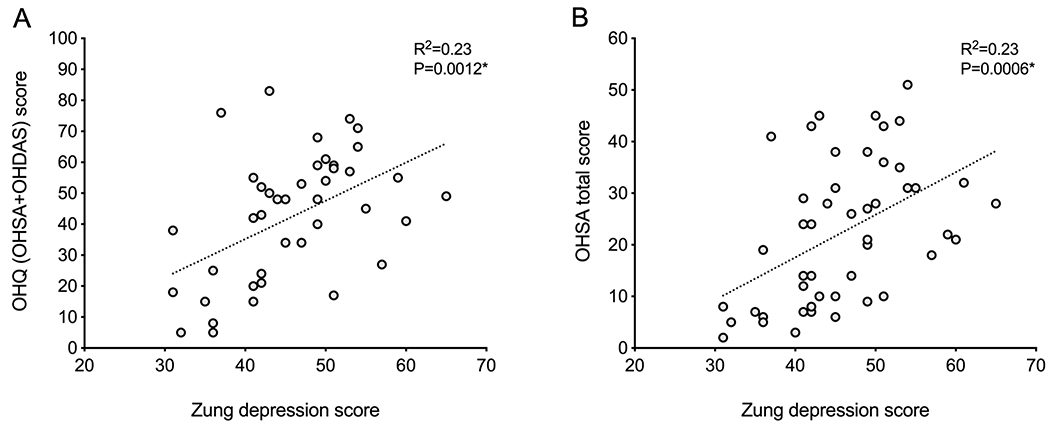
A. Higher Orthostatic Hypotension Questionnaire (OHQ) total scores were associated with higher Zung self-reported depressive symptom scores. B. Higher Orthostatic Hypotension Symptoms Assessment (OHSA) scores were associated with higher Zung self-reported depressive symptom scores.
Because the average reported severity of orthostatic hypotension symptoms was higher in patients with more burden of depressive symptoms, but orthostatic blood pressure falls and blood pressure on standing were similar (Table 2), we analyzed whether patients with depressive symptoms reported more severe orthostatic symptoms with similar fall in blood pressure. Indeed, as shown in Figure 1B, a simple regression analysis between the OHQ score and the drop (Δ) of the systolic blood pressure from supine to 3-minutes of head-up tilt in each patient showed that, for the same magnitude of blood pressure fall, patients with depression had higher OHQ score compared to patients without depression. Equivalent findings were obtained when regressing the OHQ score with the systolic blood pressure after 3-min of head-up tilt (Figure 1C), indicating that, in spite of a similar magnitude in their blood pressure fall, patients with symptoms of depression rated their orthostatic symptoms worse than non-depressed patients.
To confirm this, we used a multiple regression model with the total OHQ score as dependent variable and the Zung depression score, reduction in systolic blood pressure from supine to 3-minutes of head-up tilt, age, sex and the UMSARS-1 and UMSARS-2 scores at baseline as independent variables. This model explained 58% of the variance in the dependent variable (R2=0.583; P<0.0001), and disclosed that the total OHQ was dependent on the magnitude of the drop in blood pressure on head-tilt (as expected; P=0.008, beta=0.28 indicating that for every unit that systolic blood pressure decreases upon standing, there is a 0.28-increase in the OHQ score) and also on the Zung depression score (P=0.004, beta=1.181, indicating that, for every unit that the Zung depression score increases, there is a 1.181-increase in the total OHQ). The other independent variables entered in the model (sex, age, and UMSARS) were not predictive of the total OHQ score.
DISCUSSION
Our main finding is that symptoms of depression in patients with MSA are independently associated with higher self-perceived severity of autonomic symptoms, particularly orthostatic hypotension. Compared to those with low burden of depressive symptoms in the Zung scale, patients with high burden of depressive symptoms reported significantly more severe orthostatic symptoms for the same magnitude of blood pressure fall and similar blood pressure level when standing.
Symptoms of orthostatic hypotension and autonomic dysfunction, as measured by validated self-reported scales (OHQ and COMPASS-31), worsened with increasing depression scores, but there were no differences in hemodynamic parameters, strongly suggesting that patients with depression rate similar symptoms worse. This was confirmed by a multivariable regression model including age, sex, UMSARS and drop in systolic blood pressure upon head-up tilt as covariates, showing that the burden of orthostatic hypotension symptoms (i.e., the OHQ score) was independently associated with the burden of depressive symptoms: for every 1-unit increase in the Zung depression score, there was a 1.181-increase in the total OHQ score.
In this study, two thirds of patients with MSA had symptoms of depression as measured by the Zung Self-Rating Depression Scale. Published studies in patients with MSA showed a prevalence of depression as low as 15% to as high as 85%. The 69% prevalence of depression in our study and the lack of differences in depression or anxiety scores between parkinsonian and cerebellar phenotypes are all in agreement with previous reports [2, 17, 19, 21]. Depressive symptoms in patients with MSA may be a surrogate marker of a widespread involvement of neurodegeneration affecting neuronal circuits involving emotional functioning, such as the prefrontal cortex [11]. In our study, most patients who had a high burden of depressive symptoms were not receiving antidepressants. However, one third of the patients who were on antidepressant medications still scored as having a high burden of depressive symptoms. Recognizing and successfully treating depression should improve quality of life.
Patient-reported outcomes such as the OHQ are typically used as primary endpoint in clinical trials for orthostatic hypotension [13]. Therefore, the finding that symptoms of depression influence the patient’s subjective assessment of the severity of autonomic symptoms has implications for clinical trial design.
Supplementary Material
Acknowledgments
Funded by: National Institutes of Health (U54NS065736) and Familial Dysautonomia Foundation
Conflict of interests
J.M: Consulting fees from Theravance Biopharma. Funding from the NIH, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and FDA.
J.A.P.: Funding from the NIH, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and FDA. He is an advisory Board Member for Lundbeck, Biogen; he is the managing editor of Clinical Autonomic Research.
LNK: J.M: Consulting fees from Theravance Biopharma. Funding from the NIH, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and FDA.
A.G.: No conflict of interests.
H.K.: Funding from the NIH, Michael J. Fox Foundation, MSA Coalition, Familial Dysautonomia Foundation, and FDA. Advisory Board Member for Lunbeck, Biogen, Theravance, Biohaven, Eli Lilly, Pfizer, and AstraZeneca; Editor-in-Chief of Clinical Autonomic Research.
REFERENCESUncategorized References
- 1.Balas M, Balash Y, Giladi N, Gurevich T (2010) Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm 117:369–375 [DOI] [PubMed] [Google Scholar]
- 2.Benrud-Larson LM, Sandroni P, Schrag A, Low PA (2005) Depressive symptoms and life satisfaction in patients with multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society 20:951–957 [DOI] [PubMed] [Google Scholar]
- 3.Biggs JT, Wylie LT, Ziegler VE (1978) Validity of the Zung Self-rating Depression Scale. The British journal of psychiatry : the journal of mental science 132:381–385 [DOI] [PubMed] [Google Scholar]
- 4.Bowling A, Bond M, Jenkinson C, Lamping DL (1999) Short Form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. Journal of public health medicine 21:255–270 [DOI] [PubMed] [Google Scholar]
- 5.Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372:249–263 [DOI] [PubMed] [Google Scholar]
- 6.Fetoni V, Soliveri P, Monza D, Testa D, Girotti F (1999) Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry 66:541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 21:69–72 [DOI] [PubMed] [Google Scholar]
- 8.Gill CE, Khurana RK, Hibler RJ (1999) Occurrence of depressive symptoms in Shy-Drager syndrome. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 9:1–4 [DOI] [PubMed] [Google Scholar]
- 9.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein DS, Cheshire WP, Jr. (2017) Beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 27:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herting B, Beuthien-Baumann B, Pottrich K, Donix M, Triemer A, Lampe JB, von Kummer R, Herholz K, Reichmann H, Holthoff VA (2007) Prefrontal cortex dysfunction and depression in atypical parkinsonian syndromes. Movement disorders : official journal of the Movement Disorder Society 22:490–497 [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R (2012) The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 22:79–90 [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann H, Norcliffe-Kaufmann L, Palma JA (2015) Droxidopa in neurogenic orthostatic hypotension. Expert Rev Cardiovasc Ther 13:875–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kvaal K, Laake K, Engedal K (2001) Psychometric properties of the state part of the Spielberger State-Trait Anxiety Inventory (STAI) in geriatric patients. International journal of geriatric psychiatry 16:980–986 [DOI] [PubMed] [Google Scholar]
- 15.Pilo L, Ring H, Quinn N, Trimble M (1996) Depression in multiple system atrophy and in idiopathic Parkinson’s disease: a pilot comparative study. Biol Psychiatry 39:803–807 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt NB, Lerew DR, Trakowski JH (1997) Body vigilance in panic disorder: evaluating attention to bodily perturbations. Journal of consulting and clinical psychology 65:214–220 [DOI] [PubMed] [Google Scholar]
- 17.Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C, Litvan I, Lang AE, Bower JH, Burn DJ, Low P, Jahanshahi M (2010) A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society 25:1077–1081 [DOI] [PubMed] [Google Scholar]
- 18.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W (2012) COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 87:1196–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tison F, Yekhlef F, Chrysostome V (2006) Depression and self-reported depressive symptoms in multiple system atrophy compared to Parkinson’s disease. Mov Disord 21:1056–1057 [DOI] [PubMed] [Google Scholar]
- 20.Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, Ghorayeb I, Ory F, Galitzky M, Scaravilli T, Bozi M, Colosimo C, Gilman S, Shults CW, Quinn NP, Rascol O, Poewe W, Multiple System Atrophy Study G (2004) Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Movement disorders : official journal of the Movement Disorder Society 19:1391–1402 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LY, Cao B, Zou YT, Wei QQ, Ou RW, Zhao B, Wu Y, Shang HF (2018) Depression and anxiety in multiple system atrophy. Acta Neurologica Scandinavica 137:33–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


