Highlights
-
•
We designed an RT-PCR assay that utilizes newly designed double-quencher probes.
-
•
The new assay decreased background fluorescence and improved dynamic range.
-
•
Double-quencher probes improved the SARS-CoV-2 detection rate in clinical samples.
Keywords: SARS-CoV-2, 2019-nCoV, COVID-19, RT-PCR, Quencher, Coronavirus, Diagnosis
Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which emerged in the city of Wuhan, Hubei Province, China, has spread worldwide and is threatening human life. The detection of SARS-CoV-2 is critical for preventing new outbreaks, curbing disease spread, and managing patients. Currently, a reverse-transcription polymerase chain reaction (RT-PCR) assay is used to detect the virus in clinical laboratories. However, although this assay is considered to have high specificity, its sensitivity is reportedly as low as 60–70 %. Improved sensitivity is, therefore, urgently required.
Methods
We used the primers and single-quencher probes recommended by the CDC (N1, N2 and N3) in the USA and the NIID (N1 and N2) in Japan. In addition, we designed double-quencher probes according to the virus sequence provided by the NIID to develop a further assay (termed the YCH assay [N1 and N2]). Using these assays, we conducted RT-PCR with serially diluted DNA positive controls to assess and compare the detection sensitivity of the three assays. Furthermore, 66 nasopharyngeal swabs were tested to determine the diagnostic performances.
Results
The threshold cycle (Ct) value of the RT-PCR was relatively low for the CDC and YCH assays compared with the NIID assay. Serial dilution assays showed that both the CDC and YCH assays could detect low copy numbers of the DNA positive control. The background fluorescence signal at the baseline was lower for the YCH assay compared with the NIID assay. We assessed the diagnostic performance between single- (NIID) and double-quencher (YCH) probes using 66 nasopharyngeal swabs. When the results of YCH-N2 assay were used as a reference, each assay detected SARS-CoV-2 with positive percent agreements of 56 % for NIID-N1, 61 % for YCH-N1, and 94 % for NIID-N2, and 100 % negative percent agreements for NIID-N1, YCH-N1 and NIID-N2.
Conclusion
Double-quencher probes decreased the background fluorescence and improved the detection sensitivity of RT-PCR for SARS-CoV-2.
1. Introduction
In early December 2019, the first cases of pneumonia of unknown origin were suspected in Wuhan, the capital of Hubei, China (Huang et al., 2020). This novel human-infecting coronavirus was tentatively named “2019 novel coronavirus” (2019-nCoV) by the World Health Organization (WHO) and then later renamed as “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) (Coronaviridae Study Group of the International Committee on Taxonomy of V, 2020). As of July 7, 2020, more than 11.5 million cases of coronavirus disease 2019 (COVID-19) had been confirmed, with 0.53 million deaths globally (WHO Situation Report). Human-to-human transmission of the virus accounts for the virus spreading throughout the world (Li et al., 2020; Lillie et al., 2020; Arashiro et al., 2020; Cheng et al., 2020; Nkengasong and Mankoula, 2020; Albarello et al., 2020).
SARS-CoV-2 belongs to a group of SARS-like coronaviruses (genus Betacoronavirus, subgenus Sarbecovirus) that had previously been found in bats in China (Hu et al., 2018). Phylogenetic analysis has indicated that bats may have also been the original host of SARS-CoV-2 (Lu et al., 2020). SARS-CoV-2 has a single-stranded, positive-sense RNA genome that is 29,903 base pairs in length (NCBI Reference Sequence: NC_045512.2) (Wu et al. (2020); Kim et al. (2020)). An early viral genome sequence was published to inform and assist public health control, followed by the genome sequencing data of 60,000 samples deposited in the database curated by the Global Initiative on Sharing All Influenza Data (GISAID) (Elbe and Buckland-Merrett, 2017). These data indicated how the SARS-CoV-2 outbreaks had occurred worldwide based on the observed genetic variations.
Human infections with SARS-CoV-2 have become a global health concern. The diagnosis of COVID-19 is of high priority for patient and public health management to minimize spread of the infection. Chest computed tomography (CT) imaging can be used to diagnose patients with COVID-19 (Zu et al., 2020). In addition, a reverse-transcription polymerase chain reaction (RT-PCR) has been developed that uses throat swabs or sputum specimens; however, although this assay is considered to have high specificity, its sensitivity is reportedly as low as 60–70 % compared to CT examination (Ai et al., 2020; Kanne et al., 2020; Fang et al., 2020). Ai et al. showed RT-PCR test was positive at 59 % (601/1014), whereas chest CT was positive at 88 % (888/1014) in Wuhan, China (Ai et al., 2020). Fang et al. showed the initial RT-PCR analysis detected at 71 % (36/51) in patients who had abnormality at the rate of 98 % (50/51) by the chest CT. Even though the initial RT-PCR test was negative, repeated RT-PCR tests have increased the cumulative positive rate for COVID-19 (Al-Tawfiq and Memish, 2020).
There is significant demand for a SARS-CoV-2 diagnostic with improved sensitivity that can be used for testing suspected cases. WHO documented the interim guidance of laboratory testing, which denotes nucleic acid amplification test (i.e. RT-PCR) should be conducted to COVID-19 suspected cases (World Health Organization, 2020a). Low sensitivity in RT-PCR can be the result of several factors including insensitivity inherent to the detection method, variation in the types of detection method used, low initial viral load, types of specimen, and improper clinical sampling (Al-Tawfiq and Memish, 2020). The viral load in nasopharyngeal swabs is higher than that in throat swabs as determined by RT-PCR, suggesting that upper respiratory specimens are superior for genetic testing (Zou et al., 2020). To date, protocols of RT-PCR assay to detect SARS-CoV-2 are designed in several countries including USA (Centers for Disease Control and Prevention [CDC]), China (China CDC), Japan (National Institute of Infectious Diseases [NIID]), Germany (Charité), France (Institut Pasteur), Hong Kong (The University of Hong Kong) and Thailand (National Institute of Health) (World Health Organization, 2020b).
The USA CDC assay shows positive percent agreement (100 %, 13/13 clinical samples) and negative percent agreement (100 %, 104/104) using the primers/probe sets targeting the different regions of nucleocapsid (N) gene (World Health Organization, 2020b). The NIID assay also shows positive percent agreement (100 %, 10/10) and negative percent agreement (100 %, 15/15) compared to the result of LightMix Modular SARS and Wuhan CoV E-gene assay (Okamaoto et al., 2020).
In the present study, we looked at improving assay sensitivity with the use of an improved detection method. We compared the detection sensitivity among the primer/probe sets designed by the NIID in Japan and the CDC in the USA. Both of these assays use single-quencher probes. We further designed an RT-PCR assay using double-quencher probes based on the same SARS-CoV-2 sequence released by the NIID. The findings indicated that the double-quencher probes reduced the background signal and improved the detection sensitivity of RT-PCR for SARS-CoV-2.
2. Materials and methods
2.1. Clinical samples
We collected 66 nasopharyngeal swabs between March 11 and April 20, 2020 at our hospital. All samples were obtained with cotton swab and universal transport media (Copan, Murrieta, CA). The Institutional Review Board at Yamanashi Central Hospital (YCH) approved this study and the use of an opt-out consent method (C2019-30). The requirement for written informed consent was waived. Participation in the study by patients was optional.
2.2. Primer and probe sets
The CDC has designed an RT-PCR assay for SARS-CoV-2 and published a protocol for the detection of this virus (https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html). We purchased the 2019-nCoV CDC qPCR Probe Assays (renamed the 2019-nCoV RUO Kit) from Integrated DNA Technologies (IDT, Coralville, IA, USA), which contains research-use-only primer and probe sets based on the protocol announced by the CDC (hereafter called the CDC assay). The CDC assay includes three sets of primers, as well as probes containing a 5′-FAM dye and a 3′-Black Hole Quencher (BHQ) (Table 1 ).
Table 1.
Sequences of the primers and probes used in the CDC, NIID, and YCH assays.
| Institute | Primer name | Description | Oligonucleotide Sequence (5’>3’) | Modification |
|---|---|---|---|---|
| CDC | CDC-N1-F | 2019-nCoV_N1 Forward Primer | 5’-GACCCCAAAATCAGCGAAAT-3’ | None |
| CDC | CDC-N1-R | 2019-nCoV_N1 Reverse Primer | 5’-TCTGGTTACTGCCAGTTGAATCTG-3’ | None |
| CDC | CDC-N1-P | 2019-nCoV_N1 Probe | 5’-FAM-ACCCCGCATTACGTTTGGTGGACC-BHQ1-3’ | FAM/BHQ1 |
| CDC | CDC-N2-F | 2019-nCoV_N2 Forward Primer | 5’-TTACAAACATTGGCCGCAAA-3’ | None |
| CDC | CDC-N2-R | 2019-nCoV_N2 Reverse Primer | 5’-GCGCGACATTCCGAAGAA-3’ | None |
| CDC | CDC-N2-P | 2019-nCoV_N2 Probe | 5’-FAM-ACAATTTGCCCCCAGCGCTTCAG-BHQ1-3’ | FAM/BHQ1 |
| CDC | CDC-N3-F | 2019-nCoV_N3 Forward Primer | 5’-GGGAGCCTTGAATACACCAAAA-3’ | None |
| CDC | CDC-N3-R | 2019-nCoV_N3 Reverse Primer | 5’-TGTAGCACGATTGCAGCATTG-3’ | None |
| CDC | CDC-N3-P | 2019-nCoV_N3 Probe | 5’-FAM-AYCACATTGGCACCCGCAATCCTG-BHQ1-3’ | FAM/BHQ1 |
| CDC | RP-F | RNAse P Forward Primer | 5’-AGATTTGGACCTGCGAGCG-3’ | None |
| CDC | RP-R | RNAse P Reverse Primer | 5’-GAGCGGCTGTCTCCACAAGT-3’ | None |
| CDC | RP-P | RNAse P Probe | 5’-FAM–TTCTGACCTGAAGGCTCTGCGCG–BHQ1-3’ | FAM/BHQ1 |
| NIID | NIID-N1-F | N_Sarbeco_Forward Primer | 5’-CACATTGGCACCCGCAATC-3' | None |
| NIID | NIID-N1-R | N_Sarbeco_Reverse Primer | 5’-GAGGAACGAGAAGAGGCTTG-3' | None |
| NIID | NIID-N1-P | N_Sarbeco_Probe | 5’-FAM-ACTTCCTCAAGGAACAACATTGCCA-TAMRA-3' | FAM/TAMRA |
| NIID | NIID-N2-F | NIID_2019-nCOV_N_Forward Primer | 5’-AAATTTTGGGGACCAGGAAC-3' | None |
| NIID | NIID-N2-R | NIID_2019-nCOV_N_Reverse Primer | 5’-TGGCAGCTGTGTAGGTCAAC-3' | None |
| NIID | NIID-N2-P | NIID_2019-nCOV_N_Probe | 5’-FAM-ATGTCGCGCATTGGCATGGA-TAMRA-3' | FAM/TAMRA |
| YCH | YCH-N1-F | YCH_N1 Forward Primer | 5’-CACATTGGCACCCGCAATC-3' | None |
| YCH | YCH-N1-R | YCH_N1 Reverse Primer | 5’-GAGGAACGAGAAGAGGCTTG-3' | None |
| YCH | YCH-N1-P | YCH_N1 Probe | 5’-FAM/ACTTCCTCA/ZEN/AGGAACAACATTGCCA-IBFQ-3' | FAM/ZEN/IBFQ |
| YCH | YCH-N2-F | YCH_N1 Forward Primer | 5’-AAATTTTGGGGACCAGGAAC-3' | None |
| YCH | YCH-N2-R | YCH_N1 Reverse Primer | 5’-TGGCAGCTGTGTAGGTCAAC-3' | None |
| YCH | YCH-N2-P | YCH_N1 Probe | 5’-FAM/ATGTCGCGC/ZEN/ATTGGCATGGA-IBFQ-3' | FAM/ZEN/IBFQ |
NIID, National Institute of Infectious Diseases; YCH, Yamanashi Central Hospital; CDC, Centers for Disease Control and Prevention.
FAM, 6-carboxyfluorescein; BHQ1, Black Hole Quencher 1; IBFQ, Iowa Black Fluorescent Quencher.
The NIID has also designed an RT-PCR assay and published associated data (Shirato et al., 2020). We obtained these primer and probe sets from the NIID (hereafter called the NIID assay). The NIID assay includes two sets of primers, as well as probes containing a 5′-FAM dye and a 3’-TAMRA dye (Table 1).
We further designed double-quencher probes (purchased from IDT) based on the same primers/probe sequence reported in the NIID protocol (hereafter called the YCH assay). These probes each incorporate a 5′-FAM dye, an internal ZEN quencher, and a 3′-Iowa Black Fluorescent Quencher (IBFQ) (Table 1). The internal ZEN quencher was incorporated between bases 9 and 10 from the 5′ end of the probe. This design decreased the distance between the dye and the quencher and was expected to reduce the background signal and achieve an improved dynamic range. All of the primer/probe sets in the CDC, NIID, and YCH assays target the N gene of SARS-CoV-2. The CDC assay targets three sites along the N gene, and both of the NIID and YCH assays target two sites along this gene.
2.3. DNA positive control
For the DNA positive control, we purchased the 2019-nCoV_N_Positive Control (IDT, catalog #10006625), which consists of a plasmid containing the complete N gene (1,260 base pairs) of SARS-CoV-2 (Supplemental Table 1). We prepared a serial dilution of the DNA positive control using nuclease-free water (Thermo Fisher Scientific, Waltham, MA, USA) to assess the detection sensitivity of the CDC, NIID, and YCH assays. We also purchased the Hs_RPP30 Positive Control (IDT, catalog #10006626), which contains a portion of the ribonuclease P 30 subunit (RPP30) gene of the human genome (Supplemental Table 1), and this was used to control for non-specific amplification or internal control.
2.4. Nucleic acid extraction
Total nucleic acids were extracted from nasopharyngeal swabs using the MagMax Viral/Pathogen Nucleic Acid Isolation Kit (ThermoFisher Scientific) on automated machine KingFisher Duo Prime as previously described (Hirotsu et al., 2020a; Hirotsu et al., 2020b). Briefly, we added 400 μL of viral transport media, 10 μL of Proteinase K, 530 μL Binding Solution, 20 μL Total Nucleic Acid Binding Beads, 1 mL Wash Buffer, and 1 mL or 0.5 mL of 80 % Ethanol to each well of a Deep-well 96-well plate. 100 μL of Elution solution was added to Elution Strip. Total nucleic acids were stored at −80 °C until further RT-PCR analysis.
2.5. RT-PCR analysis
To detect SARS-CoV-2, we performed RT-PCR with each of the three assays. For the CDC and YCH assays, the reaction mixtures comprised 5 μL of 4× TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher Scientific), 1.5 μL of probe/primer mixtures (IDT), 11.5 μL of nuclease-free water, and 2 μL of serially diluted DNA positive control (2019-nCoV_N_Positive Control) in a 20 μL total volume. We also performed RT-PCR for SARS-CoV-2 using the NIID assay according to the protocol (version 2.7) announced by the NIID. Briefly, for N1 detection with the NIID assay, the reaction mixture comprised 5 μL of 4× TaqMan Fast Virus 1-Step Master Mix, 1.2 μL of forward primer, 1.6 μL of reverse primer, 0.8 μL of probe, 9.4 μL of nuclease-free water, and 2 μL of serially diluted of DNA positive control in a 20 μL total volume. For N2 detection with the NIID assay, the reaction mixture comprised 5 μL of 4× TaqMan Fast Virus 1-Step Master Mix, 1.0 μL of forward primer, 1.4 μL of reverse primer, 0.8 μL of probe, 9.8 μL of nuclease-free water, and 2 μL of serially diluted positive control in a 20 μL total volume.
When we tested the nasopharyngeal swabs, 5 μL of total nucleic acids, 5 μL of 4× TaqMan Fast Virus 1-Step Master Mix and primers/probe of each assay as aforementioned were added in a reaction mixture. Nuclease-free water was added to a final volume of 20 μL.
The RT-PCR assays were conducted on a ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) with the following cycling conditions: 50 °C for 5 min for reverse transcription, 95 °C for 20 s, and 45 cycles of 95 °C for 3 s and 60 °C for 30 s. The data were analyzed using the ViiA 7 software v2.2.2 (Thermo Fisher Scientific). The threshold cycle (Ct) value was assigned to each PCR reaction and the amplification curve was visually assessed.
3. Results
3.1. Design of the primers and probes for SARS-CoV-2 detection
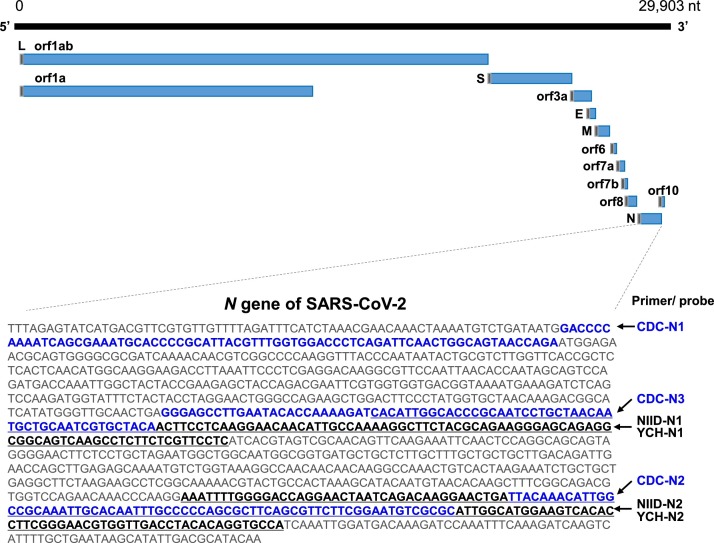
All three of the CDC, NIID, and YCH assays amplified the N gene of SARS-CoV-2 (Fig. 1 ). The CDC assay targets three sites along this gene (N1, N2, and N3), and the NIID and YCH assays target two sites (N1 and N2) (Fig. 1). A previous study showed that the reverse primer for N1 detection in the NIID assay has one mismatch with the sequence in the current database because it was constructed based on another reported sequence (GenBank: MN908947.1) (Shirato et al., 2020). This mismatch in the reverse primer did not influence the detection sensitivity compared with the perfectly matched reverse primer (Shirato et al., 2020).
Fig. 1.
Genomic structure of SARS-CoV-2 and the design of the primer/probe sets.
SARS-CoV-2 is a single-stranded RNA virus with a genome of 29,903 base pairs (NC_045512.2). Primers and probes were previously designed to detect the nucleocapsid (N) gene. The CDC assay (USA) targets three sites along the N gene (CDC-N1, CDC-N2, CDC-N3). The NIID assay (Japan) and YCH assay (present study) target the same two sites along the N gene (NIID-N1, YCH-N1, NIID-N2, and YCH-N2). The CDC and NIID assays both utilize single-quencher probes, whereas the YCH assay uses newly designed double-quencher probes. Blue bold letter and underlined letter indicate the amplification sites by CDC and NIID/YCH assays, respectively. The gray box in each transcript indicates the leader (L) sequence. Abbreviations: orf – open reading frame, S – spike protein, E – envelope protein, M – membrane, N - nucleocapsid.
The CDC and NIID assays both use single-quencher probes; however, in the present study, we designed an RT-PCR assay (the YCH assay) using new probes incorporating double-quencher technology to reduce the background signal. The expected amplicon sizes for the CDC assay are 72, 67, and 72 bp for the N1, N2, and N3 genes, respectively. The expected amplicon sizes for the NIID and YCH assays are 128 bp and 158 bp for the N1 and N2 genes, respectively.
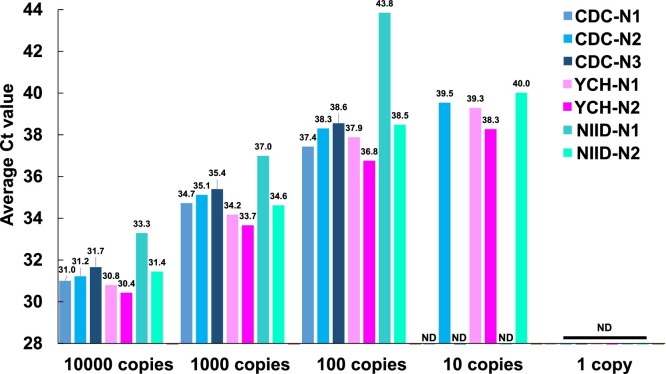
3.2. Serial dilution analysis to determine detection sensitivity
To assess the detection sensitivity of each of the three RT-PCR assays, we conducted assays using the serially diluted positive control (1, 10, 100, 1,000, and 10,000 copies) as the template (Fig. 2 ). We set the threshold value to 0.2 for all assays to determine the threshold cycle (Ct). With over 100 copies of the positive control, amplification was observed in all assays. With 10 copies, we could detect amplification signals for CDC-N2, YCH-N1, YCH-N2, and NIID-N2. None of the three assays could detect one copy of the positive control. There was no non-specific amplification of the human RPP30 control by any of the assays (data not shown). Overall, the YCH assay achieved the lowest Ct value and, hence, the highest sensitivity when using the serially diluted DNA positive control.
Fig. 2.
Serial dilution assays to determine the detection sensitivity toward SARS-CoV-2.
The DNA positive control was serially diluted from 10,000 copies to 1 copy and used as the template to compare the detection sensitivity of the CDC, NIID, and YCH assays The threshold value was set to 0.2. The bar plot shows the average threshold cycle (Ct) obtained with each assay (n = 4). The value above each bar indicates the average Ct value. ND, not detected.
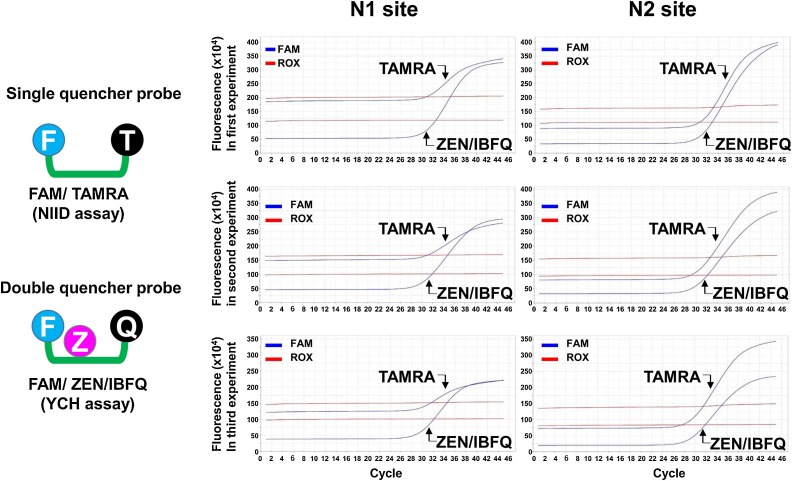
3.3. Double-quencher probes reduce the background signal
We observed that the Ct values obtained for both the N1 and N2 sites with the NIID assay were relatively high compared with those obtained with the YCH assay (Fig. 2), despite the primer and probe sequences being equivalent. We reasoned that the double-quencher probes (ZEN/IBFQ) reduced the background signal and lowered the Ct value compared with the single-quencher probes (TAMRA). To test this, we analyzed the fluorescence signals produced during the RT-PCR cycles of both assays across three independent experiments. As expected, the double-quencher probes decreased the background of the FAM fluorescence signals at the baseline (Fig. 3 ). The baseline fluorescence signal obtained for the N1 site with the NIID assay ranged from 150 × 104 to 200 × 104 relative fluorescence units (RFU), whereas that obtained with the YCH assay was ≤50 × 104 RFU. Similarly, the signal obtained for the N2 site with the NIID assay ranged from 125 × 104 to 200 × 104 RFU, while that obtained with the YCH assay was 75 × 104 to 100 × 104 RFU. These results suggested that the double-quencher probes improved the detection sensitivity for SARS-CoV-2 using RT-PCR owing to the reduction of background signal.
Fig. 3.
Double-quencher probes reduce the background fluorescence signal.
The fluorescence signals obtained from RT-PCR of the N1 and N2 sites along the N gene of SARS-CoV-2 were compared between assays using single- vs the double-quencher probes. The single-quencher probes incorporate a FAM dye and the TAMRA quencher (NIID assay). The double-quencher probes contain a FAM dye, the ZEN internal quencher, and the IBFQ quencher (YCH assay). Each experiment was conducted independently and in triplicate. The ZEN/IBFQ double-quencher reduced the background fluorescence signal compared with the TAMRA single-quencher for both of the N1 and N2 sites.
3.4. Comparison of the diagnostic performance between single- and double-quencher probes
To compare the assay performances of single- and double-quencher probes, we tested nasopharyngeal swabs from 66 patients using NIID and YCH assays. Of the 66 samples, positive signals were detected in 10, 11, 17 and 18 samples by NIID-N1, YCH-N1, NIID-N2 and YCH-N2, respectively (Table 2 ). Amplification signal of the human RPP30 as an internal control was observed in all samples. These results suggested the detection rate of N2 site was higher than that of N1 site.
Table 2.
Sixty six nasopharyngeal swabs were tested by RT-PCR using single- (NIID) and double-quencher (YCH) probes.
| Sample No. | NIID-N1 (single- quencher) |
YCH-N1 (double-quencher) |
NIID-N2 (single- quencher) |
YCH-N2 (double-quencher) |
RPP30 |
|---|---|---|---|---|---|
| #1 | + | + | + | + | + |
| #2 | + | + | + | + | + |
| #3 | + | + | + | + | + |
| #4 | + | + | + | + | + |
| #5 | + | + | + | + | + |
| #6 | + | + | + | + | + |
| #7 | + | + | + | + | + |
| #8 | + | + | + | + | + |
| #9 | + | + | + | + | + |
| #10 | + | + | + | + | + |
| #11 | ND | + | + | + | + |
| #12 | ND | ND | + | + | + |
| #13 | ND | ND | + | + | + |
| #14 | ND | ND | + | + | + |
| #15 | ND | ND | + | + | + |
| #16 | ND | ND | + | + | + |
| #17 | ND | ND | + | + | + |
| #18 | ND | ND | ND | + | + |
| #19–66 | ND | ND | ND | ND | + |
+, detected; ND, not detected; NIID, National Institute of Infectious Diseases; YCH, Yamanashi Central Hospital; RPP30, ribonuclease P 30 subunit.
If the YCH-N2 assay was used as reference, the percent positive agreements were 56 % (10/18) for NIID-N1, 61 % (11/18) for YCH- N1 and 94 % (17/18) for NIID-N2. The percent negative agreements were 100 % for NIID-N1, YCH-N1 and NIID-N2.
4. Discussion
SARS-CoV-2 is the cause of an ongoing international outbreak of respiratory illness known as COVID-19. The rapid and sensitive detection of this virus is important to prevent further outbreaks and to manage patient care. In this study, we compared RT-PCR assays with three different sets of primers and probes, including two previously published and one newly designed here. The assay designed in this study incorporated double-quencher probes, which decreased the background signal compared with the single-quencher probes used in the CDC and NIID assays. This reduction in the background signal improved the dynamic range of fluorescence detection and reduced the Ct value. Thus, the double-quencher probes increased the sensitivity of detection toward SARS-CoV-2.
Several companies have developed commercially available kits for the RT-PCR detection of SARS-CoV-2. IDT launched an assay kit targeting three sites along the N gene and the human RPP30 gene according to the CDC protocol in USA, and this kit was used in this study. Thermo Fisher Scientific has provided an assay targeting Orf1ab, the spike (S) gene, the N gene, and human RNase P; and Roche Diagnostics provides an assay targeting RdRP (in Orf1ab), the E gene and the N gene. Jung et al. recently compared several primer and probe sets developed in China, Germany, Hong Kong, Japan, Thailand, and the USA (Jung et al., 2020). They showed that the CDC-N2 (USA), CDC-N3 (USA), and NIID-N2 (Japan) sets were the most sensitive and reliable laboratory tests for SARS-CoV-2. We also observed that the CDC-N2 (USA) and NIID-N2 (Japan) assays could detect a low copy number of the DNA positive control.
Assessment of the N1 site was originally reported by researchers in Germany. Previous studies showed that the N1 site of the NIID assay is less sensitive when using the RNA positive control (Shirato et al., 2020; Ishige et al., 2020). In this study, we also confirmed that the N1 site of the NIID assay has a limit of detection of 10 copies of the DNA positive control. In addition, we observed that the Ct value was high for the N1 site with the NIID assay. We determined the detection sensitivity using clinical samples and observed the rate of positivity of N2 was higher than that of N1 site (Table 2) (Hirotsu et al., 2020b). All positive samples were detected by N1/N2 sites or N2 site only, but not N1 site only.
During the course of the manuscript submission, CDC recommended the removal of CDC-N3 (USA) primers/probe sets in the kit at March 15, 2020 (World Health Organization, 2020b), because CDC-N3 is intended to detect SARS-like coronavirus and is not specific for SARS-CoV-2. The amplification region of CDC-N3 partially overlapped with that of NIID-N1 and YCH-N1. When the amplification signal was detected in only NIID-N1 but not in NIID-N2, we think it is better to repeat test and confirm the reproducibility. Furthermore, we have to carefully interpret whether the RT-PCR specifically detects SARS-CoV-2 and validate the results by orthologous method (e.g. antigen test and RT-PCR with different primers/probe set).
The background fluorescence signal is mainly determined by two factors: (1) Probe length. Shorter probes have lower background fluorescence, as the shorter the distance between the fluorescent dye and the quencher, the greater the quenching performance owing to fluorescence resonance energy transfer. (2) Probe capacity for self-quenching. Probes that self-quench through the formation of a three-dimensional structure tend to produce lower background fluorescence.
To detect low virus copy numbers in human specimen, the reduction of background signal is important. To this end, we applied the double-quencher system (YCH assay) for detecting SARS-CoV-2 with RT-PCR. As expected, the use of YCH assay with double-quencher probe decreased the background signal at the baseline and led to a greater dynamic range compared to the NIID assay. Therefore, to improve detection sensitivity, we recommend the use of double-quencher probes for the detection of SARS-CoV-2, especially when targeting the N1 site of NIID assay.
The Japanese government announced on 6th March 2020 that the use of NIID assay for SARS-CoV-2 detection approved by the Ministry of Health, Labour and Welfare in Japan and test fee was covered by the National Health Insurance.
Overall, our findings indicate that YCH assays using double-quencher probes will enable us to detect the low viral loads in COVID-19 patients compared to NIID assay in routine clinical practice.
CRediT authorship contribution statement
Yosuke Hirotsu: Conceptualization, Methodology, Investigation, Writing - original draft, Funding acquisition. Hitoshi Mochizuki: Supervision. Masao Omata: Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
None.
Acknowledgments
We thank all of the medical and ancillary hospital staff and the patients for consenting to participate. This study was supported by a Grant-in-Aid for the Genome Research Project from Yamanashi Prefecture (to M.O. and Y.H.), the Japan Society for the Promotion of Science (JSPS) KAKENHI Early-Career Scientists (grant number JP18K16292 to Y.H.), Grant-in-Aid for Scientific Research (B) 20H03668 (to Y.H.), a Research Grant for Young Scholars (to Y.H.), the YASUDA Medical Foundation (to Y.H.), the Uehara Memorial Foundation (to Y.H.), and Medical Research Grants from the Takeda Science Foundation (to Y.H.). We thank Natasha Beeton-Kempen, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2020.113926.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarello F., Pianura E., Di Stefano F., Cristofaro M., Petrone A., Marchioni L., Palazzolo C., Schinina V., Nicastri E., Petrosillo N. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int. J. Infect. Dis. 2020;93:192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Memish Z.A. Diagnosis of SARS-CoV-2 infection based on CT scan vs. RT-PCR: reflecting on experience from MERS-CoV. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.C., Chang Y.C., Fan Chiang Y.L., Chien Y.C., Cheng M., Yang C.H., Huang C.H., Hsu Y.N. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J. Formos. Med. Assoc. 2020;119:747–751. doi: 10.1016/j.jfma.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob. Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y., Maejima M., Nakajima M., Mochizuki H., Omata M. Environmental cleaning is effective for the eradication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in contaminated hospital rooms: a patient from the Diamond Princess cruise ship. Infect. Control Hosp. Epidemiol. 2020:1–8. doi: 10.1017/ice.2020.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Omata M. Pooling RT-PCR test of SARS-CoV-2 for large cohort of’ healthy’ and infection-suspected patients: a prospective and consecutive study on 1,000 individuals. medRxiv. 2020 [Google Scholar]
- Hu D., Zhu C., Ai L., He T., Wang Y., Ye F., Yang L., Ding C., Zhu X., Lv R. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg. Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige T., Murata S., Taniguchi T., Miyabe A., Kitamura K., Kawasaki K., Nishimura M., Igari H., Matsushita K. Highly sensitive detection of SARS-CoV-2 RNA by multiplex rRT-PCR for molecular diagnosis of COVID-19 by clinical laboratories. Clin. Chim. Acta. 2020;507:139–142. doi: 10.1016/j.cca.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.J., Park G.-S., Moon J.H., Ku K., Beak S.-H., Kim S., Park G.-S., Park G.-S., Lee J.-H., Byeon C.W. Comparative analysis of primer-probe sets for the laboratory confirmation of SARS-CoV-2. bioRxiv. 2020 [Google Scholar]
- Kanne J., Little B., Chung J., Elicker B., Ketai L. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020 doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., H Chang. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.011. 914-921 e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie P.J., Samson A., Li A., Adams K., Capstick R., Barlow G.D., Easom N., Hamilton E., Moss P.J., Evans A. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J. Infect. 2020;80:578–606. doi: 10.1016/j.jinf.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395:841–842. doi: 10.1016/S0140-6736(20)30464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamaoto K., Shirato K., Nao N., Saito S., Kageyama T., Hasegawa H., Suzuki T., Matsuyama S., Takeda M. An assessment of real-time RT-PCR kits for SARS-CoV-2 detection. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.108. [DOI] [PubMed] [Google Scholar]
- Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., Katoh H., Sakata M., Nakatsu Y., Mori Y. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Arashiro T., Furukawa K., Nakamura A. COVID-19 in 2 persons with mild upper respiratory tract symptoms on a cruise ship. Japan Emerg. Infect. Dis. J. 2020:26. doi: 10.3201/eid2606.200452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases.https://wwwwhoint/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 [Google Scholar]
- World Health Organization . 2020. Summary Table of Available Protocols in This Document.https://wwwwhoint/who-documents-detail/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z., Jiang M., Xu P., Chen W., Ni Q., Lu G., Zhang L.J. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020 doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.