Abstract
Transgenic maize plants expressing dsRNA targeting western corn rootworm (WCR, Diabrotica virgifera virgifera) DvSSJ1 mRNA, a Drosophila snakeskin (ssk) ortholog, show insecticidal activity and significant plant protection from WCR damage. The gene encodes a membrane protein associated with the smooth sepate junction (SSJ) which is required for intestinal barrier function. To understand the active RNA form that leads to the mortality of WCR larvae by DvSSJ1 RNA interference (RNAi), we characterized transgenic plants expressing DvSSJ1 RNA transcripts targeting WCR DvSSJ1 mRNA. The expression of the silencing cassette results in the full-length transcript of 901 nucleotides containing a 210 bp inverted fragment of the DvSSJ1 gene, the formation of a double-stranded RNA (dsRNA) transcript and siRNAs in transgenic plants. Our artificial diet-feeding study indicates that dsRNAs greater than or equal to approximately 60 base-pairs (bp) are required for DvSSJ1 insecticidal activity. Impact of specificity of dsRNA targeting DvSSJ1 mRNA on insecticidal activities was also evaluated in diet bioassay, which showed a single nucleotide mutation can have a significant impact or abolish diet activities against WCR. These results provide insights as to the functional forms of plant-delivered dsRNA for the protection of transgenic maize from WCR feeding damage and information contributing to the risk assessment of transgenic maize expressing insecticidal dsRNA.
Subject terms: Biotechnology, Plant sciences
Introduction
RNA interference (RNAi) is a sequence-specific gene silencing mechanism in eukaryotes that is triggered by double-stranded RNA (dsRNA)1 and has become an important tool for reverse functional genomics and applications in biomedicine and agriculture2,3. The dsRNA is first processed by the nuclease Dicer in an ATP-dependent reaction that cleaves long dsRNA into small interfering RNA (siRNA) fragments of 21–26 nucleotides (nt) in length with a 2 nt overhang on the 3′ end. These siRNA fragments are further processed by the RNA-induced silencing complex (RISC) into two single-stranded RNAs, the passenger strand and the guide strand. The guide strand binds with an Argonaute (AGO) protein, the catalytic component of RISC, while the passenger strand is degraded by AGO. The guide strand complex is then paired with its homologous messenger RNA sequence by precise complementation resulting in the endonucleolytic cleavage of the target mRNA4.
The western corn rootworm (WCR), Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae), is one of the most economically important and invasive pests of maize throughout the US Corn Belt5. WCR shows a robust systemic RNAi response induced by direct injection of dsRNA6, oral feeding of dsRNA provided in artificial diet7,8, or transgenic plants expressing dsRNA7,8. By its very nature, RNAi insect control technology represents a different mode of action than protein-based traits (i.e. Cry proteins from Bacillus thuringiensis). The putative Drosophila snakeskin (ssk) homolog of WCR (DvSSJ1) has previously been shown to be an effective RNAi target for corn rootworm control8,9. The target gene, DvSSJ1, encodes a membrane protein associated with the smooth sepate junction (SSJ) which is required for intestinal barrier function. Three SSJ-associated membrane proteins, Ssk, Mesh, and Tsp2A, have been reported in Drosophila10 and their homologous counterparts were identified from WCR9. These proteins play a crucial role in maintaining tissue homeostasis through the regulation of stem cell proliferation and enterocyte behavior in the Drosophila adult midgut11. When the western corn rootworm feeds on DvSSJ1 dsRNA, the smooth septate junction gene is down-regulated by the insect’s RNAi machinery resulting in pest mortality9.
Transgenic plants expressing dsRNA targeting DvSSJ1 show insecticidal activity and significant plant protection from WCR damage8. Diet based insect feeding assays using dsRNA targeting DvSSJ1 mRNA demonstrate that SSJ protein accumulation and mortality was negatively correlated and that the interactions of SSJ proteins and barrier function are impaired in RNAi-compromised or knockout insects9. These findings support that the malfunction of SSJ complexes in the midgut triggered by DvSSJ1 RNAi were the main effects leading to the death of WCR. The DvSSJ1 is a midgut-specific gene in WCR and its functions are consistent with the biological functions described for Drosophila9. Two forms of transgenic DvSSJ1 dsRNAs present in plant tissues are long dsRNAs and siRNAs8. Similar expression patterns have been reported for Snf77 and v-ATPase C12 in transgenic maize plants. Diet-based studies of Snf713 and v-ATPase C12 showed that dsRNA of at least 60 bp in length resulted in high levels of larval mortality, while dsRNAs with lengths shorter than 60 bp did not cause a significant RNAi response. Although the mechanism of uptake at the cellular level is still unknown, an inefficient RNAi response with short dsRNA or 21-bp siRNA demonstrated less uptake in larval midgut cells for both snf713 and DvSSJ19.
The goal of the current study was to understand the active RNA form of transgenic plants expressing DvSSJ1 targeting the SSJ of WCR by analyzing: (1) sequences and expression of DvSSJ1 transcripts in transgenic plants; (2) functional forms of RNAs targeting the SSJ of WCR in planta; and (3) impacts of length of dsRNA and specificity (mismatches) of 21 bp siRNAs embedded in the Green Fluorescence Protein (GFP) sequence on insecticidal activity in artificial diet. Our data indicate that a single mismatch in siRNA (21mer) embedded in GFP sequence can significantly reduce insecticidal activity and dsRNAs greater than or equal to approximately 60 base-pairs (bp) are required for larval mortality in the artificial diet bioassays. Furthermore, transgenic plants only expressing certain 21 bp siRNAs matching the target sequence (DvSSJ1) were not efficacious. These results provide additional implications for the effectiveness of dsRNA for transgenic maize to control WCR feeding damage and contribute to the risk assessment of transgenic maize expressing insecticidal dsRNA.
Results and discussion
Molecular characterization of the transgenic plants expressing DvSSJ1 and understanding the active form of DvSSJ1 dsRNA against WCR in planta is important for the risk assessment of transgenic maize expressing insecticidal dsRNA and the effective design of RNAi hairpin constructs for WCR control. To gain insight into the functional forms of dsRNA effective against WCR artificial diet-based feeding and transgenic plant-based testing were performed to identify the active forms of DvSSJ1 dsRNA against WCR. Northern blot and sequence analyses of DvSSJ1 dsRNA derived from transgenic maize indicated the presence of detectable levels of both intact dsRNA and plant-processed siRNAs. This posed the question of which RNA form in planta, full-length dsRNA or siRNA, or both, caused the RNAi response in WCR.
Characterization of DvSSJ1 dsRNA expression in transgenic maize
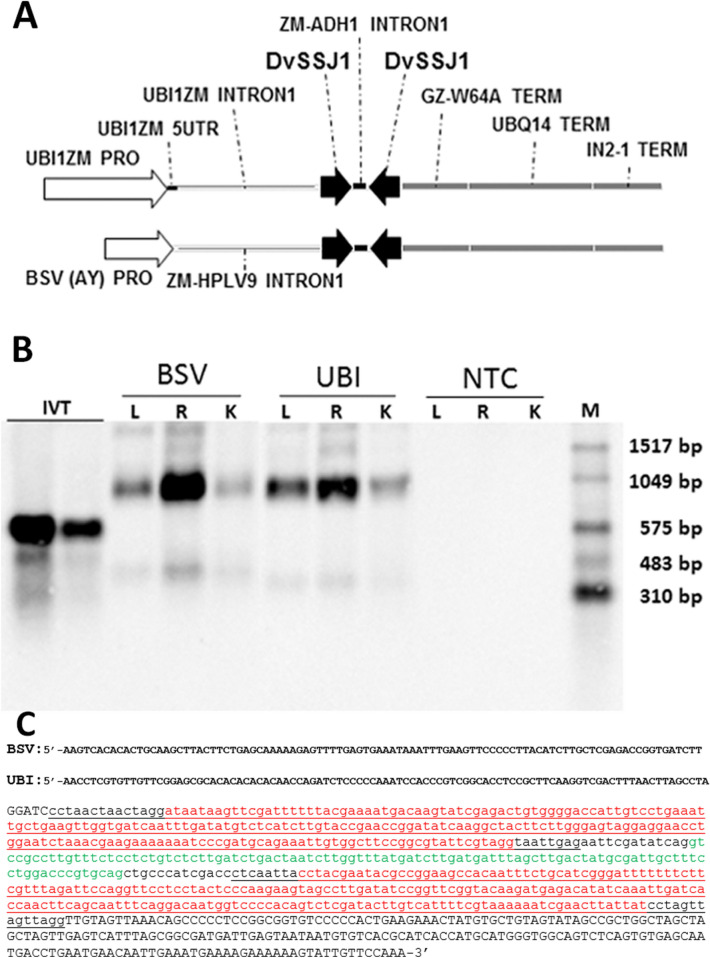
We previously reported that transgenic plants expressing dsRNA targeting DvSSJ1 show insecticidal activity and significant plant protection from WCR damage8. In this study, the DvSSJ1 expression cassette (Fig. 1A) contained the DvSSJ1 inverted stem-loop configuration under control of either a ZmUBI or BSV(AY)14 promoter with a triple terminator set as described in the Methods. These terminators were used to reduce any impact on transcriptional interference15 of a downstream expression cassette. Single copy T0 transformants showing efficacy against WCR8 were selected for further characterization at the T1 generation. To understand the active form of the DvSSJ1 RNA molecule triggering WCR mortality, we characterized the full-length DvSSJ1 transcript produced in T1 transgenic plants using 5′and 3′ rapid amplification of cDNA ends (RACE) (Supplementary Fig. 1 and 2A) and subsequent cDNA sequencing. The results from the sequencing of cDNA representing the long dsRNA transcripts revealed additional 5′sequence upstream of the expected DvSSJ1 dsRNA region (Fig. 1C). The presence of this additional sequence was confirmed by treatment of the transcript with RNase If which resulted in the removal of the single-strand region and produced a lower migrating band in Northern blots (Supplementary Fig. 1B). The molar ratio of the full-length transcript (top band = 901nt [UBI] and 900nt [BSV]) to the dsRNA hairpin ( bottom band = 590nt) was variable and likely influenced by different RNA isolation and probe preparation methods (Fig. 1B). The 901-nucleotides (nt) of the ZmUBI::DvSSJ1 transcript contains additional nucleotide sequences at the 5′ and 3′ ends, shown in black and capital letters (Fig. 1C), that match to a portion of the 5′ UTR of the maize polyubiquitin (S94464.1) gene and to the maize zein Zc2 gene (X53514.1) terminator both of which are elements found in the expression construct (Supplementary Fig. 2B and 2C). As expected, the only differences identified between ZmUBI and BSV promoter-driven constructs were at the 5′ end of the transcript (Fig. 1C). A probe targeting the 3′ end of the DvSSJ1 transcript (Supplementary Fig. 1A) was used to visualize DvSSJ1 expression in the transgenic root tips using in situ hybridization (Fig. 2 and Supplementary Fig. 3–4). The results showed the strongest expression/accumulation of DvSSJ1 transcript in the root apical meristem region (Supplementary Fig. 4A) while the mature root region showed weaker expression mostly near vascular bundle cells (Supplementary Fig. 4B).
Figure 1.
Charactering expression of DvSSJ1 transcript in T1 transgenic plants. (A) Diagram of DvSSJ1 expression cassette containing a promoter and terminators; Intron region from the Zea mays ortholog of an Oryza sativa (rice) hypothetical protein (ZM-HPLV9); (B) Northern analysis of DvSSJ1 expression in transgenic tissues collected from leaf (L), root (R), and kernel (K); purified mRNAs (200 ng) were compared to in vitro transcription (IVT; 10 pg and 5 pg) of DvSSJ1 control and RNA size marker (M). Plants of non-transgenic control (NTC) were included. The blot was probed with the 210 bp DvSSJ1 antisense riboprobe labeled with digoxigenin. The estimated amount (pg) of the top band were analyzed by ImageJ. (C) The sequence of the full DvSSJ1 transcript was analyzed by both cDNA sequencing and 5′ and 3′ RACE (Supplementary Fig. 2). The 901-nucleotide (nt) of full DvSSJ1 transcript (UBI promoter; 900 nt for BSV) contains the 5′ or 3′ end of additional sequences shown in black and capital letter, which are partially matched to 5UTR of maize polyubiquitin (C; S94464.1) and terminator of maize zein Zc2 gene (D; X53514.1) as part of the construct elements. Underline part of the sequence (232 bp) can form dsRNA stem and loop region in the middle (590 nt). DvSSJ1 210 bp was highlighted in red and truncated maize ADH1 intron1 in green.
Figure 2.

Visualization of DvSSJ1 transcript in the transgenic root tips. Transgenic root samples were collected at the V6 stage in the greenhouse and hybridized with DvSSJ1 probe. Single red dot presents one DvSSJ1 transcript in the root cell. Scale bar = 60 µm.
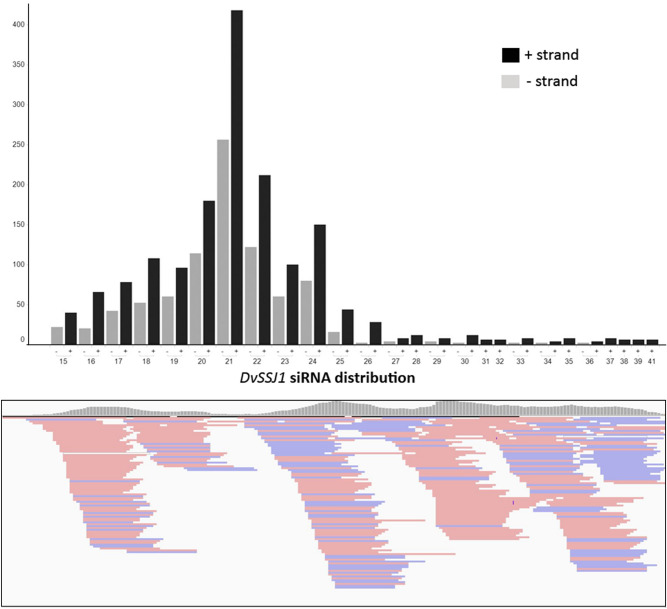
DvSSJ1 siRNAs in plants were detected by small RNA sequencing (Fig. 3) and siRNA northern analysis (Fig. 4B). The 18–41-nt siRNA sequences mapped to the sense and antisense strands of the DvSSJ1 fragment sequence (Fig. 3). The prevalent species of siRNA appeared to be 21-nt fragments, consistent with previous findings in transgenic maize plants containing RNAi constructs7,12. These siRNAs were generally distributed across the 210 bp-length of the DvSSJ1 fragment sequence but there were specific sequence regions accumulating higher siRNA species than others. There were no significant changes in patterns of DvSSJ1 siRNA accumulation between the different evaluated promoters or terminators (Supplementary Fig. 5 and 6B), and leaf or root tissue types (Supplementary Fig. 6A). The contribution of the promoter, hairpin dsRNA sequences and/or relative dynamics of dsRNA formation, Dicer processing, and degradation may impact the level of intact full-length DvSSJ1 dsRNA and siRNAs in transgenic maize plants.
Figure 3.
Analyses of DvSSJ1 siRNA expression in transgenic plants. Size distribution and strand orientation of DvSSJ1 small RNAs between 15 and 41 nucleotides were collected from root tissue under the control of the UBI promoter and showed in the top panel. Sense (red) and antisense (blue) of DvSSJ1 siRNAs were mapped to 210 bp DvSSJ1 fragment (Bottom panel). DvSSJ1 siRNA reads were visualized using Integrative Genomics Viewer software 2.8 (Broad Institute, Cambridge, MA, USA) (https://software.broadinstitute.org/software/igv/).
Figure 4.
Northern blot analyses of T0 DvSSJ1 root samples. (A) Northern blots of eight DvSSJ1 dsRNA events and a non-transgenic control (NTC). A top diagram illustrates the dsRNA expression cassette. Zm-actin (Accession #: EU952376) was included as a reference gene for northern analysis (top panel). Plants containing 210 bp DvSSJ1 cassette expressed both long dsRNA transcripts (middle panel) and siRNAs (bottom panel). There were two long dsRNA bands representing full DvSSJ1 transcript (top arrow) and dsRNA hairpin (bottom arrow). (B) Northern blots of DvSSJ1 siRNA containing events and a non-transgenic control (NTC). Two separate artificial miRNA constructs were designed to express 21-bp DvSSJ1 siRNA-1 (TCCTTGATATCCGGTTCGGTA) and siRNA-2 (TAGTAGCCTTGATATCCGGTT). Exiqon LNA 5′ Biotin-labelled DNA probes (5 ng ml−1) were used for siRNA northern analyses and zm-miR168 was included as an internal control (Supplementary Table 3).
Comparison of homologous sequences of DvSSJ1 from other insect groups
A bioinformatics approach was used to assess how conserved the SSJ gene was across different organisms with varied evolutionary distance from western corn rootworm. We identified and selected homologs of DvSSJ1 from published data8,16 and carried out transcriptome sequencing for six additional insects (Supplementary Fig. 7) representing four different orders (Supplementary Table 1). The sequences of twenty species, representing four families within the order Coleoptera, four families within the order Lepidoptera, and one family each from the order Hymenoptera and Hemiptera were compared to the 210 bp sequence from the WCR SSJ gene to determine the percent similarity, number of single nucleotide polymorphisms (SNPs), and the number of 21nt matches. In silico analysis was focused primarily within the order Coleoptera, with a total of 13 species assessed, because the DvSSJ1 dsRNA is targeted to match the sequence of the smooth septate junction protein 1 (DvSSJ1) gene from WCR. The closest genomic sequence match (percent identity to the 210 bp DvSSJ1 dsRNA sequence) was WCR (D. virgifera virgifera), which as expected had a 100% sequence match, 0 SNPs, and 190 21nt matches to the DvSSJ1 dsRNA sequence (Table 1). The ssj1 gene from the closely related species, northern corn rootworm (NCR; D. barberi), shared 97.1% identity with the DvSSJ1 dsRNA sequence, with 6 SNPs and 135 21nt matches. The ssj1 gene from the southern corn rootworm (SCR; D. undecimpunctata) shared 92.9% identity with the DvSSJ1 dsRNA sequence, with 15 SNPs and 79 21nt matches. The ssj genes from the other Coleoptera within the family Chrysomelidae [Colorado potato beetle (Leptinotarsa decemlineata), Crucifer flea beetle (Phyllotreta cruciferae), and Striped flea beetle (Phyllotreta striolata)] as well as species within the family Tenebrionidae, the family Coccinellidae, and the family Staphylinidae had decreasing percent identity with the DvSSJ1 dsRNA sequence, ranging from 77.6 to 61.9% similarity and an increasing number of SNPs (ranging from 47 to 80). All Lepidoptera species within the four familes, as well as the honey bee (Apis mellifera) and the Insidious flower bug (Orius insidiosus) also had lower percent identity with the DvSSJ1 dsRNA sequence, ranging from 64.8 to 60% identity, and an increased number of SNPs (ranging from 74 to 84). There were zero 21nt matches observed across all of the non-Diabrotica species analyzed.
Table 1.
Sequence comparison of DvSSJ1 homologous.
| Order | Name | Percent identity to DvSSJ1 dsRNA | Number of SNPs | Number of 21 nt matches (or longest nt sequence)* |
|---|---|---|---|---|
| Coleoptera | Diabrotica virgifera virgifera | 100 | 0 | 190 |
| Diabrotica longicornis | 97.1 | 6 | 135 | |
| Diabrotica undecimpunctata | 92.9 | 15 | 79 | |
| Phyllotreta cruciferae | 77.6 | 47 | 0 (20) | |
| Phyllotreta striolata | 76.2 | 50 | 0 (19) | |
| Leptinotarsa decemlineata | 73.3 | 56 | 0 (12) | |
| Tribolium castaneum | 69.5 | 64 | 0 (11) | |
| Zophobas morio | 69 | 65 | 0 (10) | |
| Epilachna varivestis | 67.6 | 68 | 0 (12) | |
| Tenebrio molitor | 65.2 | 73 | 0 (10) | |
| Dalotia coriaria | 64.3 | 75 | 0 (8) | |
| Cryptolaemus montrouzieri | 63.3 | 77 | 0 (8) | |
| Coleomegilla maculata | 61.9 | 80 | 0 (13) | |
| Hymenoptera | Apis mellifera | 68.1 | 67 | 0 (10) |
| Hemiptera | Orius insidious | 61.9 | 80 | 0 (11) |
| Lepidoptera | Vanessa cardui | 64.8 | 74 | 0 (8) |
| Ostrinia nubilalis | 64.2 | 79 | 0 (9) | |
| Spodoptera fugiperda | 62.9 | 78 | 0 (11) | |
| Cydia pomonella | 60.5 | 83 | 0 (8) | |
| Helicoverpa zea | 60 | 84 | 0 (8) |
The sequences of twenty species, representing four families within the order Coleoptera, four families within the order Lepidoptera, and one family each from the order Hymenoptera and Hemiptera were compared to the 210 bp sequence from the WCR SSJ gene to determine the percent similarity, number of single nucleotide polymorphisms (SNPs), and the number of 21nt matches.
*Longest nucleotide sequence showing 100% match to DvSSJ1 210 sequence.
Toxicity of DvSSJ1 dsRNA against the target organism
dsRNA length requirements
To assess the relationship of dsRNA fragment length and insecticidal activity, a series of seven different lengths of DvSSJ1 dsRNA was generated as described in the Method and Supplementary Fig. 9A and 10. WCR neonates were provided with seven different lengths of DvSSJ1 dsRNA incorporated into a standard artificial diet as described in Method. Acceptable control mortality of WCR associated with this diet was set at 30% given the variability of WCR performance in laboratory bioassays17. WCR mortality was significantly greater than the control for each test substance at 60 base pairs (bp) of length and greater (Table 2). There was a statistically significant decrease in weight of WCR fed the DvSSJ1 40 bp fragment (P = 0.0448) though mortality was not affected (P = 0.3457). While the mortality associated with the 21 bp treatment exceeded the 30% acceptability criteria, this was not significant (P = 0.1988). A minimum length of dsRNA for efficacious RNAi activity in WCR diet was estimated at about 60-bp. This result is similar to that reported for Snf713 and Dv v-ATPase C12 in WCR. This result also corroborated with those from previous studies that the WCR midgut had efficient uptake 210-bp long DvSSJ19 and 240-bp-long Snf713 dsRNAs but did not have efficient uptake 21-bp siRNAs.
Table 2.
Analysis of WCR mortality fed various lengths of DvSSJ1 dsRNA.
| Treatment description | Mortality (%) | 95% Confidence limit for mortality | Fisher's test P value for mortality | Mean weight (mg) (95% confidence interval) | Weight range (mg) | P value for weight |
|---|---|---|---|---|---|---|
| Bioassay control diet | 17.9 | 6.06–36.9 | – | 1.28 (0.972–1.58) | 0.1–2.5 | – |
| 21 bp | 31.0 | 15.3–50.8 | 0.1988 | 1.52 (1.19–1.85) | 0.2–3.0 | 0.8561 |
| 40 bp | 25.9 | 11.1–46.3 | 0.3457 | 0.890 (0.561–1.22) | 0.1–2.6 | 0.0448* |
| 60 bp | 50.0 | 31.3–68.7 | 0.0101* | 0.927 (0.547–1.31) | 0.3–2.0 | 0.0778 |
| 80 bp | 82.8 | 64.2–94.2 | < 0.0001* | 0.300 (-0.358–0.958) | 0.1–0.5 | 0.0044* |
| 100 bp | 96.6 | 82.2–99.9 | < 0.0001* | 0.100 | NA | – |
| 150 bp | 96.6 | 82.2–99.9 | < 0.0001* | 0.500 | NA | – |
| 210 bp | 93.1 | 77.2–99.2 | < 0.0001* | 0.250 ± 0.212 | 0.1–0.4 | – |
A series of seven different lengths of DvSSJ1 dsRNA was incorporated into a standard artificial diet and WCR mortality was recorded as described in Method.
*A statistically significant difference (P value < 0.05) was observed.
dsRNA specificity test
To assess the relationship between dsRNA fragment specificity and insecticidal activity, a 21 bp DvSSJ1 siRNA (TACCGAACCGGATATCAAGGC) was selected based on previous dsRNA feedings9 and was flanked by the GFP nucleotide sequence to ensure dsRNA uptake in the WCR gut9,12. The purpose was to better understand what level of complementarity was required to elicit biological activity in WCR. Increasing single nucleotide polymorphisms (SNPs) were introduced in various locations within the DvSSJ1 21-mer (Supplementary Fig. 9B) and fed to WCR over 14 days to assess biological activity. Acceptable WCR control mortality for these bioassays was considered to be less than or equal to 30%. For the 210 bp GFP sequence 34.5% mortality was observed (Table 3) however, it was not statistically different from the RNase free water control (P = 0.2299) and was significantly different when compared with the 21 bp DvSSJ1 embedded in the GFP nucleotide sequence (P = 0.0361). An additional bioassay was conducted to further establish whether the GFP sequence had an effect on WCR mortality or weight, and no activity was observed in this second study (P = 1.000 and 0.6546 for mortality and weight, respectively, Supplementary Table 2).
Table 3.
Analysis of WCR mortality for dsRNA specificity data.
| Treatment description | Mortality (%) | 95% Confidence limit | FDR adjusted P value versus 21 bp DvSSJ1 in GFP | Fisher's test P value versus GFP control |
|---|---|---|---|---|
| RNAse free H2O | 17.2 | 5.85–35.8 | 0.0006* | 0.2299 |
| GFP control (210 bp GFP) | 34.5 | 17.9–54.3 | 0.0361* | – |
| DvSSJ1 21 bp embedded within 210 bp GFP | 68.0 | 46.5–85.1 | – | 0.0281* |
| DvSSJ1 1 bp point mutation (loc 1) | 55.2 | 35.7–73.6 | 0.4073 | 0.1864 |
| DvSSJ1 2 bp point mutations (locs 1, 3) | 20.0 | 7.71–38.6 | 0.0008* | 0.2516 |
| DvSSJ1 3 bp point mutations (locs 1, 3, 5) | 23.3 | 9.93–42.3 | 0.0018* | 0.3985 |
| DvSSJ1 4 bp point mutations (locs 1, 3, 5, 7) | 17.2 | 5.85–35.8 | 0.0006* | 0.2299 |
| DvSSJ1 5 bp point mutations (locs 1, 3, 5, 7, 9) | 17.9 | 6.06–36.9 | 0.0006* | 0.2299 |
| DvSSJ1 1 bp point mutation (loc 11) | 16.7 | 5.64–34.7 | 0.0006* | 0.1432 |
| DvSSJ1 210 bp dsRNA | 92.9 | 76.5–99.1 | 0.0378* | < 0.0001* |
Increasing single nucleotide polymorphisms (SNPs) were introduced in various locations (loc) within the DvSSJ1 21-mer and fed to WCR over 14 days to assess WCR mortality.
*A statistically significant difference (P value < 0.05) was observed.
The 21-bp DvSSJ1 fragment embedded in the GFP sequence demonstrated the activity to WCR with significant differences from the GFP control (P = 0.0361) and the RNase free water control (P = 0.0006). Introducing 1 SNP at the first of the 21 base pairs (location 1 or loc 1) did not significantly reduce the biological activity as compared with the exact 21 bp DvSSJ1 fragment (P = 0.4073). Introducing a second SNP at location 3, however, ablated the activity of the DvSSJ1 fragment (P = 0.0008) when compared to the original 21-mer embedded in GFP nucleotide sequence. Similar observations were noted with additional SNPs (3–5) accumulated in the native 21 bp DvSSJ1 fragment (Table 3). Introducing a single SNP at location 11 also eliminated bioactivity of the dsRNA fragment (P = 0.0006). Similar observations were noted for the weight endpoint (Table 4). Therefore, not only does the number of SNPs affect the activity of dsRNA actives, but the relative location of those SNPs is also an important factor in determining activity. The number of SNPs in various locations of the 21nt DvSSJ1 siRNA were designed in this study based on a preliminary experiment (Supplementary Fig. 8). These results confirm that the siRNA seed region (bases 2–8) and splice site (bases 10–11) are the least tolerant of mismatches because of their active role in gene silencing18.
Table 4.
Summary analysis of WCR weight results for dsRNA specificity data.
| Treatment description | Mean weight (mg) (95% confidence interval) | Range (mg) | Adjusted P value versus 21 bp DvSSJ1 in GFP | P value versus GFP control |
|---|---|---|---|---|
| RNAse free H2O | 1.93 (1.61–2.26) | 0.4–3.5 | < 0.0001* | 0.9374 |
| GFP control (210 bp GFP) | 1.95 (1.59–2.31) | 0.2–3.3 | < 0.0001* | – |
| DvSSJ1 21 bp embedded within 210 bp GFP | 0.375 (-0.183–0.933) | 0.1–1.1 | – | < 0.0001* |
| DvSSJ1 1 bp point mutation (loc 1) | 1.03 (0.593–1.47) | 0.3–2.3 | 0.2387 | 0.0016* |
| DvSSJ1 2 bp point mutations (locs 1, 3) | 1.23 (0.903–1.55) | 0.1–2.3 | 0.0445* | 0.0034* |
| DvSSJ1 3 bp point mutations (locs 1, 3, 5) | 1.85 (1.52–2.18) | 0.3–3.2 | < 0.0001* | 0.6728 |
| DvSSJ1 4 bp point mutations (locs 1, 3, 5, 7) | 1.62 (1.29–1.94) | 0.2–3.0 | 0.0012* | 0.1727 |
| DvSSJ1 5 bp point mutations (locs 1, 3, 5, 7, 9) | 2.00 (1.67–2.33) | 0.4–2.8 | < 0.0001* | 0.8486 |
| DvSSJ1 1 bp point mutation (loc 11) | 1.37 (1.05–1.68) | 0.3–3.0 | 0.0130* | 0.0173* |
| DvSSJ1 210 bp dsRNA | 1.40 ± 0.707 | 0.9–1.9 | – | – |
Increasing single nucleotide polymorphisms (SNPs) were introduced in various locations (loc) within the DvSSJ1 21-mer and fed to WCR over 14 days to assess WCR weight.
*A statistically significant difference (P value < 0.05) was observed.
Long dsRNA is the insecticidal active form of RNA molecules in planta
To characterize which forms of DvSSJ1 RNA are the active molecule for mortality of WCR larvae, we designed and generated transgenic plants expressing only 21 bp siRNA of DvSSJ1 compared with plants expressing both long dsRNA and dicer processed siRNAs9,12. Two small RNA sequences were selected based on the results from small RNA profiling of WCR-fed 210-bp DvSSJ1 dsRNA9. DvSSJ1 21nt siRNAs were incorporated into the maize miR396h backbone19,20 as artificial miRNA (amiRNA) constructs21. A construct expressing the 210-bp DvSSJ1 cassette with the same promoter (UBI) and terminator (PINII) was included for comparison: this cassette produces both long dsRNAs and siRNAs in transgenic tissues. Eight to twelve T0 transgenic lines expressing 21-bp DvSSJ1 siRNA-1, -2 or dsRNA were selected for greenhouse assay and infested with 1,000 WCR eggs at the V6 (six-leaf) stage. Total RNA was extracted from root tissues for Northern blot and QuantiGene analysis to determine RNA expression in T0 transgenic plants. Both DvSSJ1 long dsRNA transcripts and dsRNA derived small RNAs (21 to 24-nt RNAs) were analyzed on Northern blots (Fig. 4). QuantiGene Singleplex22 siRNA probes (Supplementary Fig. 11) were designed by eBioscience (Thermo Fisher Scientific, Inc.). The probes were validated for sensitivity and specificity for mature siRNA-1 and siRNA-2 in plant samples (Supplementary Table 5). Expression analyses showed that the amiRNA constructs produced 3.6-fold (siRNA-1; 7.45 pg ug−1 total RNA) or 12.2-fold (siRNA-2; 8.38 pg ug−1 total RNA) higher expression compared to the same siRNAs from the 210 bp dsRNA construct (Fig. 5A, B). DvSSJ1 long dsRNA transcripts was only detected in transgenic plants expressing the 210-bp DvSSJ1 cassette (Fig. 5C).
Figure 5.
Characterizations of transgenic plants expressing DvSSJ1 siRNAs and dsRNA. T0 single copy plants were grown in the greenhouse and root tissues were collected for siRNA-1 (A), siRNA-2 (B) and 210-bp dsRNA (C) quantification as described in Method; (D) The DvSSJ1 transgenic lines and non-transgenic control (NTC) were selected for T0 greenhouse assay. Eight plants per DvSSJ1 dsRNA line and NTC plants were assayed for WCR feeding damage. The CRW nodal injury score (mean ± SD) of dsRNA line was significantly different (P value < 0.0004–0.004) between the NTC and siRNA transgenic lines. No significant difference between two small RNA constructs and non-transgenic control (NTC).
Plants were scored for WCR feeding damage23 three weeks after infestation. The average nodal injury score for the transgenic 210-bp DvSSJ1 events was less than 0.625, which was a significant reduction from the corresponding score of 1.6 for the non-transgenic control (NTC, P < 0.0004) and plants expressing only 21-bp small RNAs (P < 0.002 and < 0.004, siRNA1 and siRNA 2 respectively; Fig. 5D and Supplementary Table 4). There were no statistically significant differences between the two small RNA constructs and non-transgenic control (NTC). The results presented here suggest that full-length DvSSJ1 dsRNA can provide WCR protection in transgenic plants. In contrast the transgenic maize plants expressing specific DvSSJ1 siRNAs do not provide root protection from WCR feeding damage. These results are consistent with both diet-based studies that long dsRNAs are more effective than siRNAs, and previous studies that demonstrate siRNAs were not taken up by WCR midgut9,13. These results strongly support long dsRNAs in planta are the functional RNA form for control of WCR.
Conclusions
Plant-delivered long dsRNA is the functional RNA molecule for the protection of transgenic maize from WCR feeding damage. A single nucleotide mutation of 21-bp siRNA can have a significant impact or abolish diet activities against WCR. The molecular target of 210-bp DvSSJ1 dsRNA is arthropod-specific, has not been described in vertebrates, and is uniquely specific to the Diabrotica genus in the Chrysomelidae family of Coleoptera.
Method and materials
Plant expression vectors
The transgenic expression cassette (Fig. 1A) is expressed as a transcript that contains two inverted RNA fragments of the smooth septate junction protein 1 (DvSSJ1) gene from Diabrotica virgifera (WCR)8 separated by an intron connector sequence derived from the intron 1 region of the Zea mays alcohol dehydrogenase (zm-Adh1) gene24 to form a hairpin double-stranded RNA. The transcription product of this cassette is intended to knockdown the expression of the smooth septate junction in the mid-gut of the WCR via RNA interference9. Expression of the DvSSJ1 fragments is controlled by a third copy of the ubiZM1 promoter, the 5′ UTR, and intron25, in conjunction with the terminator region from the Zea mays W64 line 27-kDa gamma-zein (Z27G) gene26,27. Two additional terminators are present to prevent transcriptional interference15, which includes the terminator region from the Arabidopsis thaliana ubiquitin 14 (UBQ14) gene28 and the terminator region from the Zea mays In2-1 gene29. The BSV promoter30,31 was also used to express the same DvSSJ1 cassette. Single copy T0 transformants with a WCR damage score of < 1 were selected8 for further characterization at the T1 generation.
Molecular characterization of DvSSJ1 transgenic plants
Northern analyses and sequencing full-length transcript
Total RNA was isolated using TRIzol Reagent (Invitrogen). Following extraction, the total RNA was visualized on an agarose gel to determine the quality and was quantified on a NanoDrop spectrophotometer (Thermo Fisher Scientific). The mRNA was isolated from total RNA using a FastTrack MAG kit (Invitrogen) and quantified by an Agilent 2,100 bioanalyzer (Agilent Technologies). DvSSJ1 antisense riboprobe was in vitro transcribed from the DvSSJ1 PCR product (210 bp) and was labeled with digoxigenin-labeled nucleotides (DIG-11-UTP) according to the procedures provided in the DIG RNA Labeling Kit (Roche). The detailed mRNA northern method was described in the Supplementary Method. Additional northern analyses were conducted with total RNA as described before8. One microgram of total RNA was used to generate a cDNA library to sequence the DvSSJ1 transcript in transgenic plants. Two independent laboratories carried out 3′ and 5′ RACE for sequencing the full-length DvSSJ1 transcript using a 5′/3′ RACE kit (Roche) and RACE conditions provided in Supplementary Method.
In situ hybridization (ISH)
For ISH analyses, target probes, preamplifier, amplifier, and label probe (Supplementary Table 4) were designed by Advanced Cell Diagnostics (Hayward, CA) and samples were processed as previously described32. Root tissues were fixed in 10% neutral buffered formalin (4% formaldehyde) for 48 to 72 h and processed for paraffin embedding. Sections (4 μm) were processed for RNA in situ hybridization with the BaseScope Detection Kit (RNAscope Red Assay) according to the manufacturer’s standard protocol (Advanced Cell Diagnostics, Hayward, CA). Images were acquired using a Leica Aperio CS2 digital scanner and captured at 40× magnification with a resolution of 0.25 µm pixel−1.
siRNA profile analysis
For small RNA sequencing, 1 μg of RNA was used to generate small RNA libraries using the small RNA-Seq kit (Illumina)9. The total clean reads of each sample were normalized into reads per million (RPM) for the expression of DvSSJ1 siRNAs to determine differentially expressed sRNAs. Trimmed reads 18 to 41 nt in length were aligned to the 210-bp DvSSJ1 sequence using Bowtie 233. Only perfectly matched reads were included for analysis of sequence length distribution (Supplementary Figure 5). The 18- and 41-nt DvSSJ1 reads were visualized using Integrative Genomics Viewer software 2.8 (Broad Institute, Cambridge, MA, USA) (https://software.broadinstitute.org/software/igv/). The maize miR168 was determined by searching the miRBase (https://www.mirbase.org/, release 21) and used as a control for quantification and northern analyses of siRNAs (Supplementary Table 4).
DvSSJ1 homologous sequence comparison
The sources of DvSSJ1 210 bp homologous sequences were identified and selected from published data8,16 and listed in Supplementary Table 1. The transcriptomes of six additional insects were assembled as previously described32. Briefly, cDNAs prepared from larva were sequenced by Illumina paired-end and 454 Titanium sequencing technologies. De novo transcriptome assemblies were performed using Trinity method34. The AlignX tool of Vector NTi 10.3 (Invitrogen) was used to create alignments between the DvSSJ1 210-bp sequence and each homologous sequence. Each alignment was inspected to determine the percent identity over the length of the alignment. The longest contiguous match between the DvSSJ1 fragment and each of the homologous sequences from the various insects was determined by visual inspection. All the data were summarized in Table 1, Supplementary Table 1, and Supplementary Fig. 7.
Double-stranded RNA production by in vitro transcription
To produce DvSSJ1 dsRNA, gene-specific primers (Supplementary Table 3) containing T7 RNA polymerase sites (5´d[TAATACGACTCACTATAGGG]3´) at the 5′ end of each primer were used to amplify PCR product which served as the template for dsRNA synthesis by in vitro transcription.
Fragment length samples were treated with RNase A/T1 to remove single-strand T7 sequence from both ends (Supplementary Fig. 9and 10). All dsRNA/RNA samples for bioassays were purified by RNA spin column (Invitrogen), and then quantified by nanodrop 8,000 (Thermo Fisher Scientific).
WCR bioassay methods
Bioassays were conducted to determine the response of WCR to dsRNA exposure via oral ingestion. WCR eggs were obtained from an internal colony (Johnston, IA) and incubated in an environmental chamber until the eggs hatched. Neonates were used in each bioassay within 24 h of hatching. All bioassays were conducted in 24-well Falcon culture plates. Stonefly Heliothis Diet (SHD, Ward’s Science, Rochester NY) comprised the base dry diet for WCR. A proprietary mixture of additional ingredients was added to SHD to stimulate feeding and/or allow for the biological maturation of WCR. In internal testing, this diet typically generates less than 30% mortality in the negative control, similar to other WCR laboratory diets17. On Day 0 of each respective bioassay, the dry diet was mixed with RNase free water (negative control) or a liquid test substance of interest to achieve the desired concentration. Approximately 300 µl (~ 300 mg) of freshly prepared diet was dispensed into wells of the bioassay plates. One WCR neonate was placed in each well. Each plate was sealed with heat-sealing film, and two small holes were poked over each well to allow for ventilation. Bioassays were conducted in an environmental chamber set at 21 °C, 65% relative humidity, and continuous dark for a total of 14 days. Every 3 to 4 days, new bioassay plates were prepared with fresh diet as described for Day 0, and living WCR were transferred to the new plates and missing or dead organisms were recorded. After 14 days, each bioassay was complete, final mortality was assessed, and surviving organisms were individually weighed. Organisms recorded as missing from a well or lost in the transfer, or wells containing more than one organism, were excluded from statistical analysis.
Artificial miRNA expression vectors and transformation
Two 21-mer’s, siRNA-1 and -2 of DvSSJ1 were selected based on previous dsRNA-feeding data, which showed higher accumulation in WCR midgut9, and were designed for expressing 21-bp siRNAs according to the rules for artificial microRNA design20,35. The 21-bp siRNAs were incorporated into the zma-miR396h backbone19,20 to compare with the dsRNA 210-bp fragment of the DvSSJ1 gene8. The silencing cassettes (Fig. 4) consisted of the maize ubiquitin promoter, maize ubiquitin intron 136, siRNA unit or two 210 bp stretches of DvSSJ1 and an intervening truncated maize ADH intron1, designed to support assembly into a dsRNA hairpin, and the PIN II terminator37. The DvSSJ1 constructs were transformed via Agrobacterium tumefaciens into a commercial maize elite-inbred line, PHR0338. T0 maize transformants were screened by qPCR analyses39 and single-copy T-DNA integration events were used for further characterization. In the T0 greenhouse assay, 10–12 independent events per each construct (siRNA), eight DvSSJ1 210-bp dsRNA events (T0 plants) and non-transformed PHR03 control (NTC) were infested with 1,000 WCR eggs at the V6 (six-leaf) stage and evaluated for WCR damage23 and to support the expression of RNA’s analyses8.
For expression analyses, total RNA was extracted using the mirVana miRNA Isolation kit (Life Technologies, Carlsbad, CA) from T0 transgenic maize plants at the leaf stage V6-V7. Ten µg of total RNA was fractionated on a 1.5% denaturing formaldehyde gel for long dsRNA northern analysis. For siRNA northern blot analysis, 20 µg of total RNA was fractionated on a 15% Criterion TBE-Urea Gel (Bio-Rad, Hercules, CA). Hybridization and washing conditions were as described previously8.
Quantigene analyses for siRNA and dsRNA quantification in plants
Quantification of dsRNA transcripts was conducted as previously reported32 and siRNAs were detected by a luminometer (Promega Glomax) using a modified method40. Target specific probes (Supplementary Table 3) were designed to quantify DvSSJ1 siRNAs using a QuantiGene miRNA 2.0 assay (Supplementary Fig. 11). RNA oligo duplexes of siRNA-1 and -2 were used to generate a standard curve for the quantification and ZM-miR168 oligo duplex was included as an internal control to normalize sample variations. Aliquots of each plant RNA sample were diluted to 6.25 ng μl−1 using TE buffer. Each reaction comprised 125 ng of total RNA in a total volume of 20 μl. Standard curves comprised duplexed RNA oligo’s diluted with 10 ng μl−1 carrier yeast RNA. Standard curves covered six-points of each targeted duplex ranging from 10 pg – 0.0032 pg, at fivefold dilutions per point. Samples, controls, and curves were all run in triplicate as technical repeats. Standard curves were employed to calculate pg of siRNA per reaction which was further extrapolated to pg of siRNA per μg of total RNA. Hybridization, signal amplification, and data acquisition were carried out in a standard 96-well plate following the procedure described in Supplementary Method.
Statistical analysis
The Statistical analysis for this paper was generated using SAS software, Version 9.4. Copyright [2019] SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
WCR diet bioassays
For each treatment mortality was estimated with exact (Clopper-Pearson) 95% confidence intervals. Fisher’s exact test (SAS PROC MULTTEST) was then used to determine if the mortality rate of each treatment was greater than the mortality associated with the bioassay control.
For weight, SAS PROC GLIMMIX was used to conduct a linear model analysis to generate estimated means, 95% confidence intervals and the statistical comparisons between each treatment and the bioassay control. Error was initially assumed both independent and identically distributed and later confirmed by visual inspection of the residuals from the fitted model.
dsRNA Fragment Length data: Treatments with two or fewer surviving insects were not included in the analysis. For both mortality and weight, statistical significance was established if the P value was < 0.05. No multiplicity adjustment was used because the risk of falsely declaring that a treatment does not significantly differ from the control was considered of greatest concern.
dsRNA Specificity data: For comparison against the 21 bp RNA embedded within the 210 bp GFP, a multiple comparison adjustment was conducted using the false discovery rate (FDR) method41,42. In this instance falsely declaring a treatment differed from the 21 bp RNA embedded within the 210 GFP was considered of greatest concern, thus the FDR adjustment was appropriate. For comparisons against the 210 bp GFP control no FDR adjustment was employed as falsely declaring no significant difference was considered of greatest concern thus the FDR adjustment was not used. For weight, multiple comparison adjustments were conducted as noted above for the mortality endpoint except using Dunnett's method40 instead of FDR.
Plant bioassays
Mean values and standard deviations were calculated by linear regression using Minitab 15 statistical software. The difference in WCR nodal injury score was tested by a one-way analysis of variance taking the phenotype and the presence or absence of the transgene as the sources of variation. Tukey’s family error rate was chosen for one-way multiple comparisons with a P value level of significance equal to 0.05.
Supplementary information
Acknowledgements
We thank Kimberly Lang, Chris Linderblood, Ian Murphy, Taylor Olson, Rachel Woods, and Ashley Young for insect bioassay support; Julie Ritland and Jeff Robson for greenhouse bioassay and vector construction support; Corteva maize transformation and controlled environment groups for generating and managing transgenic plants; Jeremy Barnes, Xiping Niu and Bliss Kernodle for molecular analyses; Henry Mirsky, Mitali Nimkar and Guna Gurazada for bioinformatics support; Ian Lamb for thoughtful comments and suggestions.
Author contributions
X.H., B.J.C., and C.B. conceived and designed the studies. J.P.S., N.M.R., K.S., Y.W., C.A.W., J.Y., A.U., and C. F. performed the data analysis and interpreted the results. X.H., B.J.C., C.B., and A.L.L. with input from all of the authors wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/4/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Contributor Information
Xu Hu, Email: xu.hu@corteva.com.
Chad J. Boeckman, Email: chad.boeckman@corteva.com
Bin Cong, Email: bin.cong@corteva.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-68014-1.
References
- 1.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Kurreck J. RNA interference: From basic research to therapeutic applications. Angew. Chem. Int. Ed. Engl. 2009;48:1378–1398. doi: 10.1002/anie.200802092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price DR, Gatehouse JA. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008;26:393–400. doi: 10.1016/j.tibtech.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal N, et al. RNA interference: biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. MMBR. 2003;67:657–685. doi: 10.1128/mmbr.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 2009;54:303–321. doi: 10.1146/annurev.ento.54.110807.090434. [DOI] [PubMed] [Google Scholar]
- 6.Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect. Physiol. 2010;56:227–235. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Baum JA, et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007;25:1322–1326. doi: 10.1038/nbt1359. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, et al. Discovery of midgut genes for the RNA interference control of corn rootworm. Sci. Rep. 2016;6:30542. doi: 10.1038/srep30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, et al. Molecular characterization of the insecticidal activity of double-stranded RNA targeting the smooth septate junction of western corn rootworm (Diabrotica virgifera virgifera) PLoS ONE. 2019;14:e0210491. doi: 10.1371/journal.pone.0210491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi Y, Motoishi M, Furuse K, Furuse M. A tetraspanin regulates septate junction formation in Drosophila midgut. J. Cell Sci. 2016;129:1155–1164. doi: 10.1242/jcs.180448. [DOI] [PubMed] [Google Scholar]
- 11.Izumi, Y., Furuse, K. & Furuse, M. Septate junctions regulate gut homeostasis through regulation of stem cell proliferation and enterocyte behavior in “Drosophila”. bioRxiv, 582148, 10.1101/582148 (2019). [DOI] [PubMed]
- 12.Li H, et al. Long dsRNA but not siRNA initiates RNAi in western corn rootworm larvae and adults. J. Appl. Entomol. 2015;139:432–445. doi: 10.1111/jen.12224. [DOI] [Google Scholar]
- 13.Bolognesi R, et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte) PLoS ONE. 2012;7:e47534. doi: 10.1371/journal.pone.0047534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehn, S., Lu, A. L. & Simmons, C. R. Inventors; E.I. DuPont De Nemours And Company, Assignee. Viral Promoter, Truncations Thereof, and Methods of Use. WO2012088227 (2012).
- 15.Shearwin KE, Callen BP, Egan JB. Transcriptional interference—a crash course. Trends in genetics : TIG. 2005;21:339–345. doi: 10.1016/j.tig.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, X. et al. Inventors; E.I. DuPont De Nemours and Company, Assignee. Compositions and Methods to Control Insect Pests. WO2016043960 (2016).
- 17.Ludwick DC, et al. A new artificial diet for western corn rootworm larvae is compatible with and detects resistance to all current Bt toxins. Sci. Rep. 2018;8:5379. doi: 10.1038/s41598-018-23738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angart P, Vocelle D, Chan C, Walton PS. Design of siRNA therapeutics from the molecular scale. Pharmaceuticals. 2013 doi: 10.3390/ph6040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. A genome-wide characterization of microRNA genes in maize. PLoS Genet. 2009;5:e1000716. doi: 10.1371/journal.pgen.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brian McGonigle Inventor; E.I. DuPont De Nemours and Company, Assignee. Down-regulation of gene expression using artificial microRNAs. US patent 8,115,055 B2 (2012).
- 21.Meng X, Muszynski MG, Danilevskaya ON. The FT-like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize. Plant Cell. 2011;23:942–960. doi: 10.1105/tpc.110.081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcantara KMM, Garcia RL. MicroRNA-92a promotes cell proliferation, migration and survival by directly targeting the tumor suppressor gene NF2 in colorectal and lung cancer cells. Oncol. Rep. 2019;41:2103–2116. doi: 10.3892/or.2019.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oleson JD, Park YL, Nowatzki TM, Tollefson JJ. Node-injury scale to evaluate root injury by corn rootworms (Coleoptera: Chrysomelidae) J. Econ. Entomol. 2005;98:1–8. doi: 10.1093/jee/98.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Dennis ES, et al. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984;12:3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- 26.Das OP, Poliak E, Ward K, Messing J. A new allele of the duplicated 27kD zein locus of maize generated by homologous recombination. Nucleic Acids Res. 1991;19:3325–3330. doi: 10.1093/nar/19.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, et al. Gene duplication confers enhanced expression of 27-kDa γ-zein for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. USA. 2016;113:4964–4969. doi: 10.1073/pnas.1601352113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callis J, Carpenter T, Sun CW, Vierstra RD. Structure and evolution of genes encoding polyubiquitin and ubiquitin-like proteins in Arabidopsis thaliana ecotype Columbia. Genetics. 1995;139:921–939. doi: 10.1093/genetics/139.2.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershey HP, Stoner TD. Isolation and characterization of cDNA clones for RNA species induced by substituted benzenesulfonamides in corn. Plant Mol. Biol. 1991;17:679–690. doi: 10.1007/BF00037053. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang J, Wang JH, Zhang X, Liu ZX. Molecular characterization of Banana streak virus isolate from Musa acuminata in China. Virol. Sin. 2011;26:393–402. doi: 10.1007/s12250-011-3212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lheureux F, Laboureau N, Muller E, Lockhart BE, Iskra-Caruana ML. Molecular characterization of banana streak acuminata Vietnam virus isolated from Musa acuminata siamea (banana cultivar) Adv. Virol. 2007;152:1409–1416. doi: 10.1007/s00705-007-0946-9. [DOI] [PubMed] [Google Scholar]
- 32.Niu X, et al. Control of western corn rootworm (Diabrotica virgifera virgifera) reproduction through plant-mediated RNA interference. Sci. Rep. 2017;7:12591. doi: 10.1038/s41598-017-12638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callis J, Fromm M, Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987;1:1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- 37.An G, et al. Functional analysis of the 3′ control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell. 1989;1:115–122. doi: 10.1105/tpc.1.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho MJ, et al. Agrobacterium-mediated high-frequency transformation of an elite commercial maize (Zea mays L.) inbred line. Plant Cell Rep. 2014;33:1767–1777. doi: 10.1007/s00299-014-1656-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhi L, et al. Effect of Agrobacterium strain and plasmid copy number on transformation frequency, event quality and usable event quality in an elite maize cultivar. Plant Cell Rep. 2015;34:745–754. doi: 10.1007/s00299-014-1734-0. [DOI] [PubMed] [Google Scholar]
- 40.Porichis F, et al. High-throughput detection of miRNAs and gene-specific mRNA at the single-cell level by flow cytometry. Nat. Commun. 2014;5:5641. doi: 10.1038/ncomms6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- 42.Westfall, P. H., Tobias, R. D., Rom, D., Wolfinger, R. D. & Hochberg, Y. Concepts and basic methods for multiple comparisons and tests. In Multiple Comparisons and Multiple Tests: Using SAS 13–40 (SAS Institute Inc., 1999).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.