Abstract
Objective
To characterize the body composition of Prader-Willi syndrome (PWS) subjects and compare with simple obesity.
Research Methods and Procedures
Seventy-two individuals (27 PWS deletion, 21 PWS uniparental disomy, and 24 obese controls) 10 to 49 years old were studied with the use of DXA. Body composition measures were obtained, and regional fat and lean mass patterns were characterized. Significant differences were assessed with Student’s t test and ANOVA adjusting for age, gender, and BMI.
Results
Significant differences between the PWS and obese groups were found for lean measures of the arms, legs, and trunk. Total lean mass was significantly lower in PWS than in obese subjects for arms, trunk, and especially legs. Furthermore, two body regions (legs and trunk) showed significant differences for fat and lean measures between PWS and obese males. However, significant differences between PWS and obese females for these measures were found only for the legs. No significant differences were identified between PWS deletion and uniparental disomy subjects.
Discussion
Our results demonstrate that PWS individuals do, in fact, have an unusual body composition and fatness patterns, characterized by reduced lean tissue and increased adiposity, with PWS males contributing most with fat patterns more similar to females.
Keywords: fat and lean ratios, percentage fat, percentage lean, Prader-Willi syndrome, genetic subtypes
Introduction
Prader-Willi syndrome (PWS)1 is characterized by minor facial anomalies, small hands and feet, growth retardation, hypotonia and poor suck reflex, hypogonadism, learning and behavioral problems, and onset of obesity in early childhood (1–6). Approximately 70% of PWS subjects present with a paternally derived 15q11-q13 deletion, maternal disomy 15 [uniparental disomy (UPD)] or both chromosome 15s from the mother in 25% of cases, and the remaining subjects have genomic imprinting defects or chromosome 15 rearrangements (6).
PWS is the most common known genetic cause of marked obesity (3), although PWS infants are generally underweight. Hyperphagia and obesity develop in early childhood. However, their body composition is disturbed in infancy with a higher level of body fat (7) and increased fat noted during all stages of life along with reduced lean mass, bone mineral content, and bone density (8–13). Therefore, we studied body composition and fatness distribution in a relatively large cohort of subjects with PWS and simple obesity to better characterize the fatness patterns in this classic genetic obesity syndrome.
Research Methods and Procedures
Subjects
The study comprised 48 PWS subjects (27 with a 15q11-q13 deletion detected by fluorescence in situ hybridization using 15q11-q13 probes and 21 individuals with UPD identified by polymorphic DNA microsatellites from this chromosome region). Methylation testing was consistent with the diagnosis of PWS in all PWS subjects (14,15). The male-to-female ratio in the PWS group was 21:27, and age range was 10 to 45 years, with a mean of 22.5 years.
The PWS deletion group consisted of 11 males and 16 females with a mean age of 22 years, whereas the PWS UPD group consisted of 10 males and 11 females with a mean age of 23 years. The obese group comprised 24 non-syndromic individuals (nine males and 15 females) with simple obesity of unknown cause with an age range of 11 to 49 years and a mean age of 26 years. All three groups consisted of mostly white individuals, and no subjects were on growth or thyroid hormone treatment. Three of the 24 obese subjects and eight of the 48 PWS individuals had a history of type 2 diabetes; nonetheless, only two obese subjects along with five PWS subjects were on insulin therapy in the past, and fewer (one obese and three PWS) were being treated with insulin at the time of study.
Height to the nearest 0.1 cm and weight to the nearest 0.1 kg were obtained on each subject along with waist circumference measured to the nearest 0.1 cm using a steel tape measure at the umbilicus level in standing position. Hip circumference was measured to the nearest 0.1 cm at the greater trochanter level. BMI was calculated (kilograms per meter squared) with obesity defined as BMI ≥30 for adults (≥18 years of age) and a BMI >95th percentile using published standards for subjects <18 years old (16).
Body Composition Determination
We measured percentage body fat, fat mass, fat-free mass, bone mineral density, and bone mineral content with DXA from Lunar Corporation (Madison, WI). Subjects were placed in supine position, and the entire body was scanned from the top of the head down to the feet. Measurements were made for four different regions (head, arms, trunk, and legs) and for the body as a whole.
Statistical Analyses
Student’s t test was used to determine whether significant differences existed for age and BMI between the PWS and obese groups or gender differences in subjects with PWS or simple obesity. When a subset of PWS subjects was selected for analysis (e.g., fatness pattern) from the entire PWS group to match for age and gender with selected obese subjects, the Student’s t test was utilized to characterize differences between the selected groups for BMI, total body fat mass, and total body lean mass and for fat mass and lean mass divided into the three body regions (trunk, arms, and legs). These results are represented in figures as percentage change or deviation from the overall mean calculated from the combined subject groups for each variable. The overall mean is subtracted from the individual averages for each variable in each group. Therefore, the result from this calculation is then divided by the overall mean to achieve the percentage difference from the overall mean as described elsewhere (17). For example, for BMI, the averages of 40.6 for the matched obese and 33.0 for the matched PWS subject groups are combined, thus resulting in an overall mean (which equals 36.8) between the two matched subject groups. The percentage change from this overall mean is then calculated by subtracting 36.8 from 40.6 (which equals 3.8) and divided by 36.8, giving an overall percentage change of −10.3% for the PWS group. Between-group comparisons of fatness patterning using all subjects were carried out with the use of the ANOVA univariate general linear model correcting for age, gender, and BMI. Confidence intervals (CIs) were used to better characterize the data and were set at 99.94% for ANOVA results and 99.44% for the Student’s t test after correcting for multiple analyses using Bonferroni adjustments. Statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL).
Results
Analysis in PWS and Obese Subjects
Demographic and anthropometric data are shown in Table 1. Significant differences between PWS and obese subjects were found for weight, height, BMI, and lean BMI (see Table 1). PWS subjects had smaller measurements than obese subjects for all four categories. Total and regional body composition of fat and lean components for PWS and obese subjects adjusting for age, gender, and BMI are shown in Table 2. Total lean mass was significantly lower in PWS than in obese subjects for arms, trunk, and especially legs. Tissue percentage fat and tissue percentage lean measurements in the PWS subjects indicate significantly more adipose tissue relative to lean tissue compared with obese subjects. Similarly, fat-to-lean mass ratios were significantly higher for arms and legs of PWS subjects than in obese subjects (see Table 2). With respect to ratio measures, the ratios with the most change were leg fat-to-lean mass and leg lean-to-total body tissue.
Table 1.
Demographic and anthropometric measurements of Prader-Willi syndrome and obese subjects
| Subject groups | M:F | Age (yr) (mean ± SD) | Weight (kg) (mean ± SD) | Height (cm) (mean ± SD) | BMI (kg/m2) (mean ± SD) | Lean BMI* (mean ± SD) | Tissue % fat (mean ± SD) | Tissue % lean (mean ± SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Prader-Willi Syndrome | Deletion | 11:16 | 21.8 ± 8.0 | 79.1 ± 22.5 | 150.1 ± 7.9 | 34.7 ± 9.0 | 15.1 ± 2.5 | 52.6 ± 7.4 | 47.4 ± 7.4 |
| UPD | 10:11 | 23.3 ± 9.3 | 76.1 ± 20.9 | 148.8 ± 8.0 | 33.8 ± 9.1 | 15.8 ± 2.8 | 49.5 ± 7.7 | 50.5 ± 7.7 | |
| Total | 21:27 | 22.5 ± 8.5 | 77.8 ± 21.6‡ | 150.4 ± 7.8‡ | 34.3 ± 9.0† | 15.4 ± 2.6‡ | 52.2 ± 0.79 | 47.8 ± 0.79 | |
| Obese | 09:15 | 26.5 ± 13.1 | 108.4 ± 24.6‡ | 162.3 ± 9.4‡ | 41.0 ± 8.0† | 18.9 ± 2.7‡ | 47.0 ± 1.1 | 53.0 ± 1.1 | |
SD, standard deviation; UPD, uniparental disomy.
Lean BMI = lean mass (kg)/height (m)2.
p < 0.01
p < 0.001, according to Student’s t test.
Table 2.
Total and regional body composition of fat and lean components of Prader-Willi syndrome and obese subjects
| Variables | Obese (adjusted mean ± SD) (N) | Prader-Willi syndrome (adjusted mean ± SD) (N) | Confidence interval (adjusted to 99.94%) |
|---|---|---|---|
| Age (years) | 26.5 ± 13.1 (24) | 22.5 ± 8.5 (48) | |
| Gender (M:F) | 9:15 (24) | 21:27 (48) | |
| BMI (kg/m2) | 41.0 ± 8.0 (24)* | 34.3 ± 9.0 (48)* | |
| Body fat distribution | |||
| Tissue % fat | 47.0 ± 1.1 (24) | 52.2 ± 0.79 (48) | −10.4 to −0.05 |
| Tissue % lean | 53.0 ± 1.1 (24) | 47.8 ± 0.79 (48) | 0.05 to 10.4 |
| Waist-to-hip ratio | 0.97 ± 0.02 (24) | 0.97 ± 0.02 (39) | −0.10 to 0.10 |
| Waist-to-height ratio | 0.73 ± 0.01 (24) | 0.77 ± 0.01 (39) | −0.11 to 0.03 |
| Waist-to-thigh ratio | 1.9 ± 0.06 (24) | 2.0 ± 0.05 (39) | −0.41 to 0.15 |
| Trunk-to-limbs fat mass ratio | 0.87 ± 0.04 (24) | 0.86 ± 0.03 (48) | −0.17 to 0.20 |
| Trunk-to-limbs lean mass ratio | 1.1 ± 0.03 (24) | 1.1 ± 0.02 (48) | −0.21 to 0.10 |
| Arms-to-legs fat mass ratio | 0.85 ± 0.08 (24) | 0.69 ± 0.06 (48) | −0.21 to 0.54 |
| Arm measures | |||
| Total arms fat (g) | 9840 ± 537 (24) | 8989 ± 369 (48) | −1568 to 3269 |
| Total arms lean (g) | 6358 ± 317 (24) | 4452 ± 218 (48) | 478 to 3333 |
| Arms % fat | 59.2 ± 1.5 (24) | 63.3 ± 1.1 (48) | −11.0 to 2.8 |
| Arms % lean | 40.9 ± 1.4 (24) | 35.9 ± 0.98 (48) | −1.3 to 11.5 |
| Arms fat-to-lean mass ratio | 1.5 ± 0.09 (24) | 1.9 ± 0.06 (48) | −0.77 to −0.001 |
| Total arms fat-to-total body tissue ratio | 0.10 ± 0.01 (24) | 0.11 ± 0.004 (48) | −0.03 to 0.02 |
| Total arms lean-to-total body tissue ratio | 0.07 ± 0.003 (24) | 0.06 ± 0.002 (48) | −0.003 to 0.03 |
| Leg measures | |||
| Total legs fat (g) | 12479 ± 568 (24) | 12751 ± 390 (48) | −2833 to 2288 |
| Total legs lean (g) | 16012 ± 530 (24) | 11870 ± 365 (48) | 1750 to 6533 |
| Legs % fat | 43.0 ± 1.3 (24) | 50.6 ± 0.87 (48) | −13.3 to −1.9 |
| Legs % lean | 56.3 ± 1.2 (24) | 49.5 ± 0.81 (48) | 1.5 to 12.1 |
| Legs fat-to-lean mass ratio | 0.78 ± 0.05 (24) | 1.1 ± 0.03 (48) | −0.49 to −0.08 |
| Total legs fat-to-total body tissue ratio | 0.14 ± 0.01 (24) | 0.16 ± 0.004 (48) | −0.05 to 0.004 |
| Total legs lean-to-total body tissue ratio | 0.18 ± 0.004 (24) | 0.16 ± 0.003 (48) | 0.001 to 0.04 |
| Trunk measures | |||
| Total trunk fat (g) | 18283 ± 715 (24) | 17355 ± 491 (48) | −2296 to 4153 |
| Total trunk lean (g) | 24195 ± 867 (24) | 18543 ± 5960 (48) | 1744 to 9559 |
| Trunk % fat | 43.2 ± 1.2 (24) | 47.7 ± 0.83 (48) | −10.0 to 0.89 |
| Trunk % lean | 56.9 ± 1.2 (24) | 52.3 ± 0.82 (48) | −0.83 to 10.0 |
| Trunk fat-to-lean mass ratio | 0.77 ± 0.04 (24) | 0.94 ± 0.03 (48) | −0.35 to 0.02 |
| Total trunk fat-to-total body tissue ratio | 0.20 ± 0.01 (24) | 0.22 ± 0.01 (48) | −0.05 to 0.01 |
| Total trunk lean-to-total body tissue ratio | 0.26 ± 0.01 (24) | 0.24 ± 0.01 (48) | −0.01 to 0.06 |
SD, standard deviation. All calculations were executed with one-way ANOVA adjusting for age, gender, and BMI, except for the variables age, gender, and BMI that were analyzed with Student’s t test. An adjusted calculated mean value was considered significant if the confidence interval did not include the number 0. Because of multiple analyses with ANOVA, confidence intervals were adjusted to 99.94% using the Bonferroni correction.
p < 0.01 with Student’s t test.
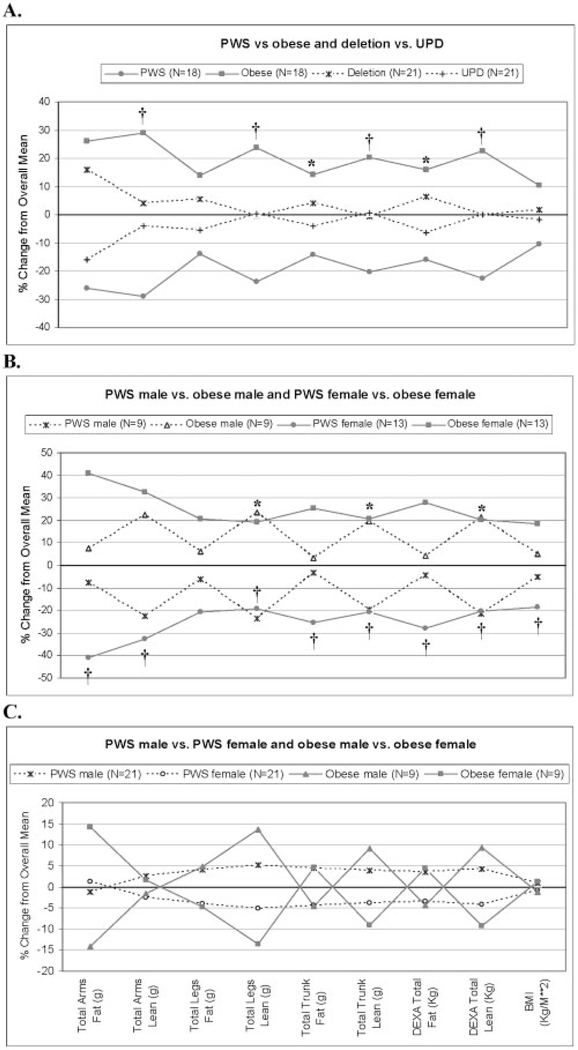
The comparison of body composition variables between a subset of PWS subjects chosen to match for age and gender with obese individuals is illustrated in Figure 1A. The obese subjects were overall heavier than the PWS subjects, but PWS subjects had significantly less lean tissue in the arms, legs, and trunk and significantly less fat mass in the trunk (Figure 1A). Total body fat and total body lean were also significantly different between the two groups.
Figure 1:
(A) Percentage change from overall mean for fat and lean mass distribution variables between PWS and obese subjects and between PWS genetic subtypes matched for age and gender. N = 9 males and 9 females for both PWS and obese groups, and N = 9 males and 11 females for both PWS genetic subtypes. PWS mean age, 24.3 ± 11.3 years; obese mean age, 24.4 ± 11.9 years; PWS deletion mean age, 23.3 ± 8.1 years; PWS UPD mean age, 23.3 ± 9.3 years. The graph represents differences seen in regional and total body composition measures between matched obese and PWS subjects, with obese subjects having a higher percentage change from overall mean between the two groups for all measures compared with PWS individuals. Little to no difference in percentage change from overall mean was seen in PWS deletion or PWS UPD subjects. * p < 0.01. † p < 0.001 with Student’s t test. (B) Percentage change from overall mean for fat and lean mass distribution variables between PWS and obese males matched for age and between PWS and obese females matched for age. PWS males (N = 9), mean age, 21.8 ± 10.4 years; obese males (N = 9), mean age, 21.1 ± 10.1 years. PWS females (N = 13), mean age, 26.9 ± 7.9 years; obese females (N = 13), mean age, 27.1 ± 12.9 years. The graph represents differences seen in regional and total body composition measures between matched obese and PWS males and females, with obese females having a higher percentage change from overall mean for all measures compared with PWS females. However, only the lean measures showed higher percentage change in obese males compared with PWS males. * p < 0.01 with Student’s t test between PWS and obese males. † p < 0.001 with Student’s t test between PWS and obese females. (C) Percentage change from overall mean for fat and lean mass distribution variables between obese males and females matched for age and between male and female PWS subjects matched for age. Obese males (N = 9), mean age, 21.1 ± 10.1 years; obese females (N = 9), mean age, 21.1 ± 10.9 years. PWS males (N = 21), mean age, 23.8 ± 9.4 years; PWS females (N = 21), mean age, 23.1 ± 8.6 years. The graph represents little to no difference in body composition measures between matched PWS male and female subjects, whereas males in the obese group showed higher percentage change for lean measures, as expected, compared with obese females. This indicates that PWS males have a body composition pattern more similar to PWS females. No significant differences were found among the variables with Student’s t test.
Few observable changes were seen in comparison of body composition measures for PWS deletion and UPD subjects (Figure 1A). Although not significant, arm fat appeared greater in the PWS deletion subjects.
Analysis in PWS and Obese Male Subjects
The obese male subjects had significantly higher tissue percentage lean than PWS males (CI, 1.5 to 17.0), whereas the opposite was observed with tissue percentage fat (CI, −17.0 to −1.5) (data not shown). This difference is seen for arms, legs, and trunk regions; however, it was only significant for legs (CI, −19.0 to −0.21 for percentage fat and CI, 0.21 to 19.0 for percentage lean) and trunk (CI, −16.0 to −1.4 for percentage fat and CI, 1.4 to 16.0 for percentage lean). The lean mass for both the legs (CI, 2128 to 11,269) and trunk (CI, 1839 to 15,406) regions were also significantly different between the two groups. The ratios of fat-to-lean masses were significantly different between the two subject groups for arms (CI, −1.2 to −0.03) and trunk (CI, −0.50 to −0.05), and the ratio of total leg lean-to-total body tissue.
The comparison of body composition variables between age-matched PWS and obese males can be seen in Figure 1B. Significantly greater differences were seen for leg lean mass, trunk lean mass, and total body lean mass in the obese males, agreeing with the results described above.
Analysis in PWS and Obese Female Subjects
The only significant difference seen between PWS and obese females was in leg lean mass (CI, 586 to 5111), which was higher in the obese group (data not shown). According to Figure 1B, obese females had significantly greater arm fat, arm lean, leg lean, trunk fat, trunk lean, total body fat, total body lean, and BMI compared with age-matched female PWS subjects.
Discussion
One of the most prominent characteristics of PWS individuals is obesity. Clinical features suggest that their body composition is different from what is observed in subjects with simple obesity, but there is a paucity of reported body composition and fatness pattern data in PWS. Therefore, we studied and characterized body composition and fatness patterning in a group of 48 PWS male and female subjects and 24 individuals with simple obesity.
Our data shows that, although obese individuals have more overall fat and lean mass compared with PWS subjects (Figure 1A), PWS subjects have increased adiposity with significantly less lean mass in all body regions studied than individuals with simple obesity (Table 2). These observations are consistent with the findings reported by Brambilla et al. (11). The ratios of fat to lean mass for the different body regions for PWS individuals were higher than for obese subjects, representing a relative increase in fat tissue and a decrease in lean mass in PWS individuals. This observation was also reported in a study by Goldstone et al. (12). Although all areas or regions of the body were similarly involved in this disparity, as can be seen by the trunk-to-limb ratios, the leg regions showed the highest level of involvement in PWS. Moreover, most of the fat accumulation tended to be in the extraabdominal areas similar to the results reported by Marzullo et al. (18).
Significant differences between obese and PWS males were seen for overall tissue percentage fat and tissue percentage lean, percentage fat and percentage lean in the legs and trunk, lean mass in the legs and trunk, and for fat-to-lean mass ratio in the legs and trunk, whereas only leg lean mass were significantly different between the obese and PWS females (data not shown). Therefore, the PWS males contributed more to disparities seen in fat and lean tissue distribution than the PWS females when comparing all of the PWS subjects with obese subjects. However, no significant differences were found when comparing PWS genetic subtypes or when looking for gender differences within the PWS and obese groups individually.
In conclusion, PWS subjects have more fat and less lean tissue relative to individuals with simple obesity, with males contributing more to these discrepancies than females. Furthermore, PWS males presented with a more feminine fat pattern similar to PWS females (Figure 1C). This could be due to delayed sexual development and small gonads seen in PWS subjects and decreased testosterone levels in males, thus interfering with muscle growth and subsequent loss of subcutaneous fat that normally occurs in adolescent boys in the general population. Another possibility is that PWS males may be more efficient at fat deposition than normal individuals. Finally, the two PWS genetic subtypes had an overall similar body fat distribution.
Hence, PWS individuals demonstrated a peculiar body composition in relationship to individuals with simple obesity. In simple obesity, there is an increase in lean tissue along with an increase in adipose tissue compared with non-obese individuals. Yet, in PWS subjects, the amount of lean tissue does not keep pace with the increase in overall body weight as it does in simple obesity. In fact, PWS subjects have a similar body composition to that of growth hormone-deficient individuals in the general population and elderly sedentary adults, even though the extent of obesity in the PWS group exceeded that of the latter two selected subject groups (11,19). Studies have also suggested that PWS individuals may have an impairment of hypothalamic regulation of growth or other hormone secretions; however, further studies are needed to find the causes of this atypical body composition seen in PWS subjects.
Acknowledgments
We thank the subjects with PWS and simple obesity and their families for participating in the study and Steve Simon for statistical advice. This study was partially funded by the National Institute of Child Health and Human Development (Grants P01HD30329 and R01HD41672), by The Hall Foundation, and by a Physician Scientist Award (Children’s Mercy Hospital and Clinics).
Footnotes
Nonstandard abbreviations: PWS, Prader-Willi syndrome; UPD, uniparental disomy; CI, confidence interval.
References
- 1.Cassidy SB. Prader-Willi syndrome. Curr Probl Pediatr. 1984;14:1–55. [DOI] [PubMed] [Google Scholar]
- 2.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91: 398–402. [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy SB. Prader-Willi syndrome. J Med Genet. 1997;34: 917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinologist. 2000;10:3–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader-Labhart-Willi syndrome. J Pediatr. 1999; 134:222–5. [DOI] [PubMed] [Google Scholar]
- 8.Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism. 1988;37:115–20. [DOI] [PubMed] [Google Scholar]
- 9.Davies PS, Joughin C. Assessment of body composition in the Prader-Willi syndrome using bioelectrical impedance. Am J Med Genet. 1992;44:75–8. [DOI] [PubMed] [Google Scholar]
- 10.van Mil EA, Westerterp KR, Gerver WJ, et al. Energy expenditure at rest and during sleep in children with Prader-Willi syndrome is explained by body composition. Am J Clin Nutr. 2000;71:752–6. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr. 1997;65: 1369–74. [DOI] [PubMed] [Google Scholar]
- 12.Goldstone AP, Brynes AE, Thomas EL, et al. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole-body magnetic resonance imaging in women with Prader-Willi syndrome. Am J Clin Nutr. 2002;75:468–75. [DOI] [PubMed] [Google Scholar]
- 13.Butler MG, Haber L, Mernaugh R, Carlson MG, Price R, Feurer ID. Decreased bone mineral density in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet. 2001;103:216–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Butler MG, Christian SL, Kubota T, Ledbetter DH. A 5-year-old white girl with Prader-Willi syndrome and a sub-microscopic deletion of chromosome 15q11q13. Am J Med Genet. 1996;65:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler MG, Bittel D, Talebizadeh Z. Prader-Willi syndrome and a deletion/duplication within the 15q11–q13 region. J Med Genet. 2002;39:202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;8:1–27. [PubMed] [Google Scholar]
- 17.Meaney FJ, Butler MG. Characterization of obesity in the Prader-Labhart-Willi syndrome: fatness patterning. Med Anthropol Q. 1989;3:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzullo P, Marcassa C, Campini R. The impact of GH/IGF-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab. 2005;90:5639–46. [DOI] [PubMed] [Google Scholar]
- 19.Forbes GB. A distinctive obesity: body composition provides the clue. Am J Clin Nutr. 1997;65:1540–1. [DOI] [PubMed] [Google Scholar]