Abstract
Background
The outbreak of coronavirus disease 2019 (COVID-19) is now becoming an enormous threat to public health. The clinical spectrum of COVID-19 is extensive, of which critical cases are with rapid disease progression and high mortality. The aim of our study is to summarize the characteristics of different subtypes and explore risk factors of illness severity for early identification and prompt treatment.
Methods
In this retrospective study, we collected data of patients confirmed COVID-19 in Zhejiang Province from 17 January to 12 February 2020. According to the definition of clinical classification, we divided confirmed cases into four types, and summarize epidemiological and clinical characteristics, laboratory and radiograph findings, treatments, and outcomes, respectively. Moreover, we used univariate and multivariate ordinal logistic regression models to explore risk factors for the severity of illness in patients with COVID-19.
Results
A total of 788 patients were enrolled in our study, of whom 52 cases (6.6%) were mild type, 658 cases (83.5%) were common type, 61 cases (7.2%) were severe type, and 17 cases (2.2%) were critical type. Multivariate ordinal logistic regression demonstrated increasing odds of the severity of illness in patients with COVID-19 associated with male (odds ratio [OR] = 1.7, 95% confidence interval [CI]: 1.2–2.6 P = 0.008), fever (OR = 3.6, 95% CI: 2.1–6.3, P < 0.001), cough (OR = 1.7, 95% CI: 1.0–2.9, P = 0.041), hemoptysis (OR = 3.4, 95% CI: 1.1–10.3, P = 0.032), gastrointestinal symptoms (OR = 1.9, 95% CI: 1.0–3.5, P = 0.047), hypertension (OR = 2.6, 95% CI: 1.2–5.6, P = 0.013). With the increase of age-grading, risk for the severity of illness was gradually higher (≤ 18 years [OR = 1.0], 19–40 years [OR = 12.7, 95% CI: 4.5–36.0, P < 0.001], 41–65 years [OR = 14.8, 95% CI: 5.2–42.1, P < 0.001], ≥ 66 years [OR = 56.5, 95% CI: 17.1–186.5, P < 0.001]).
Conclusions
Clinicians should pay close attention to these features in patients with COVID-19 including older age, male, fever, cough, hemoptysis, gastrointestinal symptoms and hypertension to identify the severity of illness as early as possible.
Keywords: COVID-19, SARS-CoV-2, Subtype, Risk factor, Gastrointestinal symptom
Background
In December, 2019, a cluster of patients with pneumonia of unknown cause appeared in Wuhan, Hubei Province, China. By 7 January 2020, China rapidly isolated the novel coronavirus and shared the viral genome sequence to World Health Organization (WHO) [1]. The novel coronavirus was identified as a novel enveloped RNA betacoronavirus and named 2019 novel coronavirus (later named as severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] by the International Committee on Taxonomy of Viruses), which has a phylogenetic similarity to severe acute respiratory syndrome coronavirus (SARS-CoV), but the contagosity is higher than SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. In order to prevent the epidemic of this contagion, Chinese government made a decision to temporarily shut down the traffic departing from Wuhan on 23 January 2020 and adopted a series of control measures.
WHO declared the spread of SARS-CoV-2 was listed as a public health emergency of international concern, and subsequently designated the pneumonia infected by SARS-CoV-2 as coronavirus disease 2019 (COVID-19) [3]. Until 19 April 2020, COVID-19 has swept across 213 countries and regions which reported 2 245 872 confirmed cases and 152 707 deaths. The largest number of confirmed cases were in the United States, followed by Spain and Italy [4]. This phenomenon signified fighting with COVID-19 is not only a matter for China, but an imperative event for global.
Nowadays, the majority of studies on COVID-19 in China are focused on Wuhan, the hardest-hit area, and little is known about the clinical features of COVID-19 outside of Wuhan [5–7]. The study of Chang et al. [8] included 13 cases in Beijing and the research of Xu et al. [9] enrolled 62 cases in Zhejiang, however, due to the small sample size, clinical characteristics might be not comprehensive. Researches with a larger number of confirmed cases were urgently needed outside of Wuhan. The clinical spectrum of COVID-19 appears to be wide, comprising mild type without pneumonia, common type with pneumonia, severe type with respiratory distress, and critical type with respiratory failure, shock or even death [10]. Diverse subtypes have their unique features, whether in epidemiology or laboratory results. A study with 72 314 cases reported by the Chinese Center for Disease Control and Prevention (China CDC) showed that the case-fatality rate was 49.0% among critical cases [11]. Therefore, mastering the characteristics of different subtypes and early identification of the severity of illness is of great significance for the treatment.
Hence, the aim of our study is to summarize the epidemiologic and clinical characteristics, laboratory and radiograph findings, treatments, and outcomes of different subtypes of patients with COVID-19 in Zhejiang Province. On this basis, we want to explore risk factors for the severity of illness in patients with COVID-19 and appeal to clinicians to attach importance to these factors.
Methods
Data sources and ethics
We conducted a retrospective study investigating on the epidemiological, clinical, laboratory, radiograph, treatments and outcomes characteristics of confirmed cases of COVID-19 in Zhejiang Province from 17 January to 12 February 2020. Diagnosis of COVID-19 was in accordance with the interim guidance from the WHO [12]. A confirmed case of COVID-19 was defined as a positive result on real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay of sputum and throat swab specimens. Only laboratory-confirmed patients were enrolled in our study. Data were uniformly collected by the Health Commission of Zhejiang Province, where all patients were allocated at specific hospitals for unified treatment according to the government emergency rule. All data enrolled in our study had been shared with WHO and the preliminary results were reported to the authority of Zhejiang Province.
Information of medical records were gathered and sent to the data collection center in Hangzhou. Demographic, epidemiological, clinical, laboratory, treatments and outcomes data were exacted from electronic medical records using a standardized data collection form. A group of doctors who have experiences in treating the patients with COVID-19 reviewed and disposed the data. When information was incomplete, the working team in Hangzhou would contact the doctor in charge for explanation.
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. IIT20200005C). Written informed consent was waived by the ethics commission of the designated hospital, and oral consent was obtained from patients.
Procedures
Sputum and throat swab specimens collected from all patients were tested by RT-PCR for SARS-CoV-2 RNA. Briefly, the CDC of Zhejiang Province and at municipal level, and the First Affiliated Hospital, School of Medicine, Zhejiang University were responsible for confirmation of SARS-CoV-2, with national authorization.
Laboratory tests were conducted on admission, including blood routine examinations, serum biochemical tests, coagulation function examinations, infection-related biomarkers, and an identification of other respiratory pathogens such as influenza A virus, influenza B virus, parainfluenza virus, adenovirus and respiratory syncytial virus. Chest radiograph or computed tomography (CT) was done for all inpatients at admission. Treatment measures and outcomes were followed up to 12 February 2020.
Case definitions
The illness severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 6.0) [13]. Patients with COVID-19 was categorized as mild, common, severe, and critical according to the illness severity. Mild type was defined as mild symptoms and no pneumonia on imaging. Common type was defined as having respiratory tract symptoms and imaging with pneumonia. Severe type was defined as satisfying any of the following items: 1) respiratory distress and respiratory frequency ≥ 30/min; 2) blood oxygen saturation ≤ 93% at rest; 3) PaO2/FiO2 ratio ≤ 300 mmHg; 4) Lung infiltrates > 50% within 24–48 h. Critical type was defined as satisfying any of the following items: 1) respiratory failure occurs and require mechanical ventilation; 2) shock occurs; 3) combined with other organ failure and requires ICU monitoring and treatment. The incubation period was defined as the time from exposure to the onset of illness, which was estimated among patients who could provide the exact data of close contact with confirmed or suspected individuals. Family cluster was defined as occurring two or more cases with fever and/or respiratory symptoms within the family in recent 2 weeks. Fever was defined as axillary temperature of at least 37.3 °C. Gastrointestinal symptoms included nausea, emesis and diarrhea.
Discharge criteria
Once the temperature returned to normal for more than 3 days, respiratory symptoms significantly improved, the imaging of lung obviously absorbed, and two consecutive negative results for SARS-CoV-2 antigen (sampling interval at least 1 day), patients could be discharged from hospital.
Statistical analysis
Continuous variables were presented as median (interquartile range [IQR]) and compared using Kruskal-Wallis. Categorical variables were presented as frequency (percentages), and compared using the χ2 test or Fisher’s exact test when appropriate.
To analyze risk factors for the severity of illness in patients with COVID-19, univariate and multivariate ordinal logistic regression models were used. Variables with P < 0.05 in the univariate models were selected into the multivariate model for calculating. A two-sided α of < 0.05 was considered statistically significant. All the analyses were done with SPSS version 25.0 (IBM Corporation, Armonk, NY, USA).
The Kaplan-Meier method was used to estimate hospitalization time, and the log rank test was applied for comparisons among mild, common, and severe type. The Kaplan-Meier analysis was performed using ‘survival’ packages in R version 3.6.1 (R Foundation, Vienna, Austria).
Results
Demographic, epidemiologic, and clinical characteristics
From 17 January 2020 to 12 February 2020, clinical data were collected on 788 patients with COVID-19 in Zhejiang Province. According to the definition of clinical classification, they were divided into 52 cases (6.6%) of mild type, 658 cases (83.5%) of common type, 61 cases (7.7%) of severe type, and 17 cases (2.2%) of critical type.
As shown in Table 1, the median age in mild, common, severe, and critical type was 37.5 years (IQR: 19.3–45.8), 45.0 years (IQR: 35.0–55.0), 55.0 years (IQR: 44.0–62.0), and 70.0 years (IQR: 55.0–73.0). The proportion of male patients was account for 50.0, 50.0, 63.9 and 76.5%, respectively. Hypertension was the most common underlying disease and there were significant differences among the four subtypes (9.6% vs 13.2% vs 31.1% vs 88.2%, P < 0.001). In both severe and critical type, the ratio of patients with gastrointestinal symptoms exceeded 15%.
Table 1.
Demographic, epidemiologic and clinical characteristics of different subtypes in patients with COVID-19
| Characteristic | Mild type (n = 52) | Common type (n = 658) | Severe type (n = 61) | Critical type (n = 17) | Pvalue |
|---|---|---|---|---|---|
| Age (years) | 37.5 (19.3–45.8) | 45.0 (35.0–55.0) | 55.0 (44.0–62.0) | 70.0 (55.0–73.0) | < 0.001 |
| Distribution | < 0.001 | ||||
| ≤ 18 | 12 (23.1) | 9 (1.4) | 0 (0.0) | 0 (0.0) | |
| 19–40 | 16 (30.8) | 241 (36.6) | 11 (18.0) | 0 (0.0) | |
| 41–65 | 24 (46.2) | 350 (53.2) | 39 (63.9) | 7 (41.2) | |
| ≥ 66 | 0 (0.0) | 58 (8.8) | 11 (18.0) | 10 (58.8) | |
| Sex (male) | 26 (50.0) | 329 (50.0) | 39 (63.9) | 13 (76.5) | 0.034 |
| BMI (kg/m2) | < 0.001 | ||||
| < 18.5 | 5/25 (20.0) | 20/370 (5.4) | 0/36 (0.0) | 0/13 (0.0) | |
| 18.5–< 25 | 17/25 (68.0) | 240/370 (64.9) | 17/36 (47.2) | 7/13 (53.8) | |
| ≥ 25 | 3/25 (12.0) | 110/370 (29.7) | 19/36 (52.8) | 6/13 (46.2) | |
| Current smoker | 2 (3.8) | 45 (6.8) | 5 (8.2) | 2 (11.8) | 0.544 |
| Exposure history in Wuhan | 23 (44.2) | 331 (50.3) | 31 (50.8) | 8 (47.1) | 0.853 |
| Incubation period (days) | 7.0 (3.0–11.5) (n = 17) | 6.0 (3.0–9.0) (n = 156) | 3.0 (2.0–5.5) (n = 14) | 7.0 (n = 1) | 0.239 |
| Family cluster | 25 (48.1) | 152 (23.1) | 11 (18.0) | 7 (41.2) | < 0.001 |
| Time from illness onset to first hospital admission (days) | 2.5 (1.0–4.0) | 3.0 (1.0–6.0) | 5.0 (2.0–8.0) | 5.0 (2.5–6.5) | < 0.001 |
| Coexisting disorder | |||||
| Any | 10 (19.2) | 161 (24.5) | 31 (50.8) | 16 (94.1) | < 0.001 |
| Hypertension | 5 (9.6) | 87 (13.2) | 19 (31.1) | 15 (88.2) | < 0.001 |
| Heart disease | 0 (0.0) | 9 (1.4) | 1 (1.6) | 1 (5.9) | 0.254 |
| Diabetes | 3 (5.8) | 42 (6.4) | 8 (13.1) | 4 (23.5) | 0.019 |
| Chronic obstructive pulmonary disease | 0 (0.0) | 0 (0.0) | 2 (3.3) | 1 (5.9) | 0.001 |
| Cancer | 0 (0.0) | 3 (0.5) | 3 (4.9) | 0 (0.0) | 0.030 |
| Chronic liver disease | 1 (1.9) | 26 (4.0) | 2 (3.3) | 2 (11.8) | 0.351 |
| Chronic renal disease | 0 (0.0) | 5 (0.8) | 1 (1.6) | 1 (5.9) | 0.150 |
| Symptoms on admission | |||||
| Fever | 28 (53.8) | 534 (81.2) | 57 (93.4) | 17 (100.0) | < 0.001 |
| Cough | 19 (36.5) | 426 (64.7) | 49 (80.3) | 12 (70.6) | < 0.001 |
| Sputum production | 8 (15.4) | 219 (33.3) | 29 (47.5) | 9 (52.9) | 0.001 |
| Hemoptysis | 0 (0.0) | 8 (1.2) | 6 (9.8) | 1 (5.9) | 0.001 |
| Sore throat | 8 (15.4) | 95 (14.4) | 7 (11.5) | 1 (5.9) | 0.796 |
| Nasal obstruction | 6 (11.5) | 41 (6.2) | 0 (0.0) | 0 (0.0) | 0.034 |
| Myalgia | 4 (7.7) | 68 (10.3) | 14 (23.0) | 5 (29.4) | 0.004 |
| Fatigue | 10 (19.2) | 105 (16.0) | 18 (29.5) | 6 (35.3) | 0.013 |
| Gastrointestinal symptom | 7 (13.5) | 63 (9.6) | 12 (19.7) | 6 (35.3) | 0.002 |
| Headache | 2 (3.8) | 61 (9.3) | 11 (18.0) | 1 (5.9) | 0.076 |
Data are presented as medians (interquartile ranges, IQR), n (%) and n/N (%)
Laboratory and radiograph findings
On admission, the majority of leucocyte in all subtypes were normal or decreased. As shown in Table 2, the four types had significant differences in neutrophil count, lymphocyte count, platelets count, albumin (ALB), aspartate aminotransferase (AST), sodium, blood urea nitrogen (BUN), creatinine (CR), creatinine kinase (CK), lactate dehydrogenase (LDH), C-reactive protein (CRP) and procalcitonin (PCT) (P < 0.05). In contrast, there were no significant differences in hemoglobin, International normalized ration (INR), alanine aminotransferase (ALT), total bilirubin (TB) and potassium (P > 0.05).
Table 2.
Laboratory and radiograph findings of different subtypes in patients with COVID-19 on admission
| Variable | Mild type (n = 52) | Common type (n = 658) | Severe type (n = 61) | Critical type (n = 17) | Pvalue |
|---|---|---|---|---|---|
| Blood routine | |||||
| Leucocyte count (× 109/L) | 5.7 (4.3–7.1) | 4.7 (3.8–5.8) | 4.9 (3.8–6.6) | 6.8 (3.8–8.8) | 0.002 |
| < 4 | 9 (17.3) | 201 (30.5) | 19 (31.1) | 5 (29.4) | |
| > 10 | 1 (1.9) | 10 (1.5) | 4 (6.6) | 3 (17.6) | |
| Neutrophil count (× 109/L) | 3.6 (2.0–5.0) | 2.9 (2.2–3.8) | 3.2 (2.6–5.0) | 5.8 (2.8–8.0) | < 0.001 |
| > 7 | 3 (5.8) | 17 (2.6) | 7 (11.5) | 7 (41.2) | |
| Lymphocyte count (× 109/L) | 1.3 (1.1–1.9) | 1.2 (0.9–1.6) | 0.9 (0.6–1.2) | 0.5 (0.4–0.8) | < 0.001 |
| < 0.8 | 8 (15.4) | 91 (13.8) | 23 (37.7) | 12 (70.6) | |
| Hemoglobin (g/L) | 139.0 (131.0–152.0) | 138.0 (127.0–150.0) | 139.0 (122.5–153.0) | 128.0 (117.0–153.5) | 0.332 |
| Platelets count (×109/L) | 206.0 (171.0–241.3) | 180.0 (147.8–221.3) | 172.0 (138.0–214.0) | 146.0 (122.0–181.5) | 0.001 |
| < 100 | 0 (0.0) | 23 (3.5) | 2 (3.3) | 2 (11.8) | |
| Coagulation function | |||||
| International normalized ration (INR) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.2) | 0.070 |
| Blood biochemistry | |||||
| Albumin (ALB, g/L) | 42.5 (40.5–45.7) | 41.7 (38.7–43.9) | 38.7 (35.8–41.6) | 35.9 (30.8–37.6) | < 0.001 |
| Alanine aminotransferase (ALT, U/L) | 20.0 (12.0–39.1) | 21.1 (15.0–33.0) | 24.0 (16.5–34.5) | 20.5 (14.0–30.8) | 0.528 |
| Aspartate aminotransferase (AST, U/L) | 22.0 (16.5–34.0) | 25.0 (19.1–32.2) | 28.0 (22.0–40.0) | 30.5 (25.0–41.8) | 0.002 |
| > 40 | 7 (13.5) | 76 (11.6) | 13 (21.3) | 5 (29.4) | |
| Total bilirubin (TB) (μmol//L) | 8.8 (6.2–11.6) | 9.5 (7.0–13.1) | 10.7 (7.8–15.6) | 10.3 (8.0–14.6) | 0.205 |
| Potassium (mmol/L) | 3.8 (3.6–4.2) | 3.8 (3.6–4.1) | 3.9 (3.6–4.2) | 3.7 (3.2–3.9) | 0.112 |
| Sodium (mmol/L) | 139.0 (138.0–141.0) | 138.5 (136.3–140.2) | 137.4 (135.0–139.3) | 136.0 (130.1–137.8) | < 0.001 |
| Blood urea nitrogen (BUN, mmol/L) | 4.2 (3.2–4.7) | 3.7 (3.0–4.6) | 4.0 (3.2–5.6) | 5.8 (3.6–12.3) | 0.001 |
| Creatinine (CR, μmol/L) | 66.0 (58.0–76.0) | 65.3 (55.0–78.0) | 71.0 (62.5–80.5) | 79.0 (65.9–106.5) | 0.002 |
| Creatinine kinase (CK, U/L) | 60.5 (42.5–75.3) | 68.5 (47.0–105.3) | 76.0 (56.5–120.5) | 146.0 (54.3–255.5) | < 0.001 |
| Lactate dehydrogenase (LDH, U/L) | 175.0 (147.0–241.0) | 207.5 (168.0–254.0) | 272.0 (221.5–366.5) | 320.5 (256.3–356.5) | < 0.001 |
| > 250 | 11 (21.2) | 167 (25.4) | 39 (63.9) | 13 (76.5) | |
| Infection-related biomarkers | |||||
| C-reactive protein (CRP, mg/L) | 1.9 (0.5–5.5) | 7.7 (2.5–19.4) | 26.2 (10.4–51.1) | 45.5 (14.7–84.9) | < 0.001 |
| Procalcitonin (PCT, ng/ml) | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | 0.1 (0.0–0.2) | 0.013 |
| Chest x-ray/CT finding | |||||
| Multiple mottling and ground-glass opacity | 0(0.0) | 183(27.8) | 37 (60.7) | 15 (88.2) | < 0.001 |
Data are presented as medians (interquartile ranges, IQR), n (%) and n/N (%)
Multiple mottling and ground-glass opacity were typical imaging manifestations of patients with COVID-19. The proportion of it in common, severe, and critical type were 27.8, 60.7, and 88.2%, respectively.
Treatments and outcomes
Patients with COVID-19 were quarantined in designated hospital and a total of 668 patients received antiviral treatments in our research. As shown in Table 3, interferon-α inhalation, lopinavir/ritonavir and arbidol were the most commonplace antiviral regimen in all subtypes. Glucocorticoids were not used in mild type, but in all critical types, and there were significant differences in the use among the four types (0.0% vs 8.2% vs 47.5% vs 100.0%, P < 0.001). Similar to glucocorticoids, there were significant differences among the four types based on the use intravenous immunoglobulin (0.0% vs 3.6% vs 42.6% vs 70.6%, P < 0.001). Till 12 February 2020, none of patients used extracorporeal membrane oxygenation (ECMO) and continuous renal-replacement therapy (CRRT).
Table 3.
Treatments, and clinical outcomes of different subtypes in patients with COVID-19
| Variable | Mild type (n = 52) | Common type (n = 658) | Severe type (n = 61) | Critical type (n = 17) | Pvalue |
|---|---|---|---|---|---|
| Shock | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.8) | < 0.001 |
| Time from illness onset to antiviral treatments (days) | 3.0 (1.0–4.5) | 4.0 (2.0–7.0) | 7.0 (4.0–9.0) | 5.0 (2.0–6.8) | < 0.001 |
| Antiviral treatments | 41 (78.8) | 552 (83.9) | 59 (98.7) | 16 (94.1) | 0.010 |
| Interferon-α inhalation + lopinavir/ritonavir | 9/41 (22.0) | 139/552 (25.2) | 16/59 (27.1) | 1/16 (6.3) | |
| Interferon-α inhalation+ arbidol | 1/41 (2.4) | 33/552 (6.0) | 7/59 (11.9) | 0/16 (0.0) | |
| Interferon-α inhalation + lopinavir/ritonavir+ arbidol | 14/41 (34.1) | 191/552 (34.6) | 23/59 (39.0) | 7/16 (43.8) | |
| Lopinavir/ritonavir + arbidol | 6/41 (14.6) | 62/552 (11.2) | 1/59 (1.7) | 4/16 (25.0) | |
| Lopinavir/ritonavir | 5/41 (12.2) | 60/552 (10.9) | 3/59 (5.1) | 0/16 (0.0) | |
| Arbidol | 2/41 (4.9) | 33/552 (6.0) | 6/61 (10.2) | 0/16 (0.0) | |
| Interferon-α inhalation only | 4/41 (9.8) | 29/552 (5.3) | 2/61 (3.4) | 0/16 (0.0) | |
| others | 0/41 (0.0) | 5/552 (0.9) | 1/61 (1.7) | 4/16 (25.0) | |
| Glucocorticoids | 0 (0.0) | 54 (8.2) | 29 (47.5) | 17 (100.0) | < 0.001 |
| Intravenous immunoglobulin | 0 (0.0) | 24 (3.6) | 26 (42.6) | 12 (70.6) | < 0.001 |
| Extracorporeal membrane oxygenation (ECMO) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Continuous renal-replacement therapy (CRRT) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Clinical outcomes at data cutoff | |||||
| Discharge from hospital | 21 (40.4) | 273 (41.5) | 27 (44.3) | 1 (5.9) | 0.029 |
| Hospitalization | 31 (59.6) | 385 (58.5) | 34 (55.7) | 16 (94.1) | 0.029 |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Data are presented as medians (interquartile ranges, IQR), n (%) and n/N (%)
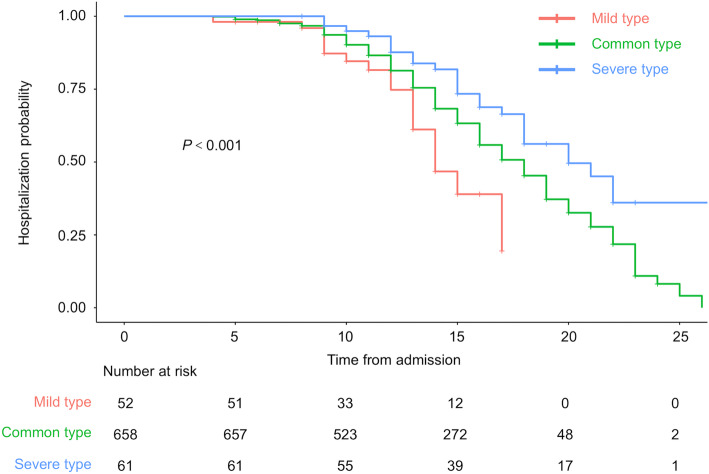
At the endpoint of our study, 21 cases (40.4%) of mild type, 273 cases (41.5%) of common type, 27 cases (44.3%) of severe type, and 1 case (5.9%) of critical type discharged from hospital, and none of the patients died. Kaplan-Meier analysis showed a significant difference in hospitalization time among mild type, common type and severe type (Fig. 1).
Fig. 1.
Kaplan–Meier analysis showed a significant difference in hospitalization time among mild type, common type and severe type
Risk factors for the severity of illness in patients with COVID-19
As shown in Table 4, the variables with P < 0.05 in the univariate ordinal logistic regression model were selected into the multivariate ordinal logistic regression model for the severity of illness in patients with COVID-19. There was no multicollinearity within the variables in the final model. Results of multivariate ordinal logistic regression showed the severity of illness was relevant to the age-grading, sex, fever, cough, hemoptysis, gastrointestinal symptoms and hypertension. Multivariate ordinal logistic regression demonstrated increasing odds of the severity of illness in patients with COVID-19 associated with male (odds ratio [OR] = 1.7, 95% confidence interval [CI]: 1.2–2.6, P = 0.008), fever (OR = 3.6, 95% CI: 2.1–6.3, P < 0.001), cough (OR = 1.7, 95% CI: 1.0–2.9, P = 0.041), hemoptysis (OR = 3.4, 95% CI: 1.1–10.3, P = 0.032), gastrointestinal symptoms (OR = 1.9, 95% CI: 1.0–3.5, P = 0.047), hypertension (OR = 2.6, 95% CI: 1.2–5.6, P = 0.013). With the increase of age-grading, the risk for the severity was gradually higher (≤ 18 years [OR = 1.0], 19–40 years [OR = 12.7, 95% CI: 4.5–36.0, P < 0.001], 41–65 years [OR = 14.8, 95% CI: 5.2–42.1, P < 0.001], ≥ 66 years [OR = 56.5, 95% CI: 17.1–186.5, P < 0.001]).
Table 4.
Risk factors for the severity of illness in patients with COVID-19
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Pvalue | OR | 95% CI | Pvalue | |
| Age (years) | ||||||
| ≥ 66 | 111.8 | 38.7–323.2 | < 0.001 | 56.5 | 17.1–186.5 | < 0.001 |
| 41–65 | 29.0 | 11.2–74.9 | < 0.001 | 14.8 | 5.2–42.1 | < 0.001 |
| 19–40 | 16.4 | 6.3–42.3 | < 0.001 | 12.7 | 4.5–35.9 | < 0.001 |
| ≤ 18 | 1.0 | 1.0 | ||||
| Sex (male) | 1.6 | 1.1–2.3 | 0.024 | 1.7 | 1.2–2.6 | 0.008 |
| Current smoker | 1.5 | 0.7–3.1 | 0.255 | |||
| Family cluster | 0.5 | 0.3–0.8 | 0.007 | 0.9 | 0.5–1.4 | 0.579 |
| Exposure History in Wuhan | 1.1 | 0.8–1.6 | 0.608 | |||
| Time from illness onset to first hospital admission | 1.1 | 1.0–1.2 | < 0.001 | 1.1 | 1.0–1.1 | 0.071 |
| Symptoms | ||||||
| Fever | 4.3 | 2.6–7.1 | < 0.001 | 3.6 | 2.1–6.3 | < 0.001 |
| Cough | 2.7 | 1.8–4.1 | < 0.001 | 1.7 | 1.0–3.0 | 0.041 |
| Sputum production | 2.2 | 1.5–3.4 | < 0.001 | 1.3 | 1.0–2.1 | 0.366 |
| Hemoptysis | 7.7 | 2.8–21.3 | < 0.001 | 3.4 | 1.1–10.3 | 0.032 |
| Sore throat | 0.8 | 0.4–1.3 | 0.345 | |||
| Nasal obstruction | 0.3 | 0.2–0.7 | 0.005 | 0.6 | 0.2–1.4 | 0.223 |
| Myalgia | 2.6 | 1.5–4.5 | 0.001 | 1.8 | 1.0–3.4 | 0.063 |
| Fatigue | 1.8 | 1.1–2.8 | 0.022 | 1.3 | 0.7–2.2 | 0.371 |
| gastrointestinal symptoms | 2.1 | 1.2–3.6 | 0.012 | 1.9 | 1.0–3.5 | 0.047 |
| Headache | 2.0 | 1.1–3.6 | 0.030 | 2.0 | 1.0–4.0 | 0.052 |
| Coexisting disorder | ||||||
| Any | 3.8 | 2.5–5.9 | < 0.001 | 1.2 | 0.6–2.4 | 0.660 |
| Hypertension | 4.9 | 3.0–7.8 | < 0.001 | 2.6 | 1.2–5.6 | 0.013 |
| Heart disease | 2.5 | 0.6–10.1 | 0.189 | |||
| Diabetes | 2.4 | 1.2–4.6 | 0.009 | 0.8 | 0.4–1.9 | 0.675 |
| Chronic obstructive pulmonary disease | 42.0 | 4.9–363.9 | 0.001 | 7.7 | 0.8–75.6 | 0.081 |
| Cancer | 7.3 | 1.5–35.2 | 0.013 | 3.7 | 0.6–22.2 | 0.149 |
| Chronic liver disease | 1.6 | 0.6–3.9 | 0.335 | |||
| Chronic renal disease | 4.3 | 0.9–20.6 | 0.065 | |||
OR Odds ratio, CI Confidence interval
Discussion
The outbreak of COVID-19 is now becoming an enormous threat to public health. With further research of the structure and infection mechanism of respiratory, scientists found angiotensin converting enzyme 2 (ACE2) might be the site of SARS-CoV-2 binding on the surface of cells, with the same route of infection of SARS-CoV [14]. It has been proved that ACE2 might play an important role in virus transmission and infection. SARS-CoV-2 not only attacked the lung but also caused damages to many other organs, including heart, kidney, liver and central nervous system [3, 6, 15, 16]. The diagnosis of COVID-19 was complicated by the diversity in symptoms, imaging findings and the severity of illness, therefore, describing features of each subtype and exploring risk factors for the severity of illness could help clinicians to better tackle with this disease.
In this respective study, the median age of critical types was higher than the other three types, with the reason of low immunity and degeneration of related physiological function in older. The length of the incubation period is related to many factors, such as the number of pathogens, the time required for toxin production and transmission, and human immunity. The incubation period was shorter in severe type, probably due to poor immunity and higher viral load [17]. Fever and cough were the dominant symptoms in all subtypes, while hemoptysis was rare. Fever is a protective mechanism by activating the immune systems to resist pathogens, and cough is a reflective defense against invaders. When an individual was infected, the above two symptoms generally appeared at early stage. Although hemoptysis was an atypical symptom, it was reported that there was COVID-19 patient admitted only with hemoptysis as the initial symptom [18]. Moreover, we should be alert to the patients who didn’t have a fever, due to 6.6% of severe type without fever in our study. Hypertension and diabetes were the most pervasive underlying diseases in all subtypes, and the proportion of them were higher in critical type. In consideration of the aged constituting the majority of the critical type, it is common that the rates of comorbidities increased.
According to the laboratory results, the decrease of lymphocyte occurred in 70.6% of critical type. The decrease of lymphocyte was a prominent feature of critical type in our cohort which was consistent with a previous study [19]. SARS-CoV-2 might mainly act on lymphocyte, especially T lymphocytes, as did SARS-CoV [6]. In addition, the increased AST and LDH implied a potential of liver and heart damage which was more common in severe and critical types.
Currently, there is no effective antiviral treatment for COVID-19 [20]. In the light of the previous clinical experience, interferon-α, lopinavir/ritonavir and arbidol were applied for antiviral therapy in our hospital, however, the therapeutic regimen was not researched a consensus among hospitals. A retrospective study identified that proper use of corticosteroid in critical type with SARS could lead to a lower mortality and shorter hospitalization stay [21]. In our study, the dosage of glucocorticoids was limited to 40–80 mg/d to avoid side effects. Until 12 February 2020, 21 cases (40.4%) of mild type, 273 cases (41.5%) of common type, 27 cases (44.3%) of severe type, and 1 case (5.9%) of critical type discharged from hospital.
Compared with initial patients infected with SARS-CoV-2 in Wuhan, the illness condition of patients in Zhejiang Province are relatively milder. None of the patients died at the end of our follow-up. This feature is obviously different from researches in Wuhan which reported a higher mortality [10, 19]. At early stage of outbreak of COVID-19 in Wuhan, shortage of local medical resources, insufficient understanding of this disease and no effective drugs might contribute to this phenomenon.
Several risk factors for the severity of illness in patients with COVID-19 were identified in our study including male, fever, cough, hemoptysis, gastrointestinal symptoms, hypertension, and higher age-grading. Several studies demonstrated that differences in COVID-19 disease prevalence and severity were associated with sex, which was similar to our results [3, 6, 19]. One study, using single-cell sequencing, found that expression of ACE2 was more predominant in Asian men [22]. During the evolution, females develop enhanced innate and adaptive immune responses than males and are less susceptible to viral infections [23]. These above two points might be the reasons for the higher prevalence and severity of COVID-19 in men than in women. Additionally, in our study, the presence of any one of the comorbidities was higher in male compared with female (30.7% vs 24.4%, P = 0.048). We thought this result might also partly explain why men are more prone to severity illness.
Our study found increasing odds of the severity of illness was associated with gastrointestinal symptoms. A bioinformatics analysis on single-cell transcriptomes demonstrated that ACE2 was not only highly expressed in the lung AT2 cells, esophagus upper and stratified epithelial cells but also in absorptive enterocytes from ileum and colon. Recently, two independent laboratories from China declared that they have successfully isolated live SARS-CoV-2 from the stool of patients [24]. An increasing number of studies remind us that digestive system might serve as an alternative route of infection for SARS-CoV-2 [25]. We consider the digestive tract transmission of SARS-CoV-2 might impair the function of intestinal mucosal barriers and increase the production of inflammatory factors, further aggravating the severity of illness.
In addition, COVID-19 patients combined with hypertension were at higher risk for the illness severity in our study. Renin-angiotensin system (RAS) plays an important role in the pathogenesis of hypertension. It could be simply summarized as two axes, one is ACE-Ang II-AT1 axis responsible for constriction of blood vessels, and the other is ACE2-Ang-(1–7)-Mas axis with the opposite effect [26, 27]. Generally, the two axes could interact with each other and maintain the blood pressure balance. However, the balance would be broken in hypertension, with the result of a lower expression of ACE2. Once infected by SARS-CoV-2, the level of ACE2 would be even lower, followed by intensified Ang II activity. Ang II would further promote vasoconstriction, increase vascular permeability and mediate inflammatory responses, leading to illness aggravation.
There are several limitations in our study. Firstly, the retrospective design of our study might affect integrality of data and diminish its credibility, and more prospective cohort studies should be on the agenda in the future. Secondly, patients enrolled in our study only come from Zhejiang Province, and large-scale researches at the national level were urgently needed which could provide more reliable and comprehensive data. Thirdly, changes of the illness in different subtypes needed to be further investigated. A model for predicting the changes of disease was necessary for clinicians to better guide treatments. Moreover, laboratory results were not included in ordinal logistic regression model to explore risk factors for the severity of illness, due to the normal range of some indicators varied from different hospitals.
Conclusions
In summary, our study reported the largest cases of patients with COVID-19 in Zhejiang Province, and indicated risk factors of illness severity which was of great significance for early identification and prompt treatment. Based on the research findings, we recommend that clinicians should pay close attention to these features in patients with COVID-19 including older age, male, fever, cough, hemoptysis, gastrointestinal symptoms and hypertension, and strengthen self-protection during the outbreak of COVID-19.
Acknowledgments
We would like to acknowledge Health Commission of Zhejiang Province, China for coordinating data collection.
Abbreviations
- WHO
World Health Organization
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- MERS-CoV
Middle East respiratory syndrome coronavirus
- COVID-19
Coronavirus disease 2019
- RT-PCR
Real-time reverse transcriptase polymerase chain reaction
- China CDC
Chinese Center for Disease Control and Prevention
- ALB
Albumin
- AST
Aspartate aminotransferase
- BUN
Blood urea nitrogen
- CR
Creatinine
- CK
Creatinine kinase
- LDH
Lactate dehydrogenase
- CRP
C-reactive protein
- PCT
Procalcitonin
- ALT
Alanine aminotransferase
- TB
Total bilirubin
- INR
International normalized ration
- ECMO
Extracorporeal membrane oxygenation
- CRRT
Continuous renal-replacement therapy
- RAS
Renin-angiotensin system
- ACE2
Angiotensin converting enzyme 2
- ACE
Angiotensin converting enzyme
- Ang II
Angiotensin II
- AT1
Angiotensin type 1
- Ang-(1–7)
Angiotensin-(1–7)
Authors’ contributions
Yi-Da Yang, Lan-Juan Li and Ji-Fang Sheng designed the study, Shan-Yan Zhang coordinated the work and took the lead in drafting the manuscript and interpreting, Jiang-Shan Lian, Jian-Hua Hu and Xiao-Li Zhang developed the statistical methods, Ying-Feng Lu, Huan Cai, Jue-Qing Gu, Chan-Yuan Ye, Ci-Liang Jin, Guo-Dong Yu, Hong-Yu Jia and Yi-Min Zhang were participated in the collection of experimental data. All authors read and approved the final manuscript for publication.
Funding
This work was supported by National Major Science and Technology Research Projects for the Control and Prevention of Major Infectious Diseases in China (2017ZX10202202).
Availability of data and materials
All datasets are presented in the main paper.
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (NO. IIT20200005C).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 4.WHO. Coronavirus (COVID-19) https://covid19.who.int/. Accessed 19 Apr 2020.
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang LM, Wei L, Xie L, Zhu G, Dela Cruz CS, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 13 Mar 2020.
- 13.China NHCotPsRo. Chinese management guideline for COVID-19(version 6.0) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed 19 Feb 2020.
- 14.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-march 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi F, Yu Q, Huang W, Tan C. 2019 novel coronavirus (COVID-19) pneumonia with hemoptysis as the initial symptom: CT and clinical features. Korean J Radiol. 2020;21(5):537–540. doi: 10.3348/kjr.2020.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 24.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonato G, Dioscoridi L, Mutignani M. Faecal-oral transmission of SARS-COV-2: practical implications. Gastroenterology. 2020;S0016–5085(20):30449–30442. doi: 10.1053/j.gastro.2020.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colafella KM, Hilliard LM, Denton KM. Epochs in the depressor/pressor balance of the renin-angiotensin system. Clin Sci (Lond) 2016;130(10):761–771. doi: 10.1042/CS20150939. [DOI] [PubMed] [Google Scholar]
- 27.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol Rev. 2018;98(1):505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets are presented in the main paper.