Visual Abstract
Keywords: ADPKD, kidney stones, tolvaptan, calcium oxalate, polycystic kidney, autosomal dominant, creatinine, glomerular filtration rate, uric acid, calcium phosphate, dibasic, dihydrate, linear models, citric acid, body mass index, alkalies, prospective studies, cohort studies, follow-up studies, kidney calculi
Abstract
Background and objectives
Nephrolithiasis is a common health problem in autosomal dominant polycystic kidney disease (ADPKD) and significantly contributes to patient morbidity. Recently, Tolvaptan has been introduced for the treatment of ADPKD, but whether it is associated with alterations of the urinary lithogenic risk profile remains unknown.
Design, setting, participants, & measurements
We conducted an analysis of participants enrolled in the Bern ADPKD registry, a prospective observational cohort study. Twenty-four-hour urine analyses were performed at baseline and then at yearly follow-ups. Relative supersaturation ratios for calcium oxalate, brushite, and uric acid were calculated with the program EQUIL2. Unadjusted and multivariable mixed-effects linear regression models, adjusted for age, sex, body mass index, eGFR, net acid excretion, and height-adjusted total kidney volume, were used to assess the association of Tolvaptan with urinary parameters relevant for kidney stone formation. The maximum individual follow-up time was 3 years, median follow-up time 1.9 years, and cumulative follow-up time 169 years.
Results
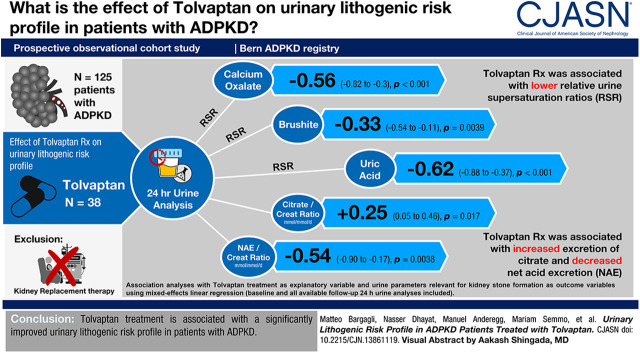
In total, 125 participants (38 with and 87 without Tolvaptan treatment) were included in the analysis. In multivariable analysis, Tolvaptan treatment was associated [adjusted estimate of the difference between Tolvaptan and no Tolvaptan; 95% confidence interval (CI)] with lower urine relative supersaturation ratios for calcium oxalate (−0.56; 95% CI, −0.82 to −0.3; P<0.001), brushite (−0.33; 95% CI, −0.54 to −0.11; P=0.004), and uric acid (−0.62; 95% CI, −0.88 to −0.37; P<0.001), and with higher urine citrate in mmol/mmol creatinine per day (0.25; 95% CI, 0.05 to 0.46; P=0.02) and calcium in mmol/mmol creatinine per day (0.31; 95% CI, 0.09 to 0.53; P=0.006) excretion. In addition, Tolvaptan treatment was associated with lower net acid excretion in mEq/mmol creatinine per day (−0.54; 95% CI, −0.90 to −0.17; P=0.004) and higher net gastrointestinal alkali absorption in mEq/mmol creatinine per day (0.57; 95% CI, 0.26 to 0.88; P<0.001).
Conclusions
Tolvaptan treatment is associated with a significantly improved urinary lithogenic risk profile in patients with ADPKD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, accounting for up to 10% of all CKD cases worldwide (1,2). ADPKD is caused by heterozygous mutations in the genes PKD1 (approximately 78%), PKD2 (approximately 15%), and very rarely GANAB (approximately 0.3%) (3–5), resulting in loss of normal differentiated kidney tubular epithelium, cyst growth, and replacement of normal kidney parenchyma by interstitial fibrosis and inflammation. This pathogenic sequence leads to a progressive decline in the GFR and is frequently associated with cyst-related complications including flank pain, urinary tract infections, episodes of gross hematuria, and abdominal fullness with early satiety. Additional ADPKD manifestations include arterial hypertension, intracranial aneurysms, liver cysts, colonic diverticular disease, abdominal hernias, and cardiac valve abnormalities.
Kidney stones are significantly more common in patients with ADPKD compared with the general population, with a reported prevalence of up to 36% in cross-sectional studies (6–9). Compared with the general population of stone formers, ADPKD is characterized by a higher frequency of uric acid stones (approximately 40%–60%), with the remaining stones being mainly composed of calcium oxalate monohydrate (8,10). Prolithogenic urinary abnormalities encountered in patients with ADPKD are low urine volume, low urine pH with low urine ammonium, hypocitraturia, hyperuricosuria, and, less commonly, hyperoxaluria (6,8,11,12). In addition, anatomic factors are likely to play an important role in stone formation, because a larger kidney volume has been shown to be an independent risk factor for the development of stones in patients with ADPKD (6,12).
Recently, the highly selective vasopressin V2 receptor antagonist Tolvaptan was approved for the treatment of ADPKD on the basis of the two large randomized, double-blind, controlled phase 3 trials Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 (TEMPO 3:4) 3:4 and Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD (13,14). Tolvaptan reduced both the annual increase in total kidney volume (TKV) and the associated decline in GFR compared with placebo. In a short-term study (1 week) with 21 idiopathic calcium stone formers, Tolvaptan was associated with lower urinary relative supersaturation ratios for calcium oxalate, brushite, and uric acid, but higher absolute urinary excretion of oxalate (15). To the best of our knowledge, changes in urinary lithogenic risk profile after Tolvaptan administration in patients with ADPKD have never been investigated. In addition, the effect of chronic (>1 week) Tolvaptan treatment on the urinary lithogenic risk profile remains unknown.
Materials and Methods
Study Population
The Bern ADPKD registry is a prospective, observational cohort study of patients with ADPKD at the Department of Nephrology and Hypertension at Bern University Hospital, Bern, Switzerland that was initiated in 2015. Inclusion criteria are: age ≥18 years, ADPKD diagnosis on the basis of the Ravine criteria (16), and signed informed consent. KRT is an exclusion criterion. To minimize selection bias, all eligible patients with ADPKD seen at the outpatient clinic (already treated at the site or newly referred) are asked to participate in the Bern ADPKD registry by one of the registry investigators (N.A.D., M.S., U.H.-D., B.V., or D.G.F.). To reduce information bias, both written and verbal communication with patients was done in their native language, if necessary, supported by professional translators. Between October 2015 and July 2019, 125 participants were enrolled in the registry and included in this analysis. During the observation time, no participant withdrew consent but 14 participants had to be excluded from the registry during follow-up because of the following reasons: death (n=1), need of KRT (n=2), emigration (n=3), and not willing to adhere to study protocol with yearly visits at study site (n=8). The Bern ADPKD registry adheres to the Declaration of Helsinki and was approved by the ethical committee of the Kanton of Bern (approval number BE 124/15).
Tolvaptan Treatment
Tolvaptan became available for patients with ADPKD in Switzerland on November 1, 2016. Treatment is reimbursed by health care insurance companies if the following criteria are met: (1) age ≥18 years, (2) typical class 1 ADPKD, (3) CKD stages 1– 3, (4) TKV ≥750 ml, and (5) evidence of rapid progression. Rapid progression is defined as Mayo class 1C–1E or eGFR decline ≥5 ml/min per 1.73 m2, or growth of kidney volume >5%/yr, or truncating PKD1 mutation, and a predicting kidney outcome in ADPKD-Score >6 (17). The decision on Tolvaptan treatment initiation was left to the responsible investigator (N.A.D., M.S., U.H.-D., B.V., or D.G.F.). Treatment was always initiated with the lowest split dose regimen of 45/15 mg, and uptitrated in monthly intervals to 60/30 mg and ultimately to 90/30 mg, as tolerated by the patient.
Data Collection and Measurements
Participants included in the registry attended a baseline visit and yearly visits thereafter. For each patient, demographic and anthropometric information (sex, age, height, and weight), clinical data regarding ADPKD complications (history of symptomatic stone events, cyst ruptures, urinary tract infections, and kidney pain), a physical examination, and office BP measurements were recorded. Office BP measurements were done in the supine position after at least 5 minutes of rest using the oscillometric method. At the baseline visit, TKV was determined by magnetic resonance imaging using the ellipsoid method, then the height-adjusted TKV was calculated and the corresponding Mayo class determined (18). Standardized blood and urine analyses, including 24-hour urine collection, were conducted at baseline and then annually. All blood analyses were performed after at least 6 hours of fasting in the morning. Urine and blood analyses were performed at the Central Laboratory of Bern University Hospital using standard laboratory methods. Data were entered manually into the registry database and double-checked by an independent database manager.
Urine relative supersaturation ratios for calcium oxalate monohydrate, brushite, and undissociated uric acid were calculated by the EQUIL2 program (19). Titratable acidity was calculated as described previously (20). Net acid excretion (NAE) was calculated using the following equation: NAE=(urine ammonium+urine titratable acidity)–urine bicarbonate (21). Urine ammonium was measured enzymatically and urine bicarbonate was calculated using the Henderson–Hasselbalch equation from the urine PCO2 and pH. Urine PCO2 was measured using a blood gas analyzer (ABL 700; Radiometer, Copenhagen, DK). The creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate the eGFR (22). Diabetes was defined as reported, treated, or fasting glycemia ≥7 mmol/L. Hypercalciuria was considered as urine calcium excretion >6.2 mmol/d for women and 7.5 mmol/d for men, hyperuricosuria was defined as urine uric acid excretion >4.5 mmol/d for women and >4.8 mmol/d for men, hyperoxaluria was defined as urine oxalate excretion >0.5 mmol/d for both men and women, and hypocitraturia was defined as urine citrate excretion <1.5 mmol/d (23).
Statistical Analyses
Continuous variables were reported as medians with 25th–75th percentiles and categorical variables were reported as counts with percentages. All 24-hour urine solute excretions were standardized to urine creatinine excretions. Regression models were created to assess the association of Tolvaptan treatment with urine composition at multiple time points. In all regression models, the repeated measures correlation within study participants was addressed by using linear mixed-effects models with participants as random effects. The unadjusted model contains Tolvaptan treatment as a fixed effect. The multivariable model contains Tolvaptan treatment, age, sex, body mass index, eGFR, NAE, and height-adjusted TKV as fixed effects. If necessary, outcome variables were square root or log transformed to ensure near normal distributions. Outcome variables were further scaled to the SD. Thus, in all models, the β coefficient indicates changes in units of SD for each outcome. Missing data were excluded from the regression analysis. The numbers of available participants and the numbers of available observations for each variable of interest are both provided in tables. Statistical tests were two sided, and P<0.05 was considered significant. Statistical analyses were performed using the software R, version 3.2.2 (24).
Results
Characteristics of the Study Population
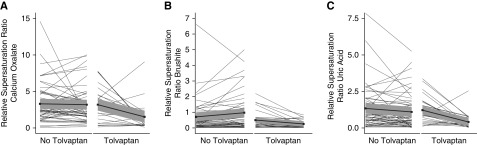
Baseline characteristics are shown in Table 1. Fifty-seven (46%) participants were men, median age was 46 years, and the median eGFR was 68 ml/min per 1.73 m2. Sixteen participants (13%) had a history of symptomatic kidney stone events. Hypocitraturia was present in 45%, hyperoxaluria in 18%, hypercalciuria in 6%, and hyperuricosuria in 3% of participants. At the baseline visit, all participants were Tolvaptan-naïve. In 38 participants, Tolvaptan treatment was initiated after the baseline visit. Participants included in the ADPKD registry performed yearly follow-up visits after baseline. At the time point of the analysis, 1-year follow-up data were available for 88 registry participants (61 without and 27 with Tolvaptan treatment) (Table 2). The maximum individual follow-up time was 3 years, the median follow-up time was 1.9 years, and the cumulative follow-up time was 169 years. Figure 1 illustrates changes in relative supersaturation ratios for calcium oxalate, brushite, and uric acid between baseline and 1-year follow-up for 61 participants without and 27 with Tolvaptan treatment.
Table 1.
Characteristics of Bern ADPKD registry participants at the baseline visit
| Characteristics | All Patients (n=125) | No Tolvaptan (n=87) | Tolvaptan (n=38) |
|---|---|---|---|
| Men, n (%) | 57 (46) | 34 (39) | 23 (61) |
| Age, yr | 46; 38–53 | 46; 36–57 | 46; 39–50 |
| Body mass index, kg/m2 | 25; 22–28 | 25; 22–28 | 25; 22–27 |
| Systolic BP, mm Hg | 136; 126–149 | 136; 125–149, [1] | 137; 130–147 |
| Diastolic BP, mm Hg | 87; 81–96 | 87; 80–96, [1] | 89; 85–94 |
| Antihypertensive medication intake, n (%) | 84 (68) | 56 (65) | 28 (74) |
| ACE-I or ARB, n (%) | 70 (56) | 43 (50) | 27 (71) |
| Calcium channel blockers, n (%) | 33 (27) | 22 (26) | 11 (29) |
| β-Blockers, n (%) | 14 (11) | 10 (12) | 4 (11) |
| Diuretics, n (%) | 31 (25) | 20 (23) | 11 (29) |
| Thiazide diuretics, n (%) | 27 (22) | 17 (20) | 10 (26) |
| Loop diuretics, n (%) | 2 (2) | 2 (2) | 0 (0) |
| Alkali therapy, n (%) | 2 (2) | 2 (2) | 0 (0) |
| Allopurinol, n (%) | 1 (1) | 1 (1) | 0 (0) |
| Diabetes, n (%) | 5 (4) | 4 (5) | 1 (3) |
| eGFR, ml/min per 1.73 m2 BSA | 68; 48–96 | 77; 46–97 | 65; 50–91 |
| eGFR subgroups, ml/min per 1.73 m2, n (%) | |||
| ≥90 | 35 (28) | 25 (29) | 10 (26) |
| 60–89 | 48 (38) | 35 (40) | 13 (34) |
| 30–59 | 27 (22) | 17 (20) | 10 (26) |
| 15–30 | 13 (10) | 8 (9) | 5 (13) |
| ≤15 | 2 (2) | 2 (2) | 0 (0) |
| Height-adjusted TKV, ml/m | 624; 366–1261 | 489; 320–1024, [3] | 986; 727–1492 |
| History of kidney stone events, n (%) | 16 (13) | 10 (12), [2] | 6 (16) |
| History of urinary tract infections, n (%) | 17 (14) | 12 (14), [2] | 5 (13) |
| History of cyst ruptures, n (%) | 16 (13) | 10 (12), [2] | 6 (16) |
| History of kidney pain, n (%) | 36 (29) | 23 (27), [2] | 13 (34) |
| Relative supersaturation ratio calcium oxalate | 2.8; 1.7–5.1 | 2.7; 1.7–5.4, [9] | 3.1; 1.7–4.7, [1] |
| Relative supersaturation ratio brushite | 0.43; 0.14–0.97 | 0.45; 0.15–1.05, [9] | 0.35; 0.12–0.71, [2] |
| Relative supersaturation ratio uric acid | 0.82; 0.33–1.53 | 0.81; 0.29–1.52, [9] | 0.83; 0.36–1.52, [1] |
| Urine calcium/creatinine ratio, mmol/mmol per d | 0.25; 0.12–0.37 | 0.27; 0.14–0.42, [5] | 0.18; 0.11–0.28 |
| Urine phosphate/creatinine ratio, mmol/mmol per d | 1.9; 1.6–2.2 | 1.9; 1.7–2.2, [6] | 1.8; 1.6–2.2, [1] |
| Urine magnesium/creatinine ratio, mmol/mmol per d | 0.31; 0.25–0.38 | 0.33; 0.25–0.38, [6] | 0.29; 0.25–0.36 |
| Urine uric acid/creatinine ratio, mmol/mmol per d | 0.22; 0.18–0.25 | 0.22; 0.19–0.26, [7] | 0.2; 0.17–0.24 |
| Urine oxalate/creatinine ratio, mmol/mmol per d | 0.03; 0.02–0.04 | 0.03; 0.02–0.04, [8] | 0.03; 0.02–0.04 |
| Urine citrate/creatinine ratio, mmol/mmol per d | 0.12; 0.06–0.22 | 0.14; 0.08–0.22, [7] | 0.10; 0.05–0.2 |
| Urine sulfate/creatinine ratio, mmol/mmol per d | 1.5; 1.2–1.7 | 1.5; 1.3–1.7, [8] | 1.4; 1.2–1.7, [1] |
| Urine pH | 5.9; 5.4–6.3 | 5.9; 5.5–6.4, [8] | 5.8; 5.5–6.2 |
| Urine ammonium/creatinine ratio, mmol/mmol per d | 1.9; 1.5–2.5 | 1.93; 1.45–2.53, [9] | 1.88; 1.55–2.55, [2] |
| Urine titratable acidity/creatinine ratio, mEq/mmol per d | 1.3; 1.0–1.7 | 1.3; 0.9–1.7, [9] | 1.3; 1.1–1.6, [2] |
| Urine NAE/creatinine ratio, mEq/mmol per d | 1.3; 0.9–1.8 | 1.3; 0.9–1.7, [11] | 1.5; 1.1–2.0, [5] |
| Urine NGIA/creatinine ratio, mEq/mmol per d | 3.9; 3.0–5.4 | 4.1; 3.1–5.5, [8] | 3.4; 2.6–5.0, [4] |
| Urine volume, L/d | 2.3; 1.8–2.9 | 2.3; 1.7–2.9, [4] | 2.4; 2.0–2.9 |
| Plasma copeptin, pmol/L | 5.3; 3.1–11.3 | 5.2; 2.9–10.0, [15] | 5.5; 3.6–13.4, [5] |
| Hypocitraturia | 53 (45) | 33 (41), [7] | 20 (53) |
| Hypercalciuria | 7 (6) | 7 (9), [5] | 0 (0) |
| Hyperuricosuria | 3 (3) | 3 (4), [7] | 0 (0) |
| Hyperoxaluria | 21 (18) | 12 (15), [8] | 9 (24) |
Characteristics are indicated for all participants (n=125) and separately for participants without (n=87) and with (n=38) future Tolvaptan treatment. Categorical variables are described by number of participants n (%), and continuous variables by their median and 25th–75th percentiles. Numbers in square brackets indicate numbers of participants with missing data for corresponding variables. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BSA, body surface area; TKV, total kidney volume; NAE, net acid excretion; NGIA, net gastrointestinal alkali absorption.
Table 2.
Urine parameters of Bern ADPKD registry participants at 1-year follow-up
| No Tolvaptan (n=61) | Tolvaptan (n=27) | |||
|---|---|---|---|---|
| Urine Parameter | 1-Yr Follow-Up | Difference from 1-Yr Follow-Up to Baseline | 1-Yr Follow-Up | Difference from 1-Yr Follow-Up to Baseline |
| Relative supersaturation ratio calcium oxalate | 2.9; 1.8–4.5, [4] | −0.10; −0.93–1.04, [7] | 1.4; 0.6–1.7 | −1.6; −3.1–0.063, [1] |
| Relative supersaturation ratio brushite | 0.67; 0.17–1.3, [4] | 0.060; −0.090–0.49, [7] | 0.13; 0.085–0.4 | −0.080; −0.26–0.020, [1] |
| Relative supersaturation ratio uric acid | 0.59; 0.25–1.59, [4] | −0.12; −0.60–0.29, [7] | 0.22; 0.085–0.54 | −0.69; −1.4–−0.25, [1] |
| Urine calcium/creatinine ratio, mmol/mmol per d | 0.27; 0.18–0.43, [2] | −0.010; −0.030–0.070, [2] | 0.15; 0.08–0.32 | 0; −0.065–0.060 |
| Urine phosphate/creatinine ratio, mmol/mmol per d | 2.1; 1.8–2.4, [2] | 0.15; −0.17–0.37, [2] | 1.7; 1.4–2 | −0.18; −0.44–0.20 |
| Urine magnesium/creatinine ratio, mmol/mmol per d | 0.32; 0.24–0.43, [2] | 0.020; −0.060–0.080, [3] | 0.32; 0.26–0.39 | 0.030; 0.010–0.080 |
| Urine uric acid/creatinine ratio, mmol/mmol per d | 0.23; 0.20–0.27, [2] | 0.010; −0.020–0.040, [3] | 0.20; 0.16–0.23 | 0; −0.035–0.035 |
| Urine oxalate/creatinine ratio, mmol/mmol per d | 0.030; 0.020–0.040, [2] | 0; −0.010–0.010, [4] | 0.03; 0.03–0.04 | 0; 0–0.010 |
| Urine citrate/creatinine ratio, mmol/mmol per d | 0.16; 0.065–0.22, [2] | 0; −0.030–0.030, [3] | 0.11; 0.065–0.17 | 0.010; −0.015–0.055 |
| Urine sulfate/creatinine ratio, mmol/mmol per d | 1.5; 1.3–1.8, [2] | 0.11; −0.090–0.37, [4] | 1.5; 1.2–1.9 | 0.11; −0.22–0.29, [1] |
| Urine pH | 6.1; 5.4–6.5, [4] | 0.060; −0.21–0.41, [6] | 6.1; 5.7–6.5 | 0.33; 0.17–0.58 |
| Urine ammonium/creatinine ratio, mmol/mmol per d | 2.0; 1.6–2.4, [3] | −0.12; −0.45–0.41, [5] | 1.7; 1.3–1.9 | −0.41; −0.78–−0.030, [2] |
| Urine titratable acidity/creatinine ratio, mEq/mmol per d | 1.4; 0.89–1.7, [4] | −0.058; −0.36–0.29, [7] | 1; 0.69–1.4 | −0.24; −0.75–0.043, [1] |
| Urine NAE/creatinine ratio, mEq/mmol per d | 1.3; 0.60–1.6, [4] | −0.12; −0.77–0.28, [8] | 1.1; 0.43–1.5, [2] | −0.72; −1.3–−0.46, [6] |
| Urine NGIA/creatinine ratio, mEq/mmol per d | 4.3; 2.9–5.8, [2] | −0.22; −0.95–1.7, [5] | 4.7; 3.9–7, [1] | 1.8; 0.64–3.7, [4] |
| Urine volume, L/d | 2.5; 1.6–3.0, [2] | 0.62; −0.34–0.34, [2] | 5.2; 3.9–5.9 | 2.6; 1.4–3.3 |
| Plasma copeptin, pmol/L | 5.1; 3.1 –12 | 0.34; −1.1–1.4, [12] | 22; 19–31, [1] | 16; 8.8–22, [5] |
One-year follow-up 24-h urine data were available for a total of 88 participants; 61 participants without and 27 participants with Tolvaptan treatment. Differences in 1-yr follow-up to baseline are indicated for each subgroup. Continuous variables are described by their median and 25th–75th percentiles. Numbers in square brackets indicate numbers of participants with missing data for corresponding variables. NAE, net acid excretion; NGIA, net gastrointestinal alkali absorption.
Figure 1.
Relative supersaturation ratios at baseline and at 1-year follow-up. Relative supersaturation ratios for calcium oxalate (A), brushite (B), and uric acid (C) in participants with or without Tolvaptan treatment at follow-up. All participants were Tolvaptan-naïve at baseline. Baseline: left side of panel, 1-year follow-up: right side of panel.
Association Analyses
In the next step, we performed association analyses with Tolvaptan treatment as an explanatory variable and urine parameters relevant for kidney stone formation as outcome variables using mixed-effects linear regression (Table 3). To this end, all baseline and, if available, follow-up 24-hour urine(s) of individual registry participants were included in the analysis. The multivariable analysis was adjusted for age, sex, body mass index, eGFR, endogenous net acid production estimated by NAE, and height-adjusted TKV. In both the unadjusted and multivariable analysis, Tolvaptan treatment was significantly associated with lower relative supersaturation ratios for calcium oxalate, brushite, and uric acid, higher urine volume, plasma copeptin, and net gastrointestinal alkali absorption (NGIA), and lower NAE. In addition, in the unadjusted analysis Tolvaptan was associated with higher urine pH and urine oxalate excretion, and with lower urine ammonium excretion, but these associations were no longer significant after multivariable adjustment. However, after multivariable adjustment, higher urine citrate and urine calcium excretion became significantly associated with Tolvaptan treatment.
Table 3.
Associations of Tolvaptan use with urinary lithogenic risk profile
| Unadjusted Models | Multivariable Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variables | NP | NO | Difference (95% CI) | P Value | NP | NO | Difference (95% CI) | P Value |
| Relative supersaturation ratio calcium oxalate (12) | 119 | 265 | −0.74 (−1 to −0.45) | <0.001 | 115 | 248 | −0.56 (−0.82 to −0.3) | <0.001 |
| Relative supersaturation ratio brushite (9) | 118 | 264 | −0.39 (−0.62 to −0.17) | <0.001 | 115 | 249 | −0.33 (−0.54 to −0.11) | 0.004 |
| Relative supersaturation ratio uric acid (14) | 119 | 266 | −0.85 (−1.1 to −0.55) | <0.001 | 115 | 249 | −0.62 (−0.88 to −0.37) | <0.001 |
| Urine volume, L/d (16) | 121 | 280 | 1.5 (1.3 to 1.7) | <0.001 | 115 | 249 | 1.5 (1.3 to 1.8) | <0.001 |
| Plasma copeptin, pmol/L (15) | 121 | 261 | 1.2 (0.99 to 1.3) | <0.001 | 112 | 227 | 1.2 (0.98 to 1.4) | <0.001 |
| Urine calcium/creatinine ratio, mmol/mmol per d (8) | 120 | 277 | 0.041 (−0.18 to 0.25) | 0.71 | 115 | 249 | 0.31 (0.09 to 0.53) | 0.006 |
| Urine phosphate/creatinine ratio, mmol/mmol per d (1) | 118 | 274 | −0.32 (−0.64 to 0.01) | 0.05 | 115 | 249 | −0.029 (−0.36 to 0.3) | 0.87 |
| Urine uric acid/creatinine ratio, mmol/mmol per d (2) | 119 | 275 | −0.057 (−0.33 to 0.21) | 0.68 | 115 | 249 | 0.048 (−0.24 to 0.33) | 0.74 |
| Urine oxalate/creatinine ratio, mmol/mmol per d (7) | 119 | 273 | 0.41 (0.07 to 0.74) | 0.02 | 115 | 247 | 0.26 (−0.07 to 0.59) | 0.14 |
| Urine citrate/creatinine ratio, mmol/mmol per d (6) | 119 | 275 | 0.13 (−0.08 to 0.35) | 0.22 | 115 | 249 | 0.25 (0.05 to 0.46) | 0.02 |
| Urine sulfate/creatinine ratio, mmol/mmol per d (10) | 119 | 272 | 0.43 (0.09 to 0.76) | 0.01 | 115 | 248 | 0.52 (0.16 to 0.88) | 0.006 |
| Urine pH (5) | 119 | 268 | 0.40 (0.09 to 0.7) | 0.01 | 115 | 249 | 0.20 (−0.03 to 0.44) | 0.10 |
| Urine ammonium/creatinine ratio, mmol/mmol per d (3) | 118 | 270 | −0.34 (−0.61 to −0.06) | 0.02 | 115 | 249 | −0.048 (−0.30 to 0.21) | 0.72 |
| Urine titratable acidity/creatinine ratio, mEq/mmol per d (4) | 118 | 264 | −0.42 (−0.75 to −0.1 | 0.01 | 115 | 249 | −0.13 (−0.40 to 0.14) | 0.35 |
| Urine NAE/urine creatinine, mEq/mmol per d (11) | 114 | 236 | −0.53 (−0.89 to −0.16) | 0.004 | 112 | 231 | −0.54 (−0.90 to −0.17) | 0.004 |
| Urine NGIA/creatinine ratio, mEq/mmol per d (13) | 118 | 266 | 0.59 (0.25 to 0.93) | <0.001 | 114 | 244 | 0.57 (0.26 to 0.88) | <0.001 |
Associations between the explanatory variable Tolvaptan treatment with risk factors of kidney stone formation as outcome variables were assessed. All continuous outcome variables were scaled to the SD, and therefore the β coefficient for the presence of Tolvaptan treatment versus no treatment corresponds to the difference of an increase of 1 SD in each outcome. The number of participants, the number of observations, differences, adjusted differences, their 95% CIs, and the corresponding P values are indicated for each model. Multivariable models are adjusted for age, sex, body mass index, eGFR, NAE, and height-adjusted total kidney volume. Numbers in brackets in each row header indicate the numeric increments in outcome to which each β coefficient in the full multivariable model three corresponds, the highest number representing the highest increment in outcome associated with Tolvaptan. P values are indicated for the presence of Tolvaptan treatment. NP, number of participants; NO, Number of observations; 95% CI, 95% confidence interval; NAE, net acid excretion; NGIA, net gastrointestinal alkali absorption.
Discussion
ADPKD is associated with a higher risk of stone formation; up to 36% of patients with ADPKD may develop kidney stones (6–9). In our registry, 13% of participants had a history of symptomatic stone events. It is likely that the prevalence of asymptomatic nephrolithiasis is significantly higher. We only had magnetic resonance imaging available as an imaging modality for our cohort, thus the prevalence of asymptomatic nephrolithiasis could not be investigated. Corroborating previous studies, we found hypocitraturia to be the most common prolithogenic abnormality in patients with ADPKD, followed by hyperoxaluria, whereas only a small fraction of patients displayed hypercalciuria or hyperuricosuria (6,25).
V2 vasopressin receptor antagonism by Tolvaptan has become a mainstay in the treatment of patients with ADPKD at high risk of progression, slowing cyst growth and GFR decline (13,14), but information regarding the effects of Tolvaptan on urinary lithogenic risk factors is lacking. Casteleijn et al. (26) performed a post hoc analysis of the TEMPO 3:4 trial and demonstrated that Tolvaptan was associated with a significant reduction in kidney pain compared with placebo (10.1% versus 16.8%, relative risk reduction of 36%). A subgroup analysis revealed that the incidence of symptomatic stone events was lower with Tolvaptan treatment compared with placebo (2.2% versus 3.5%, P<0.001), but data on kidney stone phenotypes or 24-hour urine compositions were not collected. In addition, this trial did not include patients with eGFRs between 45 and 60 ml/min per 1.73 m2, thus information on the influence of Tolvaptan treatment on stone recurrence in patients with more advanced CKD is currently lacking.
The results obtained in our ADPKD cohort reveal that Tolvaptan is associated with significantly lower relative supersaturation ratios for calcium oxalate, brushite, and uric acid in participants treated with Tolvaptan, even after adjustment for potential confounders such as sex, age, eGFR, endogenous acid production, and height-adjusted TKV. Urine relative supersaturation ratios calculated from ambulatory 24-hour urine collections accurately reflect the long-term average supersaturation values in the urine and are highly correlated with kidney stone compositions (27,28) encountered in individual kidney stone formers (29). Treatments that have been shown to reduce kidney stone events in randomized controlled trials have been highly correlated with reductions in urine supersaturations, even in the short-term (27,28,30). It is currently unknown whether the same correlation applies to patients with ADPKD and this needs to be studied prospectively. Nevertheless, our findings suggest that in addition to slowing cyst growth and eGFR decline, Tolvaptan may also be beneficial to prevent kidney stone events in patients with ADPKD.
Interestingly, even after multivariable adjustment, lower NAE remained significantly associated with Tolvaptan treatment. At the same time, we observed that Tolvaptan treatment was associated with higher NGIA (a marker of alkali intake). This finding suggests that lower NAE may be due to higher alkali intake or gut alkali absorption in patients taking Tolvaptan (31). In addition, we also observed higher urine citrate and calcium excretion associated with Tolvaptan. The underlying mechanisms for these observations, including the associations seen with NAE and NGIA, are unclear at the moment and need to be studied in more detail, including quantitative dietary data. It is possible that Tolvaptan directly influences acid, citrate, and calcium excretion by the kidney, but extrarenal effects due to systemic V2 receptor antagonism with secondarily elevated circulating vasopressin levels may also play a role in the changes observed (32,33). Higher citrate excretion may also just be a consequence of higher alkali intake in participants with Tolvaptan treatment. However, whereas Tolvaptan was associated with higher urine oxalate excretion in the unadjusted analysis, as reported by Cheungpasitporn et al. in idiopathic calcium stone formers (15), urine oxalate was no longer associated with Tolvaptan treatment after multivariable adjustment in our cohort of patients with ADPKD.
In conclusion, our data reveal that Tolvaptan treatment is associated with a significantly improved urinary lithogenic risk profile in patients with ADPKD.
Disclosures
Dr. D.G. Fuster has served as a consultant for Otsuka Pharmaceutical (Switzerland) GmbH. Dr. D.G. Fuster, Dr. N.A. Dhayat, and Dr. M. Anderegg have received an unrestricted research grant from Otsuka Pharmaceutical (Switzerland) GmbH and nonfinancial support from Sarstedt AG (biobank material) for the conduct of this study. All remaining authors have nothing to disclose.
Funding
This study was funded by an unrestricted research grant from Otsuka Pharmaceutical (Switzerland) GmbH to Dr. D.G. Fuster, Dr. N.A. Dhayat, and Dr. M. Anderegg, and by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation) grants 33IC30_166785/1, NCCR TransCure, and NCCR Kidney.CH.
Acknowledgments
We thank the former and current Bern ADPKD registry staff members C. Wyss, S. Schorer, and M. Conrad for their assistance.
We are grateful to the patients who gave their consent to participate in this study.
Part of the results were presented at the 51st Annual Meeting of the Swiss Society of Nephrology held December 5–6, 2019 in Interlaken, Switzerland.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “ADPKD, Tolvaptan, and Nephrolithiasis Risk,” on pages 923–925.
References
- 1.Reule S, Sexton DJ, Solid CA, Chen S-C, Collins AJ, Foley RN: ESRD from autosomal dominant polycystic kidney disease in the United States, 2001-2010. Am J Kidney Dis 64: 592–599, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer A, Pippias M, Noordzij M, Stel VS, Andrusev AM, Aparicio-Madre MI, Arribas Monzón FE, Åsberg A, Barbullushi M, Beltrán P, Bonthuis M, Caskey FJ, Castro de la Nuez P, Cernevskis H, De Meester J, Finne P, Golan E, Heaf JG, Hemmelder MH, Ioannou K, Kantaria N, Komissarov K, Korejwo G, Kramar R, Lassalle M, Lopot F, Macário F, Mackinnon B, Pálsson R, Pechter Ü, Piñera VC, Santiuste de Pablos C, Segarra-Medrano A, Seyahi N, Slon Roblero MF, Stojceva-Taneva O, Vazelov E, Winzeler R, Ziginskiene E, Massy Z, Jager KJ: The European renal association - European dialysis and transplant association (ERA-EDTA) registry annual report 2016: A summary. Clin Kidney J 12: 702–720, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet M-P, Ferec C, Le Meur Y, Torres VE, Harris PC; Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease : Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 98: 1193–1207, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman AB, Devuyst O, Eckardt K-U, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants : Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int 88: 17–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornec-Le Gall E, Torres VE, Harris PC: Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol 29: 13–23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiura JL, Neves RFCA, Eloi SRM, Cintra SMLF, Ajzen SA, Heilberg IP: Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 4: 838–844, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine E, Grantham JJ: Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: Clinical and CT study in 84 patients. AJR Am J Roentgenol 159: 77–81, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Torres VE, Erickson SB, Smith LH, Wilson DM, Hattery RR, Segura JW: The association of nephrolithiasis and autosomal dominant polycystic kidney disease. Am J Kidney Dis 11: 318–325, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Gambaro G, Fabris A, Puliatta D, Lupo A: Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 34: 102–107, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Daudon M, Cohen-Solal F, Lacour B, Jungers P: [Urinary stones and urinary tract abnormalities: Is the stone composition independent of the anatomical abnormality?]. Prog Urol 13: 1320–1329, 2003 [PubMed] [Google Scholar]
- 11.Torres VE, Keith DS, Offord KP, Kon SP, Wilson DM: Renal ammonia in autosomal dominant polycystic kidney disease. Kidney Int 45: 1745–1753, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Grampsas SA, Chandhoke PS, Fan J, Glass MA, Townsend R, Johnson AM, Gabow P: Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 36: 53–57, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 14.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Cheungpasitporn W, Erickson SB, Rule AD, Enders F, Lieske JC: Short-term tolvaptan increases water intake and effectively decreases urinary calcium oxalate, calcium phosphate and uric acid supersaturations. J Urol 195: 1476–1481, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Cornec-Le Gall E, Audrézet M-P, Rousseau A, Hourmant M, Renaudineau E, Charasse C, Morin M-P, Moal M-C, Dantal J, Wehbe B, Perrichot R, Frouget T, Vigneau C, Potier J, Jousset P, Guillodo M-P, Siohan P, Terki N, Sawadogo T, Legrand D, Menoyo-Calonge V, Benarbia S, Besnier D, Longuet H, Férec C, Le Meur Y: The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 942–951, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Kok DJ, Poindexter J, Pak CYC: Calculation of titratable acidity from urinary stone risk factors. Kidney Int 44: 120–126, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Relman AS: Endogenous production of fixed acid and the measurement of the net balance of acid in normal subjects. J Am Soc Nephrol 11: 2155–2164, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro PM, Robertson WG, Johri N, Nair A, Gambaro G, Shavit L, Moochhala SH, Unwin RJ: A London experience 1995-2012: Demographic, dietary and biochemical characteristics of a large adult cohort of patients with renal stone disease. QJM 108: 561–568, 2015. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2018 [Google Scholar]
- 25.Torres VE, Wilson DM, Hattery RR, Segura JW: Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis 22: 513–519, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Casteleijn NF, Blais JD, Chapman AB, Czerwiec FS, Devuyst O, Higashihara E, Leliveld AM, Ouyang J, Perrone RD, Torres VE, Gansevoort RT; TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3:4 Trial Investigators : Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: Secondary analysis from a randomized controlled trial. Am J Kidney Dis 69: 210–219, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraro PM, Ticinesi A, Meschi T, Rodgers A, Di Maio F, Fulignati P, Borghi L, Gambaro G: Short-term changes in urinary relative supersaturation predict recurrence of kidney stones: A tool to guide preventive measures in urolithiasis. J Urol 200: 1082–1087, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A: Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol 155: 839–843, 1996. [PubMed] [Google Scholar]
- 29.Parks JH, Coward M, Coe FL: Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int 51: 894–900, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A: Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Lennon EJ, Lemann J Jr., Litzow JR: The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, Colaianni G, Moonga SS, Di Benedetto A, Grano M, Colucci S, Yuen T, New MI, Zallone A, Zaidi M: Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci U S A 110: 18644–18649, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giesecke T, Himmerkus N, Leipziger J, Bleich M, Koshimizu T-A, Fähling M, Smorodchenko A, Shpak J, Knappe C, Isermann J, Ayasse N, Kawahara K, Schmoranzer J, Gimber N, Paliege A, Bachmann S, Mutig K: Vasopressin increases urinary acidification via V1a receptors in collecting duct intercalated cells. J Am Soc Nephrol 30: 946–961, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]