Abstract
Background
Plaque rupture (PR) and plaque erosion (PE) are main causes of acute myocardial infarction with different demographic and histology characteristics and need different treatment strategy. PR and PE can be identified with optical coherence tomography (OCT) accurately, but convenient and effective noninvasive markers for them are rarely found. History of diabetes mellitus (DM) was reported to be a potential predictor of PR in ST-segment elevated myocardial infarction (STEMI) patients, but the predictive value of other glucose-related variables for it is still uncertain. Present study aimed to clear the relationship between some glucose-related variables and plaque morphology in patients with STEMI.
Methods
We consecutively enrolled 872 STEMI patients and divided them into PR group (n = 616) and PE group (n = 256) based on OCT diagnostic criteria. The relationship of glucose-related variables, including random plasma glucose on admission (ARPG), glycosylated hemoglobin (HbA1c), post-PCI fasting plasma glucose (PFPG), DM history, glucose variable tendency (GVT) and the acute-to-chronic glycemic ratio (A/C), to the PR risk of STEMI patients was analyzed. The correlation between the glucose-related variables and plaque morphology was analyzed meanwhile.
Results
Among the glucose-related variables, ARPG and GVT were confirmed to be independent predictors for PR after adjusting for other traditional risk factors in nondiabetic patients. The higher the ARPG level, the more PR risk the STEMI patients had. And high HbA1c and APPG were demonstrated to have a weak and positive correlation with lipid constituents and stenosis degree of culprit vessel.
Conclusions
Compared to HbA1c, DM history, and some other glucose-related variables, ARPG and GVT were risk factors for PR in STEMI patients, especially those without DM. And high HbA1c and ARPG were positively correlated with the development of vulnerable plaque in culprit vessels.
Trial registration Present study is a retrospective one and the population came from the EROSION study of our center previously. It was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval reference number, KY2017-249), and all patients provided written informed consent prior to the inclusion in the study and the investigation conformed to the principles outlined in the Declaration of Helsinki.
Keywords: Admission glucose, Plaque erosion, Plaque rupture, ST-segment elevated myocardial infarction
Background
Acute myocardial infarction (AMI) is the main cause of life threat in patients with coronary heart disease (CHD). Many studies have shown the positive correlation between the bad outcome of AMI and glucose-related variables, such as serum glycosylated hemoglobin (HbA1c) [1, 2], the acute-to-chronic glycemic ratio (A/C) [3], both admission hyperglycemia (AH) and post-admission hyperglycemia [4, 5]. Plaque erosion (PE) and plaque rupture (PR), the two main causes of AMI, present different lesion morphology distinctively [6]. Same as hyperglycemia and diabetes mellitus (DM), lesion morphology may affect clinical outcomes of patients with ST-elevated myocardial infarction (STEMI) [7]. And different treatment strategy should be made for STEMI patients with PR and PE [8]. PR and PE can be identified with optical coherence tomography (OCT) accurately [9], but convenient and effective noninvasive markers for them are rarely found. A study in our center showed that the history of DM is a potential predictor of lesion morphology [10]. However, the association between other glucose-related variables and the lesion morphology causing STEMI patients is still unclear. Thus, we analyzed the association between some glucose-related variables [included HbA1c, admission glucose (ARPG), post-PCI fasting plasma glucose (PFPG), DM history, A/C, and glucose variation tendency (GVT)] and plaque morphology by using optical coherence tomography (OCT) in patients with ST-elevating myocardial infarction caused by PR or PE.
Methods
Design and population
This study is a subanalysis of the EROSION study [11]. For studying the association between glucose-related variables and plaque model of STEMI patients, a total of 1005 patients hospitalized within 24 h of symptom onset in the intensive cardiology care unit of the second affiliate hospital of Harbin Medical University who underwent coronary angiography and optical coherence tomography for STEMI between August 2014 and March 2017 were identified by a search strategy. Excluded those with bad images or categorized as neither PR nor PE, totally 872 patients with PR (n = 656) and PE (n = 216) were identified and divided into two groups according to optical coherence tomography (OCT) imaging. The definition of STEMI and identification of the culprit lesion were described previously [8] and identification of PR and PE was based on established OCT diagnostic criteria [11]. OCT-rupture was defined and categorized according to the imaging of fibrous cap disruption and the presence of thrombus, OCT-erosion was defined and categorized according to the absence of fibrous cap disruption and the presence of thrombus. The optimal medical therapy for STEMI was administered in every patient during hospitalization, according to the current guidelines.
Data collection
CHD risk factors of interest included age, gender, hypertension, DM history, hyperlipemia, AMI history and current smoking. All individuals had a 12-lead or 18-lead electrocardiogram measured and left ventricular ejection fraction (LVEF) was measured within 24 h of admission by an experienced practitioner using Simpson’s biplane method of disks. Blood glucose-related variables (ARPG, PFPG, and HbA1c), complete blood count, electrolytes, creatinine, lipid parameters, hypersensitive C-reactive protein (hs-CRP), and maximum Cardiac Troponin I (cTnI), as well as the data of coronary angiography and OCT images, were collected.
Quantitative coronary angiography (QCA) was performed using the Cardiovascular Angiography Analysis System (CAAS, 5.10, Pie Medical Imaging B.V., Maastricht, the Netherlands). Culprit vessels and lesion sites were identified, and the length of the culprit lesions, reference vessel diameter, minimal lesion diameter, and degree of diameter stenosis were measured. OCT data were recorded using the C7-XR OCT intravascular imaging system (OCT C7 Dragonfly, St. Jude Medical, St. Paul, MN, USA). Quantitative and qualitative analyses of underlying plaques were performed by two independent operators as described in a previous study [10]. Any discordance was resolved by consensus with a third reviewer. Using established diagnostic criteria [12], the plaque and thrombus type were identified based on established OCT diagnostic criteria, along with identification of microchannels, cholesterol crystals, macrophages, and thin-cap fibroatheroma (TCFA). The lesion length, minimal lumen area, minimal thickness of the fibrotic cap, length of the residual thrombus, length of the lipid core, maximal arc of the lipid core, and average arc of the lipid core were measured as described previously [13] (Fig. 1)
Fig. 1.
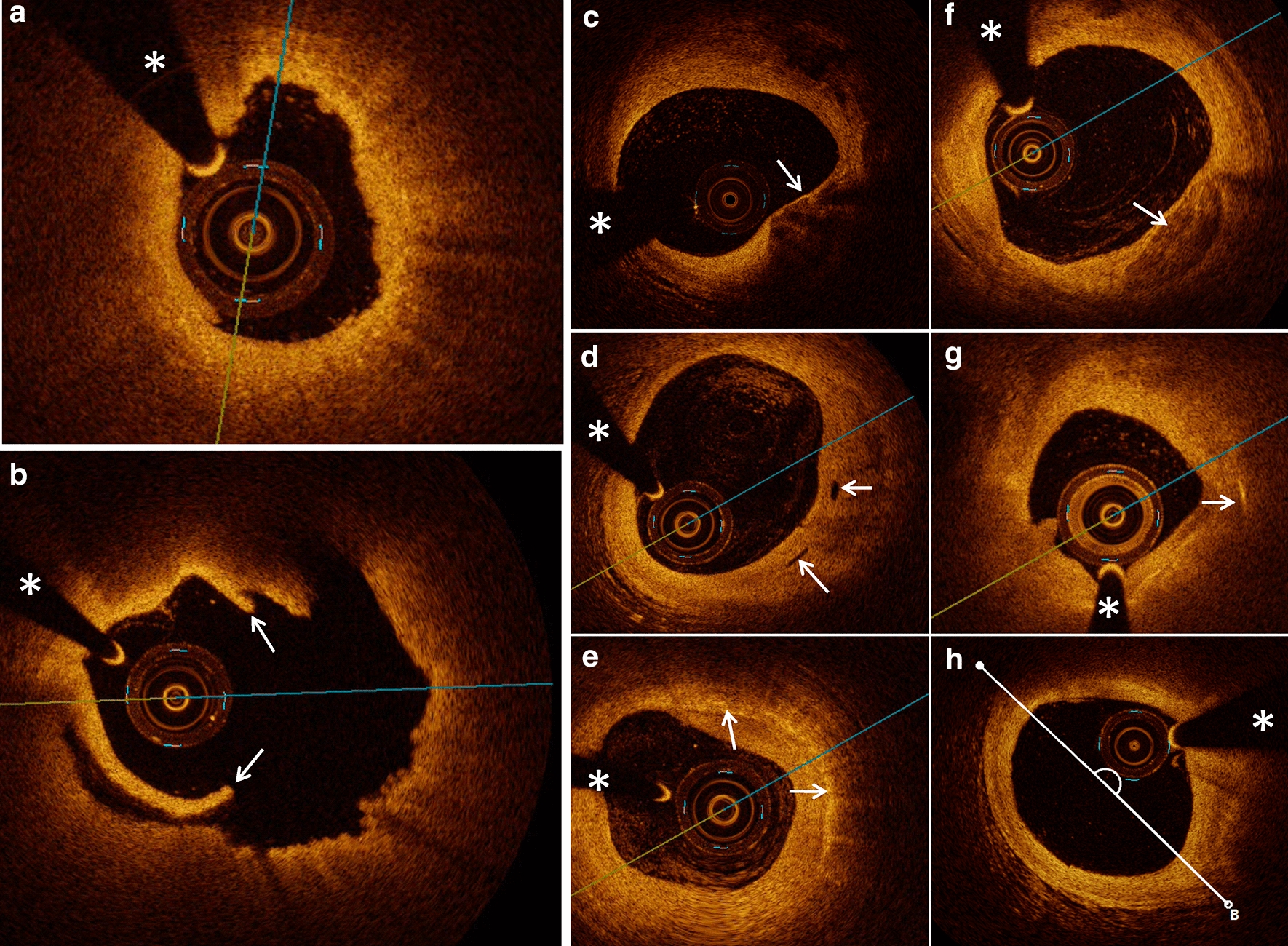
Typical optical coherence tomography (OCT) images of plaque characteristics. a A typical image of a patient with STEMI and plaque erosion characterized as intact and rouHbA1c intima attached with small white thrombus. b A typical image of a patient with STEMI and plaque rupture characterized as ruptured intima (arrow) backed with a big cavity. c–h Representative OCT images, including TCFA, micro-channel, macrophage accumulation, spotty calcification, cholesterol crystal, and lipid core measured with the lipid arc, are presented in turn (arrows). The asterisk marks the position of the OCT guide wire
Statistical analysis
Dichotomous variables were expressed as n (%) and continuous variables as mean ± SD or median (P25, P75), with normality of variables determined by the Kolmogorov–Smirnov test. For categorical data, Chi-squared or Fisher’s exact test was used, with comparisons of continuous data done by Student’s t test for normal variables or the Mann–Whitney test for non-Gaussian variables. Inter- and intra-observer reliability was assessed by Kappa statistics. A two-tailed P-value < 0.05 was considered statistically significant.
Patients were divided into four groups according to their blood ARPG levels: non AH group [ARPG < 6.1 mmol/L (< 110 mg/dL)]; mild AH group [6.1–6.9 mmol/L (110–125 mg/dL)]; moderate AH group [7.0–11.0 mmol/L (126–198 mg/dL)] and severe AH group [> 11.0 mmol/L (> 198 mg/dL)]. PFPG levels were stratified into four groups as follow: group I [PFPG < 5.6 mmol/L (< 100 mg/dL)]; group II [5.6–6.0 mmol/L (100–110 mg/dL)]; and group III [6.1–6.9 mmol/L (110–126 mg/dL)] and group IV [> 7.0 mmol/L (> 126 mg/dL)]. Patients were also divided into two groups according to their blood HbA1c levels: low HbA1c group (HbA1c < 6.5%) and high HbA1c group (HbA1c ≥ 6.5%). GVT was defined as the ARPG-to-PFPG ratio and grouped as GVT-normal group (ARPG < 6.1 mmol/L and PFPG < 5.6 mmol/L), GVT-up group (ARPG ≥ 6.1 mmol/L and/or PFPG ≥ 5.6 mmol/L, and ARPG/PFPG < 1), and GVT-down group (ARPG ≥ 6.1 mmol/L and/or PFPG ≥ 5.6 mmol/L, and ARPG/PFPG > 1). Chronic plasma glucose (CPG) was calculated by formula (CPG (mg/dL) = 28.7 × HbA1c-46.7). A/C was defined as the ARPG-to-CPG ratio (Table 2).
Table 2.
Logistic regression analysis of glucose-related variables between plaque rupture and erosion
| Exposure | Statistics | Non-adjusted | Adjustment I | Adjustment II |
|---|---|---|---|---|
| HbA1c (%) | 6.43 ± 1.44 | 1.19 (1.03, 1.36) 0.0153 | 1.13 (0.98, 1.30) 0.0915 | 1.12 (0.97, 1.30) 0.1264 |
| ARPG (mmol/L) | 9.30 ± 3.80 | 1.08 (1.03, 1.13) 0.0018 | 1.06 (1.01, 1.12) 0.0141 | 1.06 (1.01, 1.11) 0.0256 |
| PFPG (mmol/L) | 7.95 ± 3.56 | 1.03 (0.98, 1.08) 0.2441 | 1.01 (0.96, 1.06) 0.7984 | 1.01 (0.96, 1.06) 0.8090 |
| A/C | 1.25 ± 0.37 | 1.60 (0.94, 2.73) 0.0858 | 1.46 (0.85, 2.51) 0.1714 | 1.43 (0.83, 2.45) 0.1943 |
| DM history | ||||
| No | 634 (72.7%) | 1 | 1 | 1 |
| Yes | 238 (27.3%) | 1.53 (1.06, 2.21) 0.0231 | 1.42 (0.96, 2.09) 0.0800 | 1.38 (0.92, 2.05) 0.1155 |
| GVT | ||||
| Normal | 49 (6.4%) | 1 | 1 | 1 |
| Up | 210 (27.5%) | 2.83 (1.48, 5.39) 0.002 | 2.01 (1.02, 3.99) 0.045 | 2.03 (1.02, 4.07) 0.044 |
| Down | 504 (66.1%) | 2.83 (1.56, 5.14) 0.001 | 2.16 (1.15, 4.04) 0.016 | 2.04 (1.09, 3.85) 0.027 |
| Strati-HbA1c | ||||
| < 6.5 | 471 (70.2%) | 1 | 1 | 1 |
| ≥ 6.5 | 200 (29.8%) | 1.18 (0.45, 3.12) 0.7393 | 1.29 (0.47, 3.54) 0.6234 | 1.34 (0.48, 3.76) 0.5778 |
| Strati-ARPG | ||||
| < 6.1 | 117 (13.4%) | 1 | 1 | 1 |
| ≥ 6.1, < 7.1 | 144 (16.5%) | 1.41 (0.84, 2.37) 0.1909 | 1.25 (0.71, 2.20) 0.4396 | 1.17 (0.66, 2.08) 0.5945 |
| ≥ 7.1, < 11.1 | 412 (47.3%) | 2.07 (1.32, 3.24) 0.0014 | 1.63 (1.00, 2.67) 0.0517 | 1.58 (0.96, 2.61) 0.0736 |
| ≥ 11.1 | 199 (22.8%) | 2.29 (1.24, 4.21) 0.0080 | 2.05 (1.05, 3.99) 0.0345 | 1.99 (1.02, 3.90) 0.0438 |
| Strati-PFPG | ||||
| < 5.6 | 204 (26.7%) | 1 | 1 | 1 |
| ≥ 5.6, < 6.1 | 88 (11.5%) | 0.92 (0.53, 1.60) 0.7804 | 0.81 (0.46, 1.44) 0.4826 | 0.87 (0.49, 1.56) 0.6417 |
| ≥ 6.1, < 7.0 | 112 (14.7%) | 1.01 (0.61, 1.70) 0.9603 | 0.90 (0.52, 1.53) 0.6905 | 0.90 (0.52, 1.55) 0.7027 |
| ≥ 7.0 | 359 (47.1%) | 1.37 (0.93, 2.04) 0.1145 | 1.10 (0.72, 1.67) 0.6527 | 1.14 (0.74, 1.74) 0.5611 |
Data are presented as OR (95% CI) P value. Adjustment I for: age; gender; HTN history; current smoking; LDL-C. Adjustment II for: age; gender; HTN history; current smoking; LDL-C; initial TIMI flow; multivessel lesion, and culprit vessel
A/C admission/chronic glycaemia ratio, ARPG admission random plasma glucose, DM diabetes mellitus, GVT glucose variable tendency, HbA1c glycosylated hemoglobin, HTN hypertension, LDL-C low density lipoprotein cholesterol, PCI percutaneous coronary intervention, PFPG post-PCI fasting plasma glucose, Strati- stratification, TIMI thrombolysis in myocardial infarction
Univariate and multivariate logistic regression analyses were applied to screen the glucose-related variables that were significantly associated with the identification of plaque type. A P < 0.05 denoted statistical significance. Significant variables were subsequently included in a differential diagnostic model to evaluate the diagnosis value. Furthermore, the correlation between abnormal blood glucose and plaque morphology was analyzed with Spearman correlation. These analyses were all produced by EmpowerStats (www.empowerstats.com.) using R.
Results
Baseline characteristics between PR and PE are shown as Additional file 1: Table S1. Age (P < 0.001), men gender (P = 0.019), current smoking (P = 0.05), history of hypertension (HTN) (P = 0.001), low-density lipoprotein cholesterol levels (LDL-C) (P < 0.001), triglyceride levels (TC) (P = 0.012). Angiography imaging data between PR and PE are shown as Additional file 1: Table S1. The inter-observer kappa coefficients for plaque rupture and plaque erosion were 0.866 and 0.870, respectively. The intra-observer kappa coefficients for plaque rupture and plaque erosion were 0.891 and 0.905, respectively. Initial TIMI flow (P = 0.003); culprit vessel (P < 0.001), multivessel lesion (P < 0.001), stenosis degree (P < 0.001) and length of culprit lesion (P = 0.002) showed significant difference between groups. The results of the study were consistent with the results of our previous study [10]. The glucose-related variables between PR and PE are presented as Table 1. As a result, all the glucose-related variables except PFPG presented significant difference between PR and PE.
Table 1.
Glucose-related variables between plaque rupture and plaque erosion
| Glucose-related variables | Plaque rupture | Plaque erosion | P-value |
|---|---|---|---|
| N = 656 | N = 216 | ||
| DM history | 192 (29.27%) | 46 (21.30%) | 0.023 |
| HbA1c (%) | 5.90 (5.60–7.00) | 5.70 (5.40–6.30) | 0.002 |
| CG (mmol/L) | 6.81 (6.33–8.57) | 6.49 (6.02–7.45) | 0.002 |
| ARPG (mmol/L) | 8.39 (6.92–10.98) | 7.54 (6.29–9.55) | < 0.001 |
| PFPG (mmol/L) | 6.93 (5.54–9.36) | 6.38 (5.43–8.48) | 0.072 |
| A/C glycemic ratio | 1.21 (1.05–1.41) | 1.14 (0.97–1.33) | 0.013 |
| GVT | 0.002 | ||
| Normal | 26 (4.56%) | 23 (11.92%) | |
| Up | 160 (28.07%) | 50 (25.91%) | |
| Down | 384 (67.37%) | 120 (62.18%) | |
Data are presented as mean ± SD or Median (Q1–Q3)/N (%)
ARPG admission plasma glucose, A/C admission/chronic glycemic ratio, CG chronic plasma glucose, DM diabetes mellitus, GVT glucose variable tendency, HbA1c glycosylated hemoglobin, PCI percutaneous coronary intervention, PFPG post-PCI fasting plasma glucose
Logistic regression analysis showed positive and significant association between plaque rupture and the glucose-related variables included HbA1c, ARPG, DM history, and GVT. Yet PFPG and A/C showed no significant difference between the groups. After adjustment by the risk factors variables associated with plaque model, including age, gender, HTN history, current smoking, and LDL-C levels, only the difference of ARPG levels and GVT remained significantly between the groups. Added initial TIMI flow, culprit vessel and multi-vessel lesion into the adjustment variables, the difference of ARPG levels and GVT between PR and PE did not disappear either. Stratification analysis showed significant difference of both moderate and severe AH groups between groups no matter whether adjusted or not. The higher the ARPG levels of the patient, the greater the risk of PR (Table 2).
The association between the glucose variables and PR risk was then analysed in patients with or without DM history (Table 3). Logistic regression analysis showed positive and significant association of GVT and continuous ARPG with PR risk only in nondiabetic patients. Among the nondiabetic patients, stratification analysis showed that only moderate AH was positively and significantly associated with PR risk. After adjustment by the risk factors variables associated with plaque model, including age, gender, HTN history, current smoking, and LDL-C levels, the association weakened. Added initial TIMI flow, culprit vessel and multi-vessel lesion into the adjustment variables, the predictive ability for PR risk of GVT reserved but ARPG vanished. Given without enough patients, stratified ARPG of patients with DM history were failed to be analysed.
Table 3.
Logistic regression analysis of AG between plaque rupture and erosion with or without DM history
| Exposure | NDM history | DM history | Total |
|---|---|---|---|
| Crude | |||
| Continuous HbA1c | 1.62 (0.87, 3.02) 0.1303 | 1.04 (0.84, 1.30) 0.6970 | 1.10 (0.89, 1.36) 0.3874 |
| Continuous ARPG | 1.11 (1.02, 1.21) 0.0182 | 1.04 (0.96, 1.12) 0.3539 | 1.07 (1.01, 1.14) 0.0213 |
| Continuous PFPG | 1.04 (0.94, 1.14) 0.4550 | 0.96 (0.89, 1.05) 0.3658 | 0.99 (0.93, 1.06) 0.8630 |
| Continuous A/C | 1.56 (0.80, 3.08) 0.1930 | 1.36 (0.57, 3.28) 0.4880 | 1.59 (0.57, 3.28) 0.0860 |
| GVT | |||
| Normal | 1 | ||
| Up | 2.79 (1.40, 5.55) 0.0030 | 1 | |
| Down | 2.54 (1.38, 4.67) 0.0030 | 1.32 (0.67, 2.59) 0.4170 | |
| Stratified ARPG | |||
| < 6.1 | 1 | 1 | 1 |
| ≥ 6.1, < 7.0 | 1.53 (0.91, 2.59) 0.1107 | – | 1.41 (0.84, 2.37) 0.1909 |
| ≥ 7.0, < 11.1 | 2.14 (1.36, 3.38) 0.0011 | – | 2.07 (1.32, 3.24) 0.0014 |
| ≥ 11.1 | 1.96 (0.93, 4.15) 0.0781 | – | 2.29 (1.24, 4.21) 0.0080 |
| Stratified PFPG | |||
| < 5.6 | 1 | 1 | 1 |
| ≥ 5.6, < 6.1 | 0.86 (0.50, 1.51) 0.6089 | – | 0.92 (0.53, 1.59) 0.7621 |
| ≥ 6.1, < 7.0 | 1.06 (0.62, 1.80) 0.8403 | 0.40 (0.03, 5.15) 0.4822 | 1.00 (0.60, 1.68) 0.9981 |
| ≥ 7.0 | 1.25 (0.77, 2.01) 0.3685 | 0.78 (0.09, 6.87) 0.8232 | 1.20 (0.76, 1.90) 0.4250 |
| Adjust I | |||
| Continuous ARPG | 1.07 (0.97, 1.18) 0.1528 | 1.06 (0.98, 1.16) 0.1609 | 1.06 (1.00, 1.13) 0.0710 |
| Stratified ARPG | |||
| < 6.1 | 1 | 1 | 1 |
| ≥ 6.1, < 7.0 | 1.38 (0.77, 2.45) 0.2756 | – | 1.25 (0.71, 2.20) 0.4396 |
| ≥ 7.0, < 11.1 | 1.72 (1.04, 2.85) 0.0347 | – | 1.63 (1.00, 2.67) 0.0517 |
| ≥ 11.1 | 1.58 (0.70, 3.60) 0.2709 | – | 2.05 (1.05, 3.99) 0.0345 |
| GVT | |||
| Normal | 1 | ||
| Up | 2.12 (1.02, 4.41) 0.0450 | ||
| Down | 1.95 (1.03, 3.69) 0.0410 | ||
| Adjust II | |||
| Continuous ARPG | 1.07 (0.98, 1.18) 0.1408 | 1.05 (0.96, 1.15) 0.2630 | 1.05 (0.99, 1.12) 0.0983 |
| Stratified ARPG | |||
| < 6.1 | 1 | 1 | 1 |
| ≥ 6.1, < 7.0 | 1.29 (0.72, 2.33) 0.3920 | – | 1.17 (0.66, 2.08) 0.5945 |
| ≥ 7.0, < 11.1 | 1.67 (1.00, 2.80) 0.0514 | – | 1.58 (0.96, 2.61) 0.0736 |
| ≥ 11.1 | 1.67 (0.72, 3.85) 0.2313 | – | 1.99 (1.02, 3.90) 0.0438 |
| GVT | |||
| Normal | 1 | ||
| Up | 2.16 (1.03, 4.55) 0.0420 | ||
| Down | 1.89 (0.99, 3.60) 0.0550 | ||
Data are presented as OR (95% CI) P value. Adjustment I for: age; gender; HTN history; current smoking; LDL-C. Adjustment II for: age; gender; HTN history; current smoking; LDL-C; initial TIMI flow; multivessel lesion, and culprit vessel
A/C admission/chronic plasma glucose, ARPG admission random plasma glucose, DM diabetes mellitus, GVT glucose variable tendency, HbA1c glycosylated hemoglobin, HTN hypertension, LDL-C low density lipoprotein cholesterol, NDM nondiabetic mellitus, PCI percutaneous coronary intervention, PFPG post-PCI fasting plasma glucose, TIMI thrombolysis in myocardial infarction
Subsequently, we quantified the diagnostic accuracy of AG and GVT for PR using the receiver operating characteristic curve (ROC). Preliminary analysis of ROC indicated that the area under curve (AUC) of ARPG was larger than that of GVT. Combined ARPG or GVT with the risk factors (age, gender, smoking, hypertension, and LDL-C) to establish combined predictive models successively, the predictive efficiency and accuracy increased significantly. Added initial TIMI flow, culprit vessel and multi-vessel lesion into the combined model, the predictive ability increased more and to over 70% (AUC = 0.7087, 0.7022; specificity = 63.82%, 57.07%; sensitivity = 69.83%, 73.53%; and accuracy = 68.29%, 69.36%; respectively) (Additional file 1: Table S2).
Besides, the correlations between abnormal hyperglycemia variables and data of angiography and OCT were analyzed using Spearman’s correlation. As a result, ARPG, PFPG and HbA1c were determined to be positively and weakly correlated with the mean size of lipid arc. And HbA1c was also positively and weakly correlated with TCFA and maximal lipid arc (Additional file 1: Table S3). Further analysis was performed on the association between stratified ARPG and imaging characteristics of STEMI patients using logistic regression. The results indicated that the risk of PR increased as ARPG levels rose (P < 0.001). And the higher the ARPG level was, the higher risk of TCFA (P = 0.007) and the bigger size of lipid arc was (Table 4).
Table 4.
QCA and OCT characteristics among ARPG groups
| ARPG group (mmol/L) | < 6.1 | ≥ 6.1, < 7.0 | ≥ 7.1, < 11.1 | ≥ 11.1 |
|---|---|---|---|---|
| N = 117 | N = 144 | N = 412 | N = 199 | |
| QCA | ||||
| MLD (mm) | 0.79 (0.64–1.08) | 0.83 (0.66–1.10) | 0.87 (0.65–1.10) | 0.78 (0.62–0.99) |
| RVD (mm) | 2.69 ± 0.58 | 2.69 ± 0.51 | 2.70 ± 0.58 | 2.59 ± 0.50 |
| DS (%) | 69.00 (61.00–76.00) | 68.00 (61.00–74.00) | 67.00 (60.00–75.00) | 69.00 (63.00–75.00) |
| LL (mm) | 22.39 ± 8.69 | 21.21 ± 8.18 | 21.73 ± 8.42 | 22.36 ± 11.26 |
| OCT | ||||
| Plaque rupture* | 73 (62.39%) | 101 (70.14%) | 321 (77.91%) | 161 (80.90%) |
| Culprit vessel | ||||
| RCA | 34 (29.06%) | 61 (42.36%) | 171 (41.50%) | 77 (38.69%) |
| LAD | 66 (56.41%) | 70 (48.61%) | 211 (51.21%) | 102 (51.26%) |
| LCX | 17 (14.53%) | 13 (9.03%) | 30 (7.28%) | 20 (10.05%) |
| Culprit site | ||||
| Proximal | 41 (35.04%) | 55 (38.19%) | 189 (45.87%) | 90 (45.23%) |
| Middle | 54 (46.15%) | 56 (38.89%) | 160 (38.83%) | 68 (34.17%) |
| Distal | 22 (18.80%) | 33 (22.92%) | 63 (15.29%) | 41 (20.60%) |
| Multivessel lesion | 67 (57.26%) | 93 (64.58%) | 279 (67.72%) | 137 (68.84%) |
| Lesion length | 16.84 ± 6.44 | 17.49 ± 5.95 | 16.93 ± 6.53 | 17.51 ± 6.75 |
| Lipid length* | 8.95 ± 6.11 | 10.67 ± 6.04 | 9.83 ± 6.52 | 10.88 ± 6.29 |
| Mean lipid arc* | 170.45 (0.00–235.54) | 196.78 (0.00–253.35) | 188.55 (0.00–245.77) | 210.66 (158.92–257.20) |
| Max lipid arc* | 222.10 (0.00–360.00) | 299.60 (0.00–360.00) | 290.65 (0.00–360.00) | 324.60 (212.95–360.00) |
| Minimal FCT | 0.02 (0.00–0.05) | 0.03 (0.00–0.04) | 0.03 (0.00–0.04) | 0.03 (0.02–0.04) |
| TCFA* | 62 (52.99%) | 86 (60.99%) | 238 (58.33%) | 140 (70.35%) |
| Chole- crystal | 43 (36.75%) | 63 (44.68%) | 172 (42.16%) | 89 (44.95%) |
| Microchannel | 40 (34.19%) | 34 (24.11%) | 113 (27.70%) | 53 (26.63%) |
| Thrombus length | 4.80 (2.70–7.90) | 5.50 (3.40–8.90) | 5.10 (3.00–7.88) | 5.50 (2.90–8.78) |
| Thrombus type | ||||
| None | 9 (7.69%) | 12 (8.45%) | 38 (9.27%) | 20 (10.10%) |
| White | 55 (47.01%) | 73 (51.41%) | 214 (52.20%) | 84 (42.21%) |
| Red | 2 (1.71%) | 5 (3.52%) | 10 (2.44%) | 1 (0.50%) |
| Mix | 51 (43.59%) | 52 (36.62%) | 148 (36.10%) | 93 (46.73%) |
Data presented as Mean (SD) Median (Q1–Q3)/N (%)
ARPG admission random plasma glucose, Chole-crystal cholesterol crystal, DS the degree of stenosis, FCT thickness of fibrotic cap, LAD left anterior descending branch, LCX left circumflex branch, LL lesion length, MLD minimal lumen diameter, OCT optical coherence tomography, QCA quantitative coronary angiography, RCA right coronary artery, RVD reference vessel diameter, TCFA thin-cap fibroatheroma
*P value < 0.05
Discussion
To the best of our knowledge, present study is the first one to compare the association of glucose-related variables with plaque rupture and plaque erosion identified by OCT in STEMI patients. The findings are as showed below: (1) HbA1c, ARPG, GVT, A/C, and DM history were significantly and positively associated with PR risk in STEMI patients before adjustment for traditional risk factors; (2) Among the glucose-related variables, ARPG and GVT were independent predictors of PR in STEMI patients without DM rather than in those with DM; (3) The higher the ARPG levels of the STEMI patient, the greater the risk of PR; (4) High ARPG and HbA1c were positively related with plaque vulnerability; (5) The higher the ARPG levels of the STEMI patient, the higher risk of TCFA and the bigger size of lipid arc among ARPG groups.
The definite DM history has immediate association with worse cardiovascular outcomes [14]. The relationship between plaque rupture and DM was well described in the previous studies [15], but the relationship between plaque erosion and glucose condition is still unclear. High ARPG levels after AMI are common and play a role in predicting adverse events in DM and non-diabetic patients [2, 16]. Especially during hospitalization, it is a strong predictable factors for fatal outcomes in patients with AMI [5, 17]. But A/C glycemic ratio was reported to be a better predictor of in-hospital morbidity and mortality than glycaemia at admission [3]. And positive correlation of serum HbA1c with the presence of carotid plaque [1] and vulnerable plaque in ACS were reported in previous studies [16]. In present study, we compared the association and predictive ability of ARPG, HbA1c, A/C and DM history with PR and PE. Compared with PE, PR was more related with the glucose-related variables before adjustment with the traditional risks. And the association between ARPG and PR was confirmed after the adjustment. Therefore ARPG was identified as an independent predictor for PR in STEMI patients. Although the predictive ability was not strong, it raised an auxiliary factor for distinguishing PR and PE in STEMI patients clinically. It also induced our deep thought on the mechanism of the onset and development of PR and PE.
Previous studies indicated that patients with AMI caused by plaque rupture had some clinical characteristics [7] same as those with admission hyperglycemia did, such as more multivessel lesions [18], more no-reflow occurrence during PCI [19], larger infarct sizes than normoglycemic patients [20], and accompanied by long term [21] and short term poor outcomes [22]. Our results provided a strong clinical evidence for the closer association of AH and STEMI patients with PR than PE. The higher the ARPG, the greater the PR risk a STEMI patient has. Admission hyperglycemia in STEMI patients might play a role in coronary thrombosis through altering clot features and enhancing local thrombin generation and platelet activation [23]. It is associated with the large thrombus burden which causes adverse cardiac outcomes [24]. There were no relationships of ARPG to thrombus length and type in the present study which might due to that the thrombus overlying the lesion might have been dissolved before OCT imaging [11]. We guess the treatment targeting AH such as intensified insulin-based glycaemia control may benefit STEMI patients with PR via reducing thrombosis and thrombus burden. It can also explain why the intensified insulin-based glycaemia control after the onset of STEMI might improve the prognosis [25]. And the intensified insulin-based glycaemia control should get more attention in the patients with STEMI caused by PR than those by PE.
In addition, present study only indicated the association of AH with PR in STEMI patients without DM but not those with DM. Elevated ARPG in nondiabetic patients may link to impaired glucose tolerance (IGT) which was more associated with PR than normal glucose tolerance (NGT) [26]. On the other hand, although ARPG in nondiabetic AMI patients could offer an initial screening tool for those patients with high risk for future DM [27], it does not represent previously undiagnosed DM and abnormal glucose tolerance [28]. The elevated ARPG levels in nondiabetic patients with STEMI patients are independently associated with larger infarct size and higher long-term mortality rates [29]. An elevated ARPG level not only disturbed glucose metabolism that worsens the prognosis, but also accompanied by elevated stress hormone and reflected acute stress state [30, 31]. Stress hyperglycaemia with myocardial infarction is associated with a poor prognosis in DM and nondiabetic patients [32, 33] via disturbing myocardial blood flow and energetics, contributing to high monocyte chemo-attractant protein-1 levels and sFas apoptosis levels [34, 35], inducing a pro-oxidative/pro-inflammatory state [36, 37], and resulting in coronary plaque rupture eventually [38]. It might partly explain why the patients with AH continue to have a higher mortality even after rapid revascularization and treatment of hyperglycaemia [18]. Considering of the closer association between ARPG and PR than PE resulted from present study, treatment of antioxidant and antagonistic stress should benefit more and be taken more seriously in the STEMI patients with PR than in those with PE.
Although no association between HbA1c and plaque morphology in STEMI patients was found by multivariable logistic regression analysis after adjustment for traditional risk factors, both HbA1c and ARPG were determined to be positively correlated with degree of lumen stenosis and the lipid content in the plaque on the evidence of imaging, and HbA1c seemed have more correlation than ARPG. It provided a clinical evidence for the role chronic hyperglycemia might play in the developing of vulnerable plaque which characterized with large lipid core. It may partly explain why patients with DM had a higher prevalence of lipid-rich plaque but similar prevalence of plaque rupture and plaque erosion was also been detected in a study by Tomoyo Sugiyama [15]. This also helps explain why increased duration of DM combined with higher HbA1c levels is associated with negative coronary artery remodeling [39] and influences culprit-plaque characteristics in patients with AMI [40]. High HbA1c levels may partly result from insulin resistance (IR) that subsequently increased spotty calcification and plaque vulnerability [41]. And the increased spotty calcification led to large intrinsic calcification angle (ICA) that involved in the formation of vulnerable plaques that eventually lead to plaque rupture [42]. The effective control of glucose chronically before the onset of STEMI caused by PR might benefit DM patients via improving impaired beta cell function and glycometabolic derangements [43] and prevent them from significant and insidious coronary atherosclerosis which developed vulnerable plaque and subsequent high risk for plaque rupture and cardiac events [44]. And the lipid regulation therapy might benefit either STEMI patients with AH levels or those with high HbA1c, the latter seemed more than the former.
Besides, dynamic glucose fluctuation may be associated with PR via increasing CD14(bright)CD16(+) monocyte levels preferentially [38]. Considering glucose variability was reported to be correlated with PR in previous studies, we analyzed glucose variable tendency (GVT) to value it between PR and PE in present study too. Compared to PE, positive and significant association was showed between GVT and PR as well, though GVT defined as the ratio of PFPG/ARPG in present study may not represent the dynamic glucose fluctuation completely. It may not only provide a risk factor in identifying PE or PR in STEMI patients, but also helps to simplify and facilitate clinical monitoring and prognosis stratification.
Previous studies also indicated that coronary plaques in CAD patients with NGT are more stable than in those with IGT and DM [45]. However, no correlation between PFPG with plaque morphological characteristics was identified in present study. It might due to the population studied herein was enrolled from only STEMI patients whose coronary atherosclerosis developed significantly no matter caused by PR or PE. And PFPG which was interfered by acute stress and in-hospital treatment seemed unsuitable to be a variable for diagnosing IGT.
Study limitations
There are several limitations of this study. First, it’s a single center observational study based on retrospective design. In the present study, a total of 233/1005 cases were excluded due to poor quality of OCT image or identified as neither PR nor PE, therefore some patients with STEMI caused by PR and PE were failed to be included and those patients with STEMI caused by other reasons include calcified nodules were not studied. Second, the plasma glucose was not monitored after the acute event so that mean amplitude of glycemic excursion and stress hyperglycemia failed to be studied. Last, an important risk factor body mass index (BMI) has not been adjusted because of incomplete weiHbA1ct and heiHbA1ct record. Being a subanalysis of the EROSION study, present study was a retrospective study focus on cardiovascular specialty, and other glucose-related variables (such as DM duration, the kind of diabetic drugs, IRI level, and HOMA-R) were failed to have been collected.
Conclusions
The findings of present study highlight the association of ARPG and GVT with plaque rupture in STEMI patients, especially in patients with admission glucose levels ≥ 7.1 mmol/l. ARPG and GVT are independent predictors for PR in STEMI patients without DM. And high HbA1c and ARPG were positively correlated with the development of vulnerable plaque in culprit vessels, the former more than the latter.
Supplementary information
Additional file 1: Table S1. Baseline characteristics between plaque rupture and plaque erosion. Table S2. Presictive models of PR established using ARPG/GVT single or combined with traditional risk factors. Table S3. The correlation between glucose-related variables and plaque morphological characteristics using Spearman correlation.
Acknowledgements
We sincerely thank all of the people working for the interventional catheter room of the second affiliate Hospital of Harbin Medical University.
Abbreviations
- ARPG
Random plasma glucose on admission
- AH
Admission hyperglycemia
- AMI
Acute myocardial infarction
- A/C
Acute-to-chronic glycemic ratio
- AUC
Area under curve
- CPG
Chronic plasma glucose
- CHD
Coronary heart disease
- CK-MB
Creatine kinase-MB
- cTnI
Cardiac troponin
- DM
Diabetes mellitus
- HbA1c
Glycosylated hemoglobin
- hs-CRP
Highly sensitive C-reactive protein
- LDL
Low-density lipoprotein cholesterol levels
- LVEF
Left ventricular ejection fraction
- OCT
Optical coherence tomography
- PCI
Percutaneous coronary intervention
- PE
Plaque erosion
- PFPG
Post-PCI fasting plasma glucose
- PR
Plaque rupture
- proBNP
Pro-brain natriuretic peptide
- QCA
Quantitative coronary angiography
- ROC
Receiver operating characteristic curve
- STEMI
ST-segment elevation myocardial infarction
- TCFA
Thin-cap fibroatheroma
- TIMI
Thrombolysis in myocardial infarction
Authors’ contributions
BY and JL contributions to the conception and design of the work; HC, RS, WP, and JL contributions to the acquisition of the data; BY, SW and JL contributions to the analysis of the data; BY, SF and JL interpretation of data; JL have drafted the work; BY, CC and JL substantively revised it. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2016YFC1301100; awarded to B.Y.) and National Natural Fund Program of China (Grant No. 81827806; awarded to B.Y.)
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Present study is a retrospective one and the population came from the EROSION study of our center. It was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval reference number, KY2017-249), and all patients provided written informed consent prior to the inclusion in the study and the investigation conformed to the principles outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01074-9.
References
- 1.Sato Y, Nagao M, Asai A, Nakajima Y, Takaya M, Takeichi N, Takemitsu S, Sudo M, Kano-Wakakuri T, Ishizaki A, Harada T, Tanimura-Inagaki K, Okajima F, Tamura H, Sugihara H, Oikawa S. Association of glycated albumin with the presence of carotid plaque in patients with type 2 diabetes. J Diabetes Invest. 2013;4:634–639. doi: 10.1111/jdi.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avogaro A, Bonora E, Consoli A, Del Prato S, Genovese S, Giorgino F. Glucose-lowering therapy and cardiovascular outcomes in patients with type 2 diabetes mellitus and acute coronary syndrome. Diabetes Vasc Dis Res. 2019;16:399–414. doi: 10.1177/1479164119845612. [DOI] [PubMed] [Google Scholar]
- 3.Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, Rubino M, Campodonico J, Moltrasio M, Marana I, Grazi M, Lauri G, Bonomi A, Veglia F, Manfrini R, Bartorelli AL. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018;41:847–853. doi: 10.2337/dc17-1732. [DOI] [PubMed] [Google Scholar]
- 4.Goyal A, Mehta SR, Diaz R, Gerstein HC, Afzal R, Xavier D, Liu L, Pais P, Yusuf S. Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation. 2009;120:2429–2437. doi: 10.1161/CIRCULATIONAHA.108.837765. [DOI] [PubMed] [Google Scholar]
- 5.Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenstrom A, Wedel H. Mortality prediction in diabetic patients with myocardial infarction: experiences from the digami study. Cardiovasc Res. 1997;34:248–253. doi: 10.1016/s0008-6363(96)00263-5. [DOI] [PubMed] [Google Scholar]
- 6.Saia F, Komukai K, Capodanno D, Sirbu V, Musumeci G, Boccuzzi G, Tarantini G, Fineschi M, Tumminello G, Bernelli C, Niccoli G, Coccato M, Bordoni B, Bezerra H, Biondi-Zoccai G, Virmani R, Guagliumi G, Investigators O. Eroded versus ruptured plaques at the culprit site of stemi: in vivo pathophysiological features and response to primary pci. JACC Cardiovasc Imaging. 2015;8:566–575. doi: 10.1016/j.jcmg.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Shimada K, Namba M, Sano T, Sakamoto T, Akasaka T, Yoshikawa J. Ruptured plaque is associated with larger infarct size following successful percutaneous coronary intervention in st segment elevation acute myocardial infarction. Coron Artery Dis. 2009;20:260–266. doi: 10.1097/MCA.0b013e32832c456e. [DOI] [PubMed] [Google Scholar]
- 8.Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang IK. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the erosion study) Eur Heart J. 2017;38:792–800. doi: 10.1093/eurheartj/ehw381. [DOI] [PubMed] [Google Scholar]
- 9.Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 10.Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, Lin L, Ma L, Liu H, Xu M, Ren X, Yu H, Li L, Zou Y, Zhang S, Mintz GS, Hou J, Yu B. In vivo predictors of plaque erosion in patients with st-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J. 2018;39:2077–2085. doi: 10.1093/eurheartj/ehy101. [DOI] [PubMed] [Google Scholar]
- 11.Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW, Expert’s OCTRD Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Sun C, Gu X, Liu X, Wang X, Wang X, Xie Z, Tian J, Yu B. Intraplaque neovascularization attenuated statin benefit on atherosclerotic plaque in cad patients: a follow-up study with combined imaging modalities. Atherosclerosis. 2019;287:134–139. doi: 10.1016/j.atherosclerosis.2019.06.912. [DOI] [PubMed] [Google Scholar]
- 14.Akerblom A, Wojdyla D, Steg PG, Wallentin L, James SK, Budaj A, Katus HA, Himmelmann A, Huber K, Siegbahn A, Storey RF, Becker RC, Investigators P Prevalence and relevance of abnormal glucose metabolism in acute coronary syndromes: insights from the platelet inhibition and patient outcomes (plato) trial. J Thromb Thrombolysis. 2019;48:563–569. doi: 10.1007/s11239-019-01938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama T, Yamamoto E, Bryniarski K, Xing L, Fracassi F, Lee H, Jang IK. Coronary plaque characteristics in patients with diabetes mellitus who presented with acute coronary syndromes. J Am Heart Assoc. 2018;7(14):e009245. doi: 10.1161/JAHA.118.009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Dai J, Jia H, Hu S, Du H, Li N, Zou Y, Zou Y, Jing S, Wang Y, Sun R, Yu B. Non-culprit plaque characteristics in acute coronary syndrome patients with raised hemoglobina1c: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2018;17:90. doi: 10.1186/s12933-018-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinar H, Avci A, Gulen M, Avci BS, Comertpay E, Satar S. Does stress hyperglycemia affect mortality? Acute myocardial infarction—case control study. Arch Med Sci Atherosc Dis. 2019;4:e201–e207. doi: 10.5114/amsad.2019.87303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh K, Hibbert B, Singh B, Carson K, Premaratne M, Le May M, Chong AY, Arstall M, So D. Meta-analysis of admission hyperglycaemia in acute myocardial infarction patients treated with primary angioplasty: a cause or a marker of mortality? Eur Heart J Cardiovasc Pharmacother. 2015;1:220–228. doi: 10.1093/ehjcvp/pvv023. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Kimura K, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M, Ikeda Y, Shirai M, Hiraoka H, Inoue T, Saito F, Ogawa H, Japanese Acute Coronary Syndrome Study I Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005;150:814–820. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Mather AN, Crean A, Abidin N, Worthy G, Ball SG, Plein S, Greenwood JP. Relationship of dysglycemia to acute myocardial infarct size and cardiovascular outcome as determined by cardiovascular magnetic resonance. J Cardiovasc Magn Resonan. 2010;12:61. doi: 10.1186/1532-429X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Mulder M, Cornel JH, van der Ploeg T, Boersma E, Umans VA. Elevated admission glucose is associated with increased long-term mortality in myocardial infarction patients, irrespective of the initially applied reperfusion strategy. Am Heart J. 2010;160:412–419. doi: 10.1016/j.ahj.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 22.Alavi-Moghaddam M, Parsa-Mahjoob M, Ghodssi-Ghassemabadi R, Bitazar B. Association of admission blood glucose level with major adverse cardiac events in acute coronary syndrome; a cohort study. Arch Acad Emerg Med. 2019;7:e26. [PMC free article] [PubMed] [Google Scholar]
- 23.Undas A, Wiek I, Stepien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008;31:1590–1595. doi: 10.2337/dc08-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigirci S, Yildiz SS, Keskin K, Cetinkal G, Aksan G, Gurdal A, Cetin S, Kilci H, Kilickesmez KO. The predictive value of stress hyperglycemia on thrombus burden in nondiabetic patients with st-segment elevation myocardial infarction. Blood Coagul Fibrinolysis. 2019;30:270–276. doi: 10.1097/MBC.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 25.Ritsinger V, Malmberg K, Martensson A, Ryden L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised diabetes mellitus insulin glucose infusion in acute myocardial infarction (digami 1) trial. Lancet Diabetes Endocrinol. 2014;2:627–633. doi: 10.1016/S2213-8587(14)70088-9. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Takano H, Kubota Y, Inui K, Nakamura S, Tokita Y, Kato K, Asai K, Shimizu W. Plaque characteristics in coronary artery disease patients with impaired glucose tolerance. PLoS ONE. 2016;11:e0167645. doi: 10.1371/journal.pone.0167645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisinger C, Beck J, Heier M, Hormann A, Kuch B, Sietas G, Koenig W, Group KS Myocardial infarction and incidence of type 2 diabetes mellitus. Is admission blood glucose an independent predictor for future type 2 diabetes mellitus? Am Heart J. 2010;159:258–263. doi: 10.1016/j.ahj.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Hata T, Nakama Y, Kijima Y, Kagawa E. Is admission hyperglycaemia in non-diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance? Eur Heart J. 2006;27:2413–2419. doi: 10.1093/eurheartj/ehl271. [DOI] [PubMed] [Google Scholar]
- 29.Timmer JR, van der Horst IC, Ottervanger JP, Henriques JP, Hoorntje JC, de Boer MJ, Suryapranata H, Zijlstra F, Zwolle Myocardial Infarction Study G Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004;148:399–404. doi: 10.1016/j.ahj.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22:1827–1831. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs PJ, Laycock J, Alaghband-Zadeh J, Carter G, Noble MI. Circulating stress hormone and insulin concentrations in acute coronary syndromes: identification of insulin resistance on admission. Clin Sci. 1999;96:589–595. doi: 10.1042/cs0960589. [DOI] [PubMed] [Google Scholar]
- 32.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 33.Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bossini P, Distefano S, Magnanini G, Muratori L, Rossi G, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1989;64:885–888. doi: 10.1016/0002-9149(89)90836-9. [DOI] [PubMed] [Google Scholar]
- 34.Chang J, Zhang G, Zhang L, Hou YP, Liu XL, Zhang L. High admission glucose levels increase fas apoptosis and mortality in patients with acute st-elevation myocardial infarction: a prospective cohort study. Cardiovasc Diabetol. 2013;12:171. doi: 10.1186/1475-2840-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu N, Sheng J, Wang Y. Effect of stress hyperglycaemia on monocyte chemoattractant protein-1 levels and the short-term prognosis of patients with acute st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Exp Ther Med. 2019;17:3823–3829. doi: 10.3892/etm.2019.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceriello A, Zarich SW, Testa R. Lowering glucose to prevent adverse cardiovascular outcomes in a critical care setting. J Am Coll Cardiol. 2009;53:S9–S13. doi: 10.1016/j.jacc.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Marfella R, Siniscalchi M, Esposito K, Sellitto A, De Fanis U, Romano C, Portoghese M, Siciliano S, Nappo F, Sasso FC, Mininni N, Cacciapuoti F, Lucivero G, Giunta R, Verza M, Giugliano D. Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Diabetes Care. 2003;26:3129–3135. doi: 10.2337/diacare.26.11.3129. [DOI] [PubMed] [Google Scholar]
- 38.Teraguchi I, Imanishi T, Ozaki Y, Tanimoto T, Orii M, Shiono Y, Shimamura K, Ishibashi K, Yamano T, Ino Y, Yamaguchi T, Hirata K, Kubo T, Akasaka T. Impact of glucose fluctuation and monocyte subsets on coronary plaque rupture. Nutr Metab Cardiovasc Dis. 2014;24:309–314. doi: 10.1016/j.numecd.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Du R, Zhang RY, Lu L, Shen Y, Pu LJ, Zhu ZB, Zhang Q, Hu J, Yang ZK, Ding FH, Zhang JS, Shen WF. Increased glycated albumin and decreased esrage levels in serum are related to negative coronary artery remodeling in patients with type 2 diabetes: an intravascular ultrasound study. Cardiovasc Diabetol. 2018;17:149. doi: 10.1186/s12933-018-0792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng Z, Zhou P, Liu C, Li J, Chen R, Zhou J, Song L, Zhao H, Yan H. Relationships of coronary culprit-plaque characteristics with duration of diabetes mellitus in acute myocardial infarction: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2019;18:136. doi: 10.1186/s12933-019-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Liu W, Ma Q, Yu W, Guo Y, Zhao Y, Shi D, Liu Y, Zhou Z, Wang J, Liu R, Zhou Y. Association between insulin resistance and coronary plaque vulnerability in patients with acute coronary syndromes: insights from optical coherence tomography. Angiology. 2019;70:539–546. doi: 10.1177/0003319718809931. [DOI] [PubMed] [Google Scholar]
- 42.Reith S, Milzi A, Lemma ED, Dettori R, Burgmaier K, Marx N, Burgmaier M. Intrinsic calcification angle: a novel feature of the vulnerable coronary plaque in patients with type 2 diabetes: an optical coherence tomography study. Cardiovasc Diabetol. 2019;18:122. doi: 10.1186/s12933-019-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallander M, Bartnik M, Efendic S, Hamsten A, Malmberg K, Ohrvik J, Ryden L, Silveira A, Norhammar A. Beta cell dysfunction in patients with acute myocardial infarction but without previously known type 2 diabetes: a report from the gami study. Diabetologia. 2005;48:2229–2235. doi: 10.1007/s00125-005-1931-z. [DOI] [PubMed] [Google Scholar]
- 44.Park GM, Lee CH, Lee SW, Yun SC, Kim YH, Kim YG, Won KB, Ann SH, Kim SJ, Yang DH, Kang JW, Lim TH, Koh EH, Lee WJ, Kim MS, Park JY, Kim HK, Choe J, Lee SG. Impact of diabetes control on subclinical atherosclerosis: analysis from coronary computed tomographic angiography registry. Diabetes Metab J. 2020;44(3):470–479. doi: 10.4093/dmj.2019.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jing S, Gao X, Yu B, Qiao H. Evaluation of plaque characteristics in coronary artery patients with impaired glucose tolerance through optical coherence tomography. Rev Assoc Med Bras. 2018;64:433–437. doi: 10.1590/1806-9282.64.05.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics between plaque rupture and plaque erosion. Table S2. Presictive models of PR established using ARPG/GVT single or combined with traditional risk factors. Table S3. The correlation between glucose-related variables and plaque morphological characteristics using Spearman correlation.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.


