Abstract
Omecamtiv mecarbil (OM) is a putative positive inotropic tool for treatment of systolic heart dysfunction, based on the finding that in vivo it increases the ejection fraction and in vitro it prolongs the actin-bond life time of the cardiac and slow-skeletal muscle isoforms of myosin. OM action in situ, however, is still poorly understood as the enhanced Ca2+-sensitivity of the myofilaments is at odds with the reduction of force and rate of force development observed at saturating Ca2+. Here we show, by combining fast sarcomere-level mechanics and ATPase measurements in single slow demembranated fibres from rabbit soleus, that the depressant effect of OM on the force per attached motor is reversed, without effect on the ATPase rate, by physiological concentrations of inorganic phosphate (Pi) (1-10 mM). This mechanism could underpin an energetically efficient reduction of systolic tension cost in OM-treated patients, whenever [Pi] increases with heart-beat frequency.
Subject terms: Muscle contraction, Physiology, Cardiology
Omecamtiv mecarbil is a small molecule effector under clinical trial for the treatment of systolic heart failure. Here the authors define the molecular mechanisms of its inotropic action and find it can increase the efficiency of contraction in muscle fibres when the orthophosphate concentration rises with the beat frequency.
Introduction
In the heart, mutations in the myosin motor are responsible for cardiomyopathies, which consist either in an increased contractility with reduced ventricular filling or diastolic dysfunction (hypertrophic cardiomyopathy, HCM) or in a decreased contractility with reduced ejection fraction or systolic dysfunction (dilated cardiomyopathy)1. A promising approach for systolic dysfunction treatment is the development of small molecules that, acting as cardiac myosin activators, are able to increase the contractility during the systole. Among them, omecamtiv mecarbil (OM) has been found to increase the systolic ejection fraction2–6 and is currently in phase-three clinical trial7. OM acts on both α and β cardiac myosin heavy chain (MHC) isoforms and on the slow-skeletal MHC isoform8. Noteworthy, the β cardiac MHC isoform, which accounts for >95% of the myosin in the human ventricle, is encoded by the same gene (MYH7) as the slow-skeletal MHC isoform8–12 and the two isoforms will be named β/slow MHC hereafter. OM binds to the catalytic domain of the myosin molecule between the nucleotide binding pocket and the converter domain, a communicating zone between the active site and the lever arm responsible for the working stroke. As a consequence, the release of inorganic phosphate (Pi) is promoted and the lever arm of the molecular motor is stabilized in a pre-working stroke position2,13,14. Accordingly, the velocity of actin sliding in the in vitro motility assay is reduced by OM15–17. A recent in vitro mechanical study9 has significantly advanced the understanding of OM mechanism, showing that OM binding to β-cardiac myosin inhibits the working stroke and prolongs the actin-attachment life time of the motor. In this way, in situ, OM may increase thin filament activation at low Ca2+ allowing recruitment of OM-free motors, able to generate force. However, a detailed in situ analysis of the effects of OM on the mechano-kinetics and energetic of myosin motors has still to be done.
Here we determine, in Ca2+-activated demembranated fibres from rabbit soleus, the effects of OM on either the number and force of the attached myosin motors or their ATPase activity and how physiological concentrations of inorganic phosphate (Pi) influence these parameters. The slow-skeletal muscle of the rabbit has been chosen instead of the heart because their myosin isoforms exhibit similar affinity for OM8, while the required nanometre-microsecond resolution of sarcomere-level mechanics can only be achieved in demembranated myocytes from skeletal muscle18,19. Intact trabeculae dissected from the ventricle of the rat heart, on which sarcomere-level mechanics has been recently successfully exploited20,21, do not suit the present investigation because (i) only skinned myocytes allow the required manipulation of [Ca2+] and [Pi] and (ii), only ∼20% of the myosin is β/slow MHC isoform and the remaining ∼80% is α MHC isoform21. Determining the fraction of motors recruited for actin interaction during Ca2+-activated isometric contraction of demembranated soleus fibres allowed the demonstration that OM reduces the average force per attached motor but at low Ca2+ it increases the number of attached motors. The inhibitory effect of OM on force generation is reversed by increasing [Pi] (range 1–10 mM), which acts as an allosteric competitor that allows the OM-bound no-force-generating motors to release OM and re-enter the force-generating cycle without any change in the rate of ATP hydrolysis. We conclude that in the heart systole, in which the internal [Ca2+] remains below the saturating level, the inotropic action of OM occurs via two mechanisms: the first is the increase in the number of attached motors, the second, emerging at physiological levels of intracellular Pi (1–10 mM22–28), is a Pi-dependent recovery of the force per motor. This effect of Pi on the OM-bound motors represents an energetically efficient inotropic response in patients under OM treatment, whenever the increase of heart-beat frequency increases [Pi]27,28.
Results
Titration of OM effect on the isometric force development
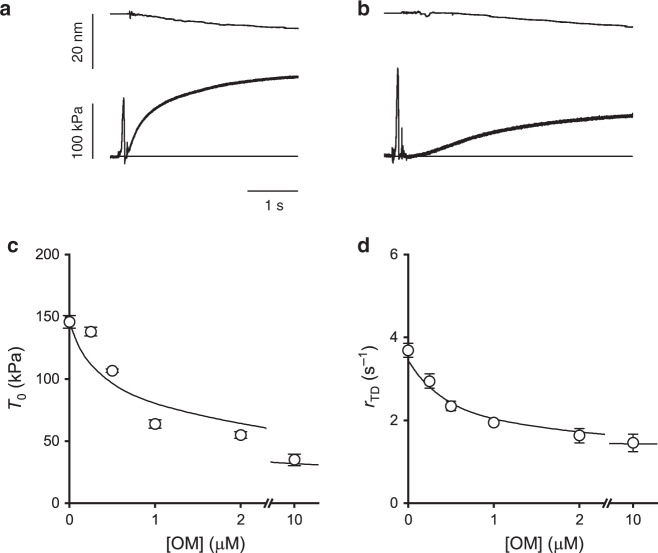
Following a temperature jump from 1 to 12 °C in the activating solution at saturating [Ca2+] (pCa 4.5), the force rises from near zero to a steady value (T0) with an almost exponential time course (Fig. 1a). Addition of 1 μM OM halves T0 and slows the force development that shows a sigmoidal shape (Fig. 1b). In eight fibres T0 decreases from 146 ± 5 kPa in the control to 64 ± 3 kPa in the presence of 1 μM OM. The reciprocal of the half-time for force development, rTD, taken as an estimate of the rate of force development, is reduced by 1 μM OM from 3.7 ± 0.2 s−1 to 1.9 ± 0.1 s−1. The reduction of both T0 and rTD by OM is dose-dependent as shown by the relations of T0 (Fig. 1c) and rTD (d) versus [OM]. The effect nearly saturates at 10 μM OM when T0 is ∼35 kPa and rTD is ∼1.4 s−1. The concentration of OM for half-maximal effect is ∼0.5 μM for both T0 and rTD.
Fig. 1. Effect of OM on isometric force development.
Force development following T-jump (from 1 to 12 °C) in control (a) and in the presence of 1 μM OM (b) on an activated fibre (pCa 4.5). Traces show the length change per half-sarcomere (upper trace) and the force (lower trace). The artefact in the traces marks the transition to the test temperature drop. The horizontal line in the lower panels indicates zero force. Fibre length, 4.5 mm; length of the segment under the striation follower, 0.8 mm; average sarcomere length within the segment, 2.3 µm; CSA, 3000 µm2; pCa, 4.5. Dependence of the isometric force T0 (c) and of the rate of force development rTD (d) on the concentration of OM. Lines, simulation with model of Fig. 6. Data are mean values ± SEM from eight fibres. Source data are provided as a Source Data file.
OM does not alter the fraction of motors at full activation
The half-sarcomere stiffness at T0 (k0) during maximal Ca2+ activation is estimated from the slope of the T1 relation determined, both in the control and in the presence of OM (range 0.1–10 μM), by plotting the force attained in response to stepwise changes in fibre length against the corresponding changes in the half-sarcomere length measured with the striation follower (see “Methods”) (Fig. 2a, b). The T1 relations are shown in Fig. 2c in both control (filled circles) and 1 μM OM (open circles). Both the ordinate intercept (T0) and the abscissa intercept (Y0, an estimate of the half-sarcomere strain at T0) reduce in OM (open circles) by a similar amount (∼60%) relative to the control (filled circles). Thus, the slope of the T1 relation, k0, is the same in the presence of OM as in control. In the eight fibres used for these experiments, k0 is 28.1 ± 0.7 kPa nm−1 in control and remains almost constant independent of [OM] in the whole range of concentrations used (0.1–10 μM, Fig. 2d, triangles), with a mean value of 29.8 ± 0.6 kPa nm−1 (dashed line). In the same range of [OM], Y0 linearly depends on the isometric force and the extrapolation of the relation to zero force crosses the origin (Fig. 2e). This result indicates that the reduction of isometric force by OM at saturating [Ca2+] is due to a proportional reduction in the strain of all the elastic elements in the half-sarcomere (myofilaments and attached myosin motors) and thus OM reduces the half-sarcomere force by reducing the average force per attached myosin motor without changing the number of attached motors.
Fig. 2. Effect of OM on the relevant mechanical parameters determined by stiffness measurements.
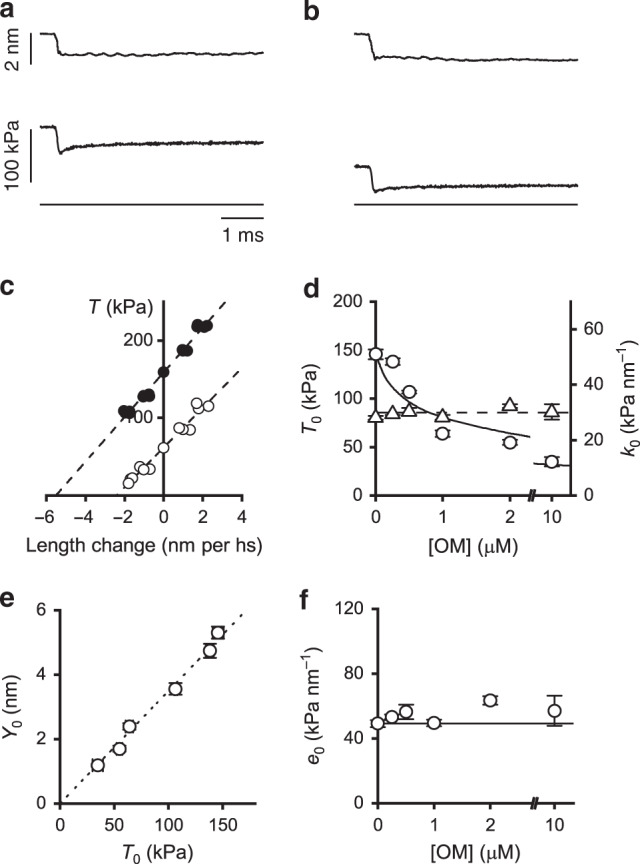
Sample records of the force response (middle trace) to a step length change (upper trace) imposed at the isometric plateau of an activated fibre (pCa 4.5) in control (a) and in the presence of 1 μM OM (b). Lower traces indicate zero force. c T1 relations in control (filled circles) and in the presence of 1 μM OM (open circles) from the same fibre as (a) and (b). Dashed lines are 1st order regression equations fitted to the data. Same fibre as in Fig. 1. d Dependence of T0 (left ordinate, circles) and half-sarcomere stiffness (right ordinate, triangles) on the concentration of OM. Circles are the same data as in Fig. 1c. Continuous line, model simulation of T0–[OM] relation; dashed line, mean value of k0 in the whole range of OM concentrations. e Dependence of half-sarcomere strain (Y0) on OM-modulated isometric force (T0). Dotted line, linear regression on data points. f Dependence of cross-bridge stiffness (e0) on the concentration of OM. Continuous line, value in the absence of OM. Data points in d–f are mean values ± SEM from eight fibres. Source data are provided as a Source Data file.
This conclusion is supported quantitatively by extracting the stiffness of the array of attached motors, e0 (Fig. 2f), from k0. e0 is determined by subtracting the contribution of the filament compliance (Cf) from the half-sarcomere compliance (see “Methods”). The procedure is based on the definition of the half-sarcomere in terms of a simple mechanical model in which the attached motors, represented by an array of in parallel springs with average strain s018, are in series with the actin and myosin filament elasticity represented by a spring with an equivalent filament compliance Cf. The values of Cf and s0 are reported in Table 1. Knowing Cf (15.2 nm MPa−1, average from Table 1), the dependence of e0 on [OM] can be calculated from the relation between k0 and [OM] (Eq. (1)). As shown in Fig. 2f, e0 remains almost constant (∼55 kPa nm−1) independent of [OM]. Under the sensible assumption that OM does not affect the stiffness of the motor, e0 is an estimate of the fraction of attached motors, β, which results constant independent of [OM]. On the other hand, in the presence of 1 μM OM s0 decreases from 3.20 to 1.57 nm (Table 1), indicating that the reduction of T0 to ∼ 1/2 by 1 μM OM (Fig. 1c) is fully accounted for by a corresponding decrease in s0. T0 and the strain of all the elastic elements decrease in proportion in the whole range of [OM] (Fig. 2e), indicating that the reduction of s0 accounts for that in T0. The average force per attached motor (F0) can be calculated from s0 if the stiffness of the myosin motor (ε) is known: F0 = s0 · ε. Taking ε for the slow myosin isoform = 0.56 ± 0.04 pN nm−119, F0 is 1.8 ± 0.2 pN in control and 0.9 ± 0.1 pN in 1 µM OM (Fig. 3c).
Table 1.
Estimates of the mechanical parameters of the half-sarcomere.
| Control | 1 μM OM | |
|---|---|---|
| T0,4.5 (kPa) | 156 ± 7 | 69 ± 5 |
| Cf (nm MPa−1) | 15.2 ± 1.3 | 15.3 ± 2.0 |
| s0 (nm) | 3.20 ± 0.15 | 1.57 ± 0.10 |
Cf and s0 are determined according to the procedure detailed in “Methods”, in control and in the presence of OM. The isometric force at saturating Ca2+ is reported in the first row. T0,4.5, mean ± SEM from four fibres. Cf and s0, slope and ordinate intercept from data in Fig. 7c. Source data are provided as a Source Data file.
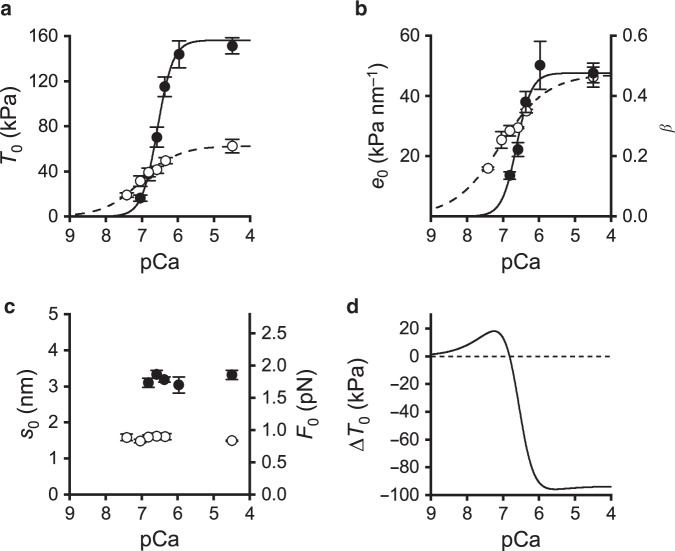
Fig. 3. Effect of OM on myofilament Ca2+ sensitivity for motor attachment.
a T0-pCa relation in control (filled circles) and in the presence of 1 μM OM (open circles). Continuous (control) and dashed (1 μM OM) lines are calculated by fitting Hill equation T0 = T0,45/( 1+ 10n(pCa−pCa50)) to data. The best fit parameters are listed in Table 2. b Dependence of the stiffness of the motor array (e0, left ordinate) and of the fraction of attached motors (β, right ordinate) on [Ca2+]. β in control at saturating [Ca2+] is assumed 0.4719. Continuous (control) and dashed (1 μM OM) lines are calculated by fitting the Hill’s sigmoidal equation to data. c Dependence of the strain per motor (s0, left ordinate) and of the force per motor (F0, right ordinate) on [Ca2+]. d Dependence of the difference (ΔT0) between the fitted T0-pCa relation in 1 μM OM (dashed line in a) and in control (continuous line in a) on [Ca2+]; dashed line indicates zero difference. In all panels, [Ca2+] is expressed in pCa units. Data in a–c are mean values ± SEM from four fibres. Source data are provided as a Source Data file.
OM increases myofilament Ca-sensitivity for motor attachment
As [Ca2+] decreases, the depressant effect of OM on T0 also decreases. As shown in Fig. 3a, at pCa between 4.5 and 6.5, T0 is lower in OM (open circles) than in control (filled circles), while at pCa > 7 T0 is higher in OM. Fitting the data with the Hill equation shows that pCa50 (the pCa at which force attains half-maximum) increases from 6.57 in the control (continuous line) to 7.02 in the presence of OM (dashed line) (Table 2), indicating a corresponding increase in Ca2+-sensitivity of the force. At the same time the slope of the relation, estimated by the parameter n of the Hill equation, which is an indicator of the degree of cooperativity in myofilament activation, decreases from 2.01 in the control to 0.84 in the presence of OM.
Table 2.
Parameters of Hill’s equation fit to force-pCa relation in control and in the presence of OM.
| Control | 1 μM OM | |
|---|---|---|
| n | 2.01 ± 0.14 | 0.84 ± 0.15 |
| pCa50 | 6.57 ± 0.01 | 7.02 ± 0.07 |
n estimates the slope of the relation, pCa50 (the pCa at which force attains half-maximum) estimates the Ca2+ sensitivity. Data are mean ± SEM from four fibres. Data from Fig. 3a.
The stiffness of the array of attached motors e0 (an estimate of β) and the average motor strain s0 at each pCa can be calculated both in control and in 1 μM OM using Eqs. (1) and (2) as detailed in “Methods”. As shown in Fig. 3b, while at saturating [Ca2+] e0 is the same with and without OM, at pCa larger than ∼6.5 e0 is higher in OM (open circles) than in control (filled circles). Instead the strain per motor s0 and thus the force per motor F0 are halved by the addition of 1 μM OM at saturating Ca2+ (pCa 4.5) (Fig. 3c), but are not affected by the reduction of [Ca2+] in either the absence (filled circles) or the presence of OM (open circles).
The larger value of β in OM at sub-saturating [Ca2+] but not at saturating [Ca2+] is the sign of a higher Ca2+ sensitivity, which underpins OM as a potentiator of contractility8,29,30. A more precise definition of the Ca2+ dependence of this effect is given in Fig. 3d, where the difference (ΔT0) between the fitted T0-pCa relations in 1 μM OM (dashed line in Fig. 3a) and in control (continuous line) is plotted versus pCa. At pCa 6.8, the reduced average force per motor (∼½) in 1 μM OM is compensated by the increased fraction of attached motors (∼twice); at pCa >6.8, 1 μM OM acts as a potentiator increasing the sarcomere force, because, while the force per motor is halved by 1 μM OM, the increased Ca-sensitivity gives a β larger than twice the control.
Effect of Pi on isometric contraction with and without OM
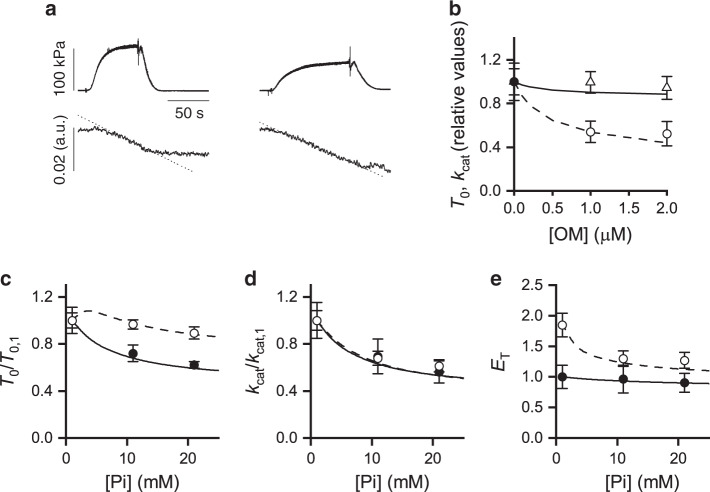
The allosteric competition between binding of OM and the presence of Pi in the catalytic site2,13,14 of the motor has been studied by determining how, at pCa 4.5, the increase of [Pi] (range 1–30 mM, where 1 mM is the [Pi] assumed for the experiments without added Pi31) influences the effects of addition of OM on the relevant mechanical parameters of the contraction, T0, rTD, e0 (and thus β) and s0 (and thus F0). In control the increase in [Pi] decreases T0 in a dose-dependent manner (Fig. 4a, filled circles) from 156 ± 1 kPa in control to 83 ± 1 kPa (−47%) in 30 mM Pi, with a [Pi] for half-maximum effect (EPi50) of 8 mM. This effect is comparable with that reported in previous papers23,32,33. Surprisingly, in 1 μM OM (Fig. 4a, open circles), T0 (77 ± 4 kPa in no added Pi, in these experiments) remains almost constant for Pi < 10 mM and attains a value of 55 ± 1 kPa (−30%) in 30 mM Pi. rTD in control (Fig. 4b, filled circles) is 3.8 ± 0.2 s−1 without added Pi (in agreement with the ordinate intercept in Fig. 1d) and increases with the increase in [Pi] with a slope that progressively decreases. A similar Pi dependence of rTD is observed in the relation determined in 1 μM OM (Fig. 4b, open circles), beyond the depressant effect reported in Fig. 1d.
Fig. 4. Effect of Pi on the isometric mechanical parameters with and without OM.
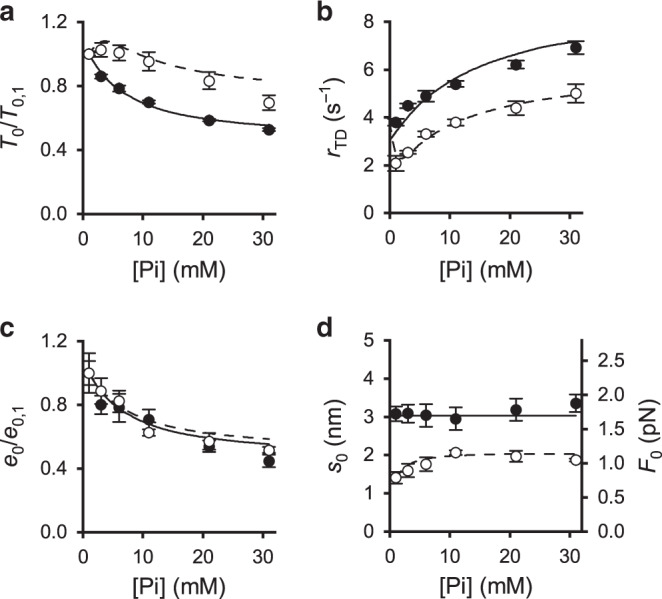
Filled circles and continuous lines, control; open circles and dashed lines, in the presence of 1 μM OM. a Pi dependence of the isometric force (T0) expressed in units relative to the value without added Pi (T0,1). b Rate of force development (rTD). c Stiffness of the motor array (e0) relative to the value without added Pi (e0,1). d Motor strain (s0, left ordinate) and motor force (F0, right ordinate). In each panel: symbols, experimental data; lines, model simulation. Data are mean values ± SEM from three fibres. Source data are provided as a Source Data file.
In these experiments, e0 without added Pi (e0,1), calculated from the half-sarcomere stiffness k0 (see Supplementary Note 1), is 54.2 ± 4.4 kPa nm−1 and 57.5 ± 7.4 kPa nm−1 without and with OM, respectively, in agreement with Fig. 2f. e0 decreases with the increase in [Pi] with a slope that progressively reduces in almost the same way without (Fig. 4c, filled circles) and with OM (open circles), attaining ∼50% the value without added Pi at 30 mM Pi. Thus, in the absence of OM, e0 (and thus β) reduces with [Pi] almost in proportion to the reduction of T0 (filled circles in Fig. 4a), indicating, in accordance with previous work in skinned fibres from fast skeletal muscle34, that Pi reduces T0 by reducing β without change in motor strain s0. In these Pi experiments s0 (calculated with Eq. (2) from data as in Supplementary Fig. 1) is 3.08 ± 0.19 nm in the absence of OM (filled circles in Fig. 4d, left ordinate). Consequently, F0 (= s0 · ε) is 1.75 ± 0.13 pN, independent of [Pi] (right ordinate in Fig. 4d, filled circles.
In the presence of 1 μM OM, the lack of the inhibitory effect of the increase in Pi on T0 for [Pi] < 10 mM (Fig. 4a, open circles), while β decreases in the same way as in control (−38% at 10 mM Pi, Fig. 4c), results in the increase of the average motor strain s0 from 1.41 ± 0.15 nm to 2.06 ± 0.06 nm (+46%, left ordinate in Fig. 4d, open circles). Above 10 mM, Pi induces, also in the presence of 1 µM OM, comparable reduction of both T0 and e0 (open circles in Fig. 4a and c), so that s0 remains roughly constant (open circles in Fig. 4d). F0 increases from 0.79 ± 0.10 to 1.16 ± 0.09 pN with the increase in [Pi] in the range 1–10 mM (right ordinate in Fig. 4d, open circles) and then, for [Pi] >10 mM, remains roughly constant at ∼1.1 pN, a value that anyway is still lower than in the absence of OM (∼1.7 pN).
OM and the rate of ATP hydrolysis in isometric contraction
Faced with the contradictory or not exhaustive results on the OM effects on the ATPase activity2,15,29,35, the effect of the increase in [Pi] (range 1–20 mM) on the ATP hydrolysis rate (kcat) in isometric contraction has been determined in the absence and in the presence of OM (Fig. 5a, b). In these experiments, in agreement with the mechanical experiments, in solution with no added Pi T0 is reduced to ∼50% the control value by the addition of 1 μM OM (circles in Fig. 5b). kcat, instead, does not change significantly (triangles), being 0.055 ± 0.007 mM s−1 in the absence of OM and 0.055 ± 0.005 mM s−1 and 0.052 ± 0.006 mM s−1 in 1 μM and 2 μM OM, respectively.
Fig. 5. Effect of OM on the rate of ATP hydrolysis in isometric contraction and its modulation by Pi.
a Sample records from an activated fibre (pCa 4.5) of the time course of force and light absorbance (in absorbance units, a.u.) in the absence (left) and in the presence (right) of 1 μM OM. The dotted line on the absorbance trace is the 1st order regression equation fitted to the trace after force has attained a steady value. The slope of the regression is used as a measure of the ATP hydrolysis rate (kcat). b Dependence on OM of T0 (circles and dashed line) and kcat (triangles and continuous line). Values are relative to the isometric values in the absence of OM (98 ± 17 kPa and 0.055 ± 0.007 mM s−1, respectively). c Dependence on Pi of the isometric force (T0) relative to the value without added Pi (T0,1) in the absence (filled circles and continuous line) and in the presence (open circles and dashed line) of 1 μM OM. d Dependence on Pi of kcat relative to the value without added Pi (kcat,1). Symbols and lines as in (c). e Tension cost of the isometric contraction (ET). Symbols and lines as in (c). ET is normalized by the value in the absence of OM and no added Pi. b–e symbols, experimental data; lines, model simulation. Data are mean values ± SEM from 12 fibres in (b) and from four fibres in (c-e). Source data are provided as a Source Data file.
Effect of Pi on ATP hydrolysis rate with and without OM
The increase of [Pi] in the absence of OM reduces by the same amount T0 (Fig. 5c, filled circles, data similar to those in Fig. 4a) and kcat (Fig. 5d, filled circles). This finding is in agreement with previous work36. In the presence of 1 μM OM, the increase in [Pi] has an inhibitory effect on T0 that emerges only at [Pi] above 10 mM (Fig. 5c, open circles, data similar to those in Fig. 4a, open circles). Instead, kcat is reduced by the increase in [Pi] (Fig. 5d, open circles) in the same way as in the absence of OM (filled circles). The tension cost of the isometric contraction (ET) can be calculated as the ratio of kcat over T0 (Fig. 5e). Without added Pi, ET in the presence of 1 μM OM is almost twice the control (points at 1 mM Pi in Fig. 5e, filled circle control, open circle 1 μM OM), as a consequence of the depressant effect of OM on the force per motor F0 without effect on kcat. In the absence of OM ET remains the same independent of [Pi] (filled circles), while in 1 μM OM (open circles) ET decreases with the increase of [Pi] approaching the value in the absence of OM.
Model simulation
A kinetic model able to explain the effects of OM and Pi on the in situ mechanics and energetics of the β/slow MHC isoform of myosin has been developed. The model is based on that originally proposed for fast skeletal muscle fibres from rabbit psoas34,37, which has been modified to adapt the rate constants of the forward and backward transitions and the corresponding equilibrium constants (k+x, k−x and Kx, respectively) to fit the bulk of kinetic data from in vitro and in situ studies of β/slow MHC isoform in control condition (without added Pi and OM)9,15,32,38 (see Table 3 for the detailed justification of the assumptions made for each kinetic step and the dedicated section in Methods for the criteria of selection of the parameters for the model fitting). In particular, relevant kinetic constraints in this study are as follows: (i) rTD ∼ 4 s−1 (Fig. 1d, ten times slower than that of fast muscle), (ii) β at T0 = 0.47 (40% larger than that of fast muscle19) and (iii) kcat = 0.055 mM s−1 (ten times slower than that of fast muscle39). The original model37 (green-highlighted part of the scheme in Fig. 6), in agreement with experimental evidence34,39–44, assumes the formation of strongly bound, force-generating motors also before Pi release, so that rise in Pi should exert a minor inhibitory effect on T0 and β than on kcat. In contrast to this view, in fast skeletal muscle fibres Pi reduces T0 and β more than kcat34,39, suggesting that the conventional chemo-mechanical cycle has to be integrated with a branch that allows motor detachment at an early stage of the ATPase cycle (step 6), with Pi still bound to the catalytic site. The finding in this work that β and kcat are reduced by the same extent by the rise in Pi (filled circles in Figs. 4c and 5d) confirms the requirements for the unconventional early detachment with the hydrolysis products still bound to the catalytic site.
Table 3.
Rate constants for the forward (k+x) and backward (k−x) transitions and corresponding equilibrium constants (Kx) for the scheme of Fig. 6.
| Step in the cycle | k+x | k−x | Kx | Justification |
|---|---|---|---|---|
| A, green-highlighted path in Fig. 6 | ||||
| (1) | 1 × 106 M−1s−1 | 1 s−1 | 1 × 106 M−1 | According to refs. 32,38,62,63 |
| (2) | 14 s−1 | 9 s−1 | 1.56 | k2, according to ref. 38; k-2, selected to adjust the distribution of detached myosin motors |
| (3) | 2.2 s−1 | 4.5 s−1 | 0.49 | k+3, adjusted to fit the fraction of attached motors in isometric contraction19 |
| (4) | 11 s−1 | 0.9 × 103 M−1s−1 | 12.2 × 10−3 M | k+4 from refs. 9,15 (assuming Q10 = 2) and K4 adjusted to fit the Pi dependence of the rate of force development assuming these steps are 50 times slower than in psoas muscle32 |
| (5) | 1 s−1 | 1 × 103 M−1 s−1 | 1 × 10−3 M | Adjusted to fit kcat in isometric contraction in the absence of OM64–66 |
| (6) | 0.8 s−1 | 2 × 10−3 s−1 | 0.4 × 103 | Adjusted to fit the Pi dependence of kcat in the absence of OM37 |
| (7) | 50 s−1 | – | – | Rapid and irreversible release of hydrolysis products and ATP binding37 |
| B, pink-highlighted path in Fig. 6 | ||||
| (8) | 400 × 106 M−1 s−1 | 100 s−1 | 4 × 106 M−1 | Adjusted to have the correct proportion of OM-bound myosin motors |
| (9) | 1.2 s−1 | 1 s−1 | 1.2 | k+9 and k−9 adjusted to account for OM-dependent increase of probability of actin attachment by motors (K9 > K3)15 and for the constant total number of attached motors in the presence of OM |
| (10) | 18 s−1 | 1.48 × 103 M−1 s−1 | 12.2 × 10−3 M | k+10 set to 1.64 k+4, to take into account the effect of OM on the rate of Pi release9,15; K10, same value as in step (4) |
| (11) | 1000 s−1 | 1000 s−1 | 1 | Rapid isomerization step; K11 set to 1 to adjust the stoichiometry of the Pi effect |
| (12) | 1.5 s−1 | 2 × 10−3 s−1 | 1 × 103 | Detachment set to fit kcat in the presence of OM |
| (13) | 3 s−1 | – | – | Irreversible release of ADP and OM followed by rapid ATP binding |
| (14) | 3 × 103 M−1 s−1 | 3 × 106 M−1 s−1 | 1 × 10−3 | Adjusted to fit the kinetics of force development and the Pi dependence of T0 and e0 in the presence of OM |
Values for the model in the absence of OM (A, green-highlighted in Fig. 6) and for the branched pathway induced by OM (B, pink-highlighted in Fig. 6). The justification of the kinetic parameters are reported in the last column. Where possible, the parameters are selected according to the relevant information from the literature. The selection of the value of the kinetic parameters concerning OM and OM-Pi actions and the sensitivity of the model fit to the selected value are achieved with an iterative procedure of model simulation by changing one parameter at a time as detailed in “Methods” and in Supplementary Figs. 2 and 3. x represents the step number, as reported in brackets in the first column. For the second-order rate constants, the apparent rate constants are calculated assuming [MgATP] = 5 mM and [ADP] = 20 μM; [Pi] and [OM] according to the experimental conditions.
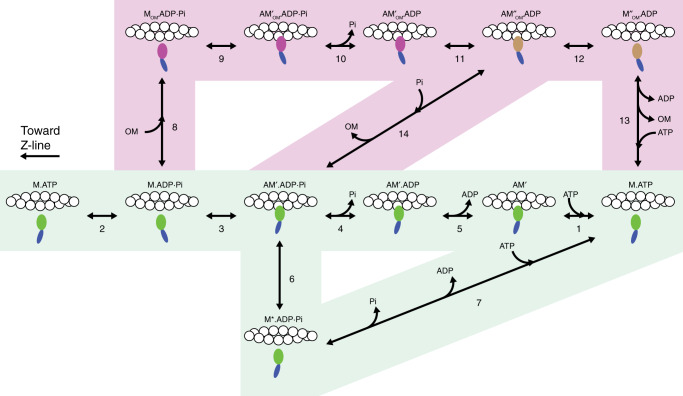
Fig. 6. Chemo-mechanical cycle of the myosin motor.
The green-highlighted part is the original model37; the pink-highlighted part is the branched pathway promoted by OM. Myosin catalytic domain is green for the motors without OM (M) and magenta and brown for the OM-bound motors (MOM) before and after the isomerization step (step 11), respectively. The working stroke in the attached head is indicated by the tilting of the light chain domain (the lever arm, blue) towards the Z-line. The system of linear differential equation for the calculation of state transitions is reported in Supplementary Note 2.
To explain the effects of OM on the mechano-kinetics of the β/slow isoform of the myosin motor in situ a branched pathway (pink-highlighted part in the scheme of Fig. 6) is required. In this way the scheme accounts for both the features present in the literature (points i, ii below) and those reported in this work (points iii–v below): (i) OM binds to the catalytic domain of the myosin motor in the conformation at the beginning of the working stroke (step 8) and increases the probability of its attachment to actin (step 9), accelerating the release of Pi (step 10) and preventing the working stroke and the force generation2,9,13–15; (ii) OM-ADP motors detach by a process that does not imply ATP binding (step 12)9; (iii) strongly bound force-generating motors and attached OM-bound motors have the same stiffness, to account for the lack of effect of OM on k0 (Fig. 2d, triangles and dashed line) and e0 (Fig. 2f); (iv) the AM′OM.ADP state generated after Pi release (step 10) undergoes an isomerization (step 11) to a new state (AM″OM.ADP) with increased affinity for Pi and thus at [Pi] > 0 (also without added Pi, when contaminating [Pi] is 1 mM) can rebind Pi, release OM and re-enter the force-generating cycle (step 14); in this way, by appropriate selection of the kinetics of steps 11 and 14 (Table 3), the model is able to account for both the OM-dependent reduction in rTD at any [Pi] > 0 (Fig. 1d, continuous line and Fig. 4b), and the Pi-dependent increase in F0 in 1 μM OM (Fig. 4d, open circles); (v) the rate limiting step in isometric contraction, that in the conventional cycle is the ADP release (step 5), in the OM cycle is detachment (step 12). Thus k+12 is set ≅k+5, to account for the finding that kcat is not affected by [OM] (Fig. 5b triangles) and is reduced by the increase in Pi in the same way in the absence as in the presence of OM (Fig. 5d).
In the presence of OM the probability for an M.ADP.Pi motor to escape the conventional cycle (green pathway in Fig. 6) by binding OM and entering the pink pathway (step 8) is controlled by the second-order equilibrium constant K8, which is set at a value 4 · 106 M−1 (Table 3) in order to fit the force—[OM] relation of Fig. 1 (continuous line). The kinetics of steps 11, the isomerization that changes Pi affinity of the OM-bound motor, and step 14, in which Pi competes with OM for binding to the attached motor after the isomerization step (step 11), are dictated by the following constraints: (i) without added Pi ([Pi] ∼1 mM) and in the presence of OM, rTD attains the minimum value ∼½ the control with a [OM]50 = 0.5 μM (Fig. 1d); (ii) increase in [Pi] reduces β in the same way in the absence and presence of OM (Fig. 4c); (iii) in 1 μM OM the force per motor increases by 50% with the increase of [Pi] in the range 1–10 mM (Fig. 4d). These results are fitted (dashed line) assuming, for step 11 an equilibrium constant (K11) = 1, and for step 14, a second-order rate constant for Pi binding (k+14) = 3 · 103 M−1 s−1 and a second-order rate constant for OM binding (k−14) = 3 · 106 M−1 s−1 (Table 3).
Discussion
Sarcomere-level mechanics of demembranated fibres from rabbit soleus muscle allow a double mechanism of OM action on the β/slow MHC isoform to be revealed, which eventually accounts for a Pi-dependent recovery of efficiency of the contractile response of OM-treated fibres.
The first mechanism of action, which confirms the conclusions of recent work in vitro9,14, implies a dose-dependent depression of the active sarcomere force by OM binding to the myosin motor in the pre-working stroke state that promotes an unconventional cycle in which the working stroke is prevented and the detachment occurs without binding of ATP.
We find that, irrespective of [Ca2+], in 1 μM OM the fraction of OM-bound attached motors is ∼50% of the total (β), as indicated by the average force per motor, F0, that remains ∼50% of that in control at any [Ca2+] tested (Fig. 3c). The generation by OM of a pathway leading to strongly actin-bound motors in parallel with that responsible for the conventional force-generating cycle increases β at sub-saturating [Ca2+] but not at saturating [Ca2+] (Fig. 3b). This increase in Ca2+ sensitivity, measured by the increase of pCa50 by 0.4 units (Table 2), is similar to that reported for demembranated cardiac myocytes8,29,30 and is attributable to the contribution of OM-motors to the cooperative effect of attached motors in switching ON the thin filament9,45. The potentiating role of this mechanism in relation to the level of Ca2+ is quantified by plotting the difference between T0 in 1 μM OM and in control (ΔT0) versus pCa (Fig. 3d): ΔT0 is negative at saturating and high [Ca2+] and crosses zero at pCa 6.8 because, at this pCa, the reduced average force per motor (∼½) in 1 μM OM is compensated by the increased total number of attached motors (∼twice); at any pCa > 6.8, 1 μM OM acts as a potentiator increasing the sarcomere force, because the increased Ca-sensitivity makes the total number of motors larger than twice the control. This mechanism applies equally well to explain the OM potentiating effects at the partial Ca2+-activation found in demembranated cardiac myocytes8,29,30. The force-pCa relation in 1 μM OM is characterized not only by the larger Ca-sensitivity but also by a reduced slope: Hill coefficient n is reduced by a factor of 2 (from 2.01 to 0.84, Table 2), in agreement with previous work on both demembranated cardiac myocytes and slow-skeletal muscle fibres8,29,30. The explanation could be related to a negative effect on the cooperativity index of OM-bound motors if the propagation of the activation along the thin filament induced by motor attachment depends on the motor force.
The second mechanism of action of OM, which is revealed in this work, is the recovery from motor force depression with increase in [Pi] in the range 1–10 mM. This finding per se scales back the relevance of previous mechanisms of action of OM defined in the absence of added Pi, since 1–10 mM is the physiological range of [Pi] in working muscle. The molecular basis of this mechanism is implicit in the effect of OM on rTD also in the absence of added Pi ([Pi] = 1 mM): rTD is depressed by OM with the same dose dependency as T0 (Fig. 1 c, d), with [OM] for half-maximum effect 0.5 μM, which noteworthy is in the range (0.1–0.6 μM) of the plasma [OM] in patients under therapeutic treatment3,5,46. This consideration suggests further work in which the mechanism revealed here is tested also for the more clinically competent range of [OM].
The kinetic model of Fig. 6 is able to fit both T0- and rTD-OM relations (continuous lines in Fig. 1 c and d, respectively) assuming that, after Pi release, the OM-bound motor in the AM′OM.ADP state undergoes an isomerization to an AM″OM.ADP state with higher affinity for Pi favouring an “allosteric” exchange Pi-OM (step 14), which allows the motor to re-enter the force-generating cycle. Noteworthy, it is not possible to simulate the depressant effect of OM on rTD confining the allosteric competition between OM and Pi to step 10, because the OM-dependent effect on force would not imply any reduction in rTD. Only introducing the pink-highlighted path that contributes to T0 with a slower process through the isomerization (step 11) and the following OM-Pi exchange (step 14) the time course of T0 is slowed and the rTD-Pi relation in 1 μM OM is shifted downward (Fig. 4b). The stoichiometry of the Pi-dependent recovery of the average force per motor in the presence of 1 μM OM (Fig. 4d, open circles) is mainly accounted for by the equilibrium constants of steps 11 (1) and 14 (10−3). For step 14, assuming a second-order rate constant for Pi binding three orders of magnitude smaller than the second-order rate constant for OM binding, the equilibrium is near the unity when the ratio between [Pi] and [OM] is about 103 (Table 3), that is, with the near millimolar physiological concentration of Pi, at micromolar [OM].
The energetics of the combined OM-Pi action on contractility has been defined by determining the effect of OM on the rate of ATP hydrolysis (kcat) during isometric contraction at saturating Ca2+ and its modulation by Pi. Increase in [OM] up to 2 μM, which decreases T0 to ½ the control, does not change kcat (Fig. 5b). This result is specific for a loaded contraction, as in both solution15,35 and low-load laser trap experiments9 on β-cardiac myosin the ATPase rate has been found to be reduced in OM with respect to the control.
In either the pink-highlighted or the conventional green-highlighted path under high load the motors accumulate in the ADP-bound state because either OM (pink path) or the high load (green path) limit the probability to go through the working stroke that would accelerate the next step, the conformation dependent ADP release44,47–49. Consequently, independent of the reason that limits the execution of the working stroke, both step 12 for OM-bound motors and step 5 for OM-free motors are slow (Table 3) and the ATPase rate is limited in the same way independent of the proportion of motors passing through either path. In this way the model can simulate the OM-dependent drop in T0 (dashed line in Fig. 5b) without effect on kcat (continuous line).
In the absence of added Pi, OM reduces T0 through a reduction in F0 at constant kcat, thus it increases the tension cost (ET) of the isometric contraction. One micromolar OM, which halves F0, doubles ET (Fig. 5e). Noteworthy, this conclusion extends from maximal contractions at saturating [Ca2+] to contractions at sub-saturating [Ca2+] because reduction in [Ca2+] reduces the total number of attached motors (Fig. 3b) without affecting F0 (Fig. 3c).
A direct test of Pi effect on OM-bound motors at pCa ∼7, where OM exerts its potentiating action via the increase in Ca2+ sensitivity (Fig. 3d), would be quite important, considering that the internal [Ca2+] in the heart systole ranges 0.2–0.6 μM50,51. However, the sarcomere-level mechanics on isometric contraction of skinned soleus fibres activated at pCa ∼7 would fail to produce data that can be interpreted in terms of Eq. (1), because the stiffness of the motor array would be too low (<20 kPa nm−1, Fig. 3b) and the half-sarcomere compliance analysis would be complicated by the significant contribution of the additional elasticity in parallel with the motor array (see “Methods”, Fig. 7b). In this respect it must be noted also that the nature and the effects of the additional parallel elasticity are likely different in the slow-skeletal muscle and in the cardiac muscle, which have different isoforms of the accessory and cytoskeleton proteins like My-BPC and titin. Consequently, the conclusions from soleus muscle could not be directly translated to cardiac muscle as it is instead possible under the condition selected in this study (stiffness of the motor array > 20 kPa nm−1), in which the half-sarcomere compliance is only determined by the number of β/slow myosin isoforms attached to the actin filament.
Fig. 7. Estimate of myofilament compliance and motor strain.
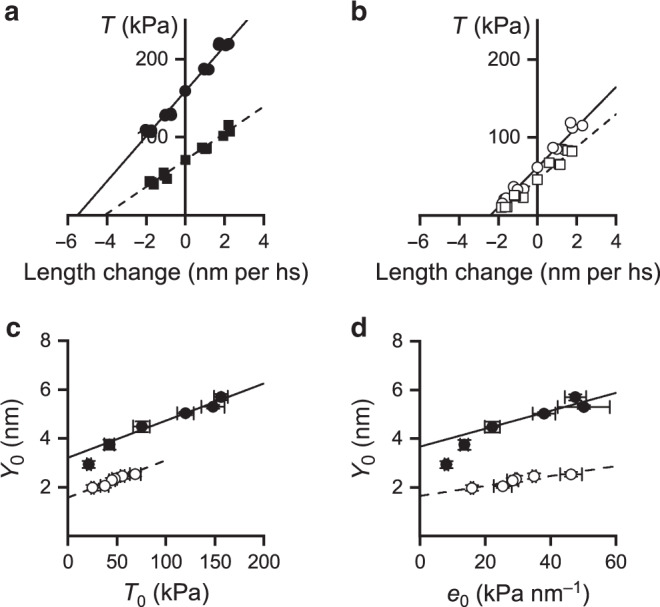
T1 relations from a fibre in control (a) and in the presence of 1 μM OM (b) at pCa 4.5 (circles) and 6.59 (squares). The relations are obtained by plotting the extreme force attained at the end of the length step, T1, versus the step size. Lines in a and b are 1st order regression equations fitted to the data (pCa 4.5, continuous; pCa 6.59, dashed). The abscissa intercept of each line is the average strain of the half-sarcomere before the length step (Y0). Fibre length, 4.2 mm; segment length under the striation follower, 0.77 mm, average segment sarcomere length, 2.34 µm, test temperature, 12.2 °C. c Relations of Y0 versus T0 at different pCa determined in control (filled circles) and in the presence of 1 μM OM (open circles); mean values ± SEM from four fibres. The lines are linear regressions on the pooled data for forces >30 kPa in control (continuous) and in the presence of OM (dashed). The slope and the ordinate intercept of the linear regressions are listed in Table 1. d Relations of half-sarcomere strain (Y0) versus the stiffness of the myosin motors at different pCa (e0) in control (filled circles) and in the presence of 1 μM OM (open circles); mean values ± SEM from four fibres. The lines are linear regressions on the pooled data for stiffness > 20 kPa nm−1 in control (continuous) and in the presence of OM (dashed). c and d, data from four fibres. Source data are provided as a Source Data file.
Under the conditions defined above, we provide the evidence for a previously unknown inotropic effect on the β/slow MHC isoform in OM-treated myocytes: increase in [Pi] within the physiological range (1–10 mM) recovers from the inhibitory effect of OM on T0 by increasing the proportion of motors re-entering the force-generating cycle and this, at constant kcat, implies a corresponding reduction in tension cost (Fig. 5e). This effect of Pi on the OM-bound motors could represent an energetically efficient response in OM-treated cardiomyopathic patients, whenever the increase of heart-beat frequency induces an increase in [Pi]27,28.
Methods
Animal and ethical approval
Experiments have been done on demembranated fibres from the soleus muscle of adult male New Zealand white rabbits at either the PhysioLab Research Unity of the Biology Department of the University of Florence (mechanical experiments), or the Department of Physiology of the VU University Medical Center in Amsterdam (ATPase rate measurements). The experiments were carried out according to the protocols approved by the Ethical Committee of the University of Florence and by the Italian Ministry of Health (authorization n. 956/2015 PR) in compliance with the Italian regulation on animal experimentation, Decreto Legislativo 26/2014 and the EU regulation (directive 2010/63). Rabbits (4–5 kg weight, 20–30 weeks old) were sacrificed by injection of an overdose of sodium pentobarbitone (150 mg kg−1) in the marginal ear vein. Two rabbits were used for this work. All animals have been kept with free access to food and water prior to use.
Fibre preparation and mechanical apparatus
Small bundles (40–80 fibres) of soleus muscle fibres were stored in skinning solution containing 50% glycerol at −20 °C for 3–4 weeks and single fibres were prepared just before the experiment as already described18,52. The osmotic agent Dextran T-500 was added to all solutions at the concentration of 4 g/100 ml (4% weight/volume) that is known to reverse the permeabilization-induced increase of interfilamentary spacing and cross-sectional area (CSA) to the value before skinning18,53–57.
A fibre segment, 4–6 mm long, was mounted between the lever arms of a loudspeaker motor able to impose steps in length complete within 80 µs and a capacitance force transducer with resonant frequency 40–50 kHz18. Sarcomere length (sl), width (w) and height (h) of the fibre were measured at 0.5 mm intervals in the 3–4 mm central segment of the relaxed fibre with a 40× dry objective (Zeiss, NA 0.60) and a 25× eyepiece. The fibre length (L0) was adjusted to have a sl within the range 2.3–2.5 µm. The fibre CSA was determined assuming the fibre cross-section as elliptical (CSA = π/4·w·h) and its value ranged between 2100 and 5300 µm2. Fibres were activated by temperature jump using a solution exchange system18. The fibre was kept in the activating solution at the test temperature (12 °C) for 4–5 s for the mechanical measurements. A striation follower58 allowed nanometre-microsecond resolution recording of length changes in a selected population of sarcomeres (range 500–1200 sarcomeres) starting at the time the optic path was permitted through the glass window in the floor of the test temperature drop (see ref. 18 for details).
Half-sarcomere stiffness measurements
Step length changes (ranging from −2 to +2 nm per hs, stretch positive, rise time 100 µs) were imposed on the isometrically contracting fibre to estimate the half-sarcomere stiffness by the slope of the relation between the force attained at the end of the step and the change in half-sarcomere length in the sarcomere population of the segment monitored by the striation follower (T1 relation) (Figs. 2a, b and 7). To enhance the precision of stiffness measurements, a train of different-sized steps at 200-ms intervals was applied during each activation and, to maintain the isometric force before the test step constant, each test step was followed, after a 50-ms pause, by a step of the same size but opposite direction18,19. Half-sarcomere stiffness measurements were done (i) at different [OM] (range 0.1–10 μM) in the absence of added Pi (that is with [Pi] ∼1 mM) and with [Ca2+] for maximum activation (pCa 4.5), (ii) at different [Ca2+] (range of pCa 4.5–7.5) without and with 1 μM OM and (iii) at different [Pi] (range 1–20 mM) at pCa 4.5 without and with 1 μM OM.
Half-sarcomere compliance analysis
The elastic characteristics of the array of myosin motors during active isometric contraction can be estimated from the stiffness of the half-sarcomere with a mechanical protocol that allows to isolate and subtract the contribution of the filament compliance (Cf) to half-sarcomere compliance (Chs, which is the reciprocal of the half-sarcomere stiffness k0). The protocol consists in determining the T1 relations during activations at different [Ca2+]. In fact, as previously demonstrated18, [Ca2+]-dependent changes in maximum isometric force (T0) are fully accounted for by changes in the number of attached motors. Under this condition the relation between the half-sarcomere strain (Y0 = Chs · T0) and T0 can be interpreted with a simple mechanical model of the half-sarcomere in which the attached motors are represented by an array of in parallel springs, the number of which, but not the strain (s0), changes in proportion to force, in series with the actin and myosin filaments represented by a spring, which accounts for an equivalent filament compliance (Cf) (Model 1 in ref. 59). According to Model 1, Chs, is expressed by the equation59:
| 1 |
where 1/e0, the compliance of the array of motors, is the reciprocal of the stiffness of the array (e0, which is proportional to the number of attached motors). From the above equation the strain of the half-sarcomere Y0 (= Chs · T0) can be derived:
| 2 |
where s0 is the average strain in the attached motors at T0. According to the model, Y0 increases linearly with the force with a slope that is explained by the increase in the strain of the myofilaments with constant compliance Cf, while s0, estimated by the ordinate intercept of the Y0 − T0 relation, is the same independent of T0.
The T1 relations were determined at different pCa in control and in the presence of 1 μM OM (which reduces T0,4.5 to ½). Figure 7 shows the relations obtained at pCa 4.5 (circles) and 6.59 (squares) in control (a) and in the presence of 1 μM OM (b). The half-sarcomere stiffness (k0, estimated by the slope of the linear fit to data, dashed line) reduces, lowering [Ca2+], from 28.8 ± 1.0 kPa nm−1 (filled circles) to 17.1 ± 0.7 kPa nm−1 (filled squares) in control (a) and from 26.3 ± 1.5 kPa nm−1 (open circles) to 20.9 ± 0.9 kPa nm−1 (open squares) in the presence of OM (b). In both conditions the half-sarcomere strain at T0 (Y0, estimated by the extrapolation of the fit to zero force) is shifted rightward with lowering [Ca2+], but less than in proportion to the reduction of the ordinate intercept, in agreement with the reduction of k0. In Fig. 7c, Y0 at different [Ca2+] in control (filled circles) and in the presence of 1 μM OM (open circles) is plotted against the corresponding T0. In control, Y0 increases linearly with the isometric force, with the exception of the lowest points (for T0 < 50 kPa) that are shifted downward. The linear relation is expected from Model 1 and the deviation from linearity at very low forces has been explained with the presence of an elastic element in parallel with the myosin motors with a stiffness that is so small that it affects the linear relation only in the region where the number of the motors, and thus their cumulative stiffness are quite low59. Noteworthy, the Y0 − T0 relation in OM (open circles in Fig. 7c) does not show deviation from linearity (see below for the explanation). The values of Cf and s0, estimated by fitting Equation (2) to Y0 − T0 values in control (in the range of T0 > 50 kPa) and in the presence of OM are reported in Table 1. The value of Cf in the presence of OM (15.3 nm MPa−1) does not differ significantly from the control value (15.2 nm MPa−1, P > 0.9, Student’s t test), indicating that also in this case the dependence of T0 on [Ca2+] is explained by the Ca2+-modulation of the number of attached motors without change in motor strain.
In OM the Y0 − T0 relation (open circles in Fig. 7c) does not show deviation from linearity for the same low forces at which the control relation shows a downward shift. This apparent contradiction is explained by considering the effect of OM that reduces the force per motor but increases the Ca-sensitivity for motor attachment, so that at the same low values of T0, in the presence of OM, the number of attached motors and thus their cumulative stiffness are higher than in control and still in the range of values that mask the contribution of the parallel elastic element. A quantitative evaluation of this interpretation is obtained in Fig. 7d by plotting Y0 versus the stiffness of the array of motors e0 at each pCa (data from Fig. 3b) for both control (filled circles) and OM (open circles). It can be seen that both control and OM relations are almost linear in the same range of e0, where the stiffness of attached motors is high enough to mask the contribution of the parallel elastic element, and that the control relation deviates from linearity, uncovering the presence of a parallel elastic element, for the small e0 values that are not attained by the OM relation.
ATPase measurements
Fibre bundles prepared in Florence lab as described above were transported to Amsterdam in ice-cold relaxing solution. ATPase activity (temperature 12 °C) at saturating [Ca2+] and at different [Pi] was measured photometrically by enzymatic coupling of the regeneration of ATP to the oxidation of NADH present in the bathing solution36,60,61 both in control and in the presence of OM (1 and 2 μM). NADH breakdown was monitored via the absorption of near UV light at 340 nm. Briefly, the set up consisted of two anodized aluminium troughs (volume 80 μl each) containing relaxing and pre-activating solution and a measuring chamber (volume about 30 μl) containing activating solution. The solution was continuously stirred via a membrane at the base of the chamber. UV light passed through the chamber beneath the fibre, and the transmitted light was monitored by two UV-enhanced photodiodes at 340 and 400 nm. The photodiode at 400 nm provided a reference signal, independent of NADH concentration. A syringe, controlled by a stepper motor, was used to add 0.05 μl of 10 mM ADP at the end of each recording to calibrate the absorbance signal. Force during the enzymatic assay was measured by means of a strain gauge transducer (AE801 SensoNor, Horten, Norway). The force and the absorbance signals were filtered at 1 kHz and 2.5 Hz, respectively. The ATPase rate was measured as the slope over time of the absorbance signal.
Procedure for the selection of the parameters of the kinetic model
The procedure followed to assign a specific value to the kinetic parameters concerning the effects of OM and Pi on the force (T0), rate of force development (rTD), stiffness of the motor array (e0) and ATPase rate (kcat) (eight relations altogether) is based on an iterative routine in which the values of the rate constants (or equilibrium constants) controlling the kinetics of the relevant steps (8–12 and 14) are changed one at a time. For each value of the rate/equilibrium constant (k) the weighted mean of residual errors for each relation is calculated according to the expression:
where Rw(k) is the mean sum of residuals (weighted for the squared value of the experimental point to allow homogeneous comparison among the eight different relations), xi is the experimental point value, xmod(k)i is the corresponding simulated value for a given k, and N is the number of points in each relation.
An example of the selection procedure for a given rate constant (k+14) in relation to its effect on each of the eight relevant relations is given in Supplementary Fig. 2, where in the left column six simulations obtained for different values of k+14 (each identified by a different colour) of four of the eight relations are superimposed on the experimental relations (open symbols) and, in the right column, the weighted mean of residuals (Rw) are plotted versus the value of k+14 assumed in each simulation (identified by the same colour as in the left column). As shown by the plots in the right column of Supplementary Fig. 2, the sensitivity of the test in showing a minimum mean residual and its dependence on the value of the rate/equilibrium constant varies depending on the relation. For this, the criterium of selection of the best value for any given rate/equilibrium constant is based on the minimization of the weighted mean of the residuals averaged over the eight relations (global mean, 〈Rw(k)〉):
In Supplementary Fig. 3 the values of 〈Rw(k)〉 are plotted versus the rate/equilibrium constant under consideration. The value selected with this global fitting for each rate/equilibrium constant, identified by the abscissa of the filled symbol, is reported in Table 3.
Solutions
The composition of the solutions, similar for the mechanical and the ATPase measurements (MgATP, 5 mM; free Mg2+, 1.2 mM) is reported in Supplementary Table 2. The activating solution at a given pCa (range 7.5–4.5) was obtained by mixing relaxing and activating solutions. OM (CK-1827452 from Selleckchem, USA) was dissolved in DMSO (Sigma D-5879) (stock solution, 17.5 mM). Different OM concentrations in the final solutions (0.1–10 μM range) were obtained for partial dilution starting from the stock solution. Four percent dextran was added to all solutions for the mechanical experiments.
Statistical analysis
All data were analyzed using dedicated programs written in LabVIEW (National Instruments) and Microsoft Excel and Origin 2018 (OriginLab Corp., Northampton, MA, USA) software. Error bars on mean data points are ±SEM.
For mean data in the figures and tables, the number of fibres is reported in the legends and the number of observations, which may vary for each data point, can be recovered from the Source Data files.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Massimo Reconditi for insightful comments on the manuscript and valuable support to the definition of the procedure for the optimization of the simulation and the staff of the mechanical workshop of the Department of Physics and Astronomy (University of Florence) for mechanical engineering support. This work was supported by the University of Florence and Fondazione Cassa di Risparmio di Firenze (Italy).
Source data
Author contributions
V.L., M.L., G.P., M.C., C.O. and G.S. designed the research. S.G., M.C. and I.P. performed the experiments and analysed the data. V.L., M.L. and G.P. wrote the paper or revised it critically for important intellectual content. All authors participated in discussions on this work and approved the final version of the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. The source data for Figs. 1, 2, 3, 4, 5, 7, Supplementary Figs. 1, 2 and 3 and for Tables 1, 2 and Supplementary Table 1 are provided as a Source Data file. All remaining data will be available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Anne Houdusse, and other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These author contributed equally: Serena Governali, Marco Caremani.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-17143-2.
References
- 1.Spudich JA. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys. J. 2014;106:1236–1249. doi: 10.1016/j.bpj.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik FI, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleland JG, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 4.Teerlink JR, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF Study. J. Am. Coll. Cardiol. 2016;67:1444–1455. doi: 10.1016/j.jacc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Morgan BP, et al. Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac Myosin. ACS Med. Chem. Lett. 2010;1:472–477. doi: 10.1021/ml100138q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplinsky E, Mallarkey G. Cardiac myosin activators for heart failure therapy: focus on omecamtiv mecarbil. Drugs Context. 2018;7:212518. doi: 10.7573/dic.212518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy L, et al. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br. J. Pharmacol. 2015;172:4506–4518. doi: 10.1111/bph.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woody MS, et al. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat. Commun. 2018;9:3838. doi: 10.1038/s41467-018-06193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Developmental Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chikuni K, Muroya S, Tanabe R-i, Nakajima I. Comparative sequence analysis of four myosin heavy chain isoforms expressed in porcine skeletal muscles: Sequencing and characterization of the porcine myosin heavy chain slow isoform. Anim. Sci. J. 2002;73:257–262. [Google Scholar]
- 13.Rohde JA, Thomas DD, Muretta JM. Heart failure drug changes the mechanoenzymology of the cardiac myosin powerstroke. Proc. Natl Acad. Sci. USA. 2017;114:E1796–E1804. doi: 10.1073/pnas.1611698114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J, Malik FI, Houdusse A. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat. Commun. 2017;8:190. doi: 10.1038/s41467-017-00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, White HD, Belknap B, Winkelmann DA, Forgacs E. Omecamtiv Mecarbil modulates the kinetic and motile properties of porcine beta-cardiac myosin. Biochemistry. 2015;54:1963–1975. doi: 10.1021/bi5015166. [DOI] [PubMed] [Google Scholar]
- 16.Aksel T, Choe YuE, Sutton S, Ruppel KM, Spudich JA. Ensemble force changes that result from human cardiac myosin mutations and a small-molecule effector. Cell Rep. 2015;11:910–920. doi: 10.1016/j.celrep.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkelmann DA, Forgacs E, Miller MT, Stock AM. Structural basis for drug-induced allosteric changes to human beta-cardiac myosin motor activity. Nat. Commun. 2015;6:7974. doi: 10.1038/ncomms8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linari M, Caremani M, Piperio C, Brandt P, Lombardi V. Stiffness and fraction of Myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J. 2007;92:2476–2490. doi: 10.1529/biophysj.106.099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percario V, et al. Mechanical parameters of the molecular motor myosin II determined in permeabilised fibres from slow and fast skeletal muscles of the rabbit. J. Physiol. 2018;596:1243–1257. doi: 10.1113/JP275404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caremani M, et al. Size and speed of the working stroke of cardiac myosin in situ. Proc. Natl Acad. Sci. USA. 2016;113:3675–3680. doi: 10.1073/pnas.1525057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinzauti F, et al. The force and stiffness of myosin motors in the isometric twitch of a cardiac trabecula and the effect of the extracellular calcium concentration. J. Physiol. 2018;596:2581–2596. doi: 10.1113/JP275579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen DG, Morris PG, Orchard CH, Pirolo JS. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J. Physiol. 1985;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kentish JC. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J. Physiol. 1986;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris P. G., Allen D. G. & Orchard C. H. High-time-resolution 31P NMR studies of the perfused ferret heart. In: Advances in Myocardiology (eds Harris, P. & Poole-Wilson, P.A.) (Springer US, 1985). [DOI] [PubMed]
- 25.Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am. J. Physiol. 1985;248:C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- 26.Bevington A, et al. A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin. Sci. 1986;71:729–735. doi: 10.1042/cs0710729. [DOI] [PubMed] [Google Scholar]
- 27.Elliott AC, Smith GL, Allen DG. The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts. J. Physiol. 1994;474:147–159. doi: 10.1113/jphysiol.1994.sp020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eijgelshoven MH, et al. Cardiac high-energy phosphates adapt faster than oxygen consumption to changes in heart rate. Circ. Res. 1994;75:751–759. doi: 10.1161/01.res.75.4.751. [DOI] [PubMed] [Google Scholar]
- 29.Kampourakis T, Zhang X, Sun YB, Irving M. Omecamtiv mercabil and blebbistatin modulate cardiac contractility by perturbing the regulatory state of the myosin filament. J. Physiol. 2018;596:31–46. doi: 10.1113/JP275050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieu TT, Awinda PO, Tanner BCW. Omecamtiv mecarbil slows myosin kinetics in skinned rat myocardium at physiological temperature. Biophys. J. 2019;116:2149–2160. doi: 10.1016/j.bpj.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pate E, Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J. Muscle Res. Cell Motil. 1989;10:181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- 32.Millar NC, Homsher E. Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am. J. Physiol. 1992;262:C1239–C1245. doi: 10.1152/ajpcell.1992.262.5.C1239. [DOI] [PubMed] [Google Scholar]
- 33.Tesi C, Colomo F, Piroddi N, Poggesi C. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J. Physiol. 2002;541:187–199. doi: 10.1113/jphysiol.2001.013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caremani M, Dantzig J, Goldman YE, Lombardi V, Linari M. Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys. J. 2008;95:5798–5808. doi: 10.1529/biophysj.108.130435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swenson AM, et al. Omecamtiv mecarbil enhances the duty ratio of human beta-cardiac myosin resulting in increased calcium sensitivity and slowed force development in cardiac muscle. J. Biol. Chem. 2017;292:3768–3778. doi: 10.1074/jbc.M116.748780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potma EJ, van Graas IA, Stienen GJ. Effects of pH on myofibrillar ATPase activity in fast and slow skeletal muscle fibers of the rabbit. Biophys. J. 1994;67:2404–2410. doi: 10.1016/S0006-3495(94)80727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linari M, Caremani M, Lombardi V. A kinetic model that explains the effect of inorganic phosphate on the mechanics and energetics of isometric contraction of fast skeletal muscle. Proc. Biol. Sci. 2010;277:19–27. doi: 10.1098/rspb.2009.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson CA, et al. The ATPase cycle of human muscle myosin II isoforms: Adaptation of a single mechanochemical cycle for different physiological roles. J. Biol. Chem. 2019;294:14267–14278. doi: 10.1074/jbc.RA119.009825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potma EJ, van Graas IA, Stienen GJ. Influence of inorganic phosphate and pH on ATP utilization in fast and slow skeletal muscle fibers. Biophys. J. 1995;69:2580–2589. doi: 10.1016/S0006-3495(95)80129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowater R, Sleep J. Demembranated muscle fibers catalyze a more rapid exchange between phosphate and adenosine triphosphate than actomyosin subfragment 1. Biochemistry. 1988;27:5314–5323. doi: 10.1021/bi00414a055. [DOI] [PubMed] [Google Scholar]
- 41.Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J. Biol. Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- 42.Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caremani M, Melli L, Dolfi M, Lombardi V, Linari M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 2013;591:5187–5205. doi: 10.1113/jphysiol.2013.257410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caremani M, Melli L, Dolfi M, Lombardi V, Linari M. Force and number of myosin motors during muscle shortening and the coupling with the release of the ATP hydrolysis products. J. Physiol. 2015;593:3313–3332. doi: 10.1113/JP270265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys. J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vu T, et al. Population pharmacokinetic-pharmacodynamic modeling of omecamtiv mecarbil, a cardiac myosin activator, in healthy volunteers and patients with stable heart failure. J. Clin. Pharmacol. 2015;55:1236–1247. doi: 10.1002/jcph.538. [DOI] [PubMed] [Google Scholar]
- 47.Dantzig JA, Hibberd MG, Trentham DR, Goldman YE. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J. Physiol. 1991;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc. Natl Acad. Sci. USA. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potma EJ, Stienen GJ. Increase in ATP consumption during shortening in skinned fibres from rabbit psoas muscle: effects of inorganic phosphate. J. Physiol. 1996;496(Pt 1):1–12. doi: 10.1113/jphysiol.1996.sp021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circ. Res. 1995;76:1036–1048. doi: 10.1161/01.res.76.6.1036. [DOI] [PubMed] [Google Scholar]
- 51.Frampton JE, Orchard CH, Boyett MR. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J. Physiol. 1991;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman YE, Hibberd MG, Trentham DR. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5’-triphosphate. J. Physiol. 1984;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner B, Yu LC. Characterization of radial force and radial stiffness in Ca(2+)-activated skinned fibres of the rabbit psoas muscle. J. Physiol. 1991;441:703–718. doi: 10.1113/jphysiol.1991.sp018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai M, Wray JS, Zhao Y. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. I. Proportionality between the lattice spacing and the fiber width. Biophys. J. 1993;64:187–196. doi: 10.1016/S0006-3495(93)81356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsubara I, Elliott GF. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J. Mol. Biol. 1972;72:657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- 56.Maughan DW, Godt RE. Stretch and radial compression studies on relaxed skinned muscle fibers of the frog. Biophys. J. 1979;28:391–402. doi: 10.1016/S0006-3495(79)85188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linari M, et al. The stiffness of skeletal muscle in isometric contraction and rigor: the fraction of myosin heads bound to actin. Biophys. J. 1998;74:2459–2473. doi: 10.1016/S0006-3495(98)77954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huxley AF, Lombardi V, Peachey D. A system for fast recording of longitudinal displacement of a striated muscle fibre. J. Physiol. 1981;317:12–13. [Google Scholar]
- 59.Fusi L, Brunello E, Reconditi M, Piazzesi G, Lombardi V. The non-linear elasticity of the muscle sarcomere and the compliance of myosin motors. J. Physiol. 2014;592:1109–1118. doi: 10.1113/jphysiol.2013.265983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glyn H, Sleep J. Dependence of adenosine triphosphatase activity of rabbit psoas muscle fibres and myofibrils on substrate concentration. J. Physiol. 1985;365:259–276. doi: 10.1113/jphysiol.1985.sp015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potma EJ, Stienen GJ, Barends JP, Elzinga G. Myofibrillar ATPase activity and mechanical performance of skinned fibres from rabbit psoas muscle. J. Physiol. 1994;474:303–317. doi: 10.1113/jphysiol.1994.sp020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White HD, Taylor EW. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976;15:5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- 63.Sleep JA, Taylor EW. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976;15:5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- 64.Goldman YE. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev. Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- 65.Kawai M, Halvorson HR. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys. J. 1991;59:329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nyitrai M, Geeves MA. Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1867–1877. doi: 10.1098/rstb.2004.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. The source data for Figs. 1, 2, 3, 4, 5, 7, Supplementary Figs. 1, 2 and 3 and for Tables 1, 2 and Supplementary Table 1 are provided as a Source Data file. All remaining data will be available from the corresponding author upon reasonable request. Source data are provided with this paper.