Introduction
Secondary hyperparathyroidism is an almost universal phenomenon of CKD that worsens as CKD progresses. It affects 40% of individuals with stage 3 CKD and 82% of individuals with stage 4 CKD (1). Early management and treatment of secondary hyperparathyroidism are imperative to preserve bone health and decrease soft tissue and vascular calcifications. Below is a discussion of the pathophysiology and treatment, including both pharmacologic and surgical options for secondary hyperparathyroidism.
Patient
A 61-year-old man with stage 4 CKD, autosomal dominant polycystic kidney disease, and hypertension is evaluated for management of mineral metabolism. His creatinine is 2.6 mg/dl, calcium is 9.2 mg/dl, phosphorus is 4.2 mg/dl, albumin is 4.1 g/dl, parathyroid hormone (PTH) is 228 pg/ml, and 25-hydroxyvitamin D [25(OH)D] is 18 ng/ml.
Pathophysiology of Secondary Hyperparathyroidism
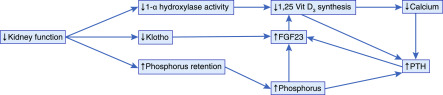
Figure 1 illustrates that the pathophysiology of secondary hyperparathyroidism is complex. With decreasing kidney function, there is a decreased filtered load of phosphate, resulting in decreased phosphate excretion (2). The retained phosphate stimulates osteocytes and osteoblasts to synthesize fibroblast growth factor-23 (FGF23), one of the hormones involved in maintaining phosphate homeostasis. FGF23 binds to fibroblast growth factor receptor 1, and in the presence of the required coreceptor αKlotho, FGF23 decreases the type II sodium–dependent phosphate cotransporters NaPi2a and NaPi2c in the proximal tubule of the kidney, thereby inhibiting phosphate reabsorption and promoting phosphaturia (3). Unfortunately, there is also decreased production of Klotho with progressive CKD; thus, the effectiveness of FGF23/Klotho to correct phosphate retention is limited, and as CKD progresses, there is the development of overt hyperphosphatemia, resulting in further stimulation of FGF23 production. In addition, both 1,25-dihydroxyvitamin D [1,25(OH)2D] and PTH increase the production of FGF23 (2).
Figure 1.
Pathophysiology of secondary hyperparathyroidism. FGF23, fibroblast growth factor-23; PTH, parathyroid hormone.
FGF23 also reduces 1,25(OH)2D production in the kidney by inhibition of the 1-α-hydroxylase (CYP27B1) and stimulates 1,25(OH)2D degradation by stimulating 24-hydroxylase (CYP24A1) (3). Because 1,25(OH)2D promotes intestinal absorption of calcium and phosphorus, this cascade of events leads to decreased gut absorption of these minerals. The reduced serum calcium and calcitriol concentrations are sensed by the vitamin D receptors and calcium sensing receptors of the parathyroid cells, leading to cell proliferation and a rise in PTH production. In addition, hyperphosphatemia also directly inhibits calcium sensing receptors and also stimulates PTH production (4). Although FGF23 binds to the Klotho-FGF23 receptor in the parathyroid to inhibit PTH, there is decreased Klotho with worsening kidney function, and thus, the ability of FGF23 to decrease PTH concentrations is lost (2). PTH promotes bone resorption with release of calcium and phosphate and increases CYP27B1 expression in the proximal tubules in an attempt to maintain normal serum calcium concentrations. The ongoing phosphate retention and hypocalcemia lead to a sustained and progressive increase in PTH production, and ultimately, they lead to parathyroid gland hyperplasia further augmenting secondary hyperparathyroidism. As parathyroid gland hyperplasia progresses and nodules develop, there is a progressive loss of both the vitamin D and calcium sensing receptors causing the loss of negative regulation of PTH production resulting in tertiary hyperparathyroidism.
Treatment
We recommend that therapy should be focused on early management and prevention of hyperparathyroidism. High phosphorus concentrations are involved in the pathogenesis of secondary hyperparathyroidism; therefore, reducing serum phosphorus concentrations by decreasing dietary intake can be used as a treatment strategy. The CKD Optimal Management with Binders and Nicotinamide trial showed that individuals with stage 3b–4 CKD on phosphate binders or nicotinamide, an inhibitor of intestinal phosphate transport, had no significant reduction in serum phosphate or FGF23 levels at 1 year when taken either alone or combined (5). Thus, further data are required for evaluating the role of phosphate binders on serum phosphate, FGF23, and PTH concentrations in patients with CKD and normophosphatemia.
Therefore, the mainstay of therapy would be correction of 25(OH)D deficiency. This can initially be approached with the use of the inactive compounds, ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Ergocalciferol is derived from plant sources, and cholecalciferol is derived from animal sources. Administration of these compounds should result in increase of 25(OH)D, which should increase 1,25(OH)2D; this should then stimulate the vitamin D receptor on parathyroid gland to suppress PTH production. As kidney function declines, the ability of these compounds to reduce PTH synthesis declines. Therapy with ergocalciferol resulted in a normalization of circulating 25(OH)D concentrations with a 13% decrease in PTH in individuals with stage 3 CKD; however, there was not a statistically significant decrease in PTH for individuals with stage 4 CKD (6). Another study using high-dose cholecalciferol for 12 weeks resulted in an almost threefold increase in circulating 25(OH)D concentrations with stabilization of PTH concentrations, compared with placebo in which circulating 25(OH)D concentrations remained stable and there was a 16% increase in PTH (7). It seems that, as kidney function deteriorates, higher 25(OH)D concentrations may be required to maximally suppress PTH (8). Thus, in many patients with advanced CKD in the absence of hypercalcemia, treatment with 1,25(OH)2D or an analog may be required to suppress PTH (6,9).
Calcitriol is a biologically active nonselective vitamin D receptor agonist that is used in the treatment of secondary hyperparathyroidism. It decreases PTH synthesis and prevents parathyroid gland hyperplasia (9). Activation of vitamin D receptors also increases serum calcium, which activates calcium sensing receptors in the parathyroid gland, further decreasing PTH production (9). Calcitriol is usually started when vitamin D supplementation is no longer adequate. Unlike ergocalciferol and cholecalciferol, calcitriol suppresses PTH regardless of CKD stage. Limitations of calcitriol include ineffective repletion of serum 25(OH)D and stimulation of CYP24A1 and FGF23, which will further decrease 25(OH)D concentrations. In addition, calcitriol and other vitamin D receptor agonists may cause hypercalcemia, hypercalciuria, and hyperphosphatemia, which can lead to vascular calcifications. Therefore, we recommend that calcitriol should be used as adjunctive therapy in patients who cannot be controlled with ergocalciferol or cholecalciferol with careful monitoring for hypercalcemia and hyperphosphatemia. Other vitamin D analogs include doxercalciferol and paricalcitol. These analogs also decrease PTH production but like calcitriol, at the risk of causing both hypercalcemia and hyperphosphatemia. In the only comparative study, there was no difference in the development of hypercalcemia and hyperphosphatemia with calcitriol and paricalcitol (10).
The third option for correcting vitamin D deficiency would be the use of extended release calcifediol [25(OH)D], which has been shown to effectively increase both circulating 25(OH)D and 1,25(OH)2D concentrations while decreasing PTH levels (1). This effect does not seem to be dependent on CKD stage, and the development of hypercalcemia was similar to placebo-treated subjects (1).
Other options to treat refractory hyperparathyroidism would include calcimimetics, such as cinacalcet, which activate calcium sensing receptors and increase sensitivity of serum calcium to inhibit PTH production and release, resulting in a reduction in PTH within a few hours. Studies have shown that treatment of secondary hyperparathyroidism with calcimimetics can decrease proliferation of parathyroid cells and subsequently suppress parathyroid hyperplasia (2). Unfortunately, calcimimetics can cause hypocalcemia and in CKD, may result in hyperphosphatemia. Therefore, calcimimetics are currently not used for patients with secondary hyperparathyroidism and CKD.
If refractory hyperparathyroidism is not responsive to medical therapy, then parathyroidectomy may be indicated. Indications for parathyroidectomy in appropriate surgical candidates include serum PTH of >800 pg/ml refractory to medical therapy, hyperplastic parathyroid gland measuring >500 mm3, or glands >1 cm in diameter (11). It is important to monitor these patients because secondary hyperparathyroidism can return due to hyperfunctioning of the remaining or autotransplanted tissue. Potential benefits of parathyroidectomy include decreased fracture risk, increased bone mineral density, and improved survival and quality of life (2,11).
This patient was treated with extended release calcifediol 30 mg for 6 months. Repeat laboratory tests demonstrated that creatinine is 2.8 mg/dl with calcium of 9.6 mg/dl, phosphorus of 4.0 mg/dl, albumin of 3.9 g/dl, PTH of 144 pg/ml, and vitamin D of 68 ng/ml. In conclusion, secondary hyperparathyroidism is important to treat early in patients with CKD to prevent mineral bone disease and maintain vascular health. Medical management may initially consist of correcting 25(OH)D deficiency with ergocalciferol or cholecalciferol, although these therapies generally do not effectively decrease PTH in later stages of CKD. If 25(OH)D deficiency persists, a trial with extended release calcifediol may be warranted. If PTH remains elevated after 25(OH)D is corrected, vitamin D receptor agonists could be added but will need to closely monitor for possible hypercalcemia and hyperphosphatemia. If medical management is not successful in treating secondary hyperparathyroidism, parathyroidectomy may be an alternative. Further clinical trials that evaluate clinical outcomes, such as fractures, cardiovascular disease, and mortality, are necessary to determine the best treatment regimen for secondary hyperparathyroidism in CKD.
Disclosures
Dr. S. Sprague reports grants and personal fees from Amgen, grants and personal fees from Opko, grants and personal fees from Vifor, and grants and personal fees from Fresenius outside the submitted work. Dr. R. Hyder has nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Sprague SM, Crawford PW, Melnick J, Strugnell SA, Ali S, Mangoo-Karim R, Lee S, Petkovich PM, Bishop CW: Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol 44: 316–325, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Martin A, David V, Quarles LD: Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 92: 131–155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centeno PP, Herberger A, Mun HC, Tu C, Nemeth EF, Chang W, Conigrave AD, Ward DT: Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun 10: 4693, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK, Sprague SM, Fried LF, Gassman JJ, Middleton JP, Flessner MF, Block GA, Wolf M: Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE trial. J Am Soc Nephrol 30: 1096–1108, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27: 36–43, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Westerberg PA, Sterner G, Ljunggren Ö, Isaksson E, Elvarson F, Dezfoolian H, Linde T: High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD stages 3-4: Results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 33: 466–471, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennis JL, Worcester EM, Coe FL, Sprague SM: Current recommended 25-hydroxyvitamin D targets for chronic kidney disease management may be too low. J Nephrol 29: 63–70, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Sprague SM, Coyne D: Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol 5: 512–518, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM: A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3-4 CKD. Clin J Am Soc Nephrol 9: 1620–1626, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau WL, Obi Y, Kalantar-Zadeh K: Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol 13: 952–961, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]