Visual Abstract
Keywords: FSGS, collapsing glomerulopathy, minimal change nephrotic syndrome; kidney biopsy, malaria infection, APOL1 protein, human, HIV-Associated Nephropathy, nephrotic syndrome, HIV Infections, immunohistochemistry, Follow-Up Studies
Abstract
Background and objectives
Malaria, a potentially life-threatening disease, is the most prevalent endemic infectious disease worldwide. In the modern era, the spectrum of glomerular involvement observed in patients after malarial infections remains poorly described.
Design, setting, participants, & measurements
We therefore performed a retrospective multicenter study to assess the clinical, biologic, pathologic, and therapeutic characteristics of patients with glomerular disease demonstrated by kidney biopsy in France within 3 months of an acute malaria episode.
Results
We identified 23 patients (12 men), all but 1 of African ancestry and including 10 patients with concomitant HIV infection. All of the imported cases were in French citizens living in France who had recently traveled back to France from an endemic area and developed malaria after their return to France. Eleven patients had to be admitted to an intensive care unit at presentation. Plasmodium falciparum was detected in 22 patients, and Plasmodium malariae was detected in 1 patient. Kidney biopsy was performed after the successful treatment of malaria, a mean of 24 days after initial presentation. At this time, all patients displayed AKI, requiring KRT in 12 patients. Nephrotic syndrome was diagnosed in 17 patients. Pathologic findings included FSGS in 21 patients and minimal change nephrotic syndrome in 2 patients. Among patients with FSGS, 18 had collapsing glomerulopathy (including 9 patients with HIV-associated nephropathy). In four patients, immunohistochemistry with an antibody targeting P. falciparum histidine-rich protein-2 demonstrated the presence of the malaria antigen in tubular cells but not in podocytes or parietal epithelial cells. An analysis of the apoL1 risk genotype showed that high-risk variants were present in all seven patients tested. After a mean follow-up of 23 months, eight patients required KRT (kidney transplantation in two patients), and mean eGFR for the other patients was 51 ml/min per 1.73 m2.
Conclusions
In patients of African ancestry, imported Plasmodium infection may be a new causal factor for secondary FSGS, particularly for collapsing glomerulopathy variants in an APOL1 high-risk variant background.
Introduction
Malaria is a major public health concern worldwide, affecting many millions of people living in tropical areas and causing a significant number of deaths annually (1). Endemic malaria has been eradicated in European countries, and the malaria cases notified in France are generally imported cases in travelers returning or migrants moving to France from countries in which malaria is endemic. AKI is one of the most feared and severe life-threatening complications, affecting 1%–4% of all patients and up to 60% of patients with severe malaria (2,3). A broad spectrum of glomerular lesions has been described in association with malaria, but the pathophysiologic relationship between these two conditions has been little investigated (4,5). Early studies, performed in patients with Plasmodium malariae (P. malariae)infection, found that immune complex–mediated membranoproliferative GN (MPGN) was one of the most frequent causes of nephrotic syndrome in Africa (5–7). The spectrum of glomerular damages associated with Plasmodium falciparum (P. falciparum) infection is less common and has not been described in detail (8). The main pathologic lesions appear to be IgA nephropathy (9), eosinophilic GN (10), minimal change nephrotic syndrome (11), and collapsing glomerulopathy (12–14). We therefore retrospectively assessed the data for 23 patients with biopsy-proven glomerular disease in a context of imported malaria, with the aim of describing the clinical, biologic, pathologic, and therapeutic characteristics of these patients and their outcome. We also performed, in four patients, immunohistochemistry studies with an antibody targeting P. falciparum histidine-rich protein-2 (HRP-2) to determine whether P. falciparum was present in the kidney tissue at the time of glomerular disease diagnosis.
Materials and Methods
Patients and Kidney Function Evaluation
We conducted this retrospective study by sending a questionnaire to all French nephrology departments asking them to identify patients with biopsy-proven glomerular disease following acute malaria episode (kidney biopsy performed within 3 months of Plasmodium infection). The main indications for kidney biopsy were significant proteinuria (urine protein-creatinine ratio [uPCR] >1.5 g/g) associated with kidney impairment. This study was performed in accordance with the ethical standards of the Helsinki Declaration and was approved by our local institutional review board (Institutional Review Board 412 Mondor No. 00003835) and by the Comité de Protection des Personnes d’Ile de France IV (No. 2016/25NICB). We included 23 adult patients from ten nephrology departments seen between 1998 and 2019. Demographic, clinical, biologic, and histologic data obtained at the time of malaria diagnosis and at kidney biopsy were assessed for each patient (Supplemental Material). Nephrotic syndrome was defined as a uPCR exceeding 3 g/g and a serum albumin concentration below 3.0 g/dl. AKI and CKD were defined according to Kidney Disease Improving Global Outcome (KDIGO) criteria (15,16). The genotyping data for two risk alleles (the G1 and G2 variant alleles) of the gene encoding apoL1 (APOL1) were systematically noted, when available (17). Follow-up data are listed in Supplemental Material.
Plasmodium Infection
The positive diagnosis of malaria was on the basis of blood tests (thick and thin blood smears Giemsa stained and observed by microscopy) (Supplemental Material). The results of parasitemia levels and species identification confirmed by PCR were noted when available. Patients were considered to have severe malaria if they met the recently updated World Health Organization (WHO) criteria for severe malaria on admission or during hospitalization (18).
Kidney Biopsy Examination and Immunohistochemistry Study
All patients underwent a kidney biopsy for the exploration of proteinuria with an associated impairment of kidney function within 3 months of acute Plasmodium infection. Minimal change nephrotic syndrome was diagnosed as previously described (19). FSGS was classified according to the Columbia classification (20). The morphologic features of collapsing glomerulopathy (including HIV-associated nephropathy [HIVAN] in HIV-infected patients) were defined as previously described (21). We investigated whether the Plasmodium directly infected some kidney cells and the possible presence of parasitized red cells in the kidney parenchyma at the time of biopsy by performing immunohistochemistry with an mAb targeting P. falciparum HRP-2 (Meridian Life Science, Inc., Memphis, TN) on frozen kidney biopsy sections fixed in ethanol (22) (Supplemental Material).
Results
Demographic, Clinical, and Biologic Characteristics of Patients with Biopsy-Proven Glomerular Disease Occurring after Imported Plasmodium Infection
We retrospectively identified 23 patients (12 men and 11 women) with a mean age of 47 years (range, 24–66 years). Their demographic, clinical, and biologic data at the time of Plasmodium infection are summarized in Table 1. All but one of the patients were of African ancestry, and ten patients had concomitant HIV infection, newly diagnosed in three patients and known for some time in the other seven. All of the patients were French citizens living in France who had recently traveled back to France from an endemic area and developed malaria after their return to France. Mean CD4+ T lymphocyte count in the ten patients with HIV infection was 345/mm3 (range, 17–910/mm3). At the time of the bout of malaria, four HIV-positive patients were on highly active antiretroviral therapy (HAART), which successfully controlled the viral infection, with undetectable levels of HIV in all patients (Table 1). Five HIV-positive patients had positive HIV viral load (2.104 to 2.106 copies per milliliter). One patient tested positive for hepatitis C virus (viral load: 5.7 viral copies per milliliter), and one patient was positive for hepatitis B virus (viral load: 260 copies per milliliter). The Plasmodium species responsible for malaria were P. falciparum in 22 patients (96%) and P. malariae in 1 patient, and mean parasitemia was 7% (range, 0.3%–29%). Severe malaria infection led to 11 patients (48%) being initially hospitalized in an intensive care unit for malaria management. The specific treatments administered for malaria are listed in Table 1. The main results for kidney function parameters, immunologic findings, and other relevant laboratory investigations at the time of kidney biopsy are shown in Table 2. All patients displayed AKI (stage 1 in 1 patient, stage 2 in 2 patients, and stage 3 in 20 patients). AKI required KRT with hemodialysis in 12 patients, and the other 11 patients presented significant alterations of kidney function with a mean creatinine level of 4.9 mg/dl (range, 1.3–9.6 mg/dl). Seventeen of the 19 patients for whom data were available (90%) presented nephrotic syndrome. Mean uPCR and serum albumin levels in the total population were 9.49 g/g (1.7–27 g/g) and 2.0 g/dl (0.7–4 g/dl), respectively. Immunologic tests, performed in 23 patients, yielded unremarkable results in all but 3 patients, 2 of whom had type II cryoglobulinemia with low levels of complement fractions, whereas the third had a monoclonal IgGκ spike.
Table 1.
Demographic and clinical data at the time of malaria
| Characteristics | Number |
|---|---|
| Mean age, yr, n=23 | 47 (range, 24–66) |
| Sex, n=23 | |
| Men | 12 (52%) |
| Women | 11 (48%) |
| Race, n=23 | |
| Black | 22 (96%) |
| White | 1 (4%) |
| HIV status, n=23 | |
| Positive | 10 (43%) |
| Mean HIV viral load, copies/ml, n=9/10 | 458,000 (range, undetectable to 2.106) |
| Mean CD4 count, /mm3, n=9/10 | 345 (range, 17–910) |
| Plasmodium species, n=23 | |
| Plasmodium falciparum | 22 (96%) |
| Plasmodium malariae | 1 (4%) |
| Mean parasitemia, %, n=16/23 | 7 (range, 0.3–29) |
| Admission unit, n=23 | |
| ICU | 11 (48%) |
| Malaria severity criteria, n=23 | |
| None | 1 (4.5%) |
| %P | 1 (4.5%) |
| AKI | 15 (64%) |
| Neurologic criteriaa | 1 (4.5%) |
| AKI and %P | 2 (9%) |
| AKI and ARDS | 1 (4.5%) |
| AKI and neurologic criteriaa | 1 (4.5%) |
| AKI, neurologic criteria, and %Pa | 1 (4.5%) |
| Specific treatment of malaria episode, n=22/23 | |
| Quinine | 4 (18%) |
| Artesunate | 10 (45%) |
| Arteminol piperaquine | 4 (18%) |
| Atovaquone proguanil | 2 (9%) |
| Atovaquone proguanil + artesunate | 1 (5%) |
| Atovaquone proguanil + artesunate + quinine | 1 (5%) |
ICU, intensive care unit; %P, parasitemia expressed as a percentage; ARDS, acute respiratory distress syndrome.
Neurologic criteria: neurologic involvement including impaired consciousness and/or prostration and/or multiple convulsions.
Table 2.
Clinical and biologic data at the time of kidney biopsy
| Characteristics | Number |
|---|---|
| AKI according KDIGO criteria, n=23 | |
| Stage 1 | 1 (4%) |
| Stage 2 | 2 (9%) |
| Stage 3 | 20 (87%) |
| Hypertension, n=23 | 10 (43%) |
| uPCR, g/g, n=21/23a | 9.49 (range, 1.7–27) |
| Serum albumin level, g/dl, n=21/23 | 2.0 (range, 0.7–4) |
| Microscopic hematuria, n=21/23a | 10 (48%) |
| Leukocyturia, n=21/23a | 9 (43%) |
| Time between malaria infection and kidney biopsy, d, n=23 | 24 d (range, 7–80) |
| Immunologic findings, n=23 | |
| None | 20 (87%) |
| IgGκ spike | 1 (4%) |
| Cryo (type II) | 2 (9%) |
| Parvovirus B19 serology, n=14/23 | |
| IgM+, IgG− | 4 (29%) |
| IgM−, IgG+ | 0 (0%) |
| IgM+, IgG+ | 0 (0%) |
| IgM−, IgG− | 10 (71%) |
| ApoL1 status, n=7/23 | |
| G1/G1 | 4 (57%) |
| G1/G2 | 3 (43%) |
KDIGO, Kidney Disease Improving Global Outcome; uPCR, urine protein-creatinine ratio; Cryo, cryoglobulinemia.
Two patients with anuria.
Underlying Glomerular Lesions
The mean time between acute malaria episode and kidney biopsy was 24 days (7–80 days). In all patients, kidney biopsy was performed after the successful management of malaria as demonstrated by an absence of parasitemia after treatment in all patients. Kidney pathology findings are shown in Table 3. The most frequent glomerular lesion, found in 21 patients (91%), was FSGS, but 2 patients (9%) had typical features of minimal change nephrotic syndrome (including 1 patient with P. malariae infection). The collapsing glomerulopathy variant was identified in 18 patients (Figure 1A), whereas lesions were classified as not otherwise specified histologic variants in 3 patients (Figure 1B). In the ten patients with concomitant HIV infection, collapsing glomerular lesions were consistent with HIVAN diagnosis in nine patients, whereas the kidney biopsy specimen from the last patient with HIV demonstrated the presence of not otherwise specified FSGS lesions. Of note, two patients had a previous history of biopsy-proven HIVAN (13 and 60 months before the malaria episode) considered to be in remission under HAART until the relapse in a context of Plasmodium infection. In one patient, HIVAN recurrence was diagnosed in a context of HAART withdrawal. Interestingly, three patients were found to have collapsing glomerulopathy despite good control of HIV infection. In one patient, HIVAN lesions were associated with glomerular damage suggestive of additional MPGN lesions with Ig monoclonal IgGκ deposits. An electron microscopy study was performed for this patient and revealed nonorganized dense subendothelial osmiophilic deposits, suggestive of proliferative GN, with monoclonal IgG deposits but no tubuloreticular inclusion. In one patient, HIVAN was associated with thrombotic microangiopathy (TMA) lesions in a context of severe hypertension. In two patients with cryoglobulinemia, no glomerular lesions suggestive of cryoglobulinemia-related GN were found on biopsy. In HIV-negative patients, collapsing glomerulopathy was the most frequent pattern of glomerular injury (nine patients; 69%). Excluding patients diagnosed with minimal change nephrotic syndrome, the percentage of glomeruli displaying segmental and sclerotic lesions was 64% (from 12.5% to 100%). In patients with collapsing glomerulopathy (regardless of HIV status), the mean percentage of glomeruli displaying segmental or global collapse was 50% (from 4% to 100%). Non-neoplastic lymphoplasmacytic infiltration was found in the interstitium in 18 patients (78%). Acute tubular necrosis (ATN) lesions were found in 15 patients (65%). Microcystic tubular dilation was observed in 15 patients, including 9 patients diagnosed with HIVAN and 6 HIV-negative patients (Figure 1C). Mild arteriolar hyalinosis was a frequent histologic finding present in 15 patients, mostly in those with collapsing glomerulopathy not associated with HIV infection (8 of 9 patients).
Table 3.
Kidney biopsy findings
| Characteristics | Number |
|---|---|
| No. of glomeruli, n=23 | 16 (range, 6–30) |
| Obsolescent glomeruli, %, n=23 | 11 (range, 0–50) |
| FSGS lesions, %, n=21/23 | 64 (range, 12.5–100) |
| Collapsing lesions, %, n=18/21 | 50 (range, 4–100) |
| Interstitial fibrosis, %, n=23 | 18 (range, 0–50) |
| Interstitial infiltrate, n=23 | 18 (78%) |
| Interstitial edema, n=23 | 7 (30%) |
| Tubular microcyst dilation, n=23 | 15 (65%) |
| Acute tubular necrosis, n=23 | 15 (65%) |
| Arteriolar hyalinosis, n=23a | 15 (65%) |
| Definitive pathologic diagnosis | |
| FSGSb | 21 (91%) |
| Collapsing glomerulopathy | 18 |
| Including HIV-associated nephropathy | 9 |
| Not otherwise specified variant | 3 |
| Minimal change disease | 2 (9%) |
Arteriolar hyalinosis was scored as follows: +, mild; ++, moderate; +++, severe.
In one patient with HIV infection, kidney biopsy findings were consistent with not otherwise specified variant. In one patient, HIV-associated nephropathy lesions were associated with additional membranoproliferative GN lesions. In one patient, HIV-associated nephropathy lesions were associated with thrombotic microangiopathy lesions.
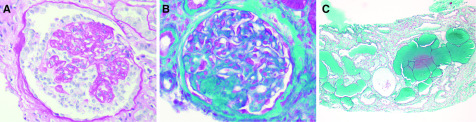
Figure 1.
FSGS and collapsing glomerulopathy variant were the main glomerular diseases occurring after imported malaria. (A) Collapsing glomerulopathy with segmental collapse of the glomerular tuft and overlying epithelial cell hypertrophy and hyperplasia (periodic acid–Schiff staining ×400) in an HIV-negative patient. (B) FSGS (not otherwise specified variant) in an HIV-negative patient (Masson trichrome staining ×400). (C) Collapsing glomerulopathy was associated with microcystic tubular dilation (Masson trichrome staining ×100) in an HIV-negative patient.
Detection of P. falciparum Protein in Kidney Parenchyma
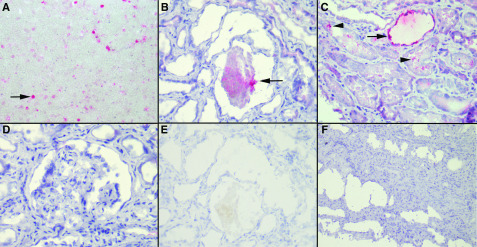
P. falciparum was the species potentially implicated in the glomerular diseases occurring after malaria in 96% of patients. We therefore used an anti–HRP-2 antibody to determine whether parasite was present in the kidney parenchyma of four of these patients. The positive control, P. falciparum–infected red blood cells, is shown in Figure 2A. Immunohistochemistry revealed the presence of the parasite antigen in the lumina of the tubules (Figure 2B) of the four patients analyzed and in the tubular cells cytoplasm (Figure 2C) in one patient. By contrast, podocytes, parietal epithelial cells, and glomerular endothelial cells were negative in all tested patients (Figure 2D). We observed no endocapillary staining suggestive of the presence of circulating infected red blood cells. The negative controls are shown in Figure 2, E and F.
Figure 2.
Immunohistochemistry with an mAb against Plasmodium falciparum histidine-rich protein-2 revealed the presence of the parasite antigen in the lumina of the tubules. (A) P. falciparum–infected red blood cells as a positive control (arrow; ×400). (B) P. falciparum parasites in the tubule lumen (arrow; ×400). (C) P. falciparum parasites in the tubule lumen (arrowheads) and cytoplasm (arrow; ×400). (D) Absence of parasites in FSGS lesions (×400). (E) Negative control, with omission of the primary antibody (×400), for comparison with (B). (F) Absence of staining in a negative control consisting of a patient with HIV-associated nephropathy without malaria (×200). Similar results were obtained for patients with acute tubular necrosis unrelated to malaria.
Treatment and Follow-Up
Some of the patients therefore underwent additional analyses to assess the likelihood of FSGS being caused by a secondary process (Table 2). APOL1 risk allele variants were studied in seven patients, all of whom tested positive (four patients were homozygous for the G1/G1 genotype, and three had the compound heterozygous G1/G2 genotype). Serologic tests for parvovirus B19 were performed in 14 patients. Four patients tested positive for specific IgM antibodies, whereas concomitant serum PCR assays yielded negative results. At the time of kidney biopsy, HAART had been started in three of the HIV-infected patients, reintroduced in one patient, and modified in six patients (Table 4). Steroid therapy was introduced in ten patients, including one with HIVAN associated with MPGN and the two patients diagnosed with minimal change nephrotic syndrome. An immunosuppressive regimen was added in two patients. After a mean follow-up of 23 months (0.5–220 months), only four of the patients were considered to present a complete remission of nephrotic syndrome. At the end of follow-up, KRT was required in eight patients: maintenance intermittent hemodialysis was performed in six patients, and the other two underwent kidney transplantation. All of these patients had severe AKI at the time of kidney biopsy (stage 3 of the KDIGO classification in all patients), and seven were already on dialysis at initial nephrologic evaluation. One patient died from septic shock 1 month after kidney biopsy. It was possible to stop KRT in 5 of the remaining 15 patients. Mean eGFR of these 15 patients was 51 ml/min per 1.73 m2 at the end of follow-up (ranging from 9.5 to 113 ml/min per 1.73 m2).
Table 4.
Treatment and follow-up
| Characteristics | Number |
|---|---|
| HAART initiation in patients with HIV, n=10 | 10 (100%) |
| Steroids, n=23 | 10 (43%) |
| RAS inhibitors, n=23 | 11 (48%) |
| Cyclosporin, n=23 | 2 (9%) |
| Mean follow-up, mo, n=23 | 23 (range, 0.5–220) |
| Complete remission of nephrotic syndrome, n=22/23 | 4 (18%) |
| Kidney status at last follow-up, n=23a | |
| Hemodialysis | 6 (27%) |
| Kidney transplant | 2 (9%) |
| Mean eGFR at last follow-up visit in those not needing KRT, ml/min per 1.73 m2, n=15 | 51 (range, 9.5–113) |
HAART, highly active antiretroviral therapy; RAS, renin-angiotensin system.
Death occurred in one patient 1 month after kidney biopsy.
Discussion
The main clinical, biologic, pathologic, and therapeutic characteristics of travelers with biopsy-proven glomerular disease after imported malaria have never been investigated in detail. In this retrospective French survey, we found that FSGS was the most frequent glomerular lesion observed in this setting. We also demonstrated that the most frequent morphologic variant of FSGS was collapsing glomerulopathy, which was observed in 18 of 23 patients.
The mean time between the onset of Plasmodium infection and the histologic diagnosis of glomerular disease was 24 days. This relatively long interval probably reflects the severity of malaria because intensive care unit admission was required for 11 patients at initial presentation, and 22 patients were considered to have severe malaria infection according to WHO criteria. AKI is a relatively frequent complication of severe malaria and is associated with higher mortality rates (23). The pathophysiologic processes underlying AKI due to malaria seem to be multifactorial, involving hemodynamic instability, immune-mediated kidney injury, metabolic disturbances, and/or the sequestration of parasitized red blood cells in the kidney vasculature leading to ischemic ATN (4). Regardless of the underlying glomerular disease, our pathologic study showed that ATN lesions were present in 65% of patients. Given the potential role of ischemic injury in collapsing glomerulopathy pathogenesis (21), we can hypothesize that ischemia related to P. falciparum infection may act as an additional factor triggering glomerular involvement in these patients. We also observed lymphoplasmocytic cell infiltration into the interstitial area in 78% of patients, suggesting that interstitial inflammation and systemic immune inflammatory processes may also contribute to AKI (4).
The predominance of collapsing glomerulopathy among the glomerular lesions in these patients may also be a key factor associated with the high frequency of AKI in our cohort (24). Collapsing glomerulopathy, a severe clinicopathologic type of glomerular injury considered to be a distinctive variant of the FSGS spectrum, was initially described in Afro-Caribbean populations, mostly in patients with HIV infection (classic HIVAN) (24) but also in some patients not infected with HIV (25). The list of known underlying processes associated with collapsing glomerulopathy is continuing to grow (21), but it has rarely been reported in a context of acute malaria (12–14). Several potential confounding causal agents of collapsing glomerulopathy could be considered in our study. In ten patients, Plasmodium infection occurred in a context of HIV infection, and collapsing glomerulopathy is the main glomerular feature in patients diagnosed with HIVAN (21). Compelling evidence has been reported, demonstrating a direct role for HIV viral proteins in affected kidney parenchymal cells, promoting HIVAN (26,27). Unfortunately, we did not perform in situ hybridization to detect HIV in kidney tissues. Interestingly, HIV infection was successfully controlled in four patients on HAART therapy at the time of HIVAN diagnosis, suggesting that the trigger was not HIV infection but malaria infection. One HIV-infected patient displayed FSGS lesions without the other pathologic characteristics of HIVAN, highlighting the specific role of P. falciparum in podocyte injury. Infection with parvovirus B19, another agent that has been implicated in FSGS and/or collapsing glomerulopathy (21,28,29), was investigated in 14 patients. Four patients (29%) tested positive for IgM antibodies, suggesting recent infection. In our series, we cannot definitively exclude the possibility of a role of parvovirus B19 infection in the pathogenesis of glomerular disease due to the absence of DNA extraction and amplification on kidney tissue. Moreover, only two of our patients displayed clinical or biologic features of autoimmune disease. These two patients had cryoglobulinemia, but their kidney lesions were consistent with collapsing glomerulopathy, associated with TMA lesions in one patient and minimal change lesions in the other.
Our findings therefore suggest that infection with P. falciparum (96% of the patients considered here) may be a crucial trigger for FSGS/collapsing glomerulopathy development in patients of African ancestry. The only patient in this cohort not of African descent was diagnosed with minimal change nephrotic syndrome. Recent studies have demonstrated that Afro-Caribbean individuals carrying APOL1 risk alleles have a higher risk of developing several types of glomerular injury, leading to CKDs (30–33). We investigated APOL1 risk genotype in seven patients from whom DNA samples were available. Strikingly, all of them were positive for high-risk alleles. There is growing experimental evidence to suggest that the kidney-specific expression of the APOL1-G1/G2 risk variants may interfere with normal podocyte homeostasis (34–36). Unfortunately, the retrospective nature of this study and the limited number of patients tested for APOL1 genetic variants are inherent limitations to the approach used here that weaken our presumptions concerning the potential role of APOL1 polymorphism in the occurrence of glomerular injury. The potential role of malaria in the two patients with P. falciparum infection and minimal change nephrotic syndrome remains more speculative. Minimal change nephrotic syndrome is generally considered to be an idiopathic glomerular disorder potentially triggered by immunologic stimuli, leading to secondary podocyte dysfunction (37). Electron microscopy analyses are not routinely performed in the context of minimal change nephrotic syndrome at French nephrology centers. Light and immunofluorescence microscopy findings were highly suggestive of this diagnosis, but differential diagnoses could not be definitively ruled out. In our two patients, it remains difficult to determine whether minimal change nephrotic syndrome and malaria should be considered as a related disorder or as a fortuitous association. Plasmodium infection is associated with a profound dysregulation of the immune response (38), and we cannot, therefore, exclude the possibility that an increase in the production of proinflammatory cytokines interferes with normal podocyte biology, leading to subsequent nephrotic syndrome like it has already been described in other settings, such as hemophagocytic syndrome (39).
In order to test the hypothesis of a direct role of the parasitic infection on kidney disease, we searched for Plasmodium in the kidney cells of our patients by performing immunohistochemistry with an antibody specifically targeting a P. falciparum protein in four patients. We detected parasite protein in the tubules but obtained no evidence of parasitized red blood cell adhesion to the capillary glomerular endothelium or of the presence of parasites in podocytes. Nevertheless, the lack of detection of a P. falciparum–specific protein in the glomeruli at the time of kidney biopsy (mean of 24 days between malaria infection and kidney biopsy) does not rule out the possibility of the parasite playing a key role in early stages of glomerular injury. Consistent with this hypothesis, cytomegalovirus infection may be considered a second hit for de novo collapsing glomerulopathy despite the absence of glomerular staining for cytomegalovirus (40). The presence of parasite antigens in the tubular lumen and tubular cell cytoplasm after the virus has been eradicated remains surprising. We can hypothesize that, as in HIV infection (41), the kidneys may act as a reservoir of malaria infection despite undetectable parasitemia. Further studies on the basis of PCR tests on urine should be considered to determine whether this noninvasive method could be used for the diagnosis of persistent Plasmodium infection in the kidneys. Furthermore, we cannot exclude the possibility that HRP-2 detection in the kidney parenchyma is related to degradation products resulting from Plasmodium lysis.
Collapsing glomerulopathy is a particularly aggressive form of FSGS that responds poorly to steroid therapy (21,42). In our study, after a mean of 23 months of follow-up, notwithstanding the initiation of steroid treatment in ten patients, eight of the patients in the total population progressed to ESKD requiring KRT, and another four patients displayed severe decreases in eGFR. Previous studies in patients with collapsing glomerulopathy showed that the higher ESKD risk was correlated with the degree of interstitial fibrosis, the number of glomeruli with collapsing lesions, and proteinuria and creatinine levels (43,44). Twenty of the patients from our cohort had stage 3 AKI at initial presentation, and all but one of the patients displayed severe kidney impairment at the time of biopsy. In addition, in 16 patients, >20% of the glomeruli presented collapsing lesions, and 12 patients had >20% interstitial fibrosis. Overall, these data may explain the poor kidney outcome of patients with glomerular disease after Plasmodium infection.
In conclusion, our study suggests that, in patients of African ancestry, imported malaria can be added to the spectrum of underlying processes promoting FSGS lesions. Our immunohistochemistry study results do not support a direct role for P. falciparum in glomerular damages. P. falciparum infection seems to act as a trigger for FSGS occurrence in patients with genetic susceptibility factors and/or preexisting viral infection.
Disclosures
Dr. V. Audard reports receiving personal fees from Addmedica outside of the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We acknowledge all of the nephrologists and kidney pathologists involved in the medical care of the patients included in this study. We thank Julie Sappa for editorial assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00590120/-/DCSupplemental.
Supplemental Material. Material and methods.
References
- 1.Askling HH, Bruneel F, Burchard G, Castelli F, Chiodini PL, Grobusch MP, Lopez-Vélez R, Paul M, Petersen E, Popescu C, Ramharter M, Schlagenhauf P; European Society for Clinical Microbiology and Infectious Diseases Study Group on Clinical Parasitology : Management of imported malaria in Europe. Malar J 11: 328, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdmann EA, Jha V: Acute kidney injury due to tropical infectious diseases and animal venoms: A tale of 2 continents. Kidney Int 91: 1033–1046, 2017. [DOI] [PubMed] [Google Scholar]
- 3.van Wolfswinkel ME, Koopmans LC, Hesselink DA, Hoorn EJ, Koelewijn R, van Hellemond JJ, van Genderen PJ: Neutrophil gelatinase-associated lipocalin (NGAL) predicts the occurrence of malaria-induced acute kidney injury. Malar J 15: 464, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barsoum RS: Malarial acute renal failure. J Am Soc Nephrol 11: 2147–2154, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Van Velthuysen ML: Glomerulopathy associated with parasitic infections. Parasitol Today 12: 102–107, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Elsheikha HM, Sheashaa HA: Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res 101: 1183–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Yashima A, Mizuno M, Yuzawa Y, Shimada K, Suzuki N, Tawada H, Sato W, Tsuboi N, Maruyama S, Ito Y, Matsuo S, Ohno T: Mesangial proliferative glomerulonephritis in murine malaria parasite, Plasmodium chabaudi AS, infected NC mice. Clin Exp Nephrol 21: 589–596, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Barsoum RS: Malarial nephropathies. Nephrol Dial Transplant 13: 1588–1597, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Yoo DE, Kim JH, Kie JH, Park Y, Chang TI, Oh HJ, Kim SJ, Yoo TH, Choi KH, Kang SW, Han SH: Immunoglobulin A nephropathy associated with Plasmodium falciparum malaria. J Korean Med Sci 27: 446–449, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker A, Ellis J, Irama M, Senkungu J, Nansera D, Axton J, Coward RJ, Peat DS, Bode HH, Mathieson PW: Eosinophilic glomerulonephritis in children in Southwestern Uganda. Kidney Int 71: 569–573, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Rangwani N, Facaros S, Wang J, Agarwal S, Shah P, Raina R: Minimal change disease and malaria. Clin Kidney J 12: 245–247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kute VB, Trivedi HL, Vanikar AV, Shah PR, Gumber MR, Kanodia KV: Collapsing glomerulopathy and hemolytic uremic syndrome associated with falciparum malaria: Completely reversible acute kidney injury. J Parasit Dis 37: 286–290, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niang A, Niang SE, Ka HF, Ka MM, Diouf B: Collapsing glomerulopathy and haemophagocytic syndrome related to malaria: A case report. Nephrol Dial Transplant 23: 3359–3361, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Sehar N, Gobran E, Elsayegh S: Collapsing focal segmental glomerulosclerosis in a patient with acute malaria. Case Rep Med 2015: 420459, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 12: 1–138, 2012 [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 17.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization : Guidelines for the Treatment of Malaria, 3rd Ed., Geneva, Switzerland, WHO Press, 2015 [Google Scholar]
- 19.Vivarelli M, Massella L, Ruggiero B, Emma F: Minimal change disease. Clin J Am Soc Nephrol 12: 332–345, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Albaqumi M, Barisoni L: Current views on collapsing glomerulopathy. J Am Soc Nephrol 19: 1276–1281, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Genrich GL, Guarner J, Paddock CD, Shieh WJ, Greer PW, Barnwell JW, Zaki SR: Fatal malaria infection in travelers: Novel immunohistochemical assays for the detection of Plasmodium falciparum in tissues and implications for pathogenesis. Am J Trop Med Hyg 76: 251–259, 2007. [PubMed] [Google Scholar]
- 23.Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TT, Mai NT, Waller DJ, White NJ: Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 15: 874–880, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JA, Markowitz GS, Valeri A, Appel GB: Collapsing glomerulopathy. Semin Nephrol 23: 209–218, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Weiss MA, Daquioag E, Margolin EG, Pollak VE: Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: A new clinicopathologic entity? Am J Kidney Dis 7: 20–28, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Ross MJ: Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int 86: 266–274, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Swanepoel CR, Atta MG, D’Agati VD, Estrella MM, Fogo AB, Naicker S, Post FA, Wearne N, Winkler CA, Cheung M, Wheeler DC, Winkelmayer WC, Wyatt CM; Conference Participants : Kidney disease in the setting of HIV infection: Conclusions from a Kidney Disease: Improving Global outcomes (KDIGO) Controversies Conference. Kidney Int 93: 545–559, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, Jordan SC, Toyoda M: Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59: 2126–2133, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Tanawattanacharoen S, Falk RJ, Jennette JC, Kopp JB: Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis 35: 1166–1174, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kormann R, Jannot AS, Narjoz C, Ribeil JA, Manceau S, Delville M, Joste V, Prié D, Pouchot J, Thervet E, Courbebaisse M, Arlet JB: Roles of APOL1 G1 and G2 variants in sickle cell disease patients: Kidney is the main target. Br J Haematol 179: 323–335, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI: Localization of APOL1 protein and mRNA in the human kidney: Nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, Boustany-Kari CM, Pullen SS, Miner JH, Hu CA, Rohacs T, Inoue K, Ishibe S, Saleem MA, Palmer MB, Cuervo AM, Kopp JB, Susztak K, Freedman BI: Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Paliwal N, Ayasolla K, Vashistha H, Jha A, Chandel N, Chowdhary S, Saleem MA, Malhotra A, Chander PN, Skorecki K, Singhal PC: Disruption of APOL1-miR193a Axis induces disorganization of podocyte actin cytoskeleton. Sci Rep 9: 3582, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahali D, Sendeyo K, Mangier M, Audard V, Zhang SY, Lang P, Ollero M, Pawlak A: Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin Immunopathol 36: 421–429, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega-Pajares A, Rogerson SJ: The rough guide to monocytes in malaria infection. Front Immunol 9: 2888, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan JL, Noël LH, Karras A: Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 69: 1892–1898, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Chang JH, Husain SA, Santoriello D, Stokes MB, Miles CD, Foster KW, Li Y, Dale LA, Crew RJ, Cohen DJ, Kiryluk K, Gharavi AG, Mohan S: Donor’s APOL1 risk genotype and “second hits” associated with de novo collapsing glomerulopathy in deceased donor kidney transplant recipients: A report of 5 cases. Am J Kidney Dis 73: 134–139, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canaud G, Dejucq-Rainsford N, Avettand-Fenoël V, Viard JP, Anglicheau D, Bienaimé F, Muorah M, Galmiche L, Gribouval O, Noël LH, Satie AP, Martinez F, Sberro-Soussan R, Scemla A, Gubler MC, Friedlander G, Antignac C, Timsit MO, Onetti Muda A, Terzi F, Rouzioux C, Legendre C: The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol 25: 407–419, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Laurinavicius A, Hurwitz S, Rennke HG: Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int 56: 2203–2213, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V: Idiopathic collapsing focal segmental glomerulosclerosis: A clinicopathologic study. Kidney Int 50: 1734–1746, 1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.