Abstract
The overall kidney survival among lupus nephritis patients has improved with currently used induction immunosuppression regimens of corticosteroids and either cyclophosphamide or mycophenolate mofetil; however, there still remains a significant number of lupus nephritis patients who do not achieve remission with these regimens. Investigators have looked at other immunosuppressive regimens for lupus nephritis, and there has been interest in the use of calcineurin inhibitors in this regard. Calcineurin inhibitors are potentially an attractive option because of their established ability to inhibit T cell function, attenuate proteinuria through non-immunologic means, and their safety in pregnancy and lactation. In this review, we discuss the findings and limitations of selected trials that evaluated the use of calcineurin inhibitors in the treatment of lupus nephritis, either with corticosteroids alone or as a component of multitarget therapy when combined with mycophenolate mofetil. There may be a role for calcineurin inhibitors among patients with heavy proteinuria, as well as younger patients with refractory lupus nephritis. The multitarget therapy trials reveal higher rates of remission compared with mycophenolate mofetil alone and cyclophosphamide; however, some trials highlight the possibility of more infectious adverse events. We discuss the need for further study of calcineurin inhibitors in more diverse patient populations and the need for trials with longer follow-up with “hard” endpoints beyond proteinuria reduction, such as worsening CKD or repeat protocol biopsies, given the calcineurin inhibitors ability to reduce proteinuria non-immunologically and thus increased rate of relapse when the drug is tapered. While there may indeed be a space for calcineurin inhibitors to help increase remission rates in lupus nephritis patients, more work is needed to help address the questions the studies available to date have yet to answer.
Keywords: Pregnancy, lupus nephritis, Calcineurin Inhibitors, Mycophenolic Acid, Follow-Up Studies, T-Lymphocytes, Cyclophosphamide, Immunosuppressive Agents, immunosuppression, proteinuria, Lactation, Adrenal Cortex Hormones, Recurrence, Biopsy, Infections, Renal Insufficiency, Chronic
Introduction
The prevalence of SLE in North America is estimated to be 23.2 per 100,000 person years (1), and half of all patients with SLE will have lupus nephritis. The 5-year kidney survival for lupus nephritis was as low as 20% before 1980; however, now only 10%–17% of patients with lupus nephritis progress to kidney failure (2). The 10-year follow-up of the Euro-Lupus Nephritis Trial (ELNT) reported 14% kidney failure rates in the low-dose cyclophosphamide group and 11% in the high-dose cyclophosphamide group (3).
Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for GN recommends corticosteroids (1A evidence) combined with either cyclophosphamide or mycophenolate mofetil (MMF; 1B evidence) as induction therapy for proliferative lupus nephritis (4). This recommendation came from several large trials, including the ELNT (5) and the Aspreva Lupus Management Study (ALMS) (6). The ELNT reported treatment failure in 16% of the low-dose cyclophosphamide group and 20% of the high-dose cyclophosphamide group, and the ALMS reported a treatment failure rate of 43.8% in the MMF group and 47% in the cyclophosphamide group. Therefore, despite improvement in kidney failure free survival, a considerable number of patients do not achieve remission with the KDIGO 2012 recommended induction regimens.
Rationale for Calcineurin Inhibition in Lupus Nephritis
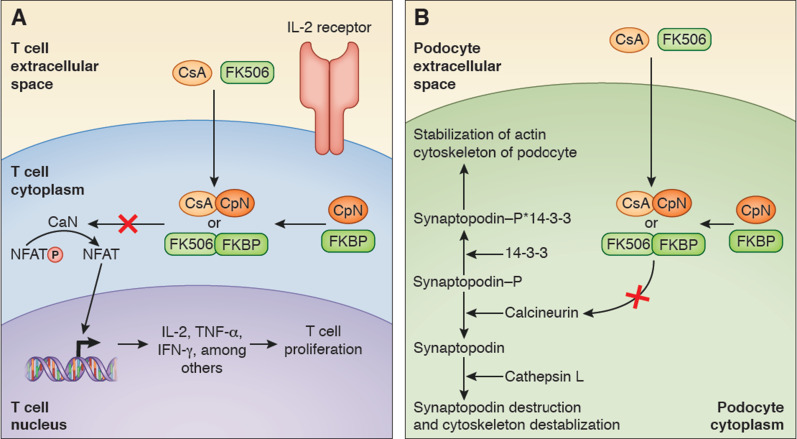
SLE is the end result of a number of immune aberrations that involve defective clearance of autoantigens and immune complexes resulting in excessive activation of T helper cells and B cells, leading to autoantibody production and complement activation and eventually, tissue inflammation and end organ injury. Calcineurin inhibitors (cyclosporin and tacrolimus) block T cell activation via suppression of the calcium- and calmodulin-dependent phosphatase calcineurin. Calcineurin inhibition leads to decreased nuclear translocation of transcription factors (e.g., NF-AT) involved with IL-2 transcription (7). In addition to its immunomodulatory effects, the calcineurin inhibitors also are able to decrease proteinuria via direct podocyte stabilization and afferent arteriole vasoconstriction (Figure 1). This antiproteinuric effect occurs independent of immune modulation, such as has been shown in patients with nephrotic syndrome due to nonimmunologic mechanisms (8). Podocyte stabilization occurs in part via preventing synaptopodin 14-3-3 degradation by cathepsin L (9). Therefore, calcineurin inhibitors are an attractive option for management of lupus nephritis because of their dual ability to inhibit T cell activation and reduce proteinuria via nonimmune-mediated mechanisms.
Figure 1.
Calcineurin inhibition results in both decreased T cell proliferation and in podocyte stabilization. (A) Cyclosporin (CsA) binds cyclophilin (CpN), and tacrolimus (FK506) binds FK binding protein (FKBP). The resulting complex (cyclosporin-cycophilin [CsA-CpN] or tacrolimus-FK binding protein [FK506-FKBP]) binds and competitively inhibits calcineurin (CaN) from dephosphorylating phosphorylated nuclear factor of activated T cells (NFAT-P) to nuclear factor of activated T cells (NFAT). When dephosphorylated, NFAT will activate translation and transcription of various cytokines (IL-2, TNF-α, and IFN-γ), which will promote T cell proliferation in part by IL-2 binding to the IL-2 receptor on the T cell. (B) Calcineurin inhibition on podocyte calcineurin activation is involved with podocyte actin cytoskeleton destabilization. Phosphorylated synaptopodin binds with 14-3-3, which in turn, stabilizes the actin cytoskeleton of the podocyte. When activated, calcineurin dephosphorylates synaptopodin, which in turn, marks it for destruction by cathepsin L, and this in turn results in podocyte cytoskeleton destabilization. Calcineurin inhibitors will, therefore, protect synaptopodin from destruction and promote actin cytoskeleton stabilization.
Experience Using Calcineurin Inhibitor Therapy in Lupus Nephritis
Trials of Calcineurin Inhibitors with Corticosteroids
The Cyclofa-Lune Study randomized 40 patients with proliferative lupus nephritis to receive 9 months of induction followed by 9 months of maintenance therapy with either cyclophosphamide or cyclosporin. Fifty-two percent of patients in the cyclophosphamide arm achieved remission compared with 43% in the cyclosporin arm after induction, and 38% of patients in the cyclophosphamide arm and 58% of patients in the cyclosporin arm remained in at least partial remission after maintenance (10). A 2006 Italian trial compared azathioprine with cyclosporin as maintenance therapy over 4 years in 75 patients who received induction with cyclophosphamide. The incidence of kidney flares between the groups, kidney function change from baseline, and drop out from adverse events were similar between groups. Concerning safety, minor infection and leukopenia were more common in the azathioprine group, whereas hypertension, arthralgias, and gastrointestinal disorders were more common in the cyclosporin group. Thirty-nine percent of patients received protocol biopsies (14 assigned to cyclosporin and 15 assigned to azathioprine) at year 2. The authors found that the activity index decreased from 5.9±3.9 to 1.4±3.2 in the cyclosporin group and from 7.4±4.2 to 0.5±1.3 in the azathioprine group, and the chronicity index increased from 2.3±1.5 to 3.7±1.8 in the cyclosporin group and from 1.7±2.0 to 3.1±2.1 in the azathioprine group (11).
Because cyclophosphamide is associated with significant toxicity, work was made toward identifying safer therapies for lupus nephritis. The ALMS capped off years of smaller studies in identifying MMF as an attractive alternative to cyclophosphamide for induction (6), and the subsequently published maintenance-phase study of the ALMS further cemented the role of MMF (12). A 2012 trial (13) enrolled 60 patients with predominantly proliferative lupus nephritis and randomized them to receive intravenous cyclophosphamide, tacrolimus, or MMF for induction along with corticosteroids. The authors published 75% overall remission rates for both MMF and tacrolimus and 60% overall remission rate for intravenous cyclophosphamide at 6 months. They also found that proteinuria decrease and serum albumin increase were 1 month faster in the tacrolimus group compared with the other groups. A larger trial from China published in 2016 (14) randomized 150 patients with active lupus nephritis to receive either MMF or tacrolimus for 6 months (followed by azathioprine for maintenance) and found that overall remission in the MMF group was 80% versus 89% in the tacrolimus group. Patients in this trial were followed for a median of 60.8 months and there were numerically more kidney flares in the tacrolimus induction group (54% had kidney flares compared with 38% in the MMF cohort). The cumulative incidence rates of a composite outcome of decline in CrCl>30%, reaching CKD stage 4/5, or death at 5 years were similar for both MMF and tacrolimus (21% and 22%, respectively). A recent retrospective study from Japan followed 26 patients with lupus nephritis over 5 years who were treated with tacrolimus for maintenance therapy. The patients had stable GFR (only one patient had a kidney flare) and reduction in their modified lupus nephritis disease activity index over the study period (15).
In class 5 lupus nephritis, immune complexes deposit in the subepithelial space and activate complement locally. The potent anaphylatoxins, C3a and C5a, are separated from the circulation by the glomerular basement membrane, and hence, no influx of inflammatory cells occurs. Injury is limited to the glomerular epithelial cells, and the primary manifestation is proteinuria. Several smaller studies have evaluated the role of calcineurin inhibitors as management for membranous lupus. A 2009 study randomized 42 patients with class 5 and nephrotic syndrome to receive intravenous cyclophosphamide, cyclosporin, or corticosteroids alone. Patients in the cyclosporin arm experienced higher rates of remission after 1 year (83% cyclosporin, 60% intravenous cyclophosphamide, and 27% prednisone alone) but had a higher relapse rate compared with the intravenous cyclophosphamide regimen arm (2 per 100 patient months for cyclosporin versus 0.2 per 100 patients months for intravenous cyclophosphamide) (16). A 2012 small, open label, randomized trial (16 patients) from China compared MMF and tacrolimus (with tapering doses in year 2) for management of pure class 5 with nephrotic syndrome and found a trend toward higher remission rates at 2 years in the MMF group (17). Sixteen patients among the 150 patients included in the previously discussed study by Mok et al. (14) had pure class 5 lupus with, on average, nephrotic-range proteinuria, and the authors found that patients enjoyed a more rapid improvement in proteinuria and numerically more remissions in the calcineurin inhibitor group after 6 months (14).
Multitarget Therapy
A 2008 prospective trial randomized 40 patients with classes 4 + 5 lupus nephritis to induction therapy with MMF + tacrolimus versus intravenous cyclophosphamide, and it found that the rate of complete remission was higher in the multitarget group compared with the intravenous cyclophosphamide group at 6 months (50% versus 5%, respectively) and 9 months (65% versus 15%, respectively) without an increase in adverse events. This study also included protocol repeat biopsies, and 15 patients (9 multitarget and 6 intravenous cyclophosphamide) underwent repeat biopsies after induction. Repeat biopsies among patients who achieved complete remission (nine patients) showed a decrease in activity index by 6.9 (95% confidence interval [95% CI], 4.1 to 9.7) and an increase in chronicity index by 1.0 (95% CI, 0.1 to 1.8). Conversely, repeat biopsies among patients who did not achieve complete remission (six patients) had increase in their chronicity index by 2.5 (95% CI, 0.3 to 4.7) (18). A randomized trial in 2015 (19) assigned 368 patients to receive either NIH dosing of intravenous cyclophosphamide (20) or MMF/tacrolimus for 6-month induction and looked at complete remission as the primary end point. It is of note that the authors used the higher NIH dosing of intravenous cyclophosphamide as opposed to the lower ELNT dosing (5). Although the authors do not provide a reason as to why the higher dosing was used, one can postulate that this may be due to the ELNT having a paucity of patients of Asian descent and few patients with more advanced CKD. The authors of this trial found that 83.5% of patients in the MMF/tacrolimus group achieved remission compared with 63.0% in the intravenous cyclophosphamide group. A continuation of this trial published in 2017 (21) evaluated MMF/tacrolimus (116 patients) versus azathioprine (90 patients) for maintenance therapy over 18 months after achieving remission in the induction trial. The complete remission rates were similar between groups by 6 months, and rates of relapse were similar between groups, with 5.47% relapse in the multitarget group versus 7.62% relapse in the azathioprine group. A prospective observational study in 2013 evaluated multitarget therapy among patients who had a suboptimal response to standard therapy. Of the 21 patients enrolled in the study, 14 of them had at least partial remission on multitarget therapy (22).
Meta-Analyses
A meta-analysis published in 2016 (23) reviewed the use of tacrolimus in lupus nephritis and incorporated nine controlled studies (eight from China). Compared with intravenous cyclophosphamide, tacrolimus alone had higher rates of overall remission (RR, 1.23; 95% CI, 1.07 to 1.41) and complete remissions (RR, 1.59; 95% CI, 1.16 to 2.19). Compared with MMF, tacrolimus alone showed no difference for achieving remission. When comparing multitarget versus intravenous cyclophosphamide, there were more overall remissions in the multitarget group (RR, 1.37; 95% CI, 1.14 to 1.63). A larger network meta-analysis in 2017 (24), which included 53 studies (45 induction trials and 8 maintenance trials) with 4222 participants of diverse backgrounds, evaluated the best first-line treatment for lupus nephritis and found that, compared with intravenous cyclophosphamide, there were more remissions with multitarget therapy (odds ratio [OR], 2.69; 95% CI, 1.74 to 4.16) and calcineurin inhibitor alone (OR, 1.74; 95% CI, 1.09 to 2.79). With respect to six maintenance trials in preventing disease relapse, calcineurin inhibitors were not different from MMF (OR, 1.13; 95% CI, 0.35 to 3.69) or azathioprine (OR, 0.64; 95% CI, 0.22 to 1.88) in preventing disease relapse. Table 1 summarizes the results of the above trials and meta-analyses evaluating calcineurin inhibitors in lupus nephritis.
Table 1.
Selected trials evaluating calcineurin inhibitors in lupus nephritis
| Trial | Design | N | Outcome Measure | Treatment Arms | Follow-Up Duration | Response Rate |
|---|---|---|---|---|---|---|
| Moroni et al. 2006 (11) | RCT | 75 | Kidney flare | Cyclosporin versus azathioprine | 4 yr | Cyclosporin: 10.6 flares/100 patient yr; azathioprine: 13.4 flares/100 patient yr |
| Bao et al. 2008 (18) | RCT | 40 | Complete remission | Tacrolimus/MMF versus iv cyclophosphamide | 9 mo | Tacrolimus/MMF: 65% complete remission; iv cyclophosphamide: 15% complete remission |
| Zavada et al. 2010 (10) | RCT | 40 | Complete remission, overall response | Cyclosporin versus cyclophosphamide | 18 mo | Cyclosporin: 37% complete remission, 58% response; cyclophosphamide: 14% complete remission, 38% response |
| Yap et al. 2012 (17) | RCT | 16 | Complete remission, partial remission, overall remission | Tacrolimus versus MMF | 24 mo | Tacrolimus: overall 55.6%; MMF: overall 71.4% |
| Li et al. 2012 (13) | RCT | 60 | Complete remission, partial remission, overall remission | Tacrolimus versus cyclophosphamide versus MMF | 24 wk | Cyclophosphamide: 60% overall; MMF: 75% overall; tacrolimus: 75% overall |
| Mok et al. 2013 (22) | Observational | 21 | Kidney response | Tacrolimus/MMF (no control group) | 12 mo | 67% kidney response, 33% no response |
| Liu et al. 2015 (19) | RCT | 362 | Complete remission | Tacrolimus/MMF versus cyclophosphamide | 24 wk | Tacrolimus/MMF: 45.9% complete remission, 83.5% overall; cyclophosphamide: 25.6% complete remission, 63.0% overall |
| Mok et al. 2016 (14) | RCT | 150 | Complete remission at 6 mo | Tacrolimus versus MMF × 6 mo; both azathioprine after | 24 wk for induction phase, 5-yr maintenance | MMF: 59% complete remission; tacrolimus: 62% complete remission after induction; MMF: 38% kidney flare versus tacrolimus: 54% kidney flare during maintenance |
| Zhang et al. 2017 (21) | RCT | 206 | Kidney relapse rate | Tacrolimus/MMF versus azathioprine (maintenance of Liu et al. 2015 [19]) | 18 mo | Tacrolimus/MMF: 5.47% kidney relapse; azathioprine: 7.62% kidney relapse |
| Rovin et al. 2019 (27) | RCT | 265 | Complete remission | High-dose voclosporin + MMF versus low-dose voclosporin + MMF versus MMF | 48 wk | Low-dose voclosporin: 49.4% complete remission; high-dose voclosporin: 39.8% complete remission; MMF: 23.9% complete remission |
RCT, randomized, control trial; MMF, mycophenolate mofetil.
The Future Landscape of Calcineurin Inhibitors in Lupus Nephritis
Despite the success of multitarget regimens for lupus nephritis in the past, both tacrolimus and cyclosporin require regular drug monitoring (25). Voclosporin is similar to cyclosporin but has a modification of a functional group that allows for potent binding to calcineurin and faster elimination of metabolites, and drug-level monitoring is not required (26). The AURA-LV study (27) was a phase 2, multicenter, randomized, control trial comparing two doses of voclosporin (23.7 or 39.5 mg twice daily) with placebo in combination with MMF and rapidly tapered corticosteroids to induce remission in proliferative lupus nephritis over 48 weeks of treatment. Compared with the previous multitarget trials, the AURA-LV study was significantly more diverse, randomizing 265 subjects across 79 centers in 20 countries. Complete remission was attained in 49.4% of patients in the low-dose voclosporin group, 39.8% of patients in the high-dose voclosporin group, and 23.9% of patients in the placebo group. The patients in the voclosporin group achieved remission faster as well. The lack of a dose effect with voclosporin alongside increased rates of adverse events in the voclosporin group tempers excitement regarding the AURA-LV study. A phase 3 trial of voclosporin, however, is pending publication, which should be more revelatory in evaluating the efficacy and safety of this agent in treating lupus nephritis.
Safety Profile of Calcineurin Inhibitor Therapy in Lupus Nephritis
Calcineurin inhibitors are associated with hypertension (partly by stimulation of the sodium chloride cotransporter [28]) and a number of metabolic side effects, including glucose intolerance and dyslipidemia (25). Moreover, long-term calcineurin inhibitor use is associated with chronic nephrotoxicity (29). The available data give mixed results regarding calcineurin inhibitor safety in lupus nephritis. In Liu et al. (19), more patients in the multitarget group dropped out because of adverse events (5.5% versus 1.7%). Moreover, there was a higher rate of serious adverse events in the multitarget group (7.2% versus 2.8%), including more pneumonias (3.9% versus 0.6%). However, in the maintenance trial by Zhang et al. (21), which was an extension of the trial by Liu et al. (19), there was actually more adverse events in the azathioprine arm (44.4%) compared with the multitarget arm (16.4%). In the AURA-LV study (27), there were more serious adverse events in the low-dose voclosporin group (28.1%) compared with the high-dose voclosporin (25%) and placebo (15.9%) groups, with the most frequent adverse event being infection. There were also more deaths in the low-dose voclosporin group (ten deaths) versus the high-dose voclosporin group (two deaths) or the placebo group (one death); however, the majority of the deaths happened at two study sites where more patients were randomized to low-dose voclosporin. With respect to AKI risk while on calcineurin inhibitor during the above trials, there were often more transient increases in serum creatinine among the calcineurin inhibitor group compared with the competitor (most frequently in the trial by Mok et al. [14]); however, there was usually no difference in kidney function at the end of the trial (with the trial by Mok et al. [14] being an exception). Nevertheless, we must keep in mind that relatively few lupus nephritis trials followed long-term use of calcineurin inhibitors to comment on long-term nephrotoxicity, and few have long-term protocol biopsies. It is of note that not all of the trials published tacrolimus and cyclosporin drug levels, and it is necessary to correlate the drug level with the observed toxicities and to use the lowest level that allows efficacy without toxicity. With respect to adverse events reported in the meta-analyses, Hannah et al. (23) report that tacrolimus compared with competitor drug has lower incidence of leukopenia, gastrointestinal side effects, and menstrual disorders and trends toward a lower incidence of infection and a higher incidence of new-onset hypertension and hyperglycemia. Moreover, Palmer et al. (24) report more frequent major infection in the MMF group compared with the calcineurin inhibitor group (although neither drug had different odds of major infection when compared with intravenous cyclophosphamide), lower odds of ovarian failure in the calcineurin inhibitor group compared with oral cyclophosphamide, and lower odds of nausea in the calcineurin inhibitor group compared with intravenous cyclophosphamide. Table 2 summarizes the most common adverse events among trials evaluating calcineurin inhibitors in lupus nephritis.
Table 2.
Summary of adverse events of calcineurin inhibitors among selected trials evaluating calcineurin inhibitors in lupus nephritis
| Trial | Calcineurin Inhibitor Used | Most Common AEs |
|---|---|---|
| Moroni et al. 2006 (11) | Cyclosporin | Hypertension (7/36), infection (7/36), arthralgia (14/36), gastrointestinal disorders (11/36) |
| Bao et al. 2008 (18) | Tacrolimus (with MMF) | GI syndrome (2/20), leukopenia (2/20), new HTN (3/20), pneumonia (1/20) |
| Zavada et al. 2010 (10) | Cyclosporin | Increased BP (10/19), transient increase in serum creatinine (3/19) |
| Li et al. 2012 (13) | Tacrolimus | Severe infection (2/20), hyperglycemia (5/20) |
| Yap et al. 2012 (17) | Tacrolimus | Infection (4/16), acute tacrolimus nephrotoxicity (2/16), worsening hypertension (1/16), new DM (1/16) |
| Mok et al. 2013 (22) | Tacrolimus (with MMF) | Infection not requiring hospitalization (12/21), diarrhea (4/21). |
| Liu et al. 2015 (19) | Tacrolimus (with MMF) | Pneumonia (13/181), any infection (91/181), upper respiratory infection (23/181), diarrhea (14/81), new-onset HTN (10/81), serious AE (13/181), withdrawn because of AE (10/181) |
| Mok et al. 2016 (14) | Tacrolimus | Minor infection (12/74), tremor (15/74), reversible increase in serum creatinine by 30% (10/74) |
| Zhang et al. 2017 (21) | Tacrolimus (with MMF) | Infection (12/116), leukopenia (9/116) |
| Rovin et al. 2019 (27) | Voclosporin (with MMF) | Low-dose VCS: infection (11/89), death (10/89), study discontinuation from AE (16/89), AKI (4/89); high-dose VCS: infection (12/88), death (2/88), study discontinuation from AE (14/88) |
AE, adverse event; MMF, mycophenolate mofetil; GI, gastrointestinal; HTN, hypertension; DM, diabetes mellitus; VCS, voclosporin.
Limitations of Published Data on Calcineurin Inhibitors in Lupus Nephritis
Many of the studies discussed above are from China, and because outcomes in lupus nephritis are heavily influenced by race and ethnicity, it is difficult to extend the positive results of Chinese studies to international populations. Moreover, remission is a heterogeneous outcome with variable definitions across lupus nephritis studies, and in the short term, it is essentially proteinuria based. Because calcineurin inhibitors lower proteinuria independent of their effect on immune function, their efficacy cannot be assessed solely by proteinuria reduction when on drug. Studies looking at 6-month remission rates are biased toward showing a calcineurin inhibitor effect, and a more appropriate assessment of these agents’ efficacy would be remission rates over a longer time horizon when the drugs have been tapered to gauge whether the remission achieved earlier is sustained. The duration of follow-up is relatively short among the induction trials included in this review, with the median follow-up time of 52 weeks and a range of 24–104 weeks. In fact, kidney flare after coming off calcineurin inhibitors is well described, and it was found in the 2016 study by Mok et al. (14), the study by Austin et al. (16), and the study by Yap et al. (17). A nonproteinuria-based outcome (e.g., progression to kidney failure, doubling of serum creatinine, or all-cause mortality) may be more appropriate for evaluating calcineurin inhibitor efficacy in lupus nephritis.
Another way to assess if the calcineurin inhibitor is having a true immunologic effect would be to follow complement and anti-dsDNA levels. It would be difficult to use this as a test of calcineurin inhibitor efficacy when multitarget therapy is used; however, some of the trials that compared calcineurin inhibitor as a single agent against one or more competitors did publish complement and dsDNA-level trends over time and found that they increased and decreased, respectively, by similar magnitudes in the calcineurin inhibitor group compared with the control group (10,13,14).
Given difficulty in using proteinuria to gauge short-term efficacy for calcineurin inhibitors, an even more robust outcome to evaluate induction-phase therapy may be repeat protocol biopsies. Early clinical and histologic outcomes can be discordant in proliferative lupus nephritis, and progression from active to chronic lesions on a biopsy remains the most consistent predictor of long-term risk for kidney failure (30). Repeat biopsies are not routinely performed in lupus nephritis trials but can be helpful in evaluating whether calcineurin inhibitors have had any effect beyond proteinuria reduction via nonimmunologic mechanisms. Although improvement in immunosuppressive regimens has led to reduced risk of reaching kidney failure among patients with lupus nephritis, there still remains a number of treatment failures with the KDIGO 2012–recommended first-line regimens. Calcineurin inhibitors along with azathioprine are among the small group of immunosuppressive agents that can be used in pregnancy and lactation. Multitarget therapy seems to offer higher rates of remission, albeit with the caveat that they have been predominately studied in a homogenous population (mostly in Asia). At this juncture, it seems that calcineurin inhibitors may have a role in lupus nephritis among younger refractory patients and patients with severe proteinuria. The increase in adverse events in some of the multitarget trials may temper excitement about calcineurin inhibitor use in lupus nephritis as does the lack of long-term nonproteinuria-based data given the known nonimmunologic effect of calcineurin inhibitors on proteinuria reduction. Publication of the phase 3 trial evaluating voclosporin will be helpful in better understanding the benefit of adding a calcineurin inhibitor to MMF in induction for lupus nephritis and in further evaluating the safety signal seen in the AURA-LV study. Larger calcineurin inhibitor studies with more diverse patient populations, longer time horizons, and protocol biopsies will help address whether the benefit of calcineurin inhibitor is largely nonimmunologic, whether race and/or ethnicity influence response to calcineurin inhibitors in lupus nephritis, and whether long-term calcineurin inhibitor exposure leads to worsening CKD in the lupus nephritis population. These trials should help further clarify the evolving role of calcineurin inhibitors in treating lupus nephritis.
Disclosures
Dr. Bomback, Dr. Peleg, and Dr. Radhakrishnan have nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W: The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology (Oxford) 56: 1945–1961, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Bomback AS: An update on therapies for proliferative lupus nephritis: How certain can we be about the evidence? Am J Kidney Dis 72: 758–760, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 69: 61–64, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, Somers MJ, Trachtman H, Waldman M: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis 62: 403–441, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed ER, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46: 2121–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J, Solomons N, Wofsy D; Aspreva Lupus Management Study Group: Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20: 1103–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok CC: Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31: 429–438, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Bensman A, Niaudet P: Non-immunologic mechanisms of calcineurin inhibitors explain its antiproteinuric effects in genetic glomerulopathies. Pediatr Nephrol 25: 1197–1199, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zavada J, Pesickova S, Rysava R, Olejarova M, Horák P, Hrncír Z, Rychlík I, Havrda M, Vítova J, Lukác J, Rovensky J, Tegzova D, Böhmova J, Zadrazil J, Hána J, Dostál C, Tesar V: Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: The Cyclofa-Lune study. Lupus 19: 1281–1289, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Doria A, Mosca M, Alberighi OD, Ferraccioli G, Todesco S, Manno C, Altieri P, Ferrara R, Greco S, Ponticelli C: A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 1: 925–932, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N; ALMS Group: Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 365: 1886–1895, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Ren H, Zhang Q, Zhang W, Wu X, Xu Y, Shen P, Chen N: Mycophenolate mofetil or tacrolimus compared with intravenous cyclophosphamide in the induction treatment for active lupus nephritis. Nephrol Dial Transplant 27: 1467–1472, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Mok CC, Ying KY, Yim CW, Siu YP, Tong KH, To CH, Ng WL: Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: A randomised controlled trial and long-term follow-up. Ann Rheum Dis 75: 30–36, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Karasawa K, Uchida K, Kodama M, Moriyama T, Nitta K: Long-term effects of tacrolimus for maintenance therapy of lupus nephritis: A 5-year retrospective study at a single center. Rheumatol Int 38: 2271–2277, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Austin HA 3rd, Illei GG, Braun MJ, Balow JE: Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20: 901–911, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yap DY, Yu X, Chen XM, Lu F, Chen N, Li XW, Tang CS, Chan TM: Pilot 24 month study to compare mycophenolate mofetil and tacrolimus in the treatment of membranous lupus nephritis with nephrotic syndrome. Nephrology (Carlton) 17: 352–357, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT, Li LS: Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 19: 2001–2010, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, Chen J, Lin H, Liu F, He Y, He Y, Miao L, Chen N, Li Y, Gu Y, Shi W, Hu W, Liu Z, Bao H, Zeng C, Zhou M: Multitarget therapy for induction treatment of lupus nephritis: A randomized trial. Ann Intern Med 162: 18–26, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Boumpas DT, Austin HA 3rd, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, Balow JE: Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 340: 741–745, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Liu Z, Zhou M, Liu Z, Chen J, Xing C, Lin H, Ni Z, Fu P, Liu F, Chen N, He Y, Liu J, Zeng C, Liu Z: Multitarget therapy for maintenance treatment of lupus nephritis. J Am Soc Nephrol 28: 3671–3678, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok CC, To CH, Yu KL, Ho LY: Combined low-dose mycophenolate mofetil and tacrolimus for lupus nephritis with suboptimal response to standard therapy: A 12-month prospective study. Lupus 22: 1135–1141, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Hannah J, Casian A, D’Cruz D: Tacrolimus use in lupus nephritis: A systematic review and meta-analysis. Autoimmun Rev 15: 93–101, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Palmer SC, Tunnicliffe DJ, Singh-Grewal D, Mavridis D, Tonelli M, Johnson DW, Craig JC, Tong A, Strippoli GFM: Induction and maintenance immunosuppression treatment of proliferative lupus nephritis: A network meta-analysis of randomized trials. Am J Kidney Dis 70: 324–336, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, Ling S, Huizinga RB, Meier-Kriesche HU; PROMISE Investigators: The PROMISE study: A phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant 11: 2675–2684, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Mayo PR, Ling SY, Huizinga RB, Freitag DG, Aspeslet LJ, Foster RT: Population PKPD of voclosporin in renal allograft patients. J Clin Pharmacol 54: 537–545, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Rovin BH, Solomons N, Pendergraft WF 3rd, Dooley MA, Tumlin J, Romero-Diaz J, Lysenko L, Navarra SV, Huizinga RB; AURA-LV Study Group: A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int 95: 219–231, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH: The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams D, Haragsim L: Calcineurin nephrotoxicity. Adv Chronic Kidney Dis 13: 47–55, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, Nagaraja H, Indrakanti D, Rovin BH: Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 32: 1338–1344, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]