Abstract
Background:
Findings on prenatal polyunsaturated fatty acid (PUFA) intake and child wheeze and asthma have been inconsistent.
Objective:
We examined associations between prenatal PUFA status and child wheeze/asthma and modifying effects of maternal asthma/atopy, child sex and maternal race.
Methods:
Analyses included 1019 mother–child dyads with n-3 and n-6 PUFAs measured in 2nd trimester plasma; n-6/n-3 ratios were calculated. Child wheeze/asthma outcomes ascertained at age 4–6 years included: ever physician-diagnosed asthma, current wheeze (symptoms past 12 months), current asthma (diagnosis and medication and/or symptoms past 12 months) and current diagnosed asthma. Each PUFA indicator and outcome was analyzed in separate models using modified Poisson regression with interaction terms.
Results:
In quartile (Q) analyses, higher n-6 PUFAs were associated with increased risk of ever (risk ratio [RR] high v. low [RR Q4 v.Q1]: 1.70; 95% CI: 1.07, 2.71) and current (RR Q4 v. Q1: 1.70; 95% CI: 1.07, 2.71) diagnosed asthma while n-3 PUFAs were associated with lower risk (RR Q4 v. Q1 0.59; 95% CI: 0.33, 1.03) of current diagnosed asthma (ptrend<0.05 for all). Higher n-6 PUFAs were associated with higher risk of all respiratory outcomes among children born to women with asthma (pinteraction<0.05 for all outcomes). A significant three-way interaction between child sex, maternal asthma and n-6/n-3 PUFA –indicated that male children born to women with asthma and a higher ratio had the highest risk across wheeze/asthma outcomes (pinteraction<0.05).
Conclusion:
Associations between prenatal PUFA status and childhood wheeze/asthma were modified by maternal history of asthma and child sex.
Keywords: polyunsaturated fatty acid, childhood asthma, sex-specific effects, prenatal
Capsule summary:
Children born to women with a history of asthma and higher prenatal n-6 PUFAs were at increased risk of wheeze/asthma, particularly boys.
Introduction
Wheezing in early life is associated with significant morbidity and healthcare costs1 and subsequent asthma development.2 Increasing prevalence of asthma and allergic disease has been linked to Western lifestyle changes, including a shift in dietary intake.3 The decrease in consumption of n-3 (omega-3) long-chain polyunsaturated fatty acids (PUFAs), abundant in oily fish, and the concomitant increase in consumption of mostly plant based n-6 (omega-6) PUFAs, has been a particular research focus. Arachidonic acid (AA), one of the n-6 derived PUFAs, is a precursor to important pro-inflammatory markers including leukotrienes, eicosanoids and prostaglandins that can promote Th2 differentiation and IgE production, immune features correlated with particular asthma phenotypes.4 Conversely, n-3 PUFAs may have anti-inflammatory properties that counteract the effects of n-6 PUFAs by competing for incorporation into cells and inhibiting the synthesis of pro-inflammatory AA.5, 6
Research has linked maternal intakes of PUFAs during pregnancy to the development of allergic and/or respiratory disorders. Most birth cohort studies examining the association between n-3 PUFA intake and offspring wheeze have relied on food frequency questionnaires to assess usual PUFA consumption, either by estimating intakes for specific PUFAs or relying on self-reported consumption of specific foods, like lean or fatty fish. These studies have reported protective7–10, null 11–13 and even detrimental associations between n-3 PUFA intake and wheeze in childhood.14 Similarly, prospective studies that have measured PUFA biomarkers in cord blood and maternal blood collected in pregnancy have yielded mixed results.15–21 In addition to these observational studies, randomized controlled trials in which women received n-3 PUFAs supplementation during pregnancy have also produced mixed results.22–27 A recent systematic review and meta-analysis of observational and trial data concluded that while findings have been inconsistent, they are suggestive that increased maternal consumption of n-3 PUFAs in pregnancy plays a beneficial role in prevention of childhood allergic disease.28 Some prospective birth cohorts have also shown associations between higher n-6 PUFA intake in pregnancy and higher risk of eczema 11, 18 and allergic rhinitis in childhood 12, while others did not find an increased risk of allergic disease in relationship to higher n-6 PUFA intakes.9, 16 The ratio of n-6 relative to n-3 PUFA has also been associated with increased risk of allergic rhinitis 12 but not asthma or wheeze16 in 2 different prospective studies.
Few investigations have assessed how factors such as a familial predisposition to asthma, race, or child sex may influence the association between prenatal PUFA status and child wheeze/asthma. Only one cross-sectional investigation of the relationship between n-3 and n-6 PUFA status and wheeze/asthma has been published within a racially-diverse population in the United States (U.S.).21 Sex differences in childhood asthma development are well documented 29 and previous work also demonstrates sexual dimorphism in fatty acid metabolism.30 The need to consider maternal history of atopic disease as an important effect modifier between the association of prenatal diet and child atopic disease has also been underscored. 31
We assessed the relationships between 2nd trimester n-3 and n-6 PUFAs and the n-6/n-3 ratio and wheeze/asthma outcomes at age 4–6 years in mother-child dyads enrolled in a primarily African-American, prenatal cohort- the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. We hypothesized that higher prenatal n-3 PUFAs would be associated with reduced risk of early childhood wheeze/asthma, while higher prenatal n-6 PUFAs and n-6/n-3 ratios would be associated with increased likelihood of wheeze and asthma. We also examined effect modification by sex, maternal race and maternal asthma.
Methods
Study Population
Pregnant women (n=1503) were recruited during the 2nd trimester of pregnancy from community obstetric practices and an obstetric clinic in Shelby County (Memphis), Tennessee from December 2006 to June 2011.32 The demographic characteristics of the CANDLE Study participants reflect the demographics of Shelby County, which is predominantly low-income and African American. Inclusion criteria included: pregnant women aged between 16 and 40 years old, residing in Shelby County, ability to speak and understand English, and 16–27 weeks of gestation with a singleton and low medical risk pregnancy. Low medical risk pregnancy was defined as participants who did not have the following: chronic hypertension requiring therapy or vascular disease requiring therapy; maternal red-cell alloimmunization except Rhesus (Rh) factor; hemoglobinopathy; insulindependent diabetes; appreciable renal or cardiopulmonary disease; prolapsed or ruptured membranes; oligohydramnios; complete placenta previa; endocrine disease; collagen disease (e.g., lupus erythematosus or scleroderma); active or chronic hepatitis; renal disease; pulmonary or heart disease requiring therapeutic medication or limitation of physical activity; major fetal anomaly (e.g., aneuploidy, major organ-system defect); and human immunodeficiency virus.33 The parent study procedures were approved by the Institutional Review Board (IRB) of the University of Tennessee Health Science Center (UTHSC) and this study was approved by the IRBs of UTHSC and Vanderbilt University (VU). Women aged 18 and older provided written informed consent and assent was given by those aged 16–17.9 years with consent provided by their legally authorized representative.
Prenatal PUFA Indicators
The primary exposures were n-3 and n-6 PUFAs measured in maternal plasma (n=1159) obtained in the 2nd trimester and the calculated n-6/n-3 ratios. Whole blood, collected from women with unknown fasting status, was centrifuged at 300 rpm for 10 minutes to separate plasma which was then stored in the UTHSC Department of Pathology until being sent in bulk to the VUMC Lipid Services Core where phospholipid PUFA levels were determined by standard techniques.34–36 In brief, lipids were extracted using the method of Folch-Lees.35 Extracts were filtered and lipids recovered in the chloroform phase. Individual lipid classes were separated by thin layer chromatography using Silica Gel 60 A plates developed in petroleum ether, ethyl ether, acetic acid (80:20:1) and visualized by rhodamine 6G. Phospholipids, diglycerides, triglycerides and cholesteryl esters were scraped from the plates and methylated using BF3 /methanol as previously described.34 Methylated fatty acids were extracted and analyzed by gas chromatography using an Agilent 7890A gas chromatograph equipped with flame ionization detectors, a capillary column (SP2380, 0.25 mm × 30 m, 0.25 µm film, Supelco, Bellefonte, PA). Fatty acid methyl esters are identified by comparing the retention times to those of known standards. Inclusion of lipid standards with odd chain fatty acids permitted quantitation of the amount of lipid in the sample. Dipentadecanoyl phosphatidylcholine (C15:0), diheptadecanoin (C17:0), trieicosenoin (C20:1), and cholesteryl eicosenoate (C20:1) were used as standards. Measurements for 10 PUFAs were available including 4 n-3 PUFAs and 6 n-6 PUFAs. All assayed n-3 and n-6 PUFAs were expressed as the percentage of total fatty acids and these percentages (%s) were summed to calculate the respective total n-3 and n-6 PUFA %s and used to compute the n-6/n-3 ratio. In quantifying lipid classes, the inter-assay coefficient of variation was less than 1.5%. The fatty acid percentage variation was less than 1%. The advantages of using the plasma phospholipid specimen pool include the stability of the sampling method and the minimal variability in relation to postprandial triacylglycerol levels.37
Child Respiratory Outcomes
Women were asked about their child’s respiratory health at the 4–6 year postnatal visit. Outcomes were assessed using standardized questionnaires based on the International Study of Asthma and Allergies in Children (ISAAC)38 and parent-report of provider diagnoses and child asthma-specific medication use.39 A hierarchy of four outcomes were considered in these analyses. Ever asthma was defined as an affirmative response to “Has a physician or other health care provider ever told your family that your child had asthma or reactive airway disease?” Current wheeze was defined as an affirmative response to “Has your child ever had wheezing or whistling in the chest in the last 12 months?” Report of current asthma was defined as an affirmative response to 2 out of 3 of the following questions: 1)“Has a physician or other health care provider ever told your family that your child had asthma or reactive airway disease?” 2) “Has your child ever had wheezing or whistling in the chest in the last 12 months?” and 3)”In the past 12 months, has your child used any type of medicines, liquids, puffers or other medication for wheezing or asthma?” Healthcare provider diagnosed current asthma was defined as an affirmative response to “Has a physician or other health care provider ever told your family that your child had asthma or reactive airway disease?” AND either report of wheezing OR asthma medication use in the past 12 months.
Covariates
Information on maternal age (continuous), race (Black or African American, White and Other), education (<high school, high school or GED and >high school), self-report of woman’s history of asthma, parity (primiparous or multiparous) and pre-pregnancy body mass index (BMI, continuous), calculated by dividing weight by height squared (kg/m2), were self-reported at enrollment. Child sex was obtained from a neonatal summary form at birth. Child’s BMI was measured at the 4–6 year visit. Data on women’s atopic disease status was also collected on the 4–6 year visit questionnaire and was defined as self-report of the woman ever having any one of the following: asthma, hay fever/allergic rhinitis or eczema/atopic dermatitis.
Statistical Analysis
Dyads with PUFAs, estimated gestational age ≥32 weeks, and complete outcome and covariate data from the 4–6 year visit (N=1019) were included in analyses. Modified Poisson regression40 was used in order to obtain risk ratios to assess the relationships between prenatal PUFA indicators and wheeze/asthma outcomes. In separate multivariable Poisson regression models, we investigated the association of total n-3 and n-6 PUFAs (continuous and categorical [quartiles]) predictors plus their ratio with wheeze/asthma outcomes. Tests of linear trend were conducted in final multivariable models by assigning the median value from each quartile of fatty acids and modeling this as a continuous variable. Models were adjusted for maternal age at enrollment, race, education, asthma history, parity, pre-pregnancy BMI and child sex and BMI at the 4–6 year visit. We then examined effect modification by maternal asthma at enrollment (yes/no), maternal atopic disease assessed at age 4–6 visit (yes/no), child sex (male/female), and maternal race (African American/white, excluding Other category) with PUFA status (as continuous variable) for child wheeze/asthma outcomes by including the relevant interaction terms. Finally, we considered 3-way interactions between these variables and the 3 PUFA predictor variables (continuous variable) (race × sex × PUFA; race × maternal asthma × PUFA; sex × asthma × PUFA). Analyses were performed in R Version 3.5.1 (Vienna, Austria) and SPSS version 24 (Chicago, IL) with statistical significance set at 0.05.
Results
Maternal and children’s characteristics are reported in Table 1 as number and percentage (n [%]) or mean and standard deviation (median [IQR]), as appropriate. The majority of women were African American (67.6%), approximately 60% of women had a high school education or less and approximately 12% reported a history of asthma at enrollment. By age 4–6 years, 14.4% of children were reported to have had healthcare provider-diagnosed asthma.
Table 1.
Characteristics of mother–child dyads in the CANDLE cohort
| n or median | % or interquartile range | |
|---|---|---|
| Maternal characteristics | ||
| Race (n,%) | ||
| White | 315 | 30.9 |
| Black | 689 | 67.6 |
| Other | 15 | 1.5 |
| Education (n,%) | ||
| <High School | 121 | 11.9 |
| High School or GED* | 484 | 47.5 |
| >High School | 414 | 40.6 |
| Smoking in pregnancy (n,%) | ||
| Yes | 92 | 9.0 |
| Parity (n,%) | ||
| Primiparous | 448 | 39.6 |
| Multiparous | 682 | 60.4 |
| Ever asthma at enrollment (n,%) | ||
| Yes | 118 | 11.6 |
| Atopic disease† (n,%) | ||
| Yes | 364 | 36 |
| Missing | 2 | |
| Age at enrollment (years) | ||
| Median, 25th–75th percentile | 26.0 | 22.0–31.0 |
| Pre-pregnancy BMI (kg/m2) | ||
| Median, 25th–75th percentile | 25.8 | 22.1–32.1 |
| Child characteristics | ||
| Male sex (n,%) | 508 | 49.9 |
| Birth weight (g) | ||
| Median, 25th–75th percentile | 3262 | 2970–3565 |
| Missing | 1 | |
| Age at follow-up (years) | ||
| Median, 25th–75th percentile | 4.17 | 4.05–4.51 |
| BMI at follow-up (kg/m2) | ||
| Median, 25th–75th percentile | 16.00 | 15.0–17.0 |
| Outcomes (n,%) | ||
| Ever diagnosis of asthma‡, yes | 147 | 14.4 |
| Current wheeze§, yes | 200 | 19.6 |
| Current asthma||, yes | 165 | 16.2 |
| Diagnosed current asthma#, yes | 121 | 11.9 |
GED=General Educational Development test
Defined as report of the mother having any one of the following conditions: asthma, hay fever/allergic rhinitis or eczema/atopic dermatitis
Report of physician/medical provider diagnosis of asthma ever
Caretaker report of wheeze or whistling of the chest in the past 12 months
At least 2 out of 3 of following: ever diagnosis of asthma, current wheeze and wheeze/asthma medication use in past 12 months
Report of ever diagnosed asthma AND wheeze and/or asthma/wheeze medication use in past 12 months
Table 2 summarizes the plasma PUFAs measured in mid-pregnancy as median (IQR), including the total n-3% and total n-6%, the specific PUFAs that contributed to the summed indicators, as well as the n-6/n-3 ratio. For n-3, docosahexanoic acid (DHA) was the predominant contributor to the sum and for the sum of n-6 PUFA, linoleic acid (LA) and AA were the predominant fatty acids. This table also summarizes the medians across quartiles for the total n-3 and total n-6 summed indicators and the n-6/n-3 ratio.
Table 2.
Maternal plasma polyunsaturated fatty acids in mid-pregnancy (n=1019)
| Weight %, Median | IQR | |
|---|---|---|
| Total n-3 PUFAs | 5.43 | 1.35 |
| n-3 PUFA Q1 | 4.36 | |
| n-3 PUFA Q2 | 5.03 | |
| n-3 PUFA Q3 | 5.63 | |
| n-3 PUFA Q4 | 6.72 | |
| α-Linoleic acid (ALA, C18:3n-3) | 0.40 | 0.30 |
| Eicosapentaenoic acid (EPA, C20:5n-3) | 0.28 | 0.16 |
| Clupanodonic/Docosapentaenoic acid (DPA, C22:5n-3) | 0.63 | 0.20 |
| Docosahexaenoic acid (DHA, C22:6n-3) | 3.96 | 1.22 |
| Total n-6 PUFAs | 41.1 | 2.28 |
| n-6 PUFA Q1 | 39.0 | |
| n-6 PUFA Q2 | 40.6 | |
| n-6 PUFA Q3 | 41.6 | |
| n-6 PUFA Q4 | 43.1 | |
| Linoleic acid (LA, C18:2n-6) | 23.4 | 3.27 |
| γ-Linolenic acid (GLA, C18:3n-6) | 0.00 | 0.00 |
| Dihomo- γ-linolenic acid (DGLA, C20:3n-6) | 3.34 | 0.96 |
| Arachidonic acid (AA, C20:4n-6) | 12.5 | 2.57 |
| Adrenic acid (C22:4n-6) | 0.57 | 0.18 |
| Osbond acid (C22:5n-6) | 1.05 | 0.51 |
| Total n-6/n-3 ratio | 7.74 | 2.02 |
| n-6/n-3 ratio Q1 | 5.99 | |
| n-6/n-3 ratio Q2 | 7.26 | |
| n-6/n-3 ratio Q3 | 8.20 | |
| n-6/n-3 ratio Q4 | 9.53 |
Main effects
Table 3 shows the linear associations between PUFAs (continuous) and each of the four respiratory outcomes in children with adjustment for covariates. Higher summed n-6 PUFAs were associated with higher risk of caregiver-report of ever asthma diagnosis at age 4–6 years (RR: 1.21, 95%CI: [1.01, 1.46] per an interquartile [IQR] increase). No other statistically significant associations were seen in analyses considering PUFAs as continuous indicators. We see similar results when adjusting for maternal atopic disease instead of maternal asthma (supplemental table E1).
Table 3.
Association of mid-pregnancy maternal plasma PUFAs (per IQR increase) with respiratory outcomes at age 4–6 years: Main Effect Models
| PUFA measure (IQR difference) | Outcome RR (95%CI) |
|||
|---|---|---|---|---|
| Ever asthma* | Current wheeze† | Current asthma‡ | Diagnosed current asthma§ | |
| n-3 PUFAs (1.35) |
0.88 (0.72, 1.08) |
0.96 (0.83, 1.12) |
0.91 (0.76, 1.09) |
0.83 (0.65, 1.05) |
| n-6 PUFAs (2.28) |
1.21 (1.01 1.46) |
0.95 (0.82, 1.11) |
1.00 (0.84, 1.18) |
1.12 (0.91, 1.37) |
| n-6/n-3 PUFA ratio (2.02) |
1.14 (0.94, 1.37) |
0.97 (0.83, 1.13) |
1.05 (0.88, 1.24) |
1.17 (0.95, 1.43) |
Modified Poisson models adjusted for child sex, mother’s educational level at enrollment, mother’s asthma ever, parity, mother’s age at enrollment, child’s BMI at age 4–6 year visit, mother’s pre-pregnancy BMI and mother’s race. Effect sizes shown per an interquartile range (IQR) increase in PUFA measure.
Report of physician/medical provider diagnosis of asthma ever
Caretaker report of wheeze or whistling of the chest in the past 12 months
At least 2 out of 3 of following: ever diagnosis of asthma, current wheeze and wheeze/asthma medication use in past 12 months
Report of ever diagnosed asthma AND wheeze and/or asthma/wheeze medication use in past 12 months
Next, we conducted analyses with the exposures characterized as quartiles. When compared to the first (lowest) quartile of total n6 PUFAs, the third (RR Q3 v. Q1: 1.83; 95% CI:1.16, 2.91) and fourth quartiles (RR Q4 v. Q1: 1.70; 95% CI: 1.07, 2.71) were associated with higher risk of ever asthma in children at age 4–6 years (Figure 1A). When compared to the first quartile of exposure, the third quartile of total n-6 PUFAs was also associated with higher risk of diagnosed and current asthma; however, the confidence limits for the comparison of the fourth and first quartiles crossed 1.0 (Figure 1B, C)). We observed linear trends for increasing total n-6 PUFAs and higher risk of ever asthma and diagnosed current asthma (p<0.05 for both). When considering total prenatal n-3 PUFAs, increasing quartiles of exposure were associated with decreased risk of respiratory outcomes with a significant trend for diagnosed current asthma (RR Q4 v. Q1 0.59; 95% CI: 0.33, 1.03, p-trend=0.04).
Figure 1 legend. Association of mid-pregnancy maternal plasma polyunsaturated fatty acids in quartiles with respiratory outcomes at age 4–6 years.
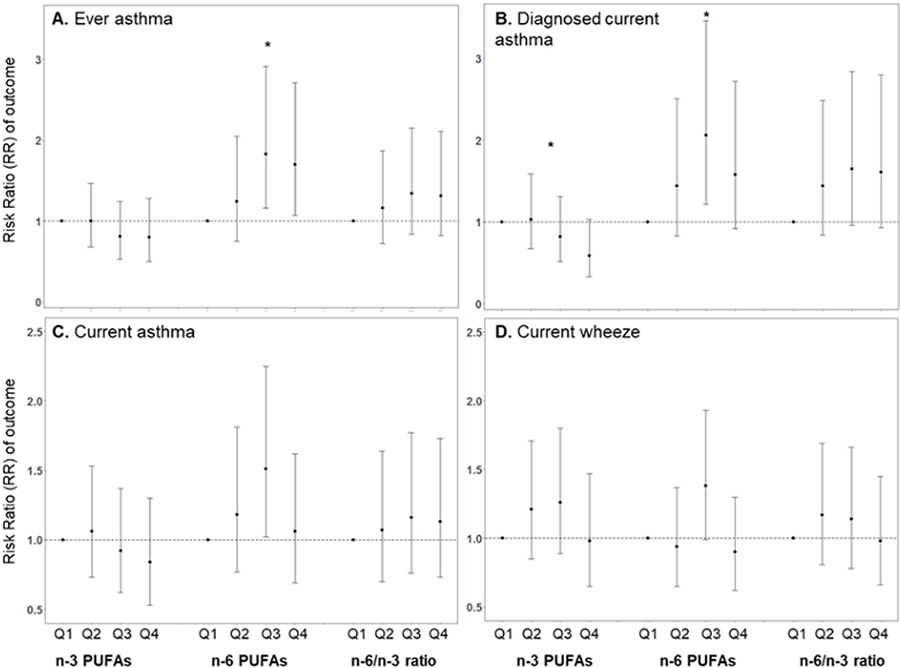
Ever asthma defined as physician/medical provider report of asthma ever (A); current wheeze defined as wheeze in the past 12 months (B); current asthma defined as 2 out of 3 ever asthma, wheeze and/or medication use in past 12 months (C); diagnosed current asthma defined as ever asthma + wheeze and/or medication use in past 12 months (D). *p for linear trend <0.05
Interaction models
In two-way interaction models, we did not find evidence for effect modification by race (all interaction p-values>0.25)) or sex (all interaction p-values>0.15). We did find evidence for effect modification by maternal asthma on associations between prenatal total n-6 PUFAs and all four child respiratory outcomes (Table 4). Increasing n-6 PUFAs were associated with higher risk of all respiratory outcomes only among children born to women who reported a history of asthma whereas in those born to women without a reported history of asthma, we did not observe statistically significant association between n-6 PUFAs and any of the respiratory outcomes. We observed similar but attenuated patterns when testing for effect modification and stratifying by maternal atopic disease (supplemental table E2). We did not find evidence that maternal asthma modified the association between n-3 PUFAs and n-6/n-3 PUFA ratio and child outcomes.
Table 4.
Association of mid-pregnancy maternal n-6 PUFAs (continuous) and respiratory outcomes stratified by maternal history of asthma
| Child Outcome | Maternal Ever Asthma Yes N=118 |
Maternal Ever Asthma No N=901 |
|
|---|---|---|---|
| RR (95% CI) |
RR (95% CI) |
P-value for interaction | |
| Ever asthma* | 1.65 (1.17, 2.30) |
1.10 (0.89, 1.38) |
0.050 |
| Current wheeze† | 1.39 (0.97, 1.98) |
0.87 (0.74, 1.03) |
0.017 |
| Current asthma‡ | 1.53 (1.07, 2.19) |
0.90 (0.74, 1.09) |
0.011 |
| Diagnosed current asthma§ | 1.70 (1.17, 2.48) |
0.96 (0.77, 1.22) |
0.005 |
Modified Poisson models adjusted for adjusted for child sex, mother’s educational level at enrollment, parity, mother’s age at enrollment, child’s BMI at age 4–6 visit, mother’s pre-pregnancy BMI and mother’s race. Effect sizes shown per an IQR (2.28) increase in n-6 PUFA measure.
Report of physician/medical provider diagnosis of asthma ever
Caretaker report of wheeze or whistling of the chest in the past 12 months
At least 2 out of 3 of following: ever diagnosis of asthma, current wheeze and wheeze/asthma medication use in past 12 months
Report of ever diagnosed asthma AND wheeze and/or asthma/wheeze medication use in past 12 months
Exploratory 3-way interactions
We also found evidence of a three-way interaction between child sex, maternal asthma and some prenatal PUFA indicators. Figures depicting all significant 3-way interactions after stratifying by the relevant covariates are shown in the Supplement (Figures E1 and E2). Male children born to women reporting a history of asthma with a higher n-6/n-3 ratio had the highest risk of ever, current and current diagnosed asthma. There were similar patterns for the association between prenatal total n-6 PUFAs and ever asthma in children, in which male children born to asthmatic women with higher n-6 PUFAs have the greatest risk. In contrast, increasing total n-3 PUFAs in the prenatal period were associated with higher risk of diagnosed asthma in girls born to women with a history of asthma, while the effect was in the opposite direction for all other groups.
Discussion
These analyses leveraged data from a large, diverse US pregnancy cohort to examine the associations between measured prenatal PUFAs and respiratory outcomes in childhood. We found that higher maternal plasma n-6 PUFAs in pregnancy were associated with higher risk of ever being diagnosed with asthma and active asthma in their children at age 4–6 years. While the associations were in the expected directions, we did not find statistically significant associations between n-6/n-3 PUFA ratios and any respiratory outcomes in our main effect models. The effects of n-6 PUFAs were modified by maternal asthma, with their effects being most pronounced in children born to women with asthma compared to those born to women without asthma. In exploratory analyses, a significant three-way interaction between child sex, maternal asthma and n-6/n-3 PUFA indicated that male children born to women with asthma and a higher n-6/n-3 ratio had the highest risk across all outcomes.
Most research on dietary fatty acids and asthma has focused on the potential protective effects of n-3 PUFA intake in pregnancy against asthma development in children, with conflicting results. Prospective birth cohorts examining n-3, n-6 and n-6/n-3 ratio PUFA measures in pregnancy in relation to allergy and asthma development in childhood have similarly produced mixed results. In an analysis of data from the Osaka Maternal and Child Health Study, higher maternal n-6 PUFA intake assessed via a semi-quantitative diet history questionnaire was associated with increased risk of eczema but not wheeze at age 2 years.11 In a similar analysis of dietary data in another Japanese cohort, the authors reported borderline protective associations between n-3 PUFA intakes and lower odds of wheeze at 23–29 months and no associations between total or individual n-6 PUFAs and wheeze. 9 A Finnish cohort reported associations between higher n-6/n-3 PUFA ratios, estimated via FFQs assessing intakes during the last month before delivery, and increased risk of allergic rhinitis by age 5 but did not find statistically significant associations with wheeze at that same age.12 In analyses from a Dutch cohort, authors reported that increasing maternal plasma n-6/n-3 PUFA ratios in maternal plasma collected in pregnancy was associated with decreased risk of eczema in the first 7 months of life but found no associations with asthma or wheeze by age 7 years.16 The differences in these findings could be due to the different instruments used to assess PUFA intakes, the timing of outcome assessment and underlying differences in demographic and lifestyle characteristics or overall dietary patterns in the samples.
Black and Sharpe first proposed that changes in consumption of n-3 and n-6 PUFAs may be related to the development of asthma and other atopic diseases.41 In the United States, dietary changes during the 20th century, particularly the increase in availability of dietary fat from soy bean derived oils, have been associated with increased linoleic acid consumption, an n-6 PUFA precursor.42 Linoleic acid is a precursor of arachidonic acid, and the principal substrate for the synthesis of prostaglandins, thromboxanes and leukotrienes which are well-characterized mediators of allergic inflammation.43 Prostaglandin E affects the Th1/Th2 balance by promoting the differentiation of naive T cells into Th2 cells, thereby increasing the production of Th2 cytokines, including interleukin (IL)-4, IL-5, and IL-13, and enhancing the production of IgE.4, 44–46
A recent review noted that maternal diet in pregnancy, including PUFA intake, may interact with the genetic predisposition to allergic disease by influencing the developing immune system.47 Most prospective studies have not examined maternal asthma/atopic disease as a potential modifier of the relationship between PUFA intakes and asthma in their children. In the Dutch Maastricht Essential Fatty Acid Birth (MEFAB) and the Greek Rhea Mother-Child Study cohorts, the authors reported no evidence of effect modification by parental atopy of the association between n-3/n-6 ratios and current asthma, rhinitis or eczema at age 6–7 years.20 PUFA levels in both cohorts were assessed in umbilical cord blood samples, rather than maternal prenatal plasma, and as expected given the study locations and differences in PUFA consumption, levels of n-3s were lower and n-6s and n-6/n-3 ratios were higher in our cohort when compared to these cohorts. Furthermore, while PUFA measures in maternal plasma and cord blood are correlated, there are differences in the distribution of specific PUFA levels.48 In maternal samples, the dominant PUFA is LA, whereas AA is the most abundant in cord blood samples.20, 48 While we do not have data on active asthma during pregnancy in the women in the study, higher n-6 intake may also affect their own asthma severity and control in pregnancy. For example, in a study of asthmatics, a higher ratio of n-6/n-3 PUFA intake was associated with greater levels of airway inflammation assessed via exhaled nitric oxide and greater likelihood of uncontrolled asthma. 49 In overlapping research, active maternal asthma in pregnancy has been associated with higher risk of offspring asthma, with risk being enhanced when asthma is not well controlled. For example, it was recently shown that higher prevalence of early-onset persistent asthma was observed among children born to women with mild uncontrolled (prevalence ratio (PR):1.2, 95% CI 1.1, 1.4), moderate-to-severe controlled (PR:1.3, 95% CI 1.1, 1.6), and moderate-to-severe uncontrolled asthma (PR:1.4, 95% CI 1.2, 1.6) compared to those born to women with mild controlled asthma. 50 Future studies examining associations between PUFA intakes in pregnancy among women with active asthma in relation to their own asthma outcomes as well as risk in their children would further inform this area of research.
Sex-differences in both fatty acid metabolism and asthma development in childhood asthma have been previously reported.29, 30 While we did not find sex differences in the association between PUFA main effects and asthma outcomes, in exploratory analyses we did find evidence of effect modification by both child sex and maternal history of asthma, particularly for the association between n-6/n-3 ratio and asthma outcomes. In examined three-way interactions, the association between n-6/n-3 ratio and n-6 PUFAs and respiratory outcomes was strongest for male children born to women with asthma. Differential maturation in lung development of males relative to females may predispose male infants to childhood respiratory diseases including asthma51–53 and they may be more susceptible to the pro-inflammatory effects of prenatal n-6 PUFAs. Conversely, we found that there was an inverse association between n-6/n-3 ratio and asthma outcomes in girls born to asthmatic women, with higher n-6/n-3 PUFA ratio being associated with lower risk of asthma in these girls. Lee Sarwar and colleagues also reported inverse associations between concurrent n-6 PUFAs and asthma outcomes in children born to parents with history of atopy.21 While the underlying mechanism of these observed effects are unclear, there are data demonstrating that males and females differ in their ability to convert ALA to other longer chain n-3 PUFAs, which may play a role.54
Strengths of our study include the prospective design, the reasonably large ethnically and demographically diverse sample facilitating our ability to examine effect modification, the objective assessment of total PUFA status in pregnancy, and our ability to adjust for important confounders. Potential limitations should also be considered. While we only measured plasma PUFAs at a single time point, it has been shown that dietary patterns do not vary much across pregnancy.55, 56 Our quartile analysis of n-6 PUFA did not reflect a linear dose response but we detected a statistically significant linear trend. Simple linear dose response relationships for dietary factors, may not be evident, in part because of limits in absorption, transport, metabolism, and storage.37 While plasma PUFAs provide an objective measure, other food components may alter these associations which were not in the realms of this investigation. In addition, we chose to use the plasma phospholipid specimen pool as opposed to alternatives such as total plasma because of the stability of the sampling method and their minimal variability in relation to postprandial levels, which is primarily seen with the triglycerides or free fatty acid fractions. Our cohort is primarily African American and demographically diverse which fills a research gap in this field, nonetheless our results may not be generalizable to other populations with differing demographics. Potential misclassification of covariates measured at baseline, such as maternal asthma, is likely non-differential relative to the measured PUFA levels and child outcomes and thus likely to move results towards the null. Child outcomes were based on questionnaire data which can lead to misclassification. However, questions were adapted from the ISAAC study and have been widely validated 57 and we used a hierarchy of wheeze/asthma definitions with consistent findings. While we found significant interactions between maternal asthma, child sex and PUFAs, our sample size within some stratum was limited which may lead to overfitting. Thus these findings warrant replication and should be examined in other populations with larger sample sizes to further explore these complex relationships. There is the possibility of unmeasured confounding or effect modifying variables that may have influenced our results.
This study adds to a growing literature underscoring the need to consider prenatal diet as an important programming factor in the development of asthma in childhood. These results demonstrate that prenatal status of long-chain PUFAs can enhance or reduce risk of children’s respiratory outcomes, depending on PUFA category. Moreover, in order to more fully understand the influence of PUFA exposures in utero on childhood asthma risk, modifying effects of maternal asthma and child sex need to be taken into account. Indeed, prior conflicting results in studies examining associations between prenatal intakes of PUFAs and child asthma and allergy risk may in part be because potential modifying factors were not considered. Understanding mechanisms underlying modifying effects of maternal asthma as well as child sex on the link between prenatal PUFAs and child respiratory outcomes will further elucidate how respiratory disease is programmed starting prenatally. Moreover, enhanced knowledge of those who may most likely benefit from prevention and intervention strategies such as n-6 consumption reduction strategies in combination with n-3 supplementation, can lead to more efficacious protocols.
Supplementary Material
Key messages:
Maternal polyunsaturated fatty acids in pregnancy are predictors of asthma risk in childhood and these associations are potentially modified by maternal asthma and child sex.
Acknowledgements
We acknowledge the contributions of the study research staff and families who enrolled in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood study.
Funding sources: This work was funded by the Urban Child Institute (FT) and the National Institutes of Health grants NHLBI HL109977 (KNC) and HL132338 (KNC and RWJ). MJR was supported by National Institute of Environmental Health Sciences grant number R00ES027496. The Lipid Core was supported by National Institutes of Health grant number DK020593.
Abbreviations:
- AA
arachidonic acid
- BMI
body mass index
- CANDLE
Conditions Affecting Neurocognitive Development and Learning in Early Childhood
- FFQ
food frequency questionnaire
- IQR
interquartile range
- ISAAC
International Study of Asthma and Allergies in Children
- PR
Prevalence ratio
- PUFA
polyunsaturated fatty acid
- RR
risk ratio
Footnotes
The authors declare that they have no relevant conflicts of interest.
References
- 1.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital Health Stat 10 2013:1–81. [PubMed] [Google Scholar]
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332:133–8. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res 2009; 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? Proceedings of the Nutrition Society 2010; 69:373–80. [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr 2012; 142:592S–9S. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 2008; 47:147–55. [DOI] [PubMed] [Google Scholar]
- 7.Chatzi L, Torrent M, Romieu I, Garcia-Esteban R, Ferrer C, Vioque J, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008; 63:507–13. [DOI] [PubMed] [Google Scholar]
- 8.Lumia M, Luukkainen P, Tapanainen H, Kaila M, Erkkola M, Uusitalo L, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatric Allergy and Immunology 2011; 22:827–35. [DOI] [PubMed] [Google Scholar]
- 9.Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal fat intake during pregnancy and wheeze and eczema in Japanese infants: the Kyushu Okinawa Maternal and Child Health Study. Annals of Epidemiology 2013; 23:674–80. [DOI] [PubMed] [Google Scholar]
- 10.Pele F, Bajeux E, Gendron H, Monfort C, Rouget F, Multigner L, et al. Maternal fish and shellfish consumption and wheeze, eczema and food allergy at age two: a prospective cohort study in Brittany, France. Environmental Health 2013; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake Y, Sasaki S, Tanaka K, Ohfuji S, Hirota Y. Maternal fat consumption during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Thorax 2009; 64:815–21. [DOI] [PubMed] [Google Scholar]
- 12.Nwaru BI, Erkkola M, Lumia M, Kronberg-Kippila C, Ahonen S, Kaila M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. British Journal of Nutrition 2012; 108:720–32. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis N, Roumeliotaki T, Oken E, Ballester F, Barros H, Basterrechea M, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. International Journal of Epidemiology 2017; 46:1465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leermakers ETM, Sonnenschein-van der Voort AMM, Heppe DHM, de Jongste JC, Moll HA, Franco OH, et al. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: The Generation R Study. European Journal of Clinical Nutrition 2013; 67:353–9. [DOI] [PubMed] [Google Scholar]
- 15.Newson RB, Shaheen SO, Henderson AJ, Emmett PM, Sherriff A, Calder PC, et al. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. Journal of Allergy and Clinical Immunology 2004; 114:531–7. [DOI] [PubMed] [Google Scholar]
- 16.Notenboom ML, Mommers M, Jansen EHJM, Penders J, Thijs C. Maternal fatty acid status in pregnancy and childhood atopic manifestations: KOALA Birth Cohort Study. Clinical and Experimental Allergy 2011; 41:407–16. [DOI] [PubMed] [Google Scholar]
- 17.Pike KC, Calder PC, Inskip HM, Robinson SM, Roberts GC, Cooper C, et al. Maternal Plasma Phosphatidylcholine Fatty Acids and Atopy and Wheeze in the Offspring at Age of 6 Years. Clinical & Developmental Immunology 2012. [DOI] [PMC free article] [PubMed]
- 18.Rucci E, den Dekker HT, de Jongste JC, Steenweg-de-Graaff J, Gaillard R, Pasmans SG, et al. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The Generation R Study. Clinical and Experimental Allergy 2016; 46:461–71. [DOI] [PubMed] [Google Scholar]
- 19.Standl M, Demmelmair H, Koletzko B, Heinrich J. Cord blood LC-PUFA composition and allergic diseases during the first 10 yr. Results from the LISAplus study. Pediatric Allergy and Immunology 2014; 25:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratakis N, Gielen M, Margetaki K, de Groot RHM, Apostolaki M, Chalkiadaki G, et al. PUFA status at birth and allergy-related phenotypes in childhood: a pooled analysis of the Maastricht Essential Fatty Acid Birth (MEFAB) and RHEA birth cohorts. British Journal of Nutrition 2018; 119:202–10. [DOI] [PubMed] [Google Scholar]
- 21.Lee-Sarwar K, Kelly RS, Lasky-Su J, Kachroo P, Zeiger RS, O’Connor GT, et al. Dietary and Plasma Polyunsaturated Fatty Acids Are Inversely Associated with Asthma and Atopy in Early Childhood. J Allergy Clin Immunol Pract 2019; 7:529–38 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. American Journal of Clinical Nutrition 2008; 88:167–75. [DOI] [PubMed] [Google Scholar]
- 23.Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood - a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J 2018; 11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013; 68:1370–6. [DOI] [PubMed] [Google Scholar]
- 25.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. British Medical Journal 2012; 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuhjelm C, Warstedt K, Fageras M, Falth-Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatric Allergy and Immunology 2011; 22:505–14. [DOI] [PubMed] [Google Scholar]
- 27.Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatrica 2009; 98:1461–7. [DOI] [PubMed] [Google Scholar]
- 28.Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr 2016; 103:128–43. [DOI] [PubMed] [Google Scholar]
- 29.Almqvist C, Worm M, Leynaert B, working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008; 63:47–57. [DOI] [PubMed] [Google Scholar]
- 30.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr 2011; 94:1914S–9S. [DOI] [PubMed] [Google Scholar]
- 31.Nurmatov U, Nwaru BI, Devereux G, Sheikh A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012; 67:1041–59. [DOI] [PubMed] [Google Scholar]
- 32.Volgyi E, Carroll KN, Hare ME, Ringwald-Smith K, Piyathilake C, Yoo W, et al. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 2013; 5:1511–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sontag-Padilla LM, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. The Urban Child Institute CANDLE Study Methodological Overview and Baseline Sample Description: RAND Corporation; 2015. Santa Monica, Calif. USA: https://www.rand.org/pubs/research_reports/RR1336.html [Google Scholar]
- 34.Morrison WR, Smith LM. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride--Methanol. J Lipid Res 1964; 5:600–8. [PubMed] [Google Scholar]
- 35.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226:497–509. [PubMed] [Google Scholar]
- 36.Brenna JT, Plourde M, Stark KD, Jones PJ, Lin YH. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr 2018; 108:211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willet W Nutritional Epidemiology. 3rd ed: Oxford University Press; 2013. [Google Scholar]
- 38.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8:483–91. [DOI] [PubMed] [Google Scholar]
- 39.Roy A, Kocak M, Hartman TJ, Vereen S, Adgent M, Piyathilake C, et al. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatr Allergy Immunol 2018; 29:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou GY. A modified Poisson regression approach to prospective studies with binary data. American Journal of Epidemiology 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 41.Black PN, Sharpe S. Dietary fat and asthma: Is there a connection? European Respiratory Journal 1997; 10:6–12. [DOI] [PubMed] [Google Scholar]
- 42.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011; 93:950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990; 323:645–55. [DOI] [PubMed] [Google Scholar]
- 44.Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, et al. Early nutrition and immunity - progress and perspectives. British Journal of Nutrition 2006; 96:774–90. [PubMed] [Google Scholar]
- 45.Calder PC. Polyunsaturated fatty acids and cytokine profiles: a clue to the changing prevalence of atopy? Clin Exp Allergy 2003; 33:412–5. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003; 38:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham MN, Bunyavanich S. Prenatal Diet and the Development of Childhood Allergic Diseases: Food for Thought. Curr Allergy Asthma Rep 2018; 18:58. [DOI] [PubMed] [Google Scholar]
- 48.Montes R, Chisaguano AM, Castellote AI, Morales E, Sunyer J, Lopez-Sabater MC. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. Eur J Clin Nutr 2013; 67:658–63. [DOI] [PubMed] [Google Scholar]
- 49.Barros R, Moreira A, Fonseca J, Delgado L, Castel-Branco MG, Haahtela T, et al. Dietary intake of alpha-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. British Journal of Nutrition 2011; 106:441–50. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Agerbo E, Schlunssen V, Wright RJ, Li J, Munk-Olsen T. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol 2018; 141:886–92 e3. [DOI] [PubMed] [Google Scholar]
- 51.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol 2015; 50:1159–69. [DOI] [PubMed] [Google Scholar]
- 52.Demissie K, Marcella SW, Breckenridge MB, Rhoads GG. Maternal asthma and transient tachypnea of the newborn. Pediatrics 1998; 102:84–90. [DOI] [PubMed] [Google Scholar]
- 53.Ishak N, Sozo F, Harding R, De Matteo R. Does lung development differ in male and female fetuses? Experimental Lung Research 2014; 40:30–9. [DOI] [PubMed] [Google Scholar]
- 54.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proceedings of the Nutrition Society 2008; 67:19–27. [DOI] [PubMed] [Google Scholar]
- 55.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s Dietary Patterns Change Little from Before to During Pregnancy. Journal of Nutrition 2009; 139:1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip HM, et al. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatric and Perinatal Epidemiology 2009; 23:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (Isaac) - Rationale and Methods. European Respiratory Journal 1995; 8:483–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


