Abstract
Polycystic ovary syndrome (PCOS), a common reproductive disorder in women, is characterized by hyperandrogenemia, chronic anovulation, cystic ovarian follicles, and luteinizing hormone (LH) hyper-pulsatility, but the pathophysiology isn’t completely understood. We recently reported a novel mouse model of PCOS using chronic letrozole (LET; aromatase inhibitor). Letrozole-treated females demonstrate multiple PCOS-like phenotypes, including polycystic ovaries, anovulation, and elevated circulating testosterone and LH, assayed in “one-off” measures. However, due to technical limitations, in vivo LH pulsatile secretion, which is elevated in PCOS women, was not previously studied, nor were the possible changes in reproductive neurons. Here, we used recent technical advances to examine in vivo LH pulse dynamics of freely moving LET female mice versus control and ovariectomized (OVX) mice. We also determined whether neural gene expression of important reproductive regulators such as kisspeptin, neurokinin B (NKB), and dynorphin, is altered in LET females. Compared to controls, LET females exhibited very rapid, elevated in vivo LH pulsatility, with increased pulse frequency, amplitude, and basal levels, similar to PCOS women. Letrozole-treated mice also had markedly elevated Kiss1, Tac2, and Pdyn expression and increased Kiss1 neuronal activation in the hypothalamic arcuate nucleus. Notably, the hyperactive LH pulses and increased kisspeptin neuron measures of LET mice were not as elevated as OVX females. Our findings indicate that LET mice, like PCOS women, have markedly elevated LH pulsatility, which likely drives increased androgen secretion. Increased hypothalamic kisspeptin and NKB levels may be fundamental contributors to the hyperactive LH pulse secretion in the LET PCOS-like condition and, perhaps, in PCOS women.
Keywords: PCOS, reproduction, infertility, GnRH, kisspeptin, Kiss1, Tac2, dynorphin, neurokinin B, androgen, aromatase
Polycystic ovary syndrome (PCOS) affects approximately 10% of reproductive-aged women (1–3). Polycystic ovary syndrome is a complex disorder encompassing multiple phenotypic parameters, with clinical diagnosis typically requiring at least 2 of the following features: polycystic ovaries, androgen excess (hyperandrogenemia), and chronic anovulation, with the latter 2 often comprising the predominant features (4). Neuroendocrine hallmarks of PCOS include increased gonadotropin-releasing hormone (GnRH) pulse frequency, increased luteinizing hormone (LH), reduced follicle-stimulating hormone (FSH), and insensitivity to progesterone (P4)-negative feedback (5–7). Along with hyperandrogenemia, disruptions in the neuroendocrine reproductive axis contribute to dysfunctional ovarian maturation and diminished fertility (1–3, 5). In many cases, PCOS is also often associated with metabolic dysfunction, including obesity, increased abdominal adiposity, insulin resistance, glucose intolerance, and a heightened risk of type 2 diabetes (3, 5, 8).
Elucidating the etiology and underlying mechanisms of PCOS has proven challenging, due in part to the heterogeneity of the disease. The development of various animal models to study PCOS has been an important research focus in this regard (9–11). Given the heterogeneity of PCOS phenotypes, different models may be useful in representing different underlying etiologies or classes of PCOS. One common model is prenatal androgenization (PNA), which has been implemented in monkeys, sheep, and rodents. The PNA model elicits many of the reproductive phenotypes associated with PCOS (11–16), including elevated androgens and LH, as well as altered steroid hormone feedback. Although the reproductive effects are quite robust, the PNA models usually lack robust metabolic effects and are often considered models of the “lean PCOS” condition. A more recent mouse model based on elevated exposure to anti-Mullerian hormone (AMH) during prenatal life (PAMH), achieved with injections of AMH given to the mothers during late pregnancy, similarly recapitulates in the female offspring a reproductive phenotype of high LH, hyperandrogenemia, and anovulation without a strong metabolic phenotype, mimicking the “lean PCOS” condition (17). In contrast to the PNA and AMH models, rodent models of PCOS using continuous treatment with letrozole (LET), a nonsteroidal aromatase inhibitor (18), recapitulate the “obese PCOS” (or overweight PCOS) condition. The LET model is based on the finding of lower estrogen/androgen ratios in follicular fluid of PCOS women, suggesting low aromatase activity (19–21), and the association of genetic variants of the aromatase gene (CYP19), which converts androgens to estrogen, with the development of PCOS and hyperandrogenism in women (22–26). Indeed, supportive of this model, ovaries and tissues of PCOS women have decreased aromatase levels (27, 28), and some overweight/obese PCOS women exhibit lower circulating E2 levels (29). The LET model was first reported in female rats, in which continuous LET treatment initiated before puberty (at doses which do not fully deplete E2 levels) develop disrupted estrous cyclicity, increased ovarian weight and cysts, hyperandrogenemia, and anovulation (18, 30). Letrozole-treated rats also exhibit several metabolic features of PCOS, including increased body weight and insulin resistance (18).
We recently developed a mouse model utilizing LET that, similar to rats, strongly recapitulates both the reproductive and metabolic hallmarks of the overweight/obese PCOS condition. Peripubertal female mice implanted with a pellet that delivered constant, long-term LET develop all the neuroendocrine features of PCOS, including elevated testosterone (T) and LH and decreased FSH (31). The latter findings were supported by increased Lhb and decreased Fshb mRNA levels in the pituitary (31). As with PCOS women, LET female mice have heavier ovaries, polycystic follicles, and diminished corpora lutea (indicative of decreased ovulation), as well as altered Fshr and steroidogenic enzyme gene expression (31). Along with the PCOS-like reproductive alterations, LET mice demonstrated several metabolic impairments, including increased body weight (BW), increased abdominal adiposity and adipose cell size, insulin resistance, and dyslipidemia (31, 32), mimicking similar phenotypes in overweight/obese women with PCOS.
Our original report (31) showed that LET female mice have elevated blood levels of LH, assayed in “one-off” measures from a single blood sample from each animal. At that time, due to long-standing technical and assay limitations in mouse models, in vivo LH pulsatile secretion, which is greatly elevated in PCOS women, was not studied in the LET mice. In addition, possible alterations in brain reproductive neural populations known to drive GnRH/LH secretion, such as kisspeptin and neurokinin B neurons, were not examined and thus, mechanistic underpinnings of the elevated LH secretion remain unknown. In the present study, we used recent technical advances (33–35) to examine in vivo LH pulse dynamics of awake, freely moving LET female mice and compared this to control mice and ovariectomized (OVX) mice, the latter of which has robustly elevated LH pulsatility due to the complete lack of ovarian hormone negative feedback. The assessment of LH pulse profiles allowed us to determine whether the elevated LH in the single “on-off” measures in the original study reflect increased LH pulse frequency, increased LH pulse amplitude, increased basal LH levels, or a combination of these various pulse parameters. In addition, the present study determined whether gene expression of known reproductive genes, such as Kiss1, Tac2, and Pdyn in the hypothalamic arcuate nucleus, or arcuate kisspeptin neuron activation levels, are enhanced in the brains of LET female mice, perhaps contributing to their increased LH and androgen secretion. We also determined if hypothalamic gene expression for the inhibitory neuropeptide, RFRP-3, was decreased in LET female mice, perhaps allowing for less inhibitory input onto the GnRH neural network and, therefore, increased LH secretion.
Materials and Methods
Animals and LET treatment
Female C57BL/6 mice were housed on a 12:12 light-dark cycle, with food and water available ad libitum. Mice were housed 2 females/cage. At 4 weeks of age, prior to pubertal completion, female mice were subcutaneously implanted with a LET (50 µg/day) or placebo control (CON) pellet. Letrozole powder was purchased from Sigma (St. Louis, Missouri) and custom 60-day continuous release LET pellets were made by Innovative Research of America (Sarasota, Florida). The 50 µg/day dose was similar to that used in previously published LET mouse studies (31, 32, 36, 37). For comparison, an additional control group of C57BL/6 females were OVX at 5 weeks of age to remove all ovarian hormone-negative feedback and induce maximally elevated GnRH/LH pulsatility. Body weight of the mice in all groups was tracked from 4 weeks of age until the end of the experiments at 9 weeks of age. All experiments were approved by the UCSD IACUC.
Experiment 1: In vivo serial LH sampling
Beginning at 6 weeks of age (2 weeks after LET implantation), LET, control, and OVX littermates underwent daily handling to habituate the mice to the tail-tip bleeding procedure used for collecting serial blood samples. Fig. 1 shows the experimental paradigm and timeline. All mice were handled daily for 3 weeks prior to the final day on which the serial bleeding occurred (9 weeks old; 5 weeks after LET implantation). All serial sampling was conducted between 9:00 am and 12:00 pm. On the day of the serial bleeding, the tail tip of each animal was cut and then ~15 minutes later serial blood samples were collected every 6 minutes for a total duration of 2.5 hours (n = 10–14/group). For each sample, 3 μl of whole blood was pipetted from the tail and mixed with 57 μl of assay buffer and then placed on ice until storage at -20°C. Animals were awake the entire time and able to freely roam their home cage in between sampling. Serial tail-tip blood samples were assayed for LH levels, as described here in the Hormone Assays section. Letrozole-treated mice are known to be acyclic and predominantly arrested in diestrus (DE) (31). In Experiment 1, most LET female mice were confirmed via vaginal smears to be in DE on the day of LH sampling, with a few mice in estrus (E). All control females were similarly in DE or E. Within each group, there was no difference between the DE and E mice for any LH measure, so group comparisons were made with DE and E mice combined to ensure high statistical power.
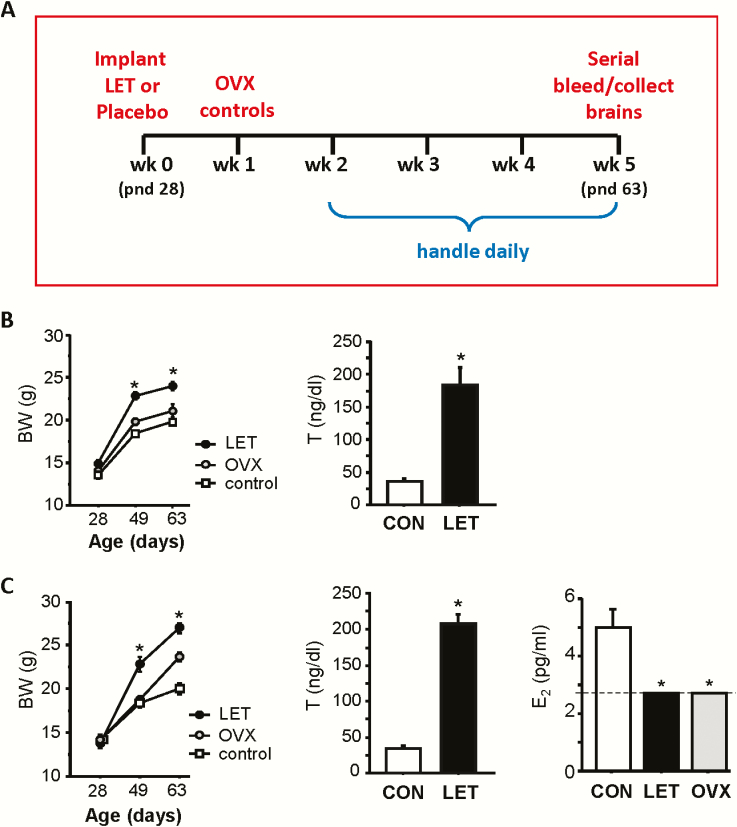
Figure 1.
A) Schematic of experimental paradigm for LET induction of PCOS-like phenotype in female mice and time line for serial bleeding (Experiment 1) and brain collection (Experiment 2). B) Mean BWs over the course of Experiment 1 and serum T at week 3 (pnd 49). C) Mean BWs over the course of Experiment 2 and serum T and E2 at week 5 (pnd 63). Unlike diestrus controls, all LET and OVX samples were below the limit of E2 assay sensitivity (2.7 pg/ml). *P < 0.05 versus control females.
Serial blood LH measures were analyzed for endogenous LH pulses. An LH value was determined to be a pulse using a similar criteria as in a previous report for mouse LH pulsatility (38): the value must show a >20% increase from 1 of the 2 previous points followed by a decrease by >10% in 1 of the next 2 subsequent points. In addition, to qualify as a pulse, the pulse amplitude must be ≥0.32 ng/ml, the detection sensitivity of the assay, as in previous reports (39). For identified LH pulses, the following parameters were calculated: (1) pulse frequency (# pulses/60 min); (2) interpulse interval, defined as the time between 2 pulses; (3) pulse amplitude, defined to be the difference between a pulse peak value and a preceding nadir, the lowest value of the 3 preceding values; (4) pulse peak, defined as the zenith LH value of each identified pulse; and (5) basal LH level, defined as the average LH of all the nadirs. In addition the overall mean LH, the average of all the LH values for an animal for the entire 2.5 h sampling period, was also calculated.
Experiment 2: Analysis of hypothalamic gene expression and neuronal activation
Letrozole-treated, control, and OVX groups (n = 6–8/group) generated similarly to Experiment 1 (Fig. 1A) were briefly anesthetized with isoflurane and rapidly decapitated (between 11:30 am and 12:30 pm) at ~9 weeks of age (5 weeks after LET implantation), similar to the age of pulse bleeding in Experiment 1. All LET and control mice were killed on DE, with OVX controls similarly sacrificed the same day. Blood and brains were collected, with the latter immediately frozen on dry ice and stored at -80°C. Frozen brains were sectioned on a cryostat into 5 sets of 20 uM coronal sections and thaw mounted on Superfrost-plus slides that were stored at -80°C until assaying.
Single-label in situ hybridization (ISH) for ARC Kiss1, Pdyn, or Tac2 (NKB) gene expression or for DMN Rfrp expression was performed, in each case, on 1 set of brain sections spanning the entire hypothalamus, which encompassed the entire ARC or DMN regions. All ISH assays were performed using published protocols and validated riboprobes (40–43). Briefly, slides with brain sections were fixed in 4% paraformaldehyde, treated with acetic anhydride, rinsed in sodium chloride (SSC), delipidated in chloroform, and dehydrated in graded ethanol. Slides were air dried before the hybridization step, where P33 radiolabeled riboprobe (Kiss, Tac2, pdyn, or Rfrp; 0.04 pmol/ml) was combined with 1/20 volume yeast tRNA in 0.1 M Tris/0.01M EDTA (pH 8) to produce the probe mix. The probe mix was heat-denatured in boiling water for 3 minutes, placed on ice for 5 minutes, and added to prewarmed hybridization buffer (60% deionized formamide, 5x hybridization salts, 0.1x Denhardt’s buffer, 0.2% SDS). 100 μl of this final hybridization mix was added to each slide, and then coverslipped and placed in a humidity chamber at 55°C for 16 hours. Following hybridization, the coverslips were removed and the slides were washed with 4x SSC at room temperature. The slides were then placed in RNAse A (10 mg/ml; Sigma) in 0.15 M sodium chloride, 10 mM Tris, and 1 mM EDTA (pH 8) for 30 minutes at 37°C followed by buffer without RNase for another 30 minutes at 37°C. Slides were then washed in 2X SSC for 30 minutes and 0.1X SSC at 62°C for 60 minutes. Slides were then washed for 3 minutes in 0.1X SSC at room temperature and dehydrated with graded ethanol. After drying, slides were dipped in Kodak NTB emulsion, air dried, and stored at 4°C for 4 to 9 days (depending on the specific assay) until they were developed and coverslipped.
Double-label ISH for cfos+Kiss1 mRNAs was performed on an additional set of ARC brain sections in order to assess neuronal activation of kisspeptin neurons. For the double-label assay, slides were treated similar to the single-label protocol, with a few modifications. Digoxigenin (DIG)-labeled antisense probe for Kiss1 was synthesized with T7 RNA polymerase and DIG labeling mix (Roche Diagnostics) and added 1:500 with a cfos P33 radiolabeled riboprobe (0.04 pmol/ml) to the probe hybridization mix. The next day, following the 62°C washes, slides were incubated in 2x SSC with 0.05% Triton X-100 containing 3% normal sheep serum for 75 minutes at room temp. The slides were then incubated for 16 hours at room temperature with anti-DIG antibody fragments conjugated to alkaline phosphatase (1:500; Roche Diagnostics). The next day, slides were washed with Buffer 1 (100mM Tris-HCl, 150 mM Nacl) and then washed in Tris buffer and incubated in a Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, California) for 1 hour at room temperature. The slides were then rinsed and air dried prior to dipping in emulsion, similar to single-label assays.
In situ hybridization slides were analyzed using previously-published techniques with a computer-automated image processing system and custom counting software (GRAINS; Dr. Don Clifton, University of Washington) by a person unaware of the treatment group of each slide (44). For single-label ISH assays, the software counted the number of silver grain clusters representing Kiss1, Tac2, Pdyn, or Rfrp cells, as well as the number of silver grains in each individual cell cluster (a semiquantitative measure of Kiss1, Tac2, Pdyn, or Rfrp mRNA expressed per cell) (45–47). Cells were considered Kiss1, Tac2, pdyn, or Rfrp positive when the number of silver grains in a cluster exceeded that of background by 3-fold. For double-label ISH, DIG-containing cells (ARC Kiss1cells) were identified under fluorescence microscopy and the custom grain-counting software was then used in dark field to quantify silver grains (representing cfos mRNA) overlying each fluorescent cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was >3, as in previous studies (43, 48, 49).
Hormone assays
In Experiment 1, serial tail-tip blood samples were measured for LH levels by the University of Virginia Ligand Assay Core using an ultrasensitive murine LH ELISA. Functional sensitivity was 0.320 ng/mL. Values are reported as ng/ml of whole blood. A subset of mice in each group also had a small retro-orbital blood collection performed at week 3 of the study to confirm that serum T was elevated in LET mice, as previously reported. Serum T was measured by the University of Virginia Ligand Assay Core using a mouse/rat T ELISA assay that has a sensitivity of 10 ng/dl (range 10.0–1600 ng/dL).
In Experiment 2, serum E2 was measured in blood collected at sacrifice to confirm functionality of the pellets. Our prior LET mouse study (31) used an E2 ELISA that was not sensitive enough on the low end of the assay, precluding proper comparison of E2 levels between LET and control females. In the present study, we used a more sensitive tandem liquid chromatography-mass spectrometry (LCMS) assay performed by Professor Brian Keevil (University Hospital of Manchester, Manchester, United Kingdom) with a lower limit of detection of 10 pmol/L (2.7 pg/ml). Given the very large volume of blood serum needed for this assay, serum samples were pooled between animals, with 2 animals of the same treatment per pooled sample (n = 3–4 pooled samples per treatment). Estradiol values that fell below the limit of assay detection were assigned a value of 2.7 pg/ml to permit its comparison to detectable values. In a subset of mice that still had enough serum volume remaining, we also measured serum T levels to confirm elevated T in the LET mice.
Statistical analysis
All data were analyzed in GraphPad Prism 6 (La Jolla, California). Mean data were analyzed as a one-way ANOVA (group), followed by post hoc Bonferroni’s multiple comparison test. All data are expressed as mean ± standard error of the mean (SEM). A value of P < 0.05 was considered to be statistically significant.
Results
Confirmation of the LET hyperandrogenemia and overweight phenotype
To confirm the previously-reported PCOS-like hyperandrogenemia, in Experiment 1 serum T levels were measured in LET and control mice at 7 weeks of age (3 weeks after LET implantation). Circulating T was significantly upregulated in LET females, as expected (P < 0.05; Fig. 1). Matching the known metabolic phenotype in this model, BWs were also greater in LET than control mice at both 3 and 5 weeks post-LET treatment (P < 0.05; (Fig. 1B). In Experiment 2, at sacrifice (5 weeks post-LET implantation), serum E2 levels were measured and found to be significantly higher in control than in LET females, as predicted (P < 0.05; Fig. 1C). Serum E2 levels of LET and OVX females could not be directly compared, as both were below the limit of the LCMS assay detection (2.7 pg/ml), unlike control mice whose values were in the detectable range (Fig. 1C). Serum T levels and BWs were both significantly elevated in LET mice versus controls, as in the Experiment 1 cohort (P < 0.05; Fig. 1C).
Experiment 1
Endogenous LH pulses are more frequent and of higher amplitude in LET mice.
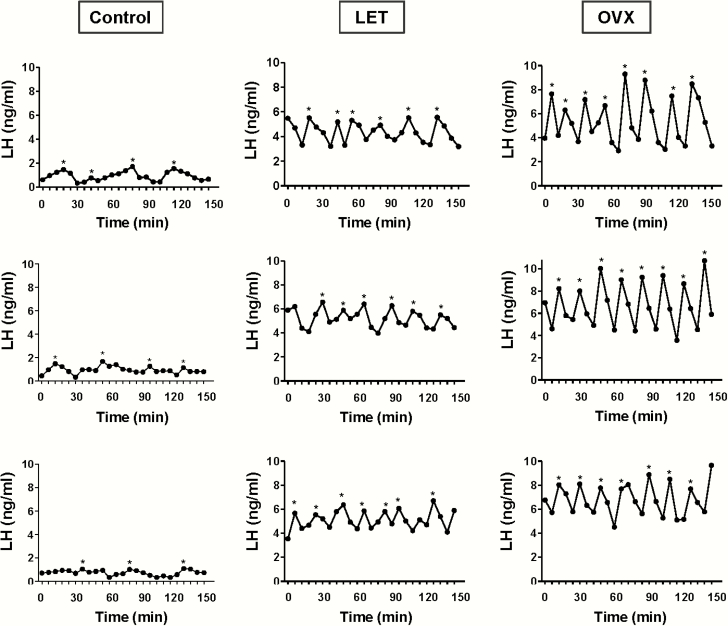
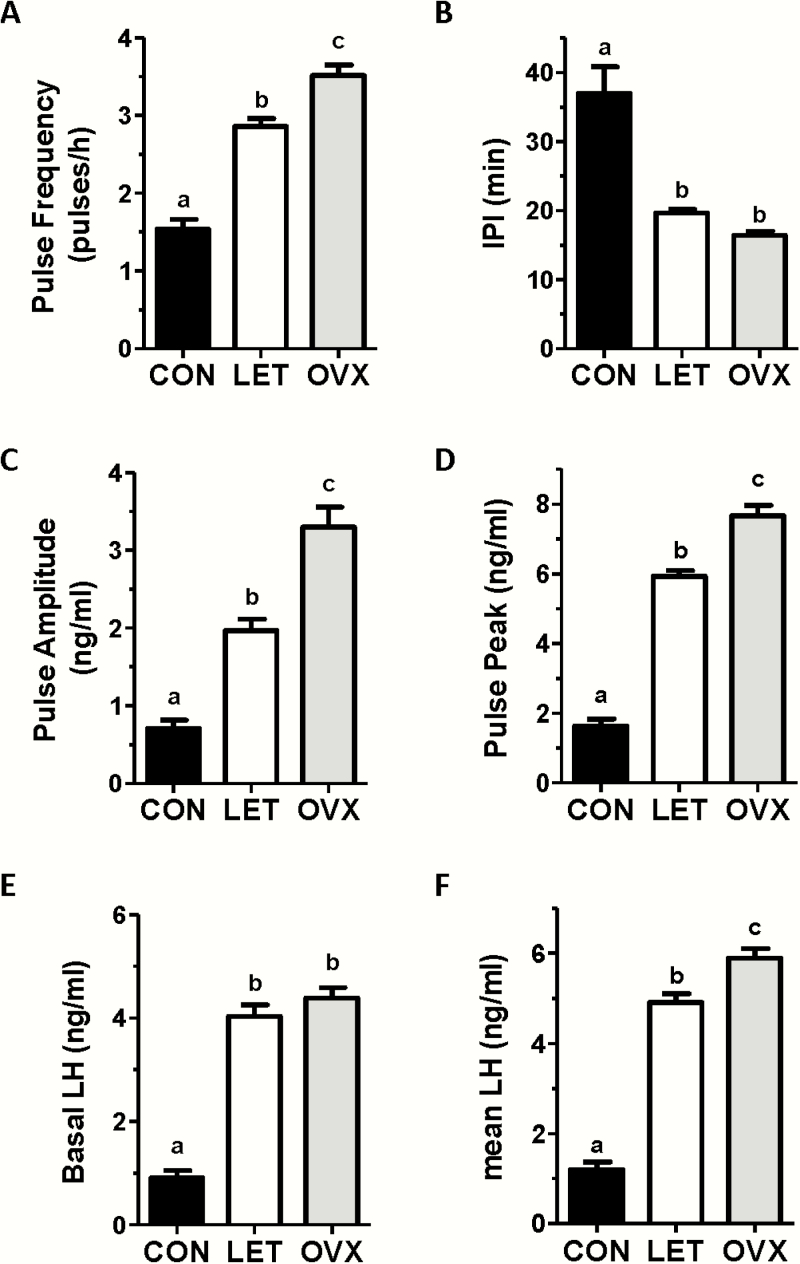
Women with PCOS have an abnormally rapid, high amplitude LH pulse profile, but the parameters of in vivo LH pulses in LET PCOS models have not been previously determined. To determine if LET mice also exhibit hyperactive LH pulses as in the PCOS condition, blood LH was measured every 6 minutes for 2.5 hours. Fig. 2 shows representative LH values during the blood collection period for 3 control females and 3 LET females. Control females exhibited overall low levels of LH with occasional small amplitude pulses. In stark contrast, LET females demonstrated a much more rapid pulsatile LH secretion pattern, along with a higher basal level and higher amplitude of each pulse. Mean analysis of individual pulse parameters indicated significant alterations in virtually all pulse parameters (Fig. 3). Specifically, the number of detectable pulses (pulse frequency) was significantly increased by 2-fold in LET mice versus controls (P < 0.01; Fig. 3A), while interpulse interval (IPI) was correspondingly decreased by ~50% in LET mice (P < 0.01; Fig. 3B). There were also large increases in mean basal levels (4-fold), mean pulse amplitude (2.5-fold), and mean pulse peak levels (ie, the zenith of a pulse; 3.5-fold) in LET mice (P < 0.01 for each parameter compared to control females; Fig. 3C–3E). Overall mean LH for the entire sampling period was significantly higher, by 4-fold, in LET versus controls (P < 0.01; Fig. 3F).
Figure 2.
Representative profiles of in vivo LH secretion in 3 LET-treated female mice (middle column), as well as 3 control female (left column) and 3 OVX female (right column) littermates. LH was measured in serial tail-tip bleeds from awake animals every 6 minutes for 2.5 hours. Identified pulses are indicated by *. Letrozole-treated females had more frequent pulses, along with higher pulse amplitude and elevated basal levels, than control mice.
Figure 3.
Mean pulse parameters of LET, control (CON), and OVX mice from 2.5 hours of in vivo serial LH sampling. Luteinizing hormone pulse frequency (pulses per hour) (A), inter-pulse interval (IPI; the number of minutes between pulses) (B), pulse amplitude (C), pulse peak (zenith value of a pulse) (D), basal LH level (E), and mean LH across the entire sampling period (F) were all significantly different in LET versus CON females. Different letters above bars indicate significantly different (P < 0.05) from each other.
The hyperactive LH pulse profile of LET mice is not identical to that of OVX mice.
To ascertain how the hyperactive LH pulses of LET mice compare to the OVX condition, in which the GnRH pulse generator is completely free of any ovarian negative feedback, we compared LET mice to OVX mice (Figs. 2 and 3). While LH pulses were rapid and high amplitude in both cases, pulse frequency was significantly lower, by 17%, in LET than OVX females (P < 0.01; Fig. 3A). Pulse amplitude and pulse peak were also significantly lower in LET mice than OVX mice, by ~40% and ~25%, respectively (P < 0.01 for each; Fig. 3C and 3D). Overall mean LH levels for the entire sampling period was 17% lower in LET females compared to OVX (P < 0.01; Fig. 3F). However, basal LH levels and interpulse interval were not statistically different between LET and OVX mice (Fig. 3B–3E). Thus, LET and OVX mice had similar baseline starting points for their LH pulses, but the pulses were slightly more frequent and achieved a higher maximum value (pulse peak) in the OVX condition (see representative profiles in Fig. 2).
Experiment 2
The brains of LET mice have greatly elevated Kiss1, Tac2, and Pdyn expression.
The mechanisms promoting the elevated LH pulse secretion in PCOS are not yet identified. We examined whether LET mice have elevated reproductive gene expression in the brain, which may be linked to increased stimulatory drive to GnRH and downstream LH secretion. We studied the genes for kisspeptin, Neurokinin B, and dynorphin (encoded by Kiss1, Tac2, and Pdyn, respectively), as these 3 neuropeptides are co-expressed together in hypothalamic arcuate (ARC) neurons that have been implicated as a key component of the GnRH pulse generator mechanism (50–52).
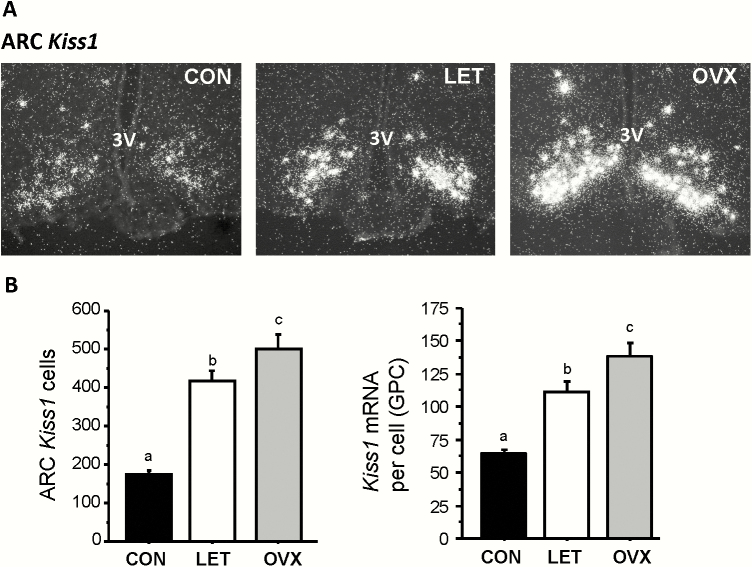
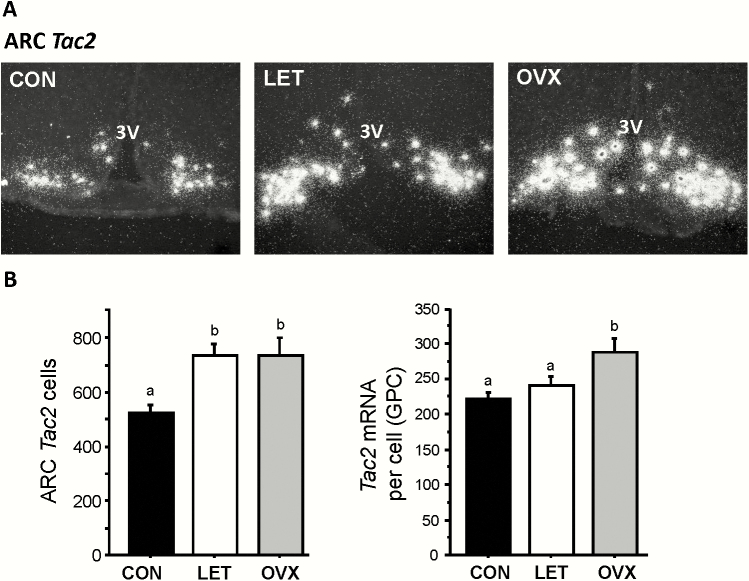
Kiss1 expression in the ARC, measured via ISH, was greatly increased in LET mice compared to control littermates (P < 0.05; Fig. 4A). Overall, Kiss1 mRNA levels were >150% higher in LET than in control females, with both the number of Kiss1 cells and levels of Kiss1 mRNA per cell being significantly elevated (P < 0.05; Fig. 4B). The levels of ARC Kiss1 expression in LET mice was significantly lower than that of OVX females (P < 0.05; Fig. 4B), correlating with the lower LH pulse profile observed in LET versus OVX females (Figs. 2 and 3). Tac2 expression levels in the ARC were similarly upregulated, by ~ 60%, in LET mice versus controls (P < 0.05; Fig. 5A and 5B), with the number of Tac2 cells being significantly elevated (P < 0.01; Fig. 5B). Unlike Kiss1, the number of identifiable ARC Tac2 cells was not significantly different between LET and OVX mice, though the level of Tac2 expression per cell was significantly higher in OVX than LET females (P < 0.05; Fig. 5B).
Figure 4.
Elevated Kiss1 levels in the arcuate nucleus of LET mice. A) Representative microscope image of Kiss1 mRNA expression in the ARC nucleus, as determined by radiolabeled in situ hybridization. B) Mean Kiss1 cell number and Kiss1 mRNA per cell in the arcuate nucleus. Different letters above bars indicate significantly different (P < 0.05) from each other.
Figure 5.
Increased Tac2 expression levels in the arcuate nucleus of LET mice. A) Representative microscope image of Tac2 mRNA expression in the ARC nucleus, as determined by radiolabeled in situ hybridization. B) Mean Tac2 cell number and Tac2 mRNA per cell in the arcuate nucleus. Different letters above bars indicate significantly different from each other.
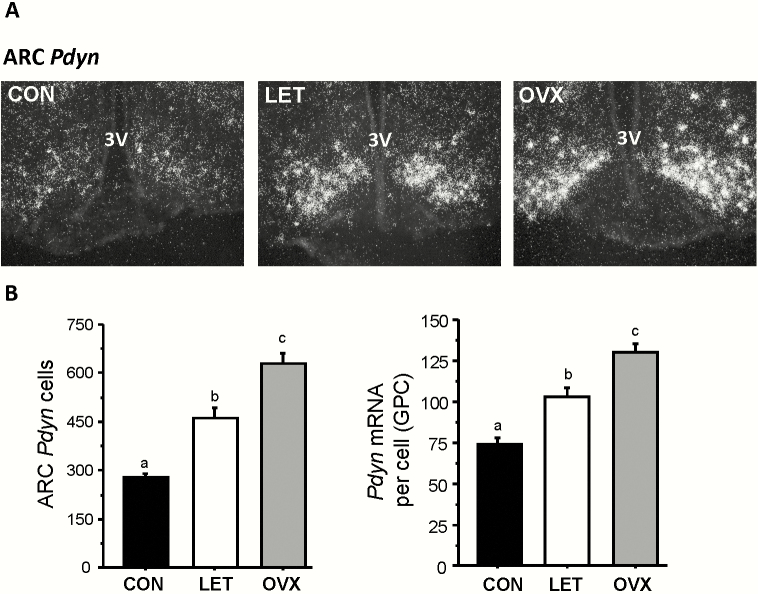
Current models of GnRH pulse generation posit that NKB stimulates neighboring ARC Kiss1/NKB cells to release kisspeptin, after which the inhibitory neuropeptide dynorphin, released from the same cells, is thought to terminate this process, thereby ending the “pulse” of kisspeptin secretion. Based on this model, we hypothesized that ARC Pdyn expression levels would be elevated in LET mice, as faster LH pulses would require both pulse start signals (NKB) and stop signals (dynorphin) to be elevated in order to generate more frequent pulse events. Indeed, we found that LET mice had markedly higher ARC Pdyn expression than control mice (P < 0.01), both in terms of identified Pdyn cells in the ARC and Pdyn mRNA levels per cell (Fig. 6A and 6B). As with Kiss1, Pdyn levels were lower in LET than OVX mice (P < 0.01), correlating with lower LH output in LET (Fig. 6B).
Figure 6.
Increased dynorphin gene expression in the arcuate nucleus of LET mice. A) Representative microscope image of Pdyn mRNA expression in the ARC nucleus, as determined by radiolabeled in situ hybridization. B) Mean Pdyn cell number and Pdyn mRNA per cell in the arcuate nucleus. Different letters above bars indicate significantly different from each other.
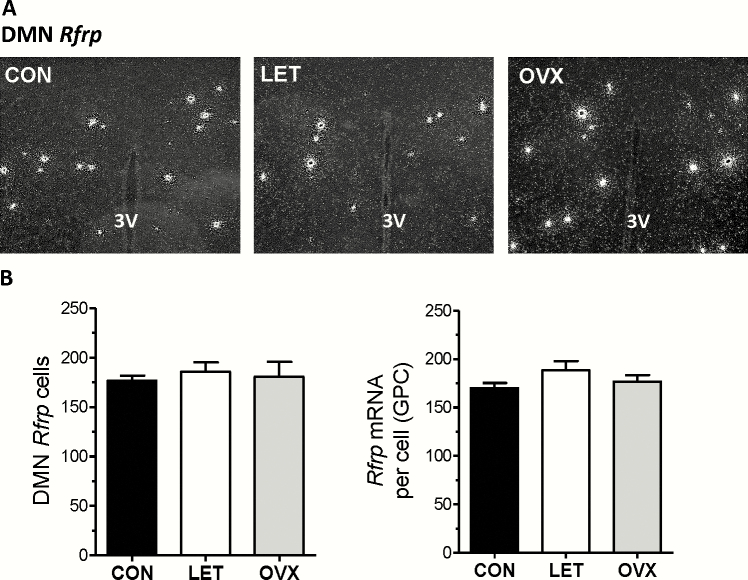
Finally, we also assessed Rfrp expression levels in the DMN (Fig. 7A), as RFRP-3 has been implicated as an inhibitor of reproductive hormone secretion (53–57). We therefore tested whether LET mice have decreased Rfrp levels, which may indicate less inhibition on the reproductive axis and, thereby, higher GnRH/LH secretion. Contrary to this hypothesis, we did not detect a significant change in Rfrp expression in LET mice versus control mice or OVX mice (Fig. 7B).
Figure 7.
Unaltered Rfrp gene expression in the DMN of LET mice. A) Representative microscope image of Rfrp mRNA expression in the DMN, as determined by radiolabeled in situ hybridization. B) Mean Rfrp cell number and Rfrp mRNA per cell in the DMN. There were no significant differences in Rfrp measures between any groups.
ARC Kiss1 neuron activation is elevated in LET female mice.
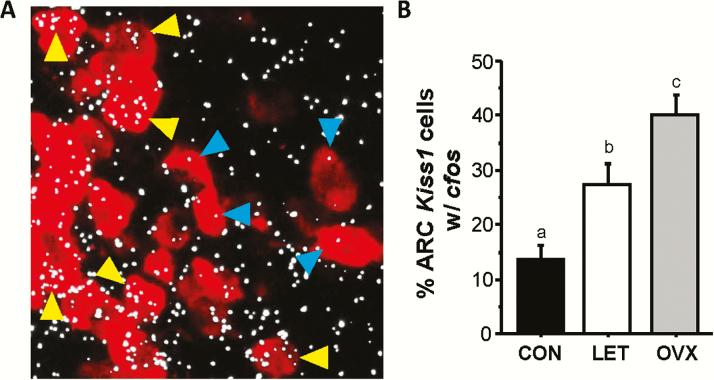
In addition to changes in reproductive neuropeptide gene expression, it is also possible that the activation and firing of reproductive neurons is altered in the LET PCOS-like condition. We hypothesized that LH levels in LET mice are elevated, in part, by enhanced ARC Kiss1/Tac2 neuron activation, which may be reflected by increased cfos mRNA induction in these neurons (a common measure of neuronal activation). We used double-label ISH to measure Kiss1+cfos mRNA coexpression in LET, control, and OVX mice. We found that LET mice had significantly elevated neuronal activation, by 2-fold, of ARC Kiss1 neurons compared to control females (P < 0.05; Fig. 8). Though elevated above controls, the degree of cfos+Kiss1 coexpression in LET brains did not reach the maximally high values observed in OVX females (Fig. 8), matching the lower LH pulse secretion and amplitude of LET versus OVX mice.
Figure 8.
Increased Kiss1 neuronal activation levels in the arcuate nucleus of LET mice. A) Representative microscope image of Kiss1 (red fluorescence) + cfos (silver grain clusters) mRNA co-expression in the ARC nucleus, as determined by double-label in situ hybridization. Yellow arrowheads denote some example co-labeled Kiss1 cells and blue arrowheads denote example Kiss1 cells lacking significant cfos co-labeling. B) Mean Kiss1+cfos co-expression in the ARC of each group, counted by investigator blind to treatment. Different letters above bars indicate significantly different from each other.
Discussion
Polycystic ovary syndrome is a complex disorder encompassing multiple phenotypic parameters, including neuroendocrine and ovarian impairments, and a high prevalence of metabolic perturbation (7, 8, 58). We recently reported a new LET mouse model that recapitulates many of the reproductive and metabolic components of the human overweight PCOS phenotype. Letrozole-treated female mice exhibit significantly elevated circulating androgens, a hallmark of women with PCOS (5, 59–61). In PCOS, hyperandrogenemia is driven by upstream increases in LH pulse secretion. We previously determined that LET mice exhibit increased pituitary Lhb mRNA expression and elevated “one-off” measures of circulating LH levels, but endogenous LH pulse patterns were not previously studied due to long-standing technical challenges. Regardless, the increased LH:FSH ratio originally reported for LET mice was suggestive of enhanced upstream pulsatile GnRH secretion, a neuroendocrine hallmark of PCOS (62). However, until now, there have been no studies in LET animals investigating the presence of persistently rapid GnRH/LH pulsatility. Moreover, the neural alterations underlying the rapid GnRH/LH pulses of PCOS women are completely unknown, and the present study is the first to assess possible brain changes in the LET PCOS-like mouse model (summarized in Fig. 9).
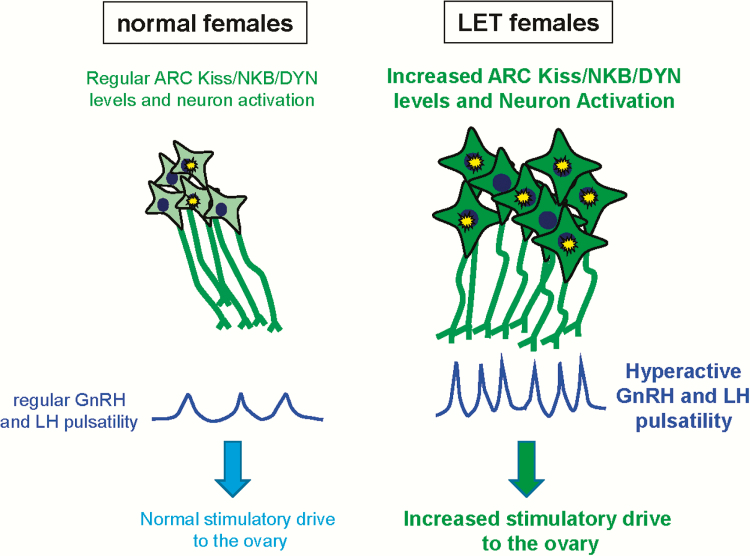
Figure 9.
Cartoon summary of neuroendocrine changes in the LET PCOS-like model. Letrozole-treated females exhibit greater numbers of detectable Kiss1/Tac2/Pdyn cells and higher Kiss1, Tac2, and Pdyn mRNA levels per cell in the ARC nucleus (represented by larger cell size and darker green color), as well as increased ARC Kiss1 neuron activation (represented by the yellow star bursts), relative to normal control females. Letrozole-treated females correspondingly demonstrate faster, higher amplitude LH pulse patterns compared to normal females, providing a greater gonadotropin drive to the ovary. Whether or not similar brain alterations are present in some PCOS women is a possibility but is currently unknown.
In the present study, we analyzed serial blood LH levels of LET mice in samples collected every 6 minutes for 2.5 hours. In clear contrast to control females, which had low LH levels with infrequent, low-amplitude pulse events (as previously reported [33, 63]), LET females demonstrated robustly elevated, high frequency LH pulses that included significant increases in pulse frequency, pulse amplitude, pulse peak levels, and basal levels. These increased LH pulse parameters, including frequency and amplitude, match similar increases reported for PCOS women (64–68). Thus, the LET model nicely recapitulates the endogenous hyperactive LH pulsatility that is a key neuroendocrine feature of PCOS.
Elevated LH pulses have also been reported for other mouse models of PCOS, in particular the PNA model and PAMH models (17, 69, 70). Interestingly, in those studies, LH pulses were more frequent in the PCOS-like condition, but basal levels and pulse amplitude were not increased relative to controls (17, 69, 70). Thus, only pulse frequency seems to be altered in the PNA and PAMH models, whereas pulse frequency, amplitude, and basal levels are modified in the LET model. It is also of interest that the elevated levels of LH in PNA mice are of lower concentration than the LH levels of LET mice. It is not clear if these differences are simply due to different hormone assay parameters or other technical differences, or if they represent real intrinsic differences in the pulse generator output in the LET versus PNA/PAMH models. If it’s the latter, this could provide valuable insights into what may be different etiologies or underlying mechanisms causing the altered LH pulses in each model, and this may relate to different mechanisms occurring across the heterogeneous spectrum of the PCOS disorder (ie, not all PCOS women are likely to have the same underlying cause for their elevated LH and T levels). Future measurement of in vivo LH pulses simultaneously in the different models within the same study will be useful to address this issue.
Women with PCOS have hyperactive LH pulses, but it is not fully understood why this is the case. Because LET females also have hyperactive LH pulses, we studied their brains to gain insight into possible underlying causes of such altered LH secretion. We previously reported no difference in GnRH mRNA levels in LET females (31), suggesting possible neural changes may be upstream of GnRH neurons. We hypothesized that arcuate kisspeptin neurons, which have been implicated as essential factors in the GnRH pulse generation mechanism, would show signs of increased activity in LET brains. Indeed, we found that both Kiss1 and Tac2 gene expression in the ARC were strongly upregulated in the LET condition, suggestive of a higher capacity for these ARC cells to make kisspeptin and NKB. Since both kisspeptin and NKB are stimulatory to GnRH pulse output, higher levels of these factors may contribute to elevated basal LH levels as well as increased LH amplitude and/or pulse frequency. We also measured the neuronal activation levels of kisspeptin cells in the ARC, using cfos mRNA induction as a proxy for neuronal activation. We found that ARC kisspeptin cells have a much greater neuronal activation in LET mice versus control mice, correlating with the faster, higher amplitude LH pulses in the former group. Collectively, these findings suggest that ARC kisspeptin/NKB neurons are “turned on” to a greater level in the LET-PCOS–like condition, thereby driving enhanced LH pulses (Fig. 9). The enhanced kisspeptin/NKB activation of GnRH secretion in LET mice suggested here may also act in concert with previously observed elevations in the pituitary GnRH receptor (31) to promote exaggerated LH synthesis, leading to heightened basal LH or pulse peak levels.
We studied gene expression for dynorphin in the ARC, as this neuropeptide plays a key role in the pulse generator process, acting to terminate kisspeptin/NKB neuron firing during pulses (51, 71). That is, for every pulse of kisspeptin secretion initiated by NKB, dynorphin signaling is thought to then terminate kisspeptin neuronal firing, acting as the “brake” to NKB’s “accelerator”. In this model, faster GnRH/LH pulses may require coordinated elevations in both NKB and dynorphin, as the latter would need to “keep up” with greater, more frequent NKB activity. This prediction was supported here by findings of elevated Pdyn levels in the ARC of LET females, relative to control females. In contrast, there was no difference in Rfrp levels in the DMN, suggesting that increased LH secretion in the LET condition is not due to diminished inhibitory RFRP-3 input onto the GnRH neural network but rather due to changes intrinsic to the GnRH pulse generator.
In contrast to our present focus on kisspeptin neurons, the PNA and PAMH models have focused on ARC gamma-aminobutyric acid (GABA) neurons, which show increased synaptic and stimulatory input onto GnRH neurons (11, 17, 69, 72). This enhanced GABA-ergic drive to GnRH neurons has been proposed to underlie increased pulsatile LH secretion in those mouse models. Future studies can assess whether GABA modulations also exist in the LET model, though it is interesting to reiterate that the reported LH pulse profiles of the 2 models do not appear similar in pulse peak or basal levels, and it is therefore possible that the different models have different underlying neural changes that impinge on GnRH output, as might occur in different cases of PCOS.
In the present study, we compared LH pulse parameters and brain gene expression of LET mice with those of OVX littermates. In the OVX condition, all ovarian steroid feedback is removed, allowing the GnRH pulse generator to be maximally active, resulting in very rapid, high amplitude LH pulses. We found that, despite being elevated and rapid, the LH pulses of LET females were not as elevated or rapid as in OVX females, with LET LH values being 18% to 40% lower than OVX (depending on the specific parameter [see Fig. 3]). Thus, the LH pulses of LET females are markedly hyperactive compared to normal controls, but they are not to maximal levels. Similarly, although they were elevated versus controls, Kiss1, pdyn, and cfos coexpression levels in LET females were all significantly lower, by 20% to 35%, than in OVX females. Collectively, these data indicate that the LET model is not just mimicking OVX, as the 2 conditions do not equate in multiple LH or brain measures. This is further supported by findings that (1) E2 is reduced but not completely absent in LET female rats (in which E2 values are above the lower limits of assay detection [18, 30); (2) certain E2-sensitive measures like Pgr mRNA levels are not radically diminished in LET mice relative to controls (31); and (3) FSH levels and Fshβ mRNA are both decreased in LET females but markedly increased in OVX females (31). Unfortunately, in mice, endogenous E2 levels are very low, and most assays are not sensitive enough to accurately detect low endogenous nonproestrus levels of E2. A previously-used E2 ELISA did not detect a difference between LET and control mice, though we did detect a significant difference using a more sensitive LCMS method. However, even that method was not sensitive enough to differentiate E2 values between LET and OVX. Without an even more sensitive assay, we cannot conclude at present that those 2 groups’ E2 differ or not. However, evidence summarized above suggests that E2 is likely reduced but not completely absent in LET mice, and that something is mechanistically different in the pulse generation process to result in faster and higher amplitude pulses, as well as higher Kiss1, Pdyn, and cfos levels in OVX versus LET females. The mechanistic underpinnings of those differences is unknown but will be an important area of future investigation.
Our findings suggest that ARC kisspeptin/NKB drive to GnRH neurons is a key facet of enhanced LH pulse secretion in the LET model, and perhaps PCOS as well. In support of this, a recent clinical study tested whether reducing NKB action would lower endogenous LH pulses in PCOS women (73). Excitingly, that study determined that the pharmacological antagonism of NK3R, the receptor for NKB, did in fact reduce LH pulsatility in PCOS subjects (73). This suggested that NKB action, which is known to stimulate kisspeptin neurons and GnRH neurons, may be exaggerated in some PCOS women; however, no direct measure of brain NKB or kisspeptin levels has been reported for PCOS women, owing to obvious technical limitations of brain analyses in humans. Our current findings of enhanced NKB levels, along with increased activation of ARC kisspeptin neurons (which contain NKB), supports the possibility that NKB (and/or kisspeptin) are good candidates for being altered in some PCOS women to drive their hyperactive LH secretion.
In summary, we show for the first time that, compared to control female mice, LET female mice exhibit very rapid, dramatically elevated in vivo LH pulsatility, with increases in pulse frequency, pulse amplitude, and basal levels, similar to LH patterns in PCOS women. Letrozole-treated mice also exhibit highly elevated Kiss1 and Tac2 expression, a corresponding increase in Pdyn levels, and enhanced Kiss1 neuronal activation in the hypothalamic ARC nucleus. These findings indicate that LET mice, like PCOS women, have markedly elevated LH pulsatility, which likely drives their increased androgen secretion. Moreover, the increased hypothalamic kisspeptin and NKB drive to downstream GnRH neurons may be a fundamental contributor to the hyperactive endogenous LH pulse secretion in the LET PCOS-like condition. Whether similar brain alterations also contribute, fully or in part, to the rapid LH pulses of some PCOS women is a possibility that currently remains unknown and an important future direction.
Acknowledgments
We thank Paige Steffen, Veronika Peterka, and Ruby Parra for their technical support.
Financial Support: This research was supported by UCSD grants NIH P50 HD012303, R01 HD090161, and R01 HD082567 and University of Virginia grant NIH P50 HD28934.
Additional Information:
Disclosure Statement: The authors have nothing to disclose.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Zadwadski J, Dunaif A. 1992. Diagnostic cirteria for polysystic ovary syndrome: toward a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystc Ovary Syndrome. Oxford: Blackwell Scientific; 377–384. [Google Scholar]
- 2. Azziz R, Carmina E, Dewailly D, et al. ; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. [DOI] [PubMed] [Google Scholar]
- 3. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 4. Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 5. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. [DOI] [PubMed] [Google Scholar]
- 6. Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25(5):352–359. [DOI] [PubMed] [Google Scholar]
- 7. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12(4):351–361. [DOI] [PubMed] [Google Scholar]
- 8. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 9. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 10. Abbott DH, Barnett DK, Levine JE, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71(9):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 16. Caldwell AS, Eid S, Kay CR, et al. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156(4):1441–1452. [DOI] [PubMed] [Google Scholar]
- 17. Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maliqueo M, Sun M, Johansson J, et al. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology. 2013;154(1):434–445. [DOI] [PubMed] [Google Scholar]
- 19. Naessen T, Kushnir MM, Chaika A, et al. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil Steril. 2010;94(6):2228–2233. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Shen S, Tan Y, et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J Ovarian Res. 2015;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franik G, Maksym M, Owczarek AJ, Chudek J, Madej P, Olszanecka-Glinianowicz M. Estradiol/testosterone and estradiol/androstenedione indexes and nutritional status in PCOS women—a pilot study. Eur J Obstet Gynecol Reprod Biol. 2019;242:166–169. [DOI] [PubMed] [Google Scholar]
- 22. Xita N, Lazaros L, Georgiou I, Tsatsoulis A. CYP19 gene: a genetic modifier of polycystic ovary syndrome phenotype. Fertil Steril. 2010;94(1):250–254. [DOI] [PubMed] [Google Scholar]
- 23. Xita N, Chatzikyriakidou A, Stavrou I, Zois Ch, Georgiou I, Tsatsoulis A. The (TTTA)n polymorphism of aromatase (CYP19) gene is associated with age at menarche. Hum Reprod. 2010;25(12):3129–3133. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Li Q, Wang T, et al. A common polymorphism in the human aromatase gene alters the risk for polycystic ovary syndrome and modifies aromatase activity in vitro. Mol Hum Reprod. 2011;17(6):386–391. [DOI] [PubMed] [Google Scholar]
- 25. Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci USA. 1993;90(24):11673–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang XL, Zhang CW, Xu P, et al. SNP rs2470152 in CYP19 is correlated to aromatase activity in Chinese polycystic ovary syndrome patients. Mol Med Rep. 2012;5(1):245–249. [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Li S, Zhao A, et al. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J Steroid Biochem Mol Biol. 2012;132(1-2):120–126. [DOI] [PubMed] [Google Scholar]
- 28. Yang F, Ruan YC, Yang YJ, et al. Follicular hyperandrogenism downregulates aromatase in luteinized granulosa cells in polycystic ovary syndrome women. Reproduction. 2015;150(4):289–296. [DOI] [PubMed] [Google Scholar]
- 29. Franik G, Madej P, Guz-Lem M, Owczarek A, Chudek J, Olszanecka-Glinianowicz M. Daytime decrease of prolactin levels is associated with PCOS regardless to nutritional status and other hormones levels. Gynecol Endocrinol. 2017;33(5):336–341. [DOI] [PubMed] [Google Scholar]
- 30. Mannerås L, Cajander S, Holmäng A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. [DOI] [PubMed] [Google Scholar]
- 31. Kauffman AS, Thackray VG, Ryan GE, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skarra DV, Hernández-Carretero A, Rivera AJ, Anvar AR, Thackray VG. Hyperandrogenemia induced by letrozole treatment of pubertal female mice results in hyperinsulinemia prior to weight gain and insulin resistance. Endocrinology. 2017;158(9):2988–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 34. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang JA, Song CI, Hughes JK, et al. Acute psychosocial stress inhibits LH pulsatility and kiss1 neuronal activation in female mice. Endocrinology. 2017;158(11):3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arroyo P, Ho BS, Sau L, Kelley ST, Thackray VG. Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome. Plos One. 2019;14(9):e0223274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torres PJ, Skarra DV, Ho BS, et al. Letrozole treatment of adult female mice results in a similar reproductive phenotype but distinct changes in metabolism and the gut microbiome compared to pubertal mice. BMC Microbiol. 2019;19(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol. 2018;239(3):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kreisman M, McCosh R, Tian K, Song C, Breen K. Estradiol enables chronic corticosterone to inhibit pulsatile LH secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2019. doi: 10.1159/000502978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stephens SBZ, Rouse ML, Tolson KP, et al. Effects of selective deletion of tyrosine hydroxylase from kisspeptin cells on puberty and reproduction in male and female mice. eNeuro. 2017;4(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology. 2016;157(10):4021–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poling MC, Luo EY, Kauffman AS. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology. 2017;158(10):3565–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chowen JA, Clifton DK. Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci. 1991;5:137–158. [Google Scholar]
- 45. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–3736. [DOI] [PubMed] [Google Scholar]
- 51. Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. Elife 2016;5:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol. 2009;30(3):371–378. [DOI] [PubMed] [Google Scholar]
- 54. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–1420. [DOI] [PubMed] [Google Scholar]
- 57. Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100(4):317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moran LJ, Lombard CB, Lim S, Noakes M, Teede HJ. Polycystic ovary syndrome and weight management. Womens Health (Lond). 2010;6(2):271–283. [DOI] [PubMed] [Google Scholar]
- 59. Escobar ME, Ropelato MG, Ballerini MG, et al. Acceleration of luteinizing hormone pulse frequency in adolescent girls with a history of central precocious puberty with versus without hyperandrogenism. Horm Res. 2007;68(6):278–285. [DOI] [PubMed] [Google Scholar]
- 60. Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95(25):14956–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carmina E, Rosato F, Jannì A, Rizzo M, Longo RA. Extensive clinical experience: relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91(1):2–6. [DOI] [PubMed] [Google Scholar]
- 62. Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology. 2019;160(6):1480–1491. [DOI] [PubMed] [Google Scholar]
- 64. Welt CK, Taylor AE, Martin KA, Hall JE. Serum inhibin B in polycystic ovary syndrome: regulation by insulin and luteinizing hormone. J Clin Endocrinol Metab. 2002;87(12):5559–5565. [DOI] [PubMed] [Google Scholar]
- 65. Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 66. Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. [DOI] [PubMed] [Google Scholar]
- 67. Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82(11):3728–3733. [DOI] [PubMed] [Google Scholar]
- 68. Bachelot A, Laborde K, Bresson JL, et al. Luteinizing hormone pulsatility in patients with major ovarian hyperandrogenism. J Endocrinol Invest. 2007;30(8):636–646. [DOI] [PubMed] [Google Scholar]
- 69. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Coutinho E, Prescott M, Hessler S, Marshall C, Herbison A, Campbell RE. Activation of a classic hunger circuit slows luteinizing hormone pulsatility. Neuroendocrinology. 2019. doi: 10.1159/000504225 [DOI] [PubMed] [Google Scholar]
- 71. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Silva MSB, Desroziers E, Hessler S, et al. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: implications for polycystic ovary syndrome. Ebiomedicine. 2019;44:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. George JT, Kakkar R, Marshall J, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–4321. [DOI] [PubMed] [Google Scholar]