Abstract
Patients with primary hyperoxaluria experience kidney stones from a young age and can develop progressive oxalate nephropathy. Progression to kidney failure often develops over a number of years, and is associated with systemic oxalosis, intensive dialysis, and often combined kidney and liver transplantation. There are no therapies approved by the Food and Drug Association. Thus, the Kidney Health Initiative, in partnership with the Oxalosis and Hyperoxaluria Foundation, initiated a project to identify end points for clinical trials. A workgroup of physicians, scientists, patients with primary hyperoxaluria, industry, and United States regulators critically examined the published literature for clinical outcomes and potential surrogate end points that could be used to evaluate new treatments. Kidney stones, change in eGFR, urine oxalate, and plasma oxalate were the strongest candidate end points. Kidney stones affect how patients with primary hyperoxaluria feel and function, but standards for measurement and monitoring are lacking. Primary hyperoxaluria registry data suggest that eGFR decline in most patients is gradual, but can be unpredictable. Epidemiologic data show a strong relationship between urine oxalate and long-term kidney function loss. Urine oxalate is reasonably likely to predict clinical benefit, due to its causal role in stone formation and kidney damage in CKD stages 1–3a, and plasma oxalate is likely associated with risk of systemic oxalosis in CKD 3b–5. Change in slope of eGFR could be considered the equivalent of a clinically meaningful end point in support of traditional approval. A substantial change in urine oxalate as a surrogate end point could support traditional approval in patients with primary hyperoxaluria type 1 and CKD stages 1–3a. A substantial change in markedly elevated plasma oxalate could support accelerated approval in patients with primary hyperoxaluria and CKD stages 3b–5. Primary hyperoxaluria type 1 accounts for the preponderance of available data, thus heavily influences the conclusions. Addressing gaps in data will further facilitate testing of promising new treatments, accelerating improved outcomes for patients with primary hyperoxaluria.
Keywords: oxalate, kidney stones, clinical trial end points, rare kidney disease, primary hyperoxaluria type 1, hyperoxaluria, primary, liver transplantation, renal dialysis, hyperoxaluria, kidney calculi, kidney, renal insufficiency, registries, biomarkers, renal insufficiency, chronic
Introduction
Primary hyperoxaluria is a rare disease, with <1000 individuals affected in the United States. There are three types of primary hyperoxaluria that differ in their severity and genetic cause. Each of the three known primary hyperoxaluria types is caused by a defect in a gene that governs the production of a different hepatic enzyme, and each result in the overproduction of oxalate by the liver. Because humans have no enzyme capable of degrading oxalate, it must be eliminated primarily by the kidneys, with a small amount by the gastrointestinal tract. Hyperoxaluria results in calcium oxalate kidney stones, progressive oxalate nephropathy, and kidney failure. To address the pressing need to evaluate new treatments for primary hyperoxaluria, the Kidney Health Initiative (KHI) (1), in partnership with the Oxalosis and Hyperoxaluria Foundation (OHF), initiated a project to identify end points that could be used in clinical trials. Published literature was examined to identify candidate end points. Workgroup members with expertise in oxalate biology and pathophysiology, clinical nephrology and urology, and drug development provided a critical analysis of each candidate. Patients with primary hyperoxaluria, their family members, and leadership of the OHF patient advocacy organization provided input throughout. This article discusses the pathophysiology of primary hyperoxaluria, current treatment strategies, approval pathways and end points in the United States, and the workgroup’s assessment of potential end points that could be used to evaluate the efficacy of new drugs for the treatment of primary hyperoxaluria. Because primary hyperoxaluria type 1 accounts for 70%–80% of all diagnosed patients with primary hyperoxaluria to date and appears to have the most severe clinical phenotype (2,3), knowledge is most extensive for this primary hyperoxaluria type. Thus, data relevant to primary hyperoxaluria type 1 influenced recommendations in this article more than other primary hyperoxaluria types.
Pathophysiology of Primary Hyperoxaluria
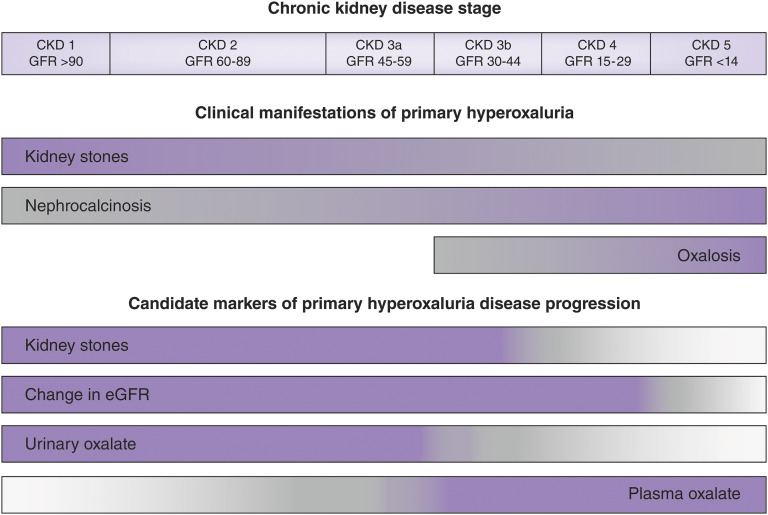
Primary hyperoxaluria is caused by deficiencies in enzymes involved in the metabolism of glycolate, glycine, and hydroxyproline within the liver. The resulting excess of glyoxylate leads to the synthesis and release of oxalate into the bloodstream. In the early stages of the disease, the increase in plasma oxalate is mitigated and compensated for by increased kidney excretion. Oxalate in the urine combines with calcium to form stones, as well as crystals that cause acute and chronic tubular injury, induce inflammation and fibrosis in the kidney parenchyma, and lead to loss of kidney function. Patients with primary hyperoxaluria typically experience kidney stones at a young age, have multiple recurrent stones, and often develop nephrocalcinosis, progressive oxalate nephropathy, and kidney failure. Because oxalate is primarily eliminated by the kidneys, advanced CKD results in high plasma oxalate concentrations which exceed the saturation threshold for calcium oxalate. Calcium oxalate crystals can then deposit in skin, retina, heart, vessels, bones, and other organ systems (oxalosis), leading to severe morbidity that may eventually cause death (3,4). In patients with primary hyperoxaluria who have kidney failure, dialysis cannot remove enough oxalate to prevent progressive systemic oxalosis, and kidney transplantation alone often fails due to recurrent oxalate injury in the allograft (3). Figure 1 illustrates clinical manifestations observed in primary hyperoxaluria by kidney disease stage (upper bars), shaded from high (blue) to low (gray) relative frequency. Of the three types of primary hyperoxaluria, primary hyperoxaluria type 1 is the most severe form with more frequent nephrocalcinosis and kidney failure at earlier ages (2,3,5). As a population, patients with primary hyperoxaluria type 1 have higher urine oxalate than those with primary hyperoxaluria type 2, followed by those with primary hyperoxaluria type 3 (2), and there is a wide variation among patients within each primary hyperoxaluria type and overlap between groups. However, the mechanisms by which oxalate causes disease are believed to be the same in all types.
Figure 1.
Clinical manifestations observed in primary hyperoxaluria and candidate markers of progression vary by kidney disease stage. The consequences of hepatic oxalate overproduction (upper bars) are shaded to reflect high (purple) to low (gray) relative frequency, as best is known at this time. The clinical and biochemical markers (lower bars) are shaded to indicate those supported by clinical and experimental evidence (purple), while those with insufficient data and need for further research are shaded lighter (gray). Figure modified from the original provided courtesy of S. Hulton.
Available Therapies and Unmet Need
Current strategies to prevent stones and kidney damage target reduction of calcium oxalate crystal formation in the urinary tract through high urine volume and medications such as citrate and magnesium. Pyridoxine is a cofactor for the alanine-glyoxylate aminotransferase (AGT) enzyme that is deficient in primary hyperoxaluria type 1. In certain primary hyperoxaluria type 1 genotypes characterized by mislocalization of AGT within hepatocytes, pharmacologic doses of pyridoxine increase enzyme activity and thus reduce oxalate production (3,4,6). Large daily fluid intake (e.g., 3.5–4 L/day in adults, with fluid prescription in children proportionate to body size) and a large pill burden compromise quality of life. Further, although these treatments may moderate the effects of hyperoxaluria, in many patients they do not prevent stone formation nor do they eliminate kidney failure in primary hyperoxaluria types 1 and 2 (3,7). The burden of frequent symptomatic kidney stones with associated hospitalizations and stone removal procedures, intensive dialysis if kidney failure ensues, systemic oxalate deposition, and transplantation are enormous. The companion paper to this article, written by patients with primary hyperoxaluria and their family members, provides insights into the experience of living with this disease (8). At this time, there are no FDA-approved pharmacologic therapies for primary hyperoxaluria. Liver transplantation can correct the metabolic deficiency in patients with primary hyperoxaluria types 1 and 2; this is an effective but extreme intervention, given that liver function is otherwise normal, and one that carries significant risks (9–11). Therefore, new treatments are urgently needed.
Surrogate End Points and Approval Pathways in the United States
In the United States, the efficacy of a product (drug or biologic) in treating, mitigating, or preventing a disease can be established by demonstrating an effect on a clinical outcome, which reflects how a patient feels, functions, or survives. Alternatively, product approval or licensure may be based on a surrogate end point. Surrogate end points, which are often biomarkers such as a laboratory measurement, radiographic image, or physical sign, are not direct measures of clinical benefit, but instead are expected to predict the effect of a product on the clinical benefit of interest. Surrogate end points can be categorized as validated, reasonably likely, or candidate, based on the strength of evidence supporting their use. A validated surrogate end point is supported by strong scientific evidence that a treatment effect on the surrogate end point predicts the treatment effect on the clinical outcome of primary interest. A reasonably likely surrogate end point is also supported by strong scientific evidence, but lacks sufficient evidence to serve as a validated surrogate end point. Reasonably likely surrogate end points can be used as a basis for accelerated approval of products intended to treat a serious or life-threatening condition and that provide a meaningful advantage over available therapies. For products granted accelerated approval, adequate and well controlled postmarketing confirmatory studies have generally been required to verify and describe the anticipated clinical benefit (12–14).
End Points for Clinical Trials in Primary Hyperoxaluria
In our evaluation of potential end points, the workgroup considered both clinical outcomes and surrogate end points. Factors considered when assessing a clinical end point included its importance to patients and whether it would be feasible to assess a treatment effect on the end point in a trial of reasonable duration. Factors considered when assessing a surrogate end point were (1) the biologic plausibility of the relationship between the surrogate end point and clinical outcome of interest, including the extent to which the causal pathway of the disease is well understood and the relationship of the surrogate to this pathway; (2) the strength and consistency of the epidemiologic data supporting the relationship; and (3) whether treatment effects on the surrogate end point have been shown to predict treatment effects on the clinical outcome of interest using different types of interventions.
Although kidney failure is a meaningful end point, it is also one that is difficult to use in primary hyperoxaluria clinical trials because of the small size of the affected population and the long and variable time course for disease progression. Of the end points identified by the workgroup (Table 1), kidney stones, rate of change in GFR, urine oxalate, and plasma oxalate were identified as the strongest end points for clinical trials (Figure 1). In the sections that follow, each of these end points is discussed.
Table 1.
Potential end points for clinical trials in primary hyperoxaluria
| End Point | Pros | Cons |
|---|---|---|
| Clinical end points | ||
| Stone events | Affects how most patients with primary hyperoxaluria feel and function | Standards for measurement and longitudinal monitoring lacking |
| Accurate methods of measurement that do not rely on ionizing radiation are needed | ||
| Reliability in CKD stages 3b–5 unknown | ||
| Kidney failure | Important to patients, easy to measure | May not have many events in a trial, particularly if trial is conducted in the available number of patients with a reasonable length of follow-up |
| Oxalosis | Important to patients, severe morbidity | May not have many events in a trial, particularly if trial is conducted in the available number of patients with a reasonable length of follow-up |
| Surrogate end points | ||
| Worsening kidney function (GFR slope) | Accepted as surrogate end point for other conditions | May not detect change in patients with slow progression |
| Easy to measure | ||
| Rapid decline would be highly supportive of clinically meaningful benefit | ||
| Urine oxalate | Causal role in stone formation and kidney damage in CKD 1–3a and relationship between baseline urine oxalate and kidney failure | Magnitude of change needed to predict clinical benefit across primary hyperoxaluria types warrants further study |
| Available data support use of substantial change as surrogate end point for traditional approval in patients with primary hyperoxaluria type 1 with CKD 1–3a | May not be useful in CKD 3b–5 due to reduced kidney function to excrete oxalate | |
| Lesser treatment effects or effects in patients with primary hyperoxaluria without very high baseline levels reasonably likely to predict clinical benefit | ||
| Plasma oxalate | Reasonably likely to predict clinical benefit due to causal role in systemic oxalosis in CKD stages 3b–5 | Magnitude of change in plasma oxalate in CKD stages 3b–5 likely to predict clinical benefit requires further study |
| Causal role in stone formation and kidney damage (CKD stages 1–3a) | Value in CKD 1–3a for prediction of clinical outcome remains to be established | |
| Limited availability of laboratory measurement. Results vary by method | ||
Data supporting these conclusions are heavily influenced by primary hyperoxaluria type 1, which accounts for the majority of patients with primary hyperoxaluria. Small numbers of patients with primary hyperoxaluria type 2 and primary hyperoxaluria type 3 in primary hyperoxaluria registries do not yet provide a similar strength of evidence for these biomarkers in other forms of primary hyperoxaluria.
Kidney Stones as a Clinical End Point
Stone burden is of particular interest in primary hyperoxaluria because stones are caused by high urine oxalate and often lead to pain, hospitalizations, and stone removal procedures. Although kidney stone disease directly affects the lives of patients with primary hyperoxaluria, work remains to develop reliable and safe methods for the assessment and quantification of stone events, and to more fully define the natural history of stone burden at all CKD stages. Assessment of stone number and size is influenced by the type of imaging modality used. Computerized tomography is recognized as the gold standard for stone detection (15,16), but longitudinal studies require technique standardization and entail recurring radiation exposure. Ultrasonography for stone measurement yields varying results depending upon the specific equipment used and sonographer technique (17). Longitudinal data documenting changes in kidney stone number or size regardless of technology have not been reported in a primary hyperoxaluria cohort. Kidney stones are often asymptomatic for long periods of time; thus, variability of stone symptoms must be considered when used as an end point for clinical trials. Further, neither definitions of symptomatic stone events nor standards for radiographic assessment of stone burden are commonly agreed upon. The effects of reduced GFR on stone burden are difficult to assess and have not been systematically described, and reduced calcium and oxalate excretion in advanced CKD could reduce stone activity. In sum, although stone burden is clinically meaningful, at the present time there are challenges related to its use as an end point in primary hyperoxaluria. As the natural history of stone disease in primary hyperoxaluria becomes informed by more robust data, its role as a feasible clinical end point should be reconsidered.
Slope of the Glomerular Filtration Rate as a Surrogate End Point
For rare causes of CKD, the efficacy of products intended to reduce the risk of progression to kidney failure may be established by showing a treatment effect on the rate of decline in kidney function (GFR slope) or a sustained 30% decline in GFR (12,18). In primary hyperoxaluria, loss of kidney function can begin in infancy and early childhood. Among those with the most severe form, primary hyperoxaluria type 1, 50% will progress to kidney failure by 33 years of age, and nearly all by 60 years of age (2). In most patients, kidney function is not typically lost at a rapid rate. In a study by Tang et al. (7), the rate of loss of kidney function in a mixed group of patients with primary hyperoxaluria type 1, primary hyperoxaluria type 2, and primary hyperoxaluria type 3 ranged from −0.4 ml/min per 1.73 m2 per year in those with no or prevalent nephrocalcinosis to −1.2 ml/min per 1.73 m2 per year in those with incident nephrocalcinosis. A small study (19) reported a change of −1.7 ml/min per 1.73 m2 per year in primary hyperoxaluria type 1 and −1.04 ml/min per 1.73 m2 per year in primary hyperoxaluria type 2. Fargue et al. (20) reported a median decrease of −1.0 ml/min per 1.73 m2 per year in 19 children with primary hyperoxaluria type 1 who were >2 years of age at diagnosis. Clinical experience suggests higher rates of GFR decline in those patients with primary hyperoxaluria who have more advanced CKD, particularly CKD stages 3b–4. Further, rapid declines in kidney function leading to kidney failure over weeks to months occasionally occur in patients with primary hyperoxaluria, sometimes precipitated by acute events such as dehydration or an obstructive kidney stone (3,21).
Additional research is needed to better define populations at greatest risk for progression and delineate the course of disease in children and adults (Table 2). Given the limited ability to identify patients at high risk of experiencing a significant loss of kidney function over the course of a trial and the size of the affected population, it is unclear whether GFR-based end points are, at present, feasible trial end points for primary hyperoxaluria.
Table 2.
Gaps in data requiring additional research
| End Point | CKD Population (Stage) | Gaps in Data |
|---|---|---|
| Kidney stones | 1–3b | Standards for definition of clinical stone event do not exist |
| Standards for radiographic assessment of stone burden are not available | ||
| Quantitative methods for stone assessments which avoid ionizing radiation not available | ||
| 4–5a | No reported studies | |
| Slope of GFR | 1–4 | Changes in rate of decline by CKD stage are not well defined |
| Patient-patient variability is not well understood | ||
| Discontinuity in eGFR formula performance as children transition to adulthood (52) | ||
| Urine oxalate excretion | 1–3a | Correlation between spot urine oxalate/creatinine and 24-h urine oxalate excretion not established |
| Confirmation of the relationship of urine oxalate excretion and incident ESKD in a validation cohort | ||
| Relationship of urine oxalate and plasma oxalate over the range of GFR not well described | ||
| Interplay between urine oxalate and plasma oxalate and the development of intrarenal calcium oxalate crystals and kidney damage are poorly understood | ||
| Quantitative effect of reduction of urine oxalate on GFR and stone burden is not known | ||
| 3b–5a | Effect of low GFR on urine oxalate has not been described; urine oxalate expected to decrease at low GFR due to declining kidney function. | |
| Plasma oxalate concentration | 1–3aa | Not widely used in clinical practice; minimal data available |
| Biologic variation not characterized | ||
| Association with later irreversible morbidity and mortality not studied | ||
| 3b–5 | Biologic variation not well characterized | |
| Standards for sample collection, processing, and measurement are lacking | ||
| Interpretation requires correction for GFR due to kidney clearance | ||
| Dynamic equilibrium between plasma oxalate and tissue oxalate stores is poorly understood | ||
| Predictive value of elevated plasma oxalate on clinical end points including risk for ESKD not defined | ||
| Magnitude of decrease in plasma oxalate needed to achieve a meaningful clinical benefit not known | ||
| Quantitative effect of plasma oxalate on systemic oxalosis is not known |
Evidence supporting the association of potential end points with disease progression differs by CKD stage. Urine oxalate is a more useful biomarker when kidney function is preserved but is expected to decrease as GFR falls to low levels. Plasma oxalate becomes the biomarker of choice when the failing kidney is no longer able to adequately excrete oxalate into the urine (CDK stages 3b–5). Additional study is needed to determine whether plasma or urine oxalate performs better as a biomarker in CKD stage 3.
Minimal data available.
Urine Oxalate as a Surrogate End Point
Urine Oxalate as a Causal Factor in Stone Formation and Loss of Kidney Function.
Persistently elevated urine oxalate levels cause kidney stone formation and also contribute to progressive kidney damage and loss of kidney function (2,3,7,21–23). In all primary hyperoxaluria types, the urine becomes supersaturated, resulting in formation of calcium oxalate crystals that aggregate within the tubular lumen. The crystals can attach to the surface of kidney tubular cells and become internalized, they then migrate into the kidney interstitium (nephrocalcinosis) where they induce inflammation, ultimately resulting in progressive damage and CKD (3). In animal models of crystal nephropathy, prolonged activation of the intrarenal inflammasome by calcium oxalate crystals appears to be one mechanism that promotes the loss of kidney function (24,25). Nephrocalcinosis is also associated with risk of kidney failure in patients with primary hyperoxaluria (7,26,27).
In all types of primary hyperoxaluria, calcium and oxalate can also crystallize upon an anchored nidus in the kidney papillum to form a stone (urolithiasis). Although higher urine oxalate correlated with a greater number of stones on radiographic images in one study (7), the relationship of urine oxalate to clinical stone events or stone burden has not been otherwise reported. Obstructing stones or stone removal procedures can contribute to the loss of kidney function in individual patients. However, in contrast to nephrocalcinosis, the number of stones and symptomatic stone events do not appear associated with kidney survival in primary hyperoxaluria at a population level (7).
Epidemiologic Data.
Epidemiologic data on the relationship between urine oxalate and loss of kidney function is derived from primary hyperoxaluria registries, observations in secondary causes of hyperoxaluria, and in a large cohort of patients with CKD with other forms of kidney disease. Analyses of primary hyperoxaluria registry data show a strong relationship between urine oxalate and loss of kidney function (21). Primary hyperoxaluria type 1 accounts for a majority of subjects. Data in primary hyperoxaluria type 2 and primary hyperoxaluria type 3 are more limited but show urine oxalate, risk of nephrocalcinosis, and risk of kidney failure to be less in primary hyperoxaluria type 2 compared with primary hyperoxaluria type 1, and even lower in primary hyperoxaluria type 3 (2,3,5,7). Nonetheless, an analysis of the OxalEurope primary hyperoxaluria type 2 registry data clearly indicates that this patient population has significant disease burden and morbidity (5). These observations suggest that more severe disease expression in primary hyperoxaluria may be related to higher urine oxalate excretion rates.
Analysis of data from the Rare Kidney Stone Consortium (RKSC) primary hyperoxaluria registry of 297 patients with all types of primary hyperoxaluria (65% primary hyperoxaluria type 1) demonstrated that kidney outcomes correlated with baseline urine oxalate excretion stratified by quartile, with a kidney failure hazard ratio for quartile 4 (Q4) versus Q1–Q3 of 3.4 (95% CI, 1.4 to 7.9). The 20-year kidney survival was 96% for patients whose oxalate excretion rate at primary hyperoxaluria diagnosis was <1.11 mmol/1.73 m2 per 24 hours, in contrast to 42% for those with excretions ≥2.45 mmol/1.73 m2 per 24 hours (21). When urine oxalate excretion rates over time were analyzed as a continuous time-dependent covariate, the risk of kidney failure was greater with increasing urine oxalate levels, yielding a hazard ratio of 1.8 (95% CI, 1.2 to 2.5) per 1 mmol/1.73 m2 per 24-hour increase. In a separate primary hyperoxaluria registry analysis (7), urine oxalate excretion rates in patients with primary hyperoxaluria were higher among those with nephrocalcinosis (2.0 mmol/24 hours) versus those without nephrocalcinosis (1.3 mmol/24 hours), P<0.01, again demonstrating a relationship between urine oxalate and kidney damage. In a large population of patients who did not have primary hyperoxaluria with baseline CKD stages 2–4, higher 24-hour urinary oxalate excretion was an independent risk factor for CKD progression and kidney failure (23). In a review of 108 patients with secondary forms of hyperoxaluria, 55% required dialysis due to biopsy-proven oxalate nephropathy (28).
Importantly, although it is difficult to draw definitive conclusions from these cross-sectional analyses, the data support the principle that those patients with the highest levels of urine oxalate are at greatest risk for primary hyperoxaluria disease progression, an important consideration for use of urine oxalate as a surrogate end point. The workgroup acknowledges that this conclusion is driven by primary hyperoxaluria type 1 data, which represent the majority of primary hyperoxaluria cases, and that direct evidence of the relationship between urine oxalate and clinical outcome for primary hyperoxaluria type 2 and primary hyperoxaluria type 3 is not yet available.
Treatment Effects.
Currently, the only treatment directly targeting urine oxalate reduction is pyridoxine, which appears to increase the enzyme activity of a subset of mutant AGT forms found in primary hyperoxaluria type 1 (29,30). Approximately 30% of patients with primary hyperoxaluria type 1 show some degree of responsiveness (31,32). A retrospective review demonstrated mean urine oxalate reduction on pyridoxine of 73% from a baseline of 1.5 mmol/1.73 m2 per day among six patients with primary hyperoxaluria type 1 who were homozygous for the most common pyridoxine-responsive AGT mutation (G170R); four achieved normal urine oxalate excretion (33). In the same study, eight patients who were heterozygous achieved a 45% reduction from a baseline of 2.2 mmol/1.73 m2 per day. Pyridoxine effect was sustained during 6.5 and 8.4 years of follow-up, respectively. Primary hyperoxaluria registry studies confirm that patients with primary hyperoxaluria type 1 who have the G170R mutation have better preservation of kidney function than those patients without this mutation (2,34). Similarly, in four of five patients with late diagnosis of primary hyperoxaluria but with urine oxalate excretions normal or near normal after initiation of pyridoxine (<0.5 mmol/1.73 m2 per day), stable kidney function was maintained during a median of 8.5 years after a kidney-alone transplant (35). The only graft loss occurred at 13.9 years post-transplant. Normalization of oxalate excretion and stabilization of kidney function has also been observed after pre-emptive liver transplantation, and nephrocalcinosis has resolved after liver-alone transplant in a few reported cases (36–38).
Recommendation for Urine Oxalate.
Available data support a substantial change in urine oxalate (e.g., near normalization) as a surrogate end point and basis for traditional approval for the treatment of primary hyperoxaluria type 1 in patients with CKD 1–3a. Lesser treatment effects or effects in patients without very high baseline levels could also translate into clinical benefit and possibly serve as a reasonably likely surrogate and basis for accelerated approval, but the magnitude of reduction needed is unclear at this time.
Urine oxalate appears to be the preferred end point in CKD 1–3a because urine oxalate is a causal factor in kidney stones and nephrocalcinosis in these patients with higher GFR. Changes in urine oxalate over the disease course have not been well described in patients who have progressed to CKD stages 3b–5. Urine oxalate is expected to decrease as GFR falls to very low levels (GFR<15 ml/min per 1.73 m2); ultimately excretion will cease with oligoanuria. The effects of very low levels of kidney function on stone burden and nephrocalcinosis have not been systematically described. Timed 24-hour urine collections are needed for the most accurate measurement of urine oxalate excretion rate but are challenging, especially in children. Additional work to quantitate the relationship between spot urine oxalate/urine creatinine measurements (39) or shorter duration collection with 24-hour urine oxalate excretion is needed. In pediatric patients, urine oxalate measurements must account for changes in reference ranges due to maturation of kidney function and growth throughout infancy and childhood.
Plasma Oxalate as a Surrogate End Point
Plasma Oxalate as a Causal Factor in Loss of Kidney Function and Systemic Oxalosis.
Plasma oxalate directly reflects hepatic synthesis of oxalate. When kidney function (GFR) is normal, the overproduction of oxalate is balanced by kidney excretion. Thus, plasma oxalate remains within the normal range, or mildly elevated, at the expense of markedly increased urine oxalate (40). As GFR declines, plasma oxalate increases due to dependence on the kidney for elimination. When plasma oxalate exceeds 35–50 µmol/L, physicochemical properties favor crystallization, leading to systemic calcium oxalate crystal deposition (41,42). Crystal injury to tissue (43) can cause bone fractures, impaired erythropoiesis, infiltrative cardiomyopathy, arrhythmias, peripheral neuropathy, retinopathy, and ischemic ulcers (4,44). Thus, in CKD stages 3b–5 (GFR<45 ml/min per 1.73 m2), elevated plasma oxalate is directly related to the pathophysiology of oxalosis. In contrast, although oxalate overproduction is a key causal factor in loss of kidney function, plasma oxalate in isolation may not track well with the loss of kidney function at higher eGFR levels (>45 ml/min per 1.73 m2) because of the ability of the kidney to excrete the excess load.
Epidemiologic Data.
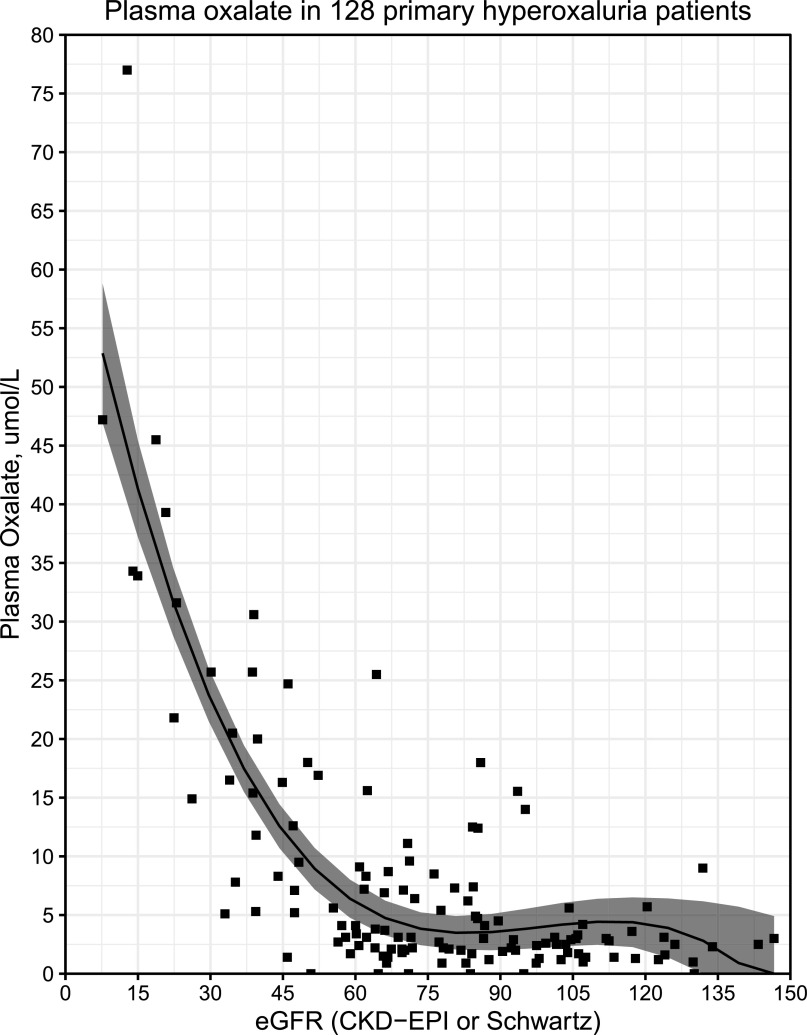
In patients with primary hyperoxaluria, plasma oxalate increases from normal/modestly increased (2–10 µmol/L; normal is 1–3 µmol/L with most assays) when GFR is well preserved (>60 ml/min per 1.73 m2) to markedly increased (>90–100 µmol/L) in patients with CKD stage 5 (3,4,40) (Figure 2). Plasma oxalate concentrations that exceed the supersaturation threshold for calcium oxalate are typically observed in patients with primary hyperoxaluria with GFR ≤30–40 ml/min per 1.73 m2 (CKD 3b–5) (40,45). Only patients with primary hyperoxaluria who have advanced CKD and markedly increased plasma oxalate experience clinically overt systemic deposition of calcium oxalate in multiple body tissues, resulting in severe disease and ultimately death (3,4,45). Although the risk and manifestations of oxalosis are most severe in primary hyperoxaluria, lesser elevations in plasma oxalate in patients with kidney failure from any cause can be associated with a mild form of systemic calcium oxalate deposition (46,47). In patients with primary hyperoxaluria who have preserved GFR (CKD 1–3a), the role of plasma oxalate in disease progression has not been studied. This may, in part, be related to the small number of laboratories performing plasma oxalate measurements, differences in measurement methods, and infrequent measurement of plasma oxalate in earlier stages of CKD.
Figure 2.
Plasma oxalate increases exponentially as eGFR declines below 60 ml/min per 1.73 m2. Plasma oxalate values and eGFR taken from the latest visit of 128 patients with primary hyperoxaluria (PH) who were >2 years of age (PH type 1, n=96; PH2, n=14; PH3, n=18) were plotted. Plasma oxalate values are relatively stable in the eGFR range of >60 ml/min per 1.73 m2, and then they increase exponentially as eGFR declines. Plasma oxalate concentration was measured by ion chromatography. The CKD Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR in adults, and the modified Schwartz equation at <18 years of age. The model was fit using a third degree polynomial regression (plotted as a black line). Both quadratic and cubic terms were statistically significant by the likelihood ratio test (P<0.001 for both). The 95% confidence interval of the regression model is indicated by the shaded region. Data shown is from the Rare Kidney Stone Consortium Primary Hyperoxaluria Registry.
Treatment Effects.
Evidence that reduction in plasma oxalate reduces the risk or severity of subsequent systemic oxalosis is limited to anecdotal experience with intensive dialysis regimens and the resolution of disease manifestations after transplantation. Plasma oxalate decreases rapidly after liver transplantation (48), with gradual resolution of oxalosis reported (37). Studies of treatment response to plasma oxalate reduction in patients with primary hyperoxaluria who have CKD stages 1–3a are lacking.
Recommendation for Plasma Oxalate.
Available data support the use of a substantial change in plasma oxalate in patients with CKD stages 3b–5 and markedly elevated plasma oxalate levels as a reasonably likely surrogate for a treatment’s effect on systemic manifestations of the disease. Among patients with CKD 1–3a, the major utility for plasma oxalate may be as a readily obtainable biomarker that reflects the net effect of oxalate burden and urinary oxalate excretion (49). Limited data are available on the magnitude of change in plasma oxalate required to confer clinical benefit. Further, in advanced systemic oxalosis, the exchange of oxalate between tissue stores and plasma is poorly understood (41,42,47). Additional research is needed to address these gaps in the data.
Other Markers Considered as Potential Surrogate End Points
Nephrocalcinosis, urine calcium oxalate supersaturation (50), and primary hyperoxaluria metabolites (glycolate, glycerate, dihydroxyglutarate, and 4-hydroxy-2-oxoglutarate) (51) were also considered as potential surrogate end points. Progression of nephrocalcinosis and its relationship with kidney function has not been studied. Further, the absence of quantitative methods for nephrocalcinosis measurement is a current barrier for its use as a marker of disease progression. Measurements of primary hyperoxaluria metabolites and calculation of calcium oxalate supersaturation are currently performed by only a few laboratories and not widely used in clinical practice. Thus, there have been no studies in primary hyperoxaluria cohorts that demonstrate a correlation of these parameters with stone burden or with progression of disease.
Among patients with primary hyperoxaluria who have CKD stages 3b–5, severity of systemic oxalosis was considered. New imaging techniques show promise for assessment of systemic calcium oxalate burden (e.g., retinal imaging with spectral domain optical coherence tomography, speckle tracking echocardiography, cardiac elastography, and quantification of calcium oxalate deposits in bone through computed tomography or magnetic resonance imaging). There is limited experience in the use of these imaging techniques in patients with primary hyperoxaluria, and no systematic studies to define their usefulness in disease progression. Thus, in the absence of standardized and reproducible measures of severity, assessment of oxalosis requires additional research.
Conclusion
To address the pressing need to evaluate new treatments for primary hyperoxaluria, KHI, in partnership with OHF, initiated a project to identify end points that could be used in clinical trials. The workgroup, which included experts in primary hyperoxaluria, patients living with primary hyperoxaluria and their families, industry, and United States regulators, considered potential clinical outcomes, as well as possible surrogate end points that could be used to establish the efficacy of new treatments. The strengths and limitations of the end points considered by the group are summarized in Table 1, and important gaps in the data are discussed in Table 2. Available evidence supports urine oxalate in CKD stages 1–3a and plasma oxalate in CKD stages 3b–5 as end points for clinical trials in primary hyperoxaluria.
We are at a tipping point in the development of new treatments for patients with primary hyperoxaluria, made possible by decades of dedicated patient participation in registries and clinical studies, the OHF in their support of primary hyperoxaluria patient and research communities, basic and clinical investigators, and the more recent investment of the pharmaceutical industry. Although work remains, patients with primary hyperoxaluria and their physicians can face the future with optimism.
Disclosures
Dr. Lieske is currently employed by Mayo Clinic. Dr. Lieske reports receiving grants from Allena, Alnylam, Dicerna, OxThera, Retrophin, and Siemens; receiving honoraria from Alnylam, American Board of Internal Medicine (ABIM), Dicerna, Orfan, OxThera, and Retrophin; serving as a consultant for ABIM, Allena, Alnylam, Dicerna, Orfan, OxThera, Retrophin, and Siemens; and serving as a scientific advisor or member of ABIM, Kidney International, and the Oxalosis and Hyperoxaluria Foundation; all outside of the submitted work. Dr. Milliner is currently employed by Mayo Clinic. Dr. Milliner reports receiving grants from Allena, Alnylam, Dicerna, and OxThera; receiving honoraria from Alnylam; serving as a consultant for Allena, Alnylam, Dicerna, and OxThera; serving in an advisory committee for Alnylam, a monitoring committee for a clinical trial conducted by Dicerna, on a data safety monitoring board for a clinical trial conducted by OxThera, and on the editorial board for Urolithiasis, all outside of the submitted work. Dr. Milliner also has ongoing work with OHF outside of the submitted work. Ms. Allain and Ms. West are currently employed by the American Society of Nephrology. Dr. Blank, Dr. Thompson, and Dr. Yang are currently employed by the FDA. Dr. Dehmel is currently employed by and reports ownership interest in OxThera outside of the submitted work. Dr. Fargue serves on the scientific advisory council for OHF and is currently employed by the University of Alabama, Birmingham, outside of the submitted work. Dr. Groothoff is currently employed by the Academic Medical Center, Amsterdam, and reports serving as a consultant for Alnylam and Dicerna, outside of the submitted work. Dr. Groothoof is supported by presentation fees from Alnylam and grants from Alnylam, Dicerna, and UniQure. Ms. Hollander is currently employed by the OHF. Ms. Hollander reports receiving grants on behalf of the OHF from Allena, Alnylam, Bridge Biopharma, Captozyme, Dicerna, EveryLife Foundation, Intella Therapeutics, Invitae Corporation, Novome Biotechnologies, Orfan, and OxThera; personal fees from Allena, Alnylam, and Dicerna; and nonfinancial support from Allena, Alnylam, Dicerna, KHI, and OxThera; all outside of the submitted work. Dr. Knight is currently employed by the University of Alabama, Birmingham. Dr. Knight reports receiving grants from Alnylam, Chinook Therapeutics, Intella Therapeutics, and Synlogic Operating Company, Inc.; receiving personal fees from Chinook Therapeutics; and has a pending patent for treating primary or idiopathic hyperoxaluria with small molecule inhibitors of lactate dehydrogenase. Dr. Lowther is currently employed by Wake Forest School of Medicine. Dr. Lowther reports receiving grants from Chinook Therapeutics and Paredox Therapeutics, having ownership interest in Chinook Therapeutics, and serving on the editorial board of the Journal of Biological Chemistry and the scientific advisory boards for Chinook Therapeutics and the Oxalosis and Hyperoxaluria Foundation, all outside of the submitted work. Dr. McGregor is currently employed by, has received travel support from, and has ownership interest in Alnylam Pharmaceuticals outside of the submitted work. Dr. Rosskamp is currently employed by and has ownership interest in Dicerna outside of the submitted work. Dr. Rumsby serves as a consultant for OxThera outside of the submitted work.
Funding
This work was supported by KHI, which is partially funded by a US FDA grant 2R18FD005283-06 and ASN. This work was also supported by the OHF and the Rare Kidney Stone Consortium of the National Institute of Diabetes and Digestive and Kidney Diseases (U54DK083908), which is a part of the Rare Diseases Clinical Research Network of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences of the National Institutes of Health.
Acknowledgments
In February 2018, the OHF conducted a workshop for members of the hyperoxaluria community, including patients and care partners, scientists, and clinicians. Clarity regarding end points for clinical trials of new agents for primary hyperoxaluria was identified as a high priority. Participants and other volunteers from the primary hyperoxaluria community, selected by the OHF and KHI as members of the KHI Workgroup, collated literature for each potential end point and presented the findings. The workgroup then deliberated and worked to a consensus on the feasibility and utility of each end point.
We would like to acknowledge workgroup members and participants of the RKSC and OxalEurope, including Kyle Wood, Barbara Cellini, Felicity Enders, Lisa Vaughan, Amanda Gentile, Elisabeth Lindner, Dayna LeSueur, Anna Twigg, Frits van Alphen, and Yaacov Frishberg for their support over the duration of this project. We would also like to acknowledge Kim Hollander and Julie Bertarelli from the OHF (www.ohf.org) for their initiation of this project and continued support along with the patients with primary hyperoxaluria and their families who motivated this work.
This work was supported by the KHI, a public-private partnership between the American Society of Nephrology (ASN), the US FDA, and >100 member organizations and companies to enhance patient safety and foster innovation in kidney disease. KHI funds were used to defray costs incurred during the conduct of the project, including project management support that was expertly provided by ASN staff members, M.A. and M.W. There was no honorarium or other financial support provided to KHI workgroup members.
The authors of this paper had final review authority and are fully responsible for its content. KHI makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the members of the workgroup. More information on KHI, the workgroup, or the conflict of interest policy can be found at www.kidneyhealthinitiative.org.
The views and opinions expressed in this publication are those of the authors and do not necessarily reflect the official policies of any KHI member organization, the US Department of Veterans Affairs, or the US Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ, Roy-Chaudhury P: Fostering innovation, advancing patient safety: the kidney health initiative. Clin J Am Soc Nephrol 8: 1609–1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC; Rare Kidney Stone Consortium: Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol 26: 2559–2570, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochat P, Rumsby G: Primary hyperoxaluria. N Engl J Med 369: 649–658, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Hoppe B, Beck BB, Milliner DS: The primary hyperoxalurias. Kidney Int 75: 1264–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrelfs SF, Rumsby G, Peters-Sengers H, Erger F, Groothoff J, Beck B, Oosterveld MJS, Pelle A, Neuhaus T, Adams B, Cochat P, Salido E, Lipkin GW, Hoppe B, Hulton SA; OxalEurope Consortium: Patients with Primary Hyperoxaluria type 2 have significant morbidity and require careful follow-up. Kidney Int 96: 1389–1399, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Fargue S, Lewin J, Rumsby G, Danpure CJ: Four of the most common mutations in primary hyperoxaluria type 1 unmask the cryptic mitochondrial targeting sequence of alanine:glyoxylate aminotransferase encoded by the polymorphic minor allele. J Biol Chem 288: 2475–2484, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Bergstralh EJ, Mehta RA, Vrtiska TJ, Milliner DS, Lieske JC: Nephrocalcinosis is a risk factor for kidney failure in primary hyperoxaluria. Kidney Int 87: 623–631, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence J, Wattenberg DJ: Primary hyperoxaluria: The patient and caregiver perspective. Clin J Am Soc Nephrol 15: XXX–XXX, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR: Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am J Transplant 10: 1420–1427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawal N, Yazigi N: Pediatric liver transplantation. Pediatr Clin North Am 64: 677–684, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Nieto R, Lykoudis P, Robertson F, Sharma D, Moore K, Malago M, Davidson BR: A simple scoring model for predicting early graft failure and postoperative mortality after liver transplantation. Ann Hepatol 18: 902–912, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Thompson A, Lawrence J, Stockbridge N: GFR decline as an end point in trials of CKD: A viewpoint from the FDA. Am J Kidney Dis 64: 836–837, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Thompson A, Carroll K, A Inker L, Floege J, Perkovic V, Boyer-Suavet S, W Major R, I Schimpf J, Barratt J, Cattran DC, S Gillespie B, Kausz A, W Mercer A, Reich HN, H Rovin B, West M, Nachman PH: Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 14: 469–481, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services: Guidance for Industry: Expedited Programs for Serious Conditions—Drugs and Biologics, Silver Spring, MD, Food and Drug Administration, 2014 [Google Scholar]
- 15.Fowler KA, Locken JA, Duchesne JH, Williamson MR: US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 222: 109–113, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Fulgham PF, Assimos DG, Pearle MS, Preminger GM: Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol 189: 1203–1213, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Dunmire B, Lee FC, Hsi RS, Cunitz BW, Paun M, Bailey MR, Sorensen MD, Harper JD: Tools to improve the accuracy of kidney stone sizing with ultrasound. J Endourol 29: 147–152, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Milliner DS, Wilson DM, Smith LH: Phenotypic expression of primary hyperoxaluria: Comparative features of types I and II. Kidney Int 59: 31–36, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Fargue S, Harambat J, Gagnadoux MF, Tsimaratos M, Janssen F, Llanas B, Berthélémé JP, Boudailliez B, Champion G, Guyot C, Macher MA, Nivet H, Ranchin B, Salomon R, Taque S, Rolland MO, Cochat P: Effect of conservative treatment on the renal outcome of children with primary hyperoxaluria type 1. Kidney Int 76: 767–773, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Zhao F, Bergstralh EJ, Mehta RA, Vaughan LE, Olson JB, Seide BM, Meek AM, Cogal AG, Lieske JC, Milliner DS; Investigators of Rare Kidney Stone Consortium: Predictors of incident ESRD among patients with primary hyperoxaluria presenting prior to kidney failure. Clin J Am Soc Nephrol 11: 119–126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS, Srivastava A, Palsson R, Shafi T, Hsu CY, Sharma K, Lash JP, Chen J, He J, Lieske J, Xie D, Zhang X, Feldman HI, Curhan GC; Chronic Renal Insufficiency Cohort study investigators: Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern Med 179: 542–551, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS: NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama T, Nagata M, Ichiyama A, Ozono S: Primary hyperoxaluria type 1 in Japan. Am J Nephrol 25: 297–302, 2005 [DOI] [PubMed] [Google Scholar]
- 27.van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA: Primary hyperoxaluria type 1 in The Netherlands: Prevalence and outcome. Nephrol Dial Transplant 18: 273–279, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P: Secondary oxalate nephropathy: A systematic review. Kidney Int Rep 3: 1363–1372, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fargue S, Rumsby G, Danpure CJ: Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim Biophys Acta 1832: 1776–1783, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Cellini B, Montioli R, Oppici E, Astegno A, Voltattorni CB: The chaperone role of the pyridoxal 5′-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin Biochem 47: 158–165, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS; OxalEurope: Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27: 1729–1736, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Hoyer-Kuhn H, Kohbrok S, Volland R, Franklin J, Hero B, Beck BB, Hoppe B: Vitamin B6 in primary hyperoxaluria I: First prospective trial after 40 years of practice. Clin J Am Soc Nephrol 9: 468–477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monico CG, Rossetti S, Olson JB, Milliner DS: Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int 67: 1704–1709, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P: Genotype-phenotype correlation in primary hyperoxaluria type 1: The p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int 77: 443–449, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Lorenz EC, Lieske JC, Seide BM, Meek AM, Olson JB, Bergstralh EJ, Milliner DS: Sustained pyridoxine response in primary hyperoxaluria type 1 recipients of kidney alone transplant. Am J Transplant 14: 1433–1438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kemper MJ, Nolkemper D, Rogiers X, Timmermann K, Sturm E, Malago M, Broelsch CE, Burdelski M, Müller-Wiefel DE: Preemptive liver transplantation in primary hyperoxaluria type 1: Timing and preliminary results. J Nephrol 11[Suppl 1]: 46–48, 1998 [PubMed] [Google Scholar]
- 37.Galanti M, Contreras A: Excellent renal function and reversal of nephrocalcinosis 8 years after isolated liver transplantation in an infant with primary hyperoxaluria type 1. Pediatr Nephrol 25: 2359–2362, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Perera MT, Sharif K, Lloyd C, Foster K, Hulton SA, Mirza DF, McKiernan PJ: Pre-emptive liver transplantation for primary hyperoxaluria (PH-I) arrests long-term renal function deterioration. Nephrol Dial Transplant 26: 354–359, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Clifford-Mobley O, Tims C, Rumsby G: The comparability of oxalate excretion and oxalate:creatinine ratio in the investigation of primary hyperoxaluria: Review of data from a referral centre. Ann Clin Biochem 52: 113–121, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Hoppe B, Kemper MJ, Bökenkamp A, Langman CB: Plasma calcium-oxalate saturation in children with renal insufficiency and in children with primary hyperoxaluria. Kidney Int 54: 921–925, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Marangella M, Cosseddu D, Petrarulo M, Vitale C, Linari F: Thresholds of serum calcium oxalate supersaturation in relation to renal function in patients with or without primary hyperoxaluria. Nephrol Dial Transplant 8: 1333–1337, 1993 [PubMed] [Google Scholar]
- 42.Hoppe B, Kemper MJ, Bökenkamp A, Portale AA, Cohn RA, Langman CB: Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int 56: 268–274, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR: Calcium oxalate stone fragment and crystal phagocytosis by human macrophages. J Urol 195: 1143–1151, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birtel J, Herrmann P, Garrelfs SF, Dulz S, Atiskova Y, Diederen RM, Gliem M, Brinkert F, Holz FG, Boon CJF, Hoppe B, Charbel Issa P: The ocular phenotype in primary hyperoxaluria type 1. Am J Ophthalmol 206: 184–191, 2019 [DOI] [PubMed] [Google Scholar]
- 45.Marangella M, Petrarulo M, Vitale C, Daniele PG, Sammartano S, Cosseddu D, Linari F: Serum calcium oxalate saturation in patients on maintenance haemodialysis for primary hyperoxaluria or oxalosis-unrelated renal diseases. Clin Sci (Lond) 81: 483–490, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Morgan SH, Purkiss P, Watts RW, Mansell MA: Oxalate dynamics in chronic renal failure. Comparison with normal subjects and patients with primary hyperoxaluria. Nephron 46: 253–257, 1987 [DOI] [PubMed] [Google Scholar]
- 47.Marangella M, Vitale C, Petrarulo M, Tricerri A, Cerelli E, Cadario A, Barbos MP, Linari F: Bony content of oxalate in patients with primary hyperoxaluria or oxalosis-unrelated renal failure. Kidney Int 48: 182–187, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, Milliner DS; IPHR Investigators: Transplantation outcomes in primary hyperoxaluria. Am J Transplant 10: 2493–2501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perinpam M, Enders FT, Mara KC, Vaughan LE, Mehta RA, Voskoboev N, Milliner DS, Lieske JC: Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem 50: 1014–1019, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 51.Clifford-Mobley O, Hewitt L, Rumsby G: Simultaneous analysis of urinary metabolites for preliminary identification of primary hyperoxaluria. Ann Clin Biochem 53: 485–494, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Rule AD, Kremers WK: What is the correct approach for comparing GFR by different methods across levels of GFR? Clin J Am Soc Nephrol 11: 1518–1521, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]