Visual Abstract
Keywords: mitochondria, hemodialysis, Skeletal muscle, chronic kidney disease, Chronic inflammation, oxidative stress, Sarcopenia, Frailty, Mitochondrial Dynamics, Phosphocreatine, Phosphorus, Walk Test, Mitochondria, Muscle, DNM1L protein, human, Mitochondrial Proteins, Microtubule-Associated Proteins, Magnetic Resonance Spectroscopy, Muscle, Skeletal, Renal Insufficiency, Chronic, Inflammation
Abstract
Background and objectives
Patients with CKD suffer from frailty and sarcopenia, which is associated with higher morbidity and mortality. Skeletal muscle mitochondria are important for physical function and could be a target to prevent frailty and sarcopenia. In this study, we tested the hypothesis that mitochondrial dysfunction is associated with the severity of CKD. We also evaluated the interaction between mitochondrial function and coexisting comorbidities, such as impaired physical performance, intermuscular adipose tissue infiltration, inflammation, and oxidative stress.
Design, setting, participants, & measurements
Sixty-three participants were studied, including controls (n=21), patients with CKD not on maintenance hemodialysis (CKD 3–5; n=20), and patients on maintenance hemodialysis (n=22). We evaluated in vivo knee extensors mitochondrial function using 31P magnetic resonance spectroscopy to obtain the phosphocreatine recovery time constant, a measure of mitochondrial function. We measured physical performance using the 6-minute walk test, intermuscular adipose tissue infiltration with magnetic resonance imaging, and markers of inflammation and oxidative stress in plasma. In skeletal muscle biopsies from a select number of patients on maintenance hemodialysis, we also measured markers of mitochondrial dynamics (fusion and fission).
Results
We found a prolonged phosphocreatine recovery constant in patients on maintenance hemodialysis (53.3 [43.4–70.1] seconds, median [interquartile range]) and patients with CKD not on maintenance hemodialysis (41.5 [35.4–49.1] seconds) compared with controls (38.9 [32.5–46.0] seconds; P=0.001 among groups). Mitochondrial dysfunction was associated with poor physical performance (r=0.62; P=0.001), greater intermuscular adipose tissue (r=0.44; P=0.001), and increased markers of inflammation and oxidative stress (r=0.60; P=0.001). We found mitochondrial fragmentation and increased content of dynamin-related protein 1, a marker of mitochondrial fission, in skeletal muscles from patients on maintenance hemodialysis (0.86 [0.48–1.35] arbitrary units (A.U.), median [interquartile range]) compared with controls (0.60 [0.24–0.75] A.U.).
Conclusions
Mitochondrial dysfunction is due to multifactorial etiologies and presents prior to the initiation of maintenance hemodialysis, including in patients with CKD stages 3–5.
Introduction
Patients with CKD frequently present with skeletal muscle atrophy and weakness, both components of sarcopenia (1,2). These factors contribute to physical frailty, a phenotype associated with fatigue, muscle weakness, and low physical activity (3). It was reported that 73% of patients with advanced CKD are frail at the time of initiation of maintenance hemodialysis (MHD) (4). Frailty and the coexisting sarcopenia are associated with greater morbidity and mortality in these patients (2,4).
Mitochondria are important for proper skeletal muscle function, and there is evidence suggesting that mitochondrial dysfunction contributes to sarcopenia and frailty (5). Recent studies have shown that mitochondrial dysfunction is associated with slow walking speed, low physical function, and fatigue in older adults without CKD (6). We and others have described muscle mitochondrial abnormalities in patients with ESKD on MHD, such as decreased mitochondrial content, increased mitophagy, and improper mitochondrial biogenesis (7−9). Other investigators have reported mitochondrial dysfunction in patients on MHD compared with healthy controls using 31P magnetic resonance spectroscopy (31P-MRS) (10−12). In contrast, there is a paucity of data in patients with CKD not on MHD, with one study showing no difference in mitochondrial function between patients with CKD not on MHD and controls (13). It is still unclear if mitochondrial dysfunction is present in patients with CKD prior to the initiation of MHD.
Fat accumulation outside of the muscle fibers, called intermuscular adipose tissue, correlates with low muscle quality, and it is associated with reduced physical performance and frailty in older adults (14−16). A previous study found increased intramuscular adipose tissue accumulation in patients on MHD compared with controls (17). Other studies have found that intramuscular adipose tissue may correlate with physical performance in patients with CKD (18,19). Fat accumulation in the muscle may induce mitochondrial stress and affect mitochondrial function (20). Thus, intramuscular adipose tissue could be an important mediator of mitochondrial dysfunction and the resultant decreased physical performance, sarcopenia, and frailty.
Inflammation and oxidative stress are common in patients with CKD (21−24). Dysfunctional mitochondria are one of the sources of oxidative stress and inflammation (25). Conversely, inflammation and oxidative stress can lead to mitochondrial dysfunction by damaging the mitochondria. Removal of damaged mitochondria relies on mitophagy and mitochondrial fission, a process of mitochondrial dynamics that segregates damaged mitochondria (26–28). Increased mitochondrial fission results in fragmented mitochondria and may induce muscle wasting (29).
In this study, we aimed to understand the complex inter-relationship between mitochondrial function and coexistent metabolic derangements in the setting of progressive CKD. We tested the hypothesis that these adverse metabolic derangements, especially mitochondrial dysfunction, are present in patients with moderate to severe CKD even prior to the initiation of MHD. We also tested the hypothesis that mitochondrial function is associated with physical performance, intramuscular adipose tissue, and systemic inflammation. The understanding of mitochondrial biology in CKD could help to understand the contribution of mitochondrial dysfunction to muscle wasting and will allow the development of therapeutic targets for frailty and sarcopenia in patients with CKD.
Materials and Methods
Participants
All participants were recruited from Vanderbilt University Medical Center Clinics from January 2015 to October 2017. We recruited 63 participants divided into three groups: (1) control, participants with no history of CKD; (2) CKD 3–5, patients with eGFR<60 ml/min per 1.73 m2 (range, 13.9–54.4 ml/min per 1.73 m2) not on MHD; and (3) MHD, patients with ESKD on hemodialysis three times per week for at least 6 months who were clinically stable, defined as not requiring hospitalization within the last month before enrollment, and adequately dialyzed (single-pool Kt/V >1.2). GFR was estimated from creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula (30). The study was approved by the Vanderbilt University Human Research Protection Program Committee.
Study Protocol
An informed consent form was given and explained to all of the participants. After the consent form was signed, participants were asked to come to the Vanderbilt General Clinical Research Center to evaluate the mitochondrial function and physical performance. We then performed muscle biopsies and blood sampling. For patients on MHD, all of these tests were performed on a nondialysis day. Quadriceps mitochondrial function was measured using a 31P-MRS protocol (31). Intramuscular adipose tissue was calculated in cross-sectional images of the midthigh region between the patella and ischial spine (32). We measured physical performance with the 6-minute walk test (6MWT) (33). Maximal static voluntary contraction of the quadriceps was measured using with the leg suspended by a Velcro strap and attached to an ergometer by rigid metallic rings. Muscle biopsies were obtained from the vastus lateralis by a percutaneous needle biopsy in a subgroup of patients (15 controls and 9 patients on MHD) (34). Samples were then prepared for electron microscopy or western blot (35). Cytokines and coenzyme Q10 (CoQ10) levels were measured in plasma (36).
Statistical Analyses
All of the participants completed 31P-MRS measurements of mitochondrial function; <5% of those (n=3) had missing 6MWT data. We used the Kruskal–Wallis test to compare the difference among the groups and implemented contrast for specific two-group comparisons. Differences in phosphocreatine (PCr) recovery time (τ) among the groups were assessed using analysis of covariance with tertile of eGFR as fixed effects and covariates, including sex, body mass index (BMI), age, and 6MWT distance, as covariates. We tested the association of mitochondrial function (PCr recovery time) with physical performance, intramuscular adipose tissue, and markers of inflammation and oxidative stress using linear regression and adjusting for potential confounders that may affect mitochondrial function. An additional comparison between control and MHD groups for mitochondrial fragmentation and markers of mitochondrial dynamics was performed using the Wilcoxon rank sum test. Hypotheses were tested at the level of α=0.05. Statistical analysis was performed using SPSS version 25 (IBM).
More details of methods are available in Supplemental Material.
Results
Demographics
Sixty-three participants were enrolled in one of the following groups: controls (n=21), CKD 3–5 not on MHD (n=20) with an eGFR between 14 and 60 ml/min per 1.73 m2, and MHD (n=22). Participant characteristics are depicted in Table 1. The causes of CKD were hypertension (52%), diabetes mellitus (10%), glomerular disease (17%), vasculitis (5%), and other (16%). Groups were matched by sex, race, BMI, history of diabetes, and hypertension. Patients with CKD stages 3–5 were older and a smaller proportion of patients was black compared with patients on MHD. Patients on MHD had a higher rate of stable cardiovascular disease (Table 1).
Table 1.
Participant characteristics
| Parameter | Controls, n=21 | CKD Stages 3–5, n=20 | Maintenance Hemodialysis, n=22 |
|---|---|---|---|
| Age, yr | 47±10 | 61±9 | 48±12 |
| Sex, men | 10 (48%) | 10 (50%) | 14 (64%) |
| Race, black | 12 (57%) | 8 (40%) | 18 (82%) |
| Body mass index, kg/m2 | 30±6 | 31±5 | 30±8 |
| History of diabetes | 2 (10%) | 2 (10%) | 4 (18%) |
| History of cardiovascular disease | 1 (5%) | 2 (10%) | 5 (23%) |
| Systolic BP, mm Hg | 128±10 | 132±10 | 137±28 |
| Hemoglobin, g/dl | 13.8±1.3 | 13.4±1.2 | 11.5±1.3 |
| Creatinine, mg/dl | 0.9±0.1 | 1.8±1.0 | 10.1±3.2 |
| Glucose, mg/dl | 85±12 | 84±12.5 | 75±12 |
| Total cholesterol, mg/dl | 199±31 | 187±46 | 176±39 |
| HDL cholesterol | 55±18 | 48±10 | 46±17 |
| Triglycerides, mg/dl | 94±42 | 118±53 | 156±96 |
Data are presented as mean ± SD.
Mitochondrial Function Measured by 31P-MRS
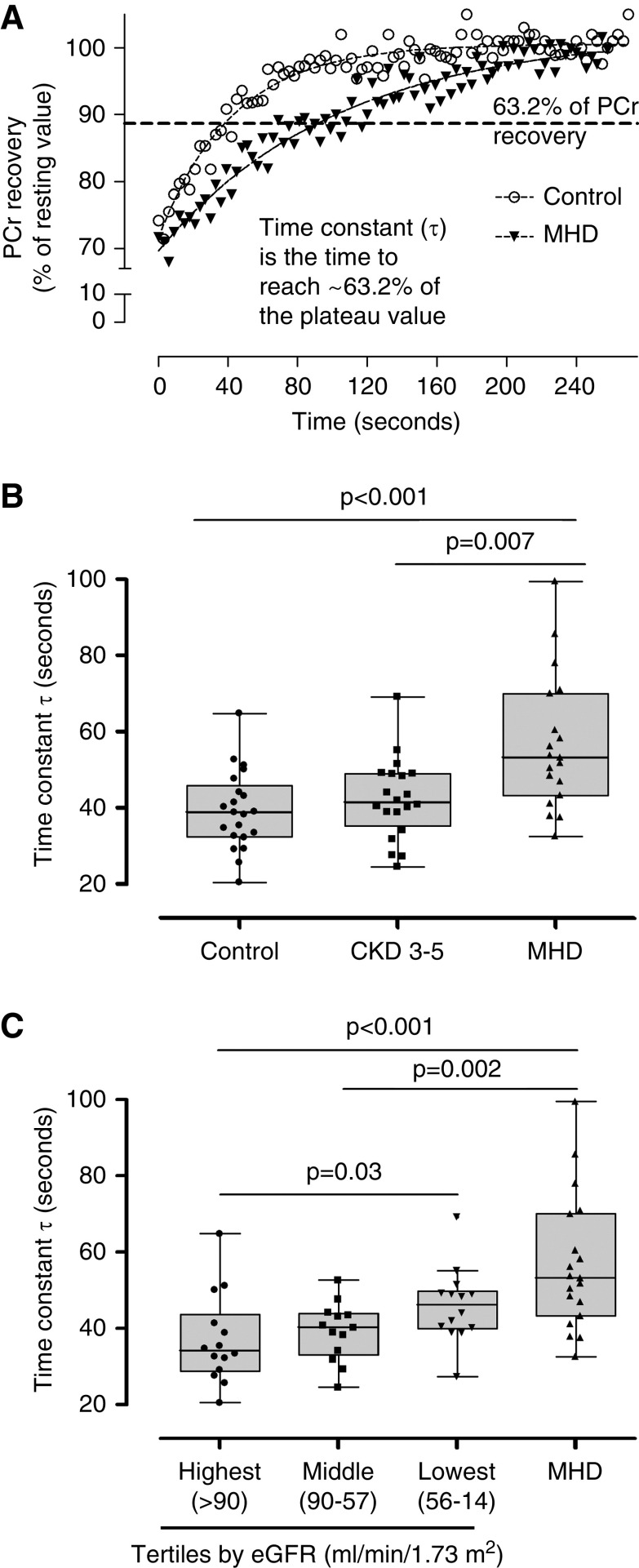
Faster PCr recovery kinetics (reflected by a shorter time constant τ) indicate better mitochondrial function and vice versa (Figure 1A) (37). We found that PCr recovery time constant (τ) was longer in patients on MHD compared with controls and patients with CKD 3–5 (Figure 1B). There was a statistically significant correlation between the PCr recovery time constant and eGFR (Rho=0.32; P=0.04) in the non-MHD group of patients. This association was also demonstrated when control and CKD 3–5 groups were divided into tertiles by the eGFR (Figure 1C), showing that patients within the lowest eGFR tertile have a longer PCr recovery time constant than individuals within the highest eGFR tertile. These results suggest that mitochondrial dysfunction is associated with the severity of CKD. When grouped by stages of kidney disease, there was no difference in mitochondrial function between controls and patients with CKD 3–5 (P=0.40). Hemoglobin levels may affect mitochondrial function. The difference in mitochondrial function among the groups persisted after adjusting for hemoglobin levels (P=0.01).
Figure 1.
Mitochondrial function in patients with CKD. During exercise, phosphocreatine (PCr) is broken down to synthesize ATP for the working muscle. During recovery, PCr is resynthesized from ATP produced by oxidative phosphorylation, and the rate of recovery of PCr is a measure of mitochondrial oxidative capacity. The recovery of PCr after light- and moderate-intensity exercise follows a monoexponential pattern, and the time constant τ of PCr recovery is frequently used as an index of mitochondrial function. (A) Representative graph showing the PCr recovery kinetics after exercise in one healthy control and a patient on maintenance hemodialysis (MHD). (B) PCr recovery time constant τ was prolonged in patients on MHD (n=22) compared with controls (n=21) and patients with CKD stages 3–5 (n=20). (C) Patients with CKD 3–5 and control participants were divided into tertiles according to the eGFR. Patients within the lowest eGFR tertile (n=14) have a prolonged PCr recovery time compared with individuals within the highest eGFR tertile (n=14).
Mitochondrial Function, Physical Performance, and Muscle Strength
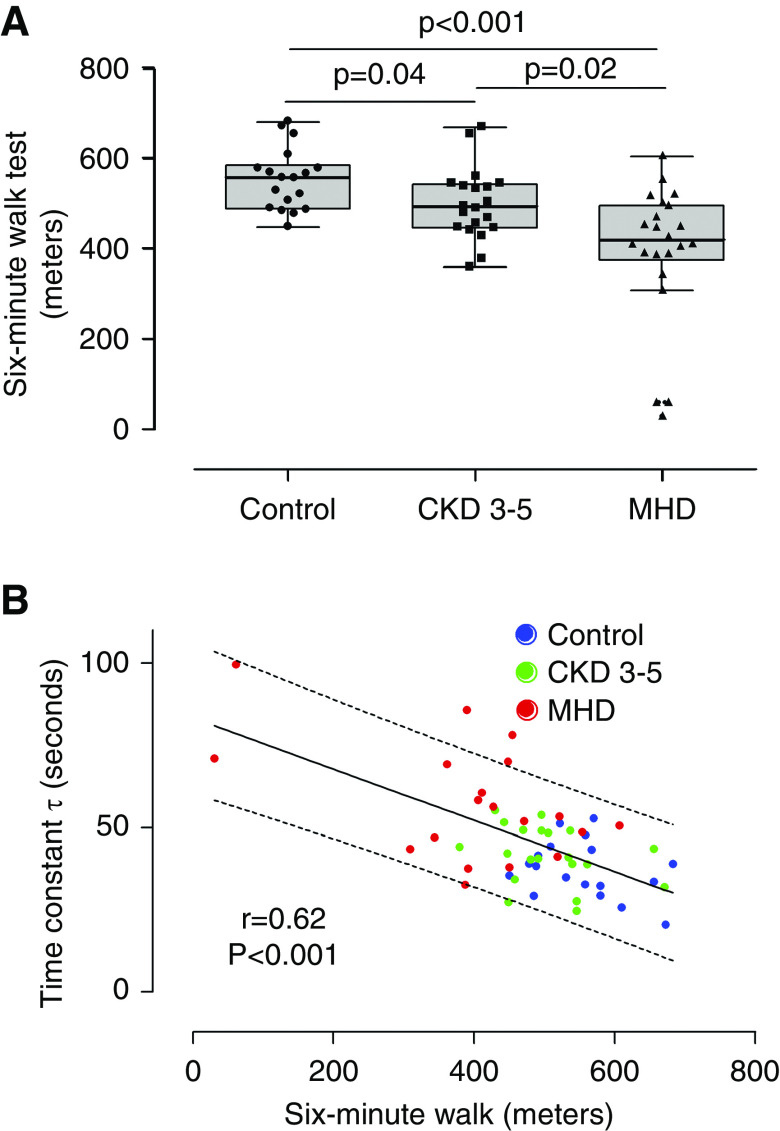
We measured physical performance using the 6MWT. We found that patients on MHD walked a significantly shorter distance compared with both controls and patients with CKD 3–5, whereas patients with CKD 3–5 walked a shorter distance compared than controls (controls: 557.5 m [489.9–586.7]; CKD 3–5: 493.0 m [447.4–544.1]; MHD: 419 m [376.3–497.2]; median [interquartile range]; P=0.001) (Figure 2A). There was a statistically significant inverse correlation between PCr recovery time constant and physical performance (Figure 2B), suggesting that mitochondrial dysfunction is associated with poor physical performance. The maximal static voluntary contraction of the knee extensors was also significantly lower in patients on MHD compared with controls and patients with CKD 3–5, but there was no difference in maximal static voluntary contraction between controls and patients with CKD 3–5 (Supplemental Figure 1). In unadjusted analysis, maximal static voluntary contraction is also inversely associated with the PCr recovery time constant (Table 2). Mulitvariable regression analysis adjusted by age, BMI, and sex showed that only the 6MWT distance remained significantly associated with PCr recovery time constant (Table 2). We also observed a statistically significant association between mitochondrial function and the cross-sectional area (CSA) of the quadriceps muscle in unadjusted (r=−0.34; P=0.02) and adjusted analysis (Supplemental Table 1).
Figure 2.
Physical performance and mitochondrial function. (A) Physical performance, measured by the 6-minute walk test, was impaired in patients with CKD 3–5 (n=20) and patients on MHD (n=22) compared with control individuals (n=18). (B) Physical performance was significantly associated with mitochondrial function (n=57).
Table 2.
Association of physical performance with mitochondrial function
| Variables | β (95% Confidence Interval) | Adjusted P Value | |
|---|---|---|---|
| Unadjusted, n=57 | Adjusted, n=57 | ||
| 6MWT, m | −0.08 (−0.11 to −0.05) | −0.06 (−0.10 to −0.02) | 0.004 |
| Maximal Static Voluntary Contraction, N | −0.10 (−0.15 to −0.05) | −0.05 (−0.13 to 0.03) | 0.22 |
| Age, yr | 0.13 (−0.13 to 0.39) | 0.32 | |
| BMI, kg/m2 | −0.51 (−1.20 to 0.18 | 0.15 | |
| Sex, women | 2.92 (−5.37 to 11.20) | 0.48 | |
| Race, white | −4.76 (−11.81 to 2.30) | 0.18 | |
Mitochondrial function (dependent variable) was measured using the τ time constant (in seconds). Each 1-m increase in the 6-minute walk test (6MWT) was negatively associated with 0.08 s less in the τ time constant. BMI, body mass index.
Mitochondrial dysfunction in CKD may be affected by different factors, such as physical performance, age, and sex. Using analysis of covariance, we showed that the difference in mitochondrial function among the tertile of eGFR groups remains significant after adjustment for 6MWT distance, sex, BMI, and age (P=0.05). Also, in a subgroup of participants with similar physical performance (i.e., those who were within the 25th and 75th percentiles for the 6MWT), we found that mitochondrial function was impaired in patients on MHD compared with controls (Supplemental Figure 2). These results suggest that the worsening of kidney function has a considerable effect on mitochondrial function independent of physical performance.
Mitochondrial Function and Intramuscular Adipose Tissue
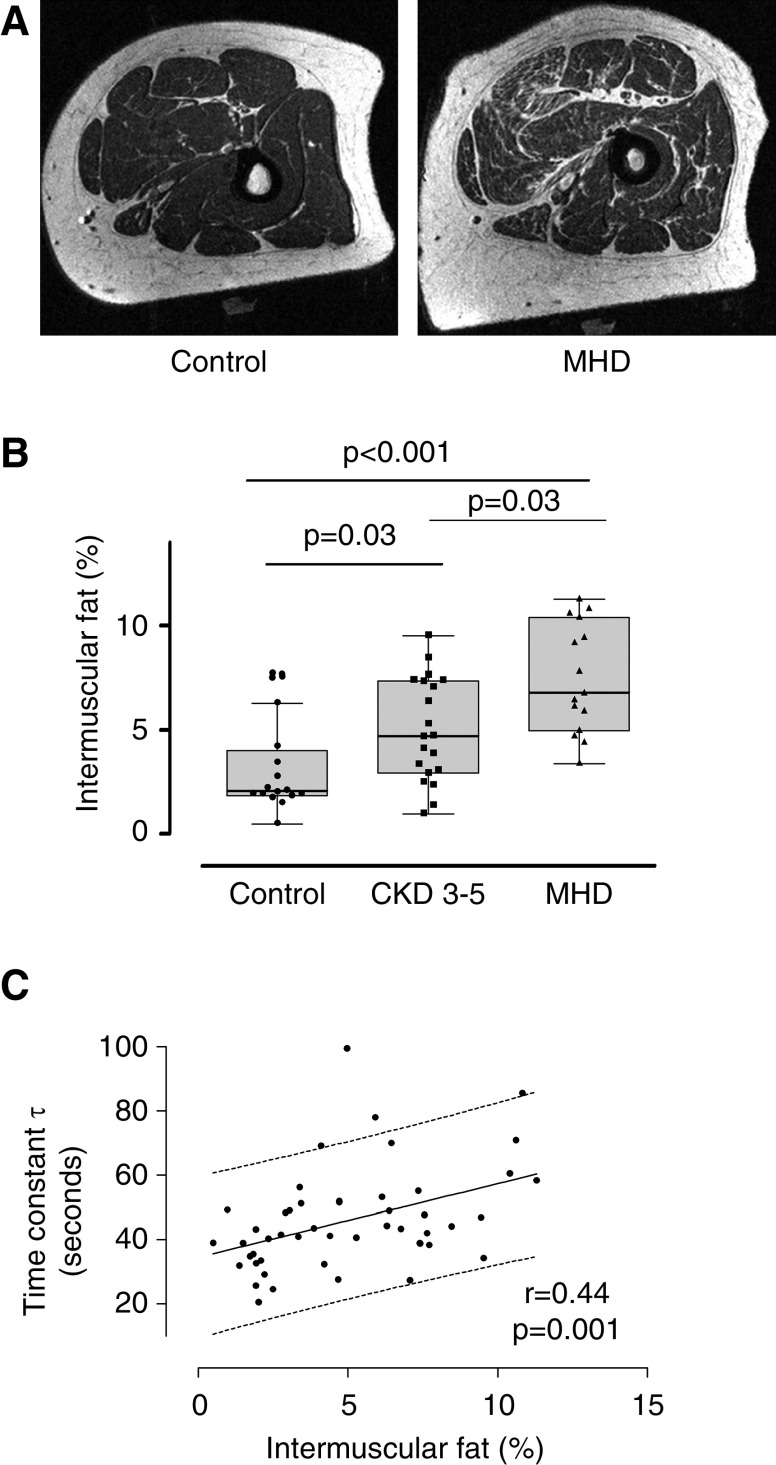
Lipid accumulation in the muscle usually coexists with mitochondrial dysfunction, suggesting that ectopic fat may damage the mitochondria. In order to examine this association, we measure intramuscular adipose tissue on 51 participants (16 controls, 20 CKD 3–5, and 15 MHD) in the quadriceps muscle, the same muscle in which mitochondrial function was measured. Intramuscular adipose tissue accumulation was significantly increased in the MHD group compared with the control and CKD 3–5 groups (Figure 3, A and B). Intramuscular adipose tissue accumulation was also greater in the CKD 3–5 group compared with controls (Figure 3B). In addition, the PCr recovery time constant also correlated with intramuscular adipose tissue, suggesting an association between mitochondrial function and intramuscular adipose tissue accumulation (Figure 3C). The association remained significant after adjusting for BMI, age, sex, and race (Table 3).
Figure 3.
Intermuscular adipose tissue and mitochondrial function. (A) Representative magnetic resonance imaging images showing increased intermuscular adipose tissue (IMAT) in a patient on MHD and a control individual. (B) IMAT infiltration (ratio of fat to muscle volume) in the quadriceps muscle was increased in patients with CKD 3–5 (n=20) and patients on MHD (n=15) compared with the control group (n=16). (C) Linear regression showing the association between mitochondrial function (time constant τ) and IMAT infiltration (n=49).
Table 3.
Association of intramuscular adipose tissue accumulation with mitochondrial function
| Variables | β (95% Confidence Interval) | Adjusted P Value | |
|---|---|---|---|
| Unadjusted, n=51 | Adjusted, n=51 | ||
| Intramuscular adipose tissue % | 2.29 (0.86 to 3.72) | 2.88 (1.35 to 4.40) | <0.001 |
| Age, yr | 0.12 (−0.28 to 0.51) | 0.56 | |
| BMI, kg/m2 | −0.95 (−1.67 to −0.24) | 0.01 | |
| Sex, women | 0.42 (−8.19 to 9.03) | 0.92 | |
| Race, white | −5.89 (−14.57 to 2.79) | 0.17 | |
Mitochondrial function (dependent variable) was measured using the τ time constant (in seconds). Each percentage increase in intramuscular adipose tissue was positively associated with 2.3 s more in the τ time constant. BMI, body mass index.
Mitochondrial Function and Markers of Inflammation and Oxidative Stress
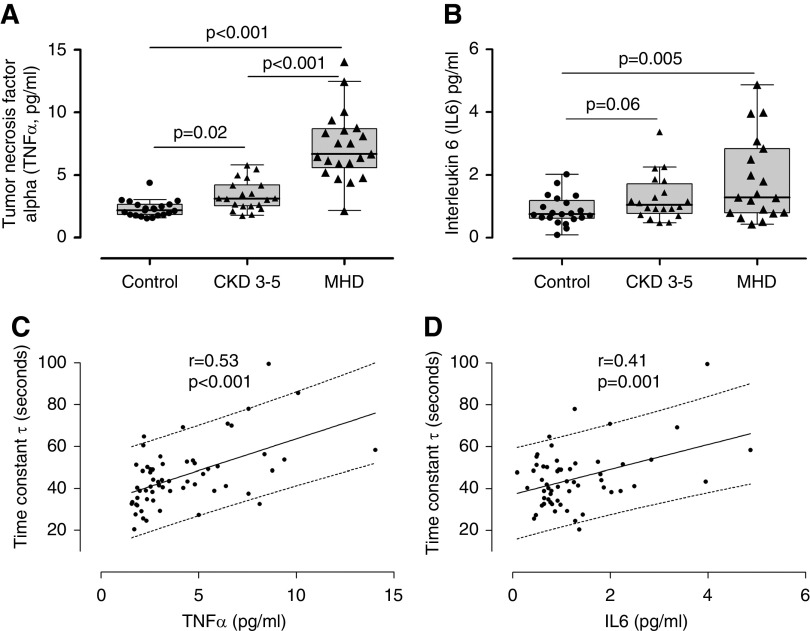
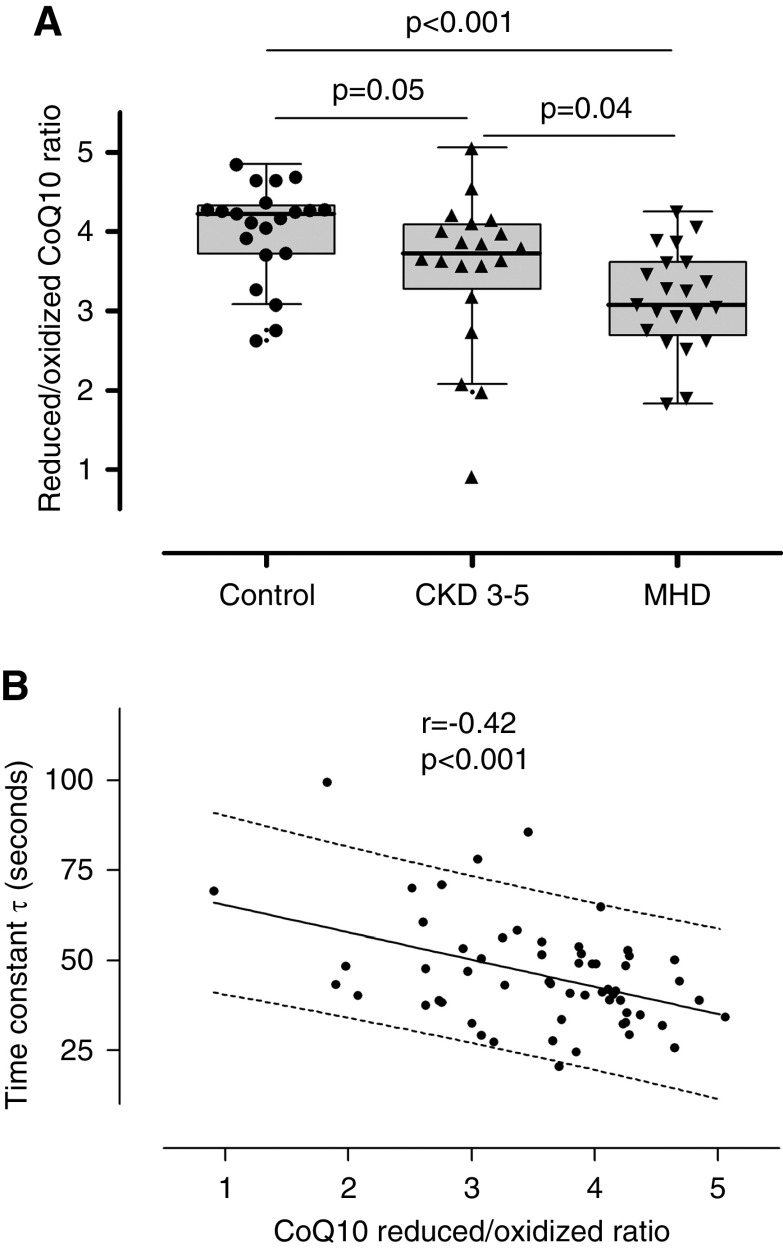
As expected, we found that serum concentrations of TNFα, IL-6, and IL-1β were elevated in patients with CKD compared with controls (Figure 4, A and B, Supplemental Figure 3). There was no difference in levels of IL-8 or -10 (Supplemental Figure 3). We also found a significant correlation between prolonged PCr recovery time constant (worse mitochondrial function) and higher levels of both TNFα and IL-6 levels (Figure 4, C and D). We also measured CoQ10 levels and the ratio of reduced to oxidized form of CoQ10 (or CoQ10 redox ratio), a validated marker of oxidative stress (38). We found that the total and reduced CoQ10 values were lower in patients on MHD compared with controls (Supplemental Figure 4). The CoQ10 redox ratio was diminished in patients with CKD and in patients on MHD compared with controls and correlated significantly with PCr recovery constant (Figure 5). The association of TNFα and CoQ10 redox ratio with mitochondrial function remains significant after adjusting for BMI, age, sex, and race (Table 4). Also, after adjusting for physical performance, the correlation between mitochondrial function and TNF remain significant, whereas the association between mitochondrial function and CoQ10 redox ratio was not significant (Supplemental Table 2).
Figure 4.
Inflammation and mitochondrial function. TNFα (A) and IL-6 (B) levels in control individuals (n=21), patients with CKD 3–5 (n=20), and patients on MHD (n=21). Association of TNFα (C) and IL-6 (D) with mitochondrial function (n=59).
Figure 5.
Oxidative stress and mitochondrial function. (A) Coenzyme Q10 (CoQ10) redox ratio (ratio of reduced to oxidized CoQ10) in control individuals (n=21), patients with CKD 3–5 (n=20), and patients on MHD (n=21). (B) Association between CoQ10 redox ratio and mitochondrial function (n=59).
Table 4.
Association of markers of inflammation and oxidative stress with mitochondrial function
| Variables | β (95% Confidence Interval) | Adjusted P Value | |
|---|---|---|---|
| Unadjusted, n=59 | Adjusted, n=59 | ||
| TNFα, pg/ml | 3.03 (1.75 to 4.31) | 3.30 (2.03 to 4.57) | <0.001 |
| Age, yr | 0.36 (−0.08 to 0.64) | 0.01 | |
| BMI, kg/m2 | −0.52 (−1.07 to −0.03) | 0.06 | |
| Sex, women | −4.18 (−10.92 to 2.55) | 0.22 | |
| Race, white | −3.772 (−10.52 to 2.97) | 0.27 | |
| CoQ10 redox ratio | −7.55 (−11.82 to −3.28) | −6.81 (−11.25 to −2.37) | 0.003 |
| Age, yr | 0.18 (−0.14 to 0.50) | 0.25 | |
| BMI, kg/m2 | −0.41 (−1.03 to −0.22) | 0.20 | |
| Sex, women | 0.74 (−6.81 to 8.28) | 0.85 | |
| Race, white | −4.79 (−12.42 to 2.85) | 0.21 | |
Mitochondrial function (dependent variable) was measured using the τ time constant (in seconds). Every 1-pg/ml increase in TNFα levels was positively associated with 3.0 s more in the τ time constant. Each unit increase in the coenzyme Q10 (CoQ10) ratio was negatively associated with 7.6 s less in the τ time constant. BMI, body mass index.
Mitochondrial Fragmentation and Markers of Mitochondrial Dynamics in Skeletal Muscle
Damaged mitochondria by factors such as inflammation and/or oxidative stress are usually segregated and fragmented by mitochondrial fission prior to being removed via mitophagy. To determine changes in mitochondrial fragmentation, we evaluated mitochondria morphology using electron micrographs. We only evaluated a group of patients with no history of CKD (controls) and patients on MHD. We found that mitochondria are smaller in patients on MHD compared with controls (Figure 6, A and B). The frequency distribution of individual mitochondrial areas showed a shift to the left in patients on MHD, reflecting an increased proportion of smaller mitochondria (Figure 6C). We also evaluated markers of mitochondrial fusion and fission in skeletal muscle samples. We found that levels of dynamin-related protein 1 (DRP1), a marker of mitochondrial fission, are increased in patients on MHD (Figure 7). There was no difference in Fis1 or on OPA-1 levels, other markers of mitochondrial dynamics.
Figure 6.
Mitochondrial fragmentation in patients on MHD in skeletal muscle biopsies from the vastus lateralis. (A) Electron microscopies showing that mitochondria (white arrows) are smaller in patients on MHD. (B) Quantification of mitochondrial areas in control individuals (n=15) and patients on MHD (n=9). Each dot represents the median area of hundreds of mitochondria in each subject. (C) Frequency distribution of individual mitochondria areas showing a greater proportion of smaller mitochondria in patients on MHD.
Figure 7.
Western blot analysis of dynamin-related protein 1 (DRP1). (A) Representative western blot of DRP1, a marker of mitochondrial fission. (B) DRP1 is increased in patients on MHD when compared with control subjects with no history of CKD. Skeletal muscle biopsies were obtained from the vastus lateralis (n=9 in each group). A.U., arbitrary units.
Discussion
In this study, we showed that mitochondrial dysfunction is present in patients with moderate CKD and occurs prior to the initiation of MHD. CKD affects mitochondrial dysfunction independent of age and physical performance, despite the known association between CKD and lower levels of physical performance. Importantly, deficits in in vivo muscle mitochondrial function are associated with clinically important limitations in physical performance measured by the 6MWT. Mitochondrial dysfunction in patients with CKD may occur as a result of mitochondrial damage caused by inflammation, oxidative stress, and intramuscular adipose tissue infiltration. We also found increased mitochondrial fragmentation and fission in skeletal muscle in patients on MHD, probably as an attempt to segregate and eliminate damaged mitochondria. Overall, these data re-emphasize the complex metabolic interplay among mitochondrial dysfunction, inflammation, and oxidative stress that coexist in moderate to advanced CKD, which collectively may culminate in poor physical performance and poor quality of life.
Mitochondrial function has been previously evaluated in patients with CKD using 31P-MRS. As in other studies, we found that mitochondrial function is impaired in patients on MHD (10−12). We now found that in vivo mitochondrial dysfunction (i.e., prolonged PCr recovery constant) correlates with lower eGFR and occurs before the initiation of MHD. This finding suggests that the uremic environment gradually damages the mitochondria. In fact, in vitro and preclinical studies have shown that uremic toxins damage the mitochondria (39−41). Thus, worsening kidney function, which leads to a gradual accumulation of uremic toxins, may result in a progressive decline in mitochondrial function. Indeed, uremic toxins, such as indoxyl sulfate and hippurate, have been shown to damage the mitochondria and alter mitochondrial function (39,40), although we did not explore this issue in detail.
Mitochondrial dysfunction may result in increased production of reactive oxygen species in CKD. Conversely, systemic oxidative stress may damage the mitochondria. Thus, we found decreased CoQ10 redox ratio (a marker of oxidative stress) in patients with CKD, which correlates with worsened mitochondrial function. Mitochondrial dysfunction may also contribute to the increased inflammatory state in CKD by different mechanisms, including the mitochondrial–reactive oxidative species-dependent activation of the NLRP3 inflammasome (42). Consistent with this, we observed a correlation between mitochondrial function and markers of inflammation. Another potential explanation is that inflammation induces mitochondrial dysfunction. In fact, TNFα and IL-1β may damage mitochondria and impair mitochondrial function in different cell types. The use of anti-inflammatory agents, such as recombinant IL receptor antagonists, or CoQ10 supplementation to increase the CoQ10 redox ratio will be necessary to evaluate causality among oxidative stress, inflammation, and mitochondrial function in patients with CKD.
Mitochondrial quality control mechanisms (i.e., mitochondrial dynamics, the continuous process of mitochondrial fission or fusion) are required to eliminate damaged mitochondria through mitophagy. We have previously shown that markers of mitophagy are increased in patients on MHD (7). We now found that mitochondria are small and fragmented in skeletal muscles from patients on MHD. We also found increased content of DRP1, a marker of mitochondrial fission. These findings suggest that mitochondrial fission is increased in patients on MHD. Recent preclinical studies suggest that uremic toxins increase mitochondrial fission through downregulation of fusion proteins and upregulation of fission proteins, including DRP1 (40,41). Although there is growing evidence showing that altered mitochondrial dynamics affect muscle function, further mechanistic research is necessary to understand the relationship between mitochondrial dynamics and frailty in patients with CKD.
The presence of frailty and the level of habitual physical activity may influence mitochondrial function. In this study, we did not measure physical activity, a major limitation. Instead, we used physical performance as a proxy for physical activity. In our study, patients on MHD have the lowest physical performance measured by the 6MWT. It is possible that the lack of physical activity contributes to the impaired mitochondrial function. Accordingly, we found that low physical performance is associated with a worse mitochondrial function. Muscle CSA has been used as a proxy of physical activity. Thus, we found an association between quadriceps CSA and mitochondrial function. This association is not as strong as the one between physical performance and mitochondrial function. This is probably because CSA is a better predictor of muscle strength than of physical function (43). To determine if the association between CKD and mitochondrial is independent of the physical performance, we evaluated mitochondrial function in a subgroup of individuals with similar physical performance. We found that, despite a similar level of physical performance, patients on MHD have impaired mitochondrial function. These results suggest that CKD affects mitochondrial function, to some extent, independently of physical performance. Further studies should measure physical activity and its relation to mitochondrial function in patients with CKD.
Intramuscular adipose tissue may affect muscle quality and function, and it is associated with poor physical activity and performance (44,45). Few studies have evaluated intramuscular adipose tissue in patients with CKD. A previous study found increased intramuscular adipose tissue accumulation in patients on MHD (17). We found a progressive intramuscular adipose tissue accumulation with increased severity of kidney disease. Intramuscular adipose tissue may damage the mitochondria, resulting in mitochondrial dysfunction. Conversely, mitochondria are the primary site for lipid metabolism, and mitochondrial dysfunction may play a role in intramuscular adipose tissue accumulation. The cross-sectional study design precludes the assessment of causality. Nevertheless, we found a strong association between mitochondrial function and intramuscular adipose tissue. Further prospective studies should evaluate changes in intramuscular adipose tissue, physical activity, and mitochondrial function as potential mechanisms to prevent frailty and sarcopenia in patients with CKD.
Lower hemoglobin levels may affect mitochondrial function. A previous study evaluated mitochondrial function in patients with CKD before and after erythropoietin treatment (46). This study found that there was no difference in mitochondrial function after treatment with erythropoietin, despite an increase in hemoglobin by 50%. The authors also showed that maximal oxygen flow from the microcirculation to the mitochondria did not increase after erythropoietin treatment. This is consistent with the observation that the difference in mitochondrial function among the groups persisted after adjusting by hemoglobin levels.
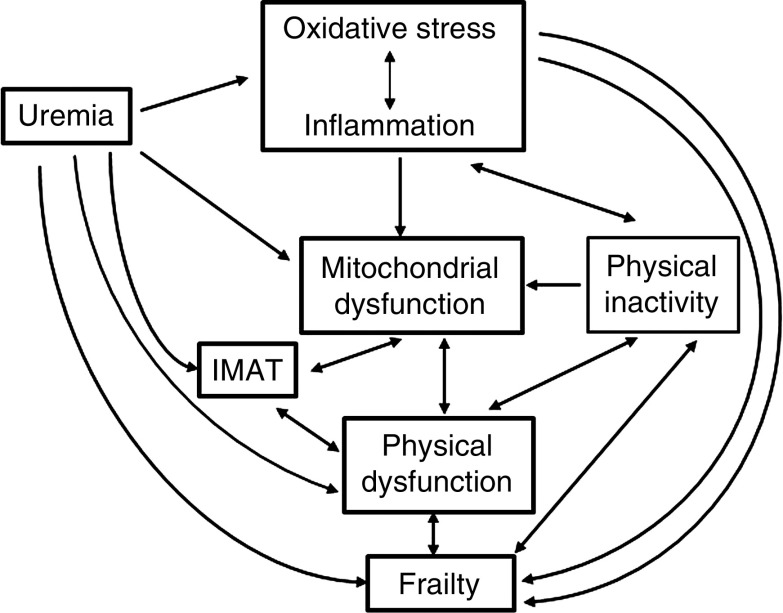
The frailty phenotype among patients with CKD may be a consequence of uremic solutes retention, which initiates a vicious cycle of impaired mitochondrial function and decreased physical functioning. Worsening of kidney disease may affect both physical and mitochondrial function. In turn, mitochondrial dysfunction may negatively affect physical functioning and vice versa, contributing to the frailty phenotype. Also, uremia may have a direct deleterious effect on frailty and physical function or indirectly by affecting muscle quality (i.e., increasing intramuscular adipose tissue infiltration). Other factors that may play a role in the frail phenotype include inflammation, oxidative stress, and intramuscular adipose tissue infiltration, either directly or by inducing mitochondrial dysfunction. Thus, the pathophysiologic link among frailty, impaired physical activity, and mitochondrial dysfunction in patients with CKD is very complex (Figure 8) and deserved further investigation. Therapeutic approaches to prevent frailty in CKD may require interventions that reverse uremia, such as kidney transplantation. Exercise is another potential intervention that may prevent or reverse frailty by improving physical and mitochondrial function. Whereas many studies show some benefit of exercise on physical function in CKD, the effect of exercise on mitochondrial function in patients with CKD remains largely unexplored. Likewise, mitochondrial-targeted therapies, which could be used in combination with exercise, may prevent frailty by improving mitochondrial bioenergetics and exercise efficiency.
Figure 8.
The possible association among factors that could be implicated in the pathogenesis of frailty in patients with CKD.
This study has several strengths. Importantly, it examines and compares mitochondrial function in patients at different stages of CKD not yet on MHD and in patients on MHD. We evaluated mitochondrial function in the quadriceps muscle whose proper function is important for daily activities, such as rising from a chair, but also for preventing falls in elderly frail individuals (47). We used 31P-MRS, a state-of-the-art technique, to measure in vivo mitochondrial function. The combination of studying in vivo mitochondrial function and muscle biopsies was important to provide mechanistic insight for the in vivo studies. There are also some limitations. This is a cross-sectional study not suitable for the evaluation of causality. Also, the CKD 3–5 group was not properly matched to the other groups; the patients were older, and there was a lower proportion of black patients. Another limitation of this study is that we did not assess physical activity, which has more clinical relevance to patients with CKD. We only evaluated mitochondrial fragmentation and makers of mitochondrial dynamics in muscles from patients on MHD and control individuals. Further analysis of muscle tissue from patients with CKD not yet on MHD would be important for a better understanding of the effect of uremia on mitochondrial dynamics.
In conclusion, we found that mitochondrial dysfunction occurs prior to the initiation of MHD. We found that mitochondrial dysfunction correlates with physical performance, intramuscular adipose tissue, oxidative stress, and inflammation. Our findings suggest that strategies aimed at improving mitochondrial function should be investigated in patients with CKD with the ultimate goal of preventing or treating frailty and sarcopenia and improving overall physical function in this high-risk patient population.
Disclosures
T.A. Ikizler reports personal fees from Fresenius Kabi and Abbott Nutrition during the conduct of the study and personal fees from Reata, ISN, Elsevier, and ABIM, outside the submitted work. B. Roshanravan reports grants from Dialysis Clinics Incorporated during the conduct of the study. All remaining authors have nothing to disclose.
Funding
The following funding was received for this study: National Center for Research Resources grant 1UL-1RR024975; National Institute of Diabetes and Digestive and Kidney Diseases grants K23DK0099442, K23DK100533, and R03DK114502; National Institute of General Medical Sciences grant T32GM07569; and US Department of Veterans Affairs grant CX001755.
Supplementary Material
Acknowledgments
We thank all of the study coordinators and participants for their dedication to the study.
The sponsors had no influence on the design, execution, and analysis of the results of the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Role of Skeletal Muscle Mitochondrial Dysfunction in CKD,” on pages 912–913.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10320819/-/DCSupplemental.
Supplemental Figure 1. PCr recovery time constant τ was prolonged in patients on MHD compared with controls and patients with CKD with similar physical performance measured by the 6-minute walk.
Supplemental Figure 2. Maximal voluntary contraction of the quadriceps muscle was diminished in the MHD group (n=18) compared with control (n=16) and CKD 3–5 (n=20) groups.
Supplemental Figure 3. IL-1β, IL-8, and IL-10 levels in control individuals (n=21), patients with CKD 3–5 (n=20), and patients on MHD (n=22).
Supplemental Figure 4. Levels of total coenzyme Q10 and reduced coenzyme Q10 in control individuals (n=21), patients with CKD 3–5 (n=20), and patients on MHD (n=21).
Supplemental Table 1. Association of total quadriceps cross-sectional area with mitochondrial function.
Supplemental Table 2. Association of physical performance with mitochondrial function after adjusting by inflammation and oxidative stress markers.
References
- 1.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O, Cederholm T, Stenvinkel P, Carrero JJ: Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 9: 1720–1728, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C: Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int J Biochem Cell Biol 45: 2288–2301, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distefano G, Standley RA, Zhang X, Carnero EA, Yi F, Cornnell HH, Coen PM: Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle 9: 279–294, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamboa JL, Billings FT 4th, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, Roberts LJ 2nd, Himmelfarb J, Ikizler TA, Brown NJ: Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep 4: e12780, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conjard A, Ferrier B, Martin M, Caillette A, Carrier H, Baverel G: Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 6: 68–74, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, Kopple JD: Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol (1985) 112: 72–78, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson CH, Kemp GJ, Taylor DJ, Ledingham JGG, Radda GK, Rajagopalan B: Effect of chronic uraemia on skeletal muscle metabolism in man. Nephrol Dial Transplant 8: 218–222, 1993 [PubMed] [Google Scholar]
- 11.Durozard D, Pimmel P, Baretto S, Caillette A, Labeeuw M, Baverel G, Zech P: 31P NMR spectroscopy investigation of muscle metabolism in hemodialysis patients. Kidney Int 43: 885–892, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Kemp GJ, Crowe AV, Anijeet HKI, Gong QY, Bimson WE, Frostick SP, Bone JM, Bell GM, Roberts JN: Abnormal mitochondrial function and muscle wasting, but normal contractile efficiency, in haemodialysed patients studied non-invasively in vivo. Nephrol Dial Transplant 19: 1520–1527, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Thompson CH, Kemp GJ, Barnes PR, Rajagopalan B, Styles P, Taylor DJ, Radda GK: Uraemic muscle metabolism at rest and during exercise. Nephrol Dial Transplant 9: 1600–1605, 1994. [PubMed] [Google Scholar]
- 14.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB: Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 15.St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, Sgarioto N, Zovilé I, Barbat-Artigas S, Reynaud O, Alkaterji F, Lemieux FC, Grenon A, Gaudreau P, Hepple RT, Chevalier S, Belanger M, Morais JA, Aubertin-Leheudre M, Gouspillou G: The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle 8: 213–228, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC: Skeletal muscle fat infiltration: Impact of age, inactivity, and exercise. J Nutr Health Aging 14: 362–366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HL, Ding TT, Lu S, Xu Y, Tian J, Hu WF, Zhang JY: Muscle mass loss and intermuscular lipid accumulation were associated with insulin resistance in patients receiving hemodialysis. Chin Med J (Engl) 126: 4612–4617, 2013. [PubMed] [Google Scholar]
- 18.Wilkinson TJ, Gould DW, Nixon DGD, Watson EL, Smith AC: Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant 34: 1344–1353, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Cheema B, Abas H, Smith B, O’Sullivan AJ, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, Berger K, Baune BT, Singh MF: Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton) 15: 454–463, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Muoio DM, Neufer PD: Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himmelfarb J, Hakim RM: Oxidative stress in uremia. Curr Opin Nephrol Hypertens 12: 593–598, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Himmelfarb J: Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial 22: 636–643, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Bergström J, Lindholm B, Lacson E Jr., Owen W Jr., Lowrie EG, Glassock RJ, Ikizler TA, Wessels FJ, Moldawer LL, Wanner C, Zimmermann J: What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial 13: 163–175, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou R, Yazdi AS, Menu P, Tschopp J: A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Ding WX, Yin XM: Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol Chem 393: 547–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer SL: Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369: 2236–2251, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Kubli DA, Gustafsson Å: Mitochondria and mitophagy: The yin and yang of cell death control. Circ Res 111: 1208–1221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M: Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes SC, Slade JM, Meyer RA: Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Physiol Nutr Metab 33: 1124–1131, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, Karampinos DC, MacLeod TD, Lin W, Nardo L, Li X, Link TM, Majumdar S, Souza RB: Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage 22: 226–234, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Statement ATS; ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories : ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Tarnopolsky MA, Pearce E, Smith K, Lach B: Suction-modified Bergström muscle biopsy technique: Experience with 13,500 procedures. Muscle Nerve 43: 717–725, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Gamboa JL, Andrade FH: Mitochondrial content and distribution changes specific to mouse diaphragm after chronic normobaric hypoxia. Am J Physiol Regul Integr Comp Physiol 298: R575–R583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivara MB, Yeung CK, Robinson-Cohen C, Phillips BR, Ruzinski J, Rock D, Linke L, Shen DD, Ikizler TA, Himmelfarb J: Effect of coenzyme Q 10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: The CoQ 10 biomarker trial. Am J Kidney Dis 69: 389–399, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paganini AT, Foley JM, Meyer RA: Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol 272: C501–C510, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Lagendijk J, Ubbink JB, Vermaak WJ: Measurement of the ratio between the reduced and oxidized forms of coenzyme Q10 in human plasma as a possible marker of oxidative stress. J Lipid Res 37: 67–75, 1996. [PubMed] [Google Scholar]
- 39.Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, Saigusa D, Miura D, Morikawa-Ichinose T, Saito R, Oba-Yabana I, Oe Y, Kisu K, Naganuma E, Koizumi K, Mokudai T, Niwano Y, Kudo T, Suzuki C, Takahashi N, Sato H, Abe T, Niwa T, Ito S: Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep 6: 36618, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang M, Wei R, Wang Y, Su T, Li P, Chen X: The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol 16: 303–313, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun CY, Cheng ML, Pan HC, Lee JH, Lee CC: Protein-bound uremic toxins impaired mitochondrial dynamics and functions. Oncotarget 8: 77722–77733, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurung P, Lukens JR, Kanneganti TD: Mitochondria: Diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med 21: 193–201, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, Caserotti P, Kritchevsky SB, Newman AB, Goodpaster BH, Satterfield S, Cummings SR, Harris TB; Health, Aging and Body Composition Study : Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc 59: 781–787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ: Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther 91: 923–930, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ, Kritchevsky SB: Associations between body composition and gait-speed decline: Results from the health, aging, and body composition study. Am J Clin Nutr 97: 552–560, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrades RM, Alonso J, Roca J, González de Suso JM, Campistol JM, Barberá JA, Diaz O, Torregrosa JV, Masclans JR, Rodríguez-Roisin R, Wagner PD: Cellular bioenergetics after erythropoietin therapy in chronic renal failure. J Clin Invest 97: 2101–2110, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmadiahangar A, Javadian Y, Babaei M, Heidari B, Hosseini S, Aminzadeh M: The role of quadriceps muscle strength in the development of falls in the elderly people, a cross-sectional study. Chiropr Man Therap 26: 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.