Abstract
Conflict with humans is a significant source of mortality for large carnivores globally. With rapid loss of forest cover and anthropogenic impacts on their habitats, large carnivores are forced to occupy multi-use landscapes outside protected areas. We investigated 857 attacks on livestock in eastern Himalaya and 375 attacks in western Himalaya by leopards between 2015 and 2018. Multivariate analyses were conducted to identify the landscape features which increased the probability of livestock depredation by leopards. The risk of a leopard killing livestock increased within a heterogeneous landscape matrix comprising of both closed and open habitats (very dense forests, moderate dense forests, open forests, scrubland and non-forests). We used the results to map potential human–leopard conflict hotspots across parts of the Indian Himalayan region. Our spatial risk maps indicate pockets in the eastern, central and western part of eastern Himalaya and the central, northern part of western Himalaya as hotspots of human–leopard conflicts. Most of the attacks occurred when livestock were grazing freely within multi-use areas without supervision of a herder. Our results suggest that awareness about high risk areas, supervised grazing, and removing vegetation cover around human settlements should be initiated to reduce predation by leopards.
Subject terms: Ecology, Zoology, Ecology
Introduction
Large carnivores are apex predators and their lethal and non-lethal effects have strong implications for ecosystem structure and functioning. They also have prominent cultural reverence in several human societies and act as flagship species for global conservation campaigns1. In areas where their populations have suffered local extinction, cascading effects have been observed on ecosystem processes either mediated by abundance or behavior of mesocarnivores and prey2, 3. Low population densities, high energetic requirements, social or solitary hunting strategies and wide-ranging behavior means that they often range beyond nature reserves for access to space and resources and seek out easy prey i.e. livestock and occasionally humans within shared landscapes. Species commonly involved in predation on livestock are canids (Canis spp.), felids (Panthera spp.) and ursids (Ursus spp.) across both Northern and Southern Hemispheres4. Government agencies, wildlife managers and conservationists spend substantial money to compensate losses to carnivores through a combination of preventative, assertive and reactive measures5, 6. In areas where the perceived risk of large carnivores is high, attacks on humans and livestock lead to retaliation by the local communities increasing their extinction risk3, and a setback for conservation efforts7.
Though protected areas are essential for conserving large carnivores, their size is often not large enough to sustain carnivore populations or even individual home ranges8. Therefore, the quality of the larger multi-use landscapes in terms of the availability of suitable habitat, abundance of wild prey, extent of human presence and tolerance of local communities determine future survival of these species. Such shared landscapes represent a major proportion of the geographic distribution of large carnivores globally9. Increasing human populations, declines in wild prey and changes in land use patterns have resulted in fragmented, heterogeneous resource limited landscapes for carnivores10. Such anthropogenic impacts on ecosystems have forced carnivores to frequent areas near human settlements, kill livestock and consequently local communities have retaliated through poisoning, shooting, snaring and electrocution11–13. A substantial proportion of large felid mortality globally (cheetah, eurasian lynx, tiger, jaguar and snow leopard) has been an outcome of human–carnivore conflicts10. Subsequently, livestock depredation has been identified as the stimuli for human–carnivore conflicts10. Retaliation also affects the overall functioning of ecosystem by removal of non-target species (avian predators, scavengers and mesocarnivores through poisoning of carcasses) and larger predators within the shared landscapes14. Conservation within shared landscapes is thus challenging especially considering the socio-cultural, political problems where it is crucial to ensure safety of human lives, property, livelihoods and well-being.
One practical approach used to mitigate human–carnivore conflicts has been to identify the spatial extent of risk zones15 and use it in developing conservation policies and planning interventions. In areas where large carnivores co-occur with humans, landscape features have been used as a proxy to determine their presence and model risk of predation on livestock16–18. Carnivores have been documented to repeatedly kill prey in areas with easy accessibility and similar landscape features. Such landscape features are a mosaic of several attributes (habitat type, prey density, human activity, availability of water)16, 19, 20. Composition and arrangement of these variables in the landscape directly impact hunting success, favoring attributes where prey is easy to detect, catch or encounter thus making certain areas hotspots of conflicts19, 21, 22. Wolves killed prey in relatively flat areas with the probability of risk being highest in scrubland, pastures and sites located at increasing distance from dense forests15, 23. Hyenas killed prey in areas with dense vegetation cover17. Predation by lions were higher within riverine, closed habitats and the vicinity of protected reserves11, 17, 24, 25. Puma, jaguar and tiger were documented to kill livestock in areas with dense vegetation cover26–29. Altitude also influenced predation risk with pumas and jaguars killing more prey in elevated mountainous regions26, 27 whereas lions, leopard and hyenas preferred flat, low lying areas25. Human infrastructure such as distribution, length and distance to roads, towns, villages and night light also influenced predation risk by large carnivores23, 28, 29. Apart from landscape features, human–carnivore conflicts are often a consequence of both human and carnivore behavior. Poor guarding practices, location of grazing pastures close to protected reserves, and lack of animal shelters also impact the extent of predation on livestock10.
Leopards are the most resilient of large felids, yet habitat loss and persecution by humans have resulted in significant reductions of their populations with only 17% of the present distribution range protected30. India has a sizeable population of leopards and they share space with humans within the majority of the agro-pastoral, forested landscapes31. Human–leopard interactions are especially intense within such shared landscapes where the size of protected areas is small, fragmented and comprise only 5% of the geographic extant of the country. Livestock and free ranging domestic dogs comprise the primary prey for leopards32, 33 outside of protected areas where crop fields, tea plantations provide cover34–36. Predation on livestock has been recognized as a major conservation problem for the species37–39 leading to increasing retaliation by the local communities. Domestic dogs and livestock also act as major attractants for leopards near human habitations and attacks on humans often occur as a consequence of leopard presence near settlements32, 33, 40. Livestock are a direct representation for the agro-pastoral societies of rural India and loss to large carnivores represents a substantial threat to human welfare and livelihoods. Hence it is crucial to identify areas where leopards are more likely to attack livestock to plan management interventions and implement mitigation measures.
Spatial risk modelling has been widely used to provide insights into habitats where carnivores kill livestock through both qualitative and graphical guides and help in prioritization of management interventions and mitigation measures15. This is essentially a quantitative tool which uses location-specific data (kills) to predict the probability of a predator attack by relating landscape attributes at kill sites and comparing them to random sites representing landscape availability. Risk models help in quantifying the realized predation risk (where livestock or wild prey were killed) especially across heterogeneous landscapes16, 22. We use a similar multivariate analytical approach to model risk of livestock depredation by leopard within the Indian Himalayan Region (IHR). We investigate patterns of leopard predation on livestock, analyze site specific conditions and map conflict hotspots. Specifically, we examine (1) extent of livestock depredation by leopard in IHR, (2) identify animal husbandry practices which increase vulnerability of livestock to leopard attacks, (3) identify high predation-risk zones based on landscape features within the human-dominated landscapes of IHR, (4) spatially map human–leopard conflict hotspots by modelling probability of livestock predation. Finally, we calculate (5) threshold values for important landscape features and use the results to recommend strategies to reduce human–leopard conflicts within IHR.
Results
Extent of livestock predation by leopards
In 2015–2018, there was a total of 857 and 375 leopard livestock predation events recorded in North Bengal and Pauri Garhwal, respectively. 54% of the livestock killed were adult cows, 16% were goats, 14% calves, 6% adult pigs, 5% piglets, 3% ox, 2% goat yearlings in North Bengal (χ2 = 312.12, df = 7, p < 0.05). In Pauri Garhwal, 70% percent of the livestock predated were adult cows followed by 9% adult goats, 8% cattle calves, 7% ox, 1% horse, 2% donkey, 2% sheep and 1% mules (χ2 = 208.28, df = 8, p < 0.05).
Characteristics of kill sites and animal husbandry practices
In North Bengal livestock killing was recorded along the foothills with an average elevation of 269 m (SE 192) (range 80–2,400) whereas they were recorded higher up at an average altitude of 1,271 m (SE 218) range (540–2,135) in Pauri Garhwal. An average of 2 houses with a range of (0–12) were present within the vicinity (50-m radius) of livestock predation sites in North Bengal. In Pauri Garhwal, an average of 3 houses range (0–30) were present within the vicinity of predation sites. Based on the vegetation types recorded within a diameter of 100 m of the conflict site, the majority of these predation events (37%) were recorded in tea gardens, 17% in scrublands, 13% in sal dominated patches, 13% in horticulture plantations, 5% in teak dominated and 4% within bamboo plantations in North Bengal. In Pauri Garhwal they were recorded in (48%) the vicinity of villages, 41% with moderate vegetation cover, 7% at roadsides and only 3% within livestock sheds. 95% and 41% of the livestock killed in North Bengal and Pauri Garhwal were unsupervised.
Seasonal and temporal patterns of livestock predation
Livestock predation peaked in the winter (November-February, 42%) and spring (March-June, 37%) seasons, whereas it was lower during monsoons (21%) in North Bengal (χ2 = 8.676, df = 2, p < 0.05). In Pauri Garhwal, the majority occurred in the summer (March-July, 45%) and winter (November–February, 31%) seasons and only 24% during monsoons (March-June) (χ2 = 6.1987, df = 2, p < 0.05). 53% of the predation events were recorded during the day (8AM–4PM), 40% between (12AM–8AM) and the rest 7% between (4PM–12PM) in North Bengal (χ2 = 33.034, df = 2, p < 0.05). 48% of the predation events were recorded during day (4PM–12AM), 46% between (8AM–4PM) and rest 6% between (12AM–8AM) in Pauri Garhwal (χ2 = 33.68, df = 2, p < 0.05).
Influence of landscape features on leopard attacks on livestock
Moran’s I identified spatial clusters of livestock predation within both North Bengal and Pauri Garhwal. The z value (13.422), Moran’s Index (0.395) and (p value < 0.01) indicate that there was a less than 1% likelihood that this pattern was due to random chance in North Bengal. The threshold distance between each neighboring livestock depredation site was estimated to be 5,000.50 m.
Results of the GLM model with binomial structure suggests that landscape features such as area of non-forest (β = 7.81E−08; 95% CI = 3.62E−09–1.4082E−07), scrubland (β = 1.88E−06; 95% CI = 7.7064E−07–2.98936E−06), open forests (β = 2.56E−07; 95% CI = 1.8152E−07–3.30284E−07), very dense forests (β = 1.98E−07; 95% CI = 1.13328E−07–2.82672E−07), area of water (β = − 1.61E−06; 95% CI = 5.0848E−07–2.71E−06), distance to protected areas (β = − 1.70E−04; 95% CI = 0.0001–0.0002), nightlight (β = − 7.45E−02; 95% CI = 0.0137–0.1352) and altitude (β = − 5.26E−03; 95% CI = 0.0033–0.0072) were the best predictors for leopard attacks on livestock in North Bengal (Table 1, Supplementary Table S1). Leopards were more likely to kill livestock within both closed (dense forests) and open habitats (non-forest, open forest and scrubland) in North Bengal. Probability of livestock killing decreased with increasing distance from protected areas. In addition, livestock killing was more likely to happen in flat low lying areas with less human presence and low availability of water. Results of the GLM model with poisson structure are similar and suggests that landscape features such as area of very dense forests (+), open forests (+), scrub (+), area of water (−), distance to protected areas (−) and altitude (−) were best predictors of livestock predation (Supplementary Table S2). Probability of livestock killing were higher within sites with dense to moderate vegetation cover and decreased with human presence, increasing distance form protected areas and water bodies.
Table 1.
Second-order Akaike Information criterion scores (AIC), (AICc), ΔAICc of generalized linear models with binomial structure predicting livestock depredation by common leopards in North Bengal landscape, using landscape predictors.
| Model | AIC | AICc | ΔAIC | BIC |
|---|---|---|---|---|
| Altitude + nightlight + distance from PAs + area of water bodies + area of non-forest + area of scrub + area of open forest + area of very dense forest | 361.01 | 361.32 | 0 | 400.59 |
| Altitude + nightlight + distance from PAs + length of roads + area of water bodies + area of non-forest + area of scrub + area of open forest + area of very dense forest | 362.56 | 362.93 | 1.61 | 406.55 |
| Altitude + nightlight + distance from PAs + area of water bodies + area of scrub + area of open forest + area of very dense forest | 365.41 | 365.65 | 4.33 | 400.59 |
| Altitude + nightlight + distance from PAs + area of water bodies + area of open forest + area of very dense forest | 376.41 | 376.59 | 15.27 | 407.20 |
| Altitude + nightlight + distance from PAs + area of open forest + area of very dense forest | 378.37 | 378.51 | 17.19 | 404.76 |
| Altitude + distance from PAs + area of open forest + area of very dense forest | 380.68 | 380.77 | 19.45 | 402.67 |
| Distance from PAs + area of open forest + area of very dense forest | 412.90 | 421.96 | 60.64 | 439.49 |
| Distance from PAs + area of open forest | 427.01 | 427.05 | 65.73 | 440.21 |
| Distance from PAs | 467.03 | 467.05 | 105.73 | 475.83 |
PAs protected areas.
The z value (11.329), Moran’s Index (0.499) and (p value < 0.01) indicate that there was less than 1% likelihood that this pattern was due to random chance in Pauri Garhwal. The threshold distance between each neighboring livestock depredation site was estimated to be 5,077.024 m.
Results of the GLM model with binomial structure suggests that landscape features such as area of moderate dense forests (β = 2.95E−07; 95% CI = 1.21E−07–4.70E−07), open forests (β = 4.62E−07; 95% CI = 2.27E−07–6.97E−07), scrub (β = 5.58E−07; 95% CI = 8.30E−08–1.03E−06), non-forests (β = 3.38E−07; 95% CI = 1.92E−07–4.85E−07) and the distance to protected areas (β = 3.11E−05; 95% CI = 7.83E−065.44E−05) were the best predictors of livestock predation by leopard in Pauri Garhwal (Table 2, Supplementary Table S3). The probability of livestock killing increased within moderate to open habitats and with distance to protected areas. Results of the GLM model with poisson structure suggests that landscape features such as area of moderate dense forests (+), open forests (+), scrubland (+), non-forest (+), water (−), distance to protected areas (+) and night light (+) were the best predictors for leopard attacks on livestock (Supplementary Table S4). The probability of livestock killing increased within moderate to open habitats. In addition, livestock killing was most likely to occur in areas with less water, more human presence and increasing distance from protected areas.
Table 2.
Second-order Akaike Information criterion scores (AIC), (AICc), ΔAICc of generalized linear models with binomial structure predicting livestock depredation by common leopards in Pauri Garhwal landscape, using landscape predictors.
| Model | AIC | AICc | ΔAIC | BIC |
|---|---|---|---|---|
| Distance from PAs + area of non-forest + area of scrub + area of open forest + area of moderate dense forest | 231.62 | 231.95 | 0 | 254.84 |
| Distance from PAs + area of water bodies + area of non-forest + area of scrub + area of open forest + area of moderate dense forest | 232.58 | 233.03 | 1.08 | 257.34 |
| Nightlight + distance from PAs + area of water bodies + area of scrub + area of open forest + area of moderate dense forest | 232.80 | 233.39 | 1.44 | 261.10 |
| Nightlight + distance from PAs + length of river + area of water bodies + area of non-forest + area of scrub + area of open forest + area of moderate dense forest + area of very dense forest | 232.82 | 233.72 | 1.77 | 268.19 |
| Nightlight + distance from PAs + length of roads + length of rivers + area of water bodies + area of non-forest + area of scrub + area of open forest + area of moderate dense forest + area of very dense forest | 234.56 | 235.65 | 3.7 | 273.47 |
| Distance from PAs + area of non-forest + area of open forest + area of moderate dense forest | 235.5 | 235.75 | 3.8 | 253.19 |
| Area of non-forest + area of open forest | 247.08 | 247.18 | 15.23 | 257.69 |
| Distance from PAs + area of non-forest + area of open forest | 247.44 | 247.60 | 15.65 | 261.59 |
| Area of open forest | 288.59 | 288.64 | 56.69 | 295.67 |
PAs protected area.
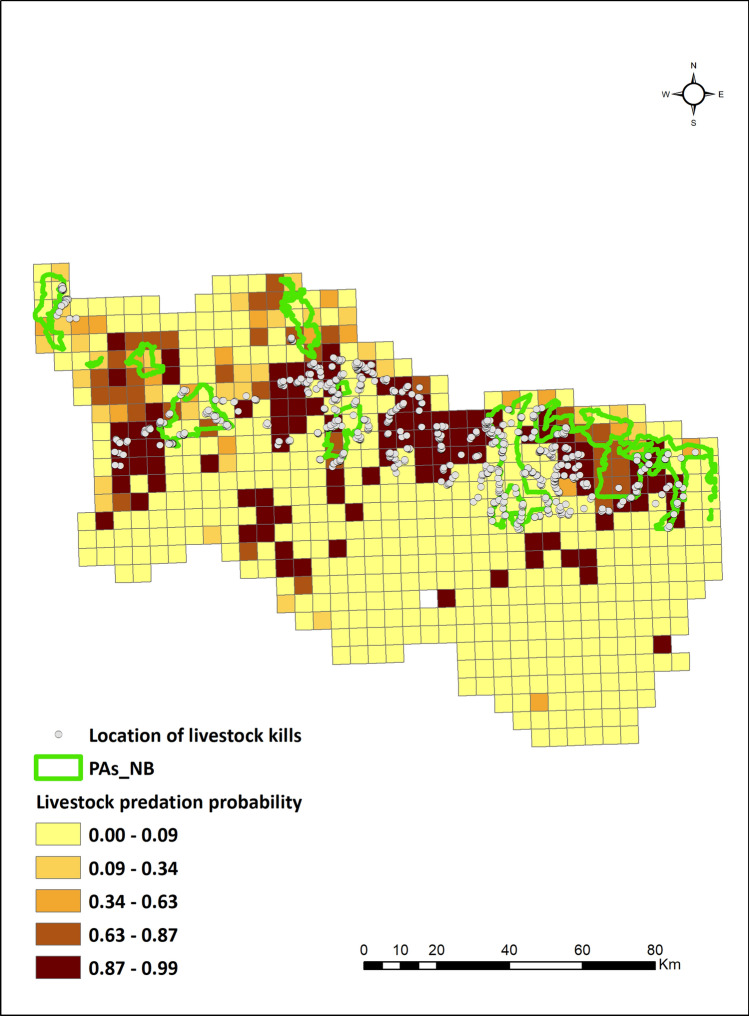
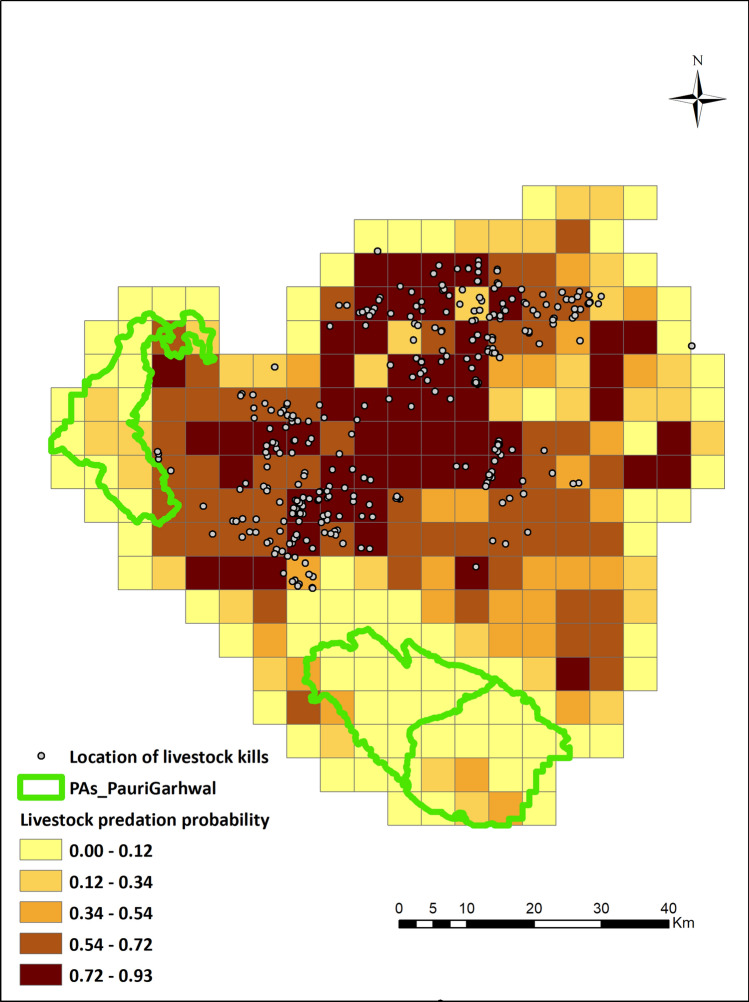
Receiver operating curves (AUC) values for the dominant model (GLM with binomial structure) were estimated to be 0.86 and 0.83 respectively for North Bengal and Pauri Garhwal. The predictive maps based on the model averaged coefficients indicated that certain pockets within eastern, central, and western parts of North Bengal were hot spots of livestock predation for leopards (Figs. 1, 2). For Pauri Garhwal, predation risk was high in the central and north eastern part of the landscape. Conflict probabilities were higher near protected areas in North Bengal whereas they were lower in Pauri Garhwal.
Figure 1.
Leopard livestock predation risk map, North Bengal prepared using Arc GIS 10.3 (https://enterprise.arcgis.com/en/portal/10.3/use/link-to-items.htm).
Figure 2.
Leopard livestock predation risk map, Pauri Garhwal prepared using Arc GIS 10.3 (https://enterprise.arcgis.com/en/portal/10.3/use/link-to-items.htm).
Wilcoxon rank sum test results indicate that majority of the predictor variables (area of very dense forests, area of moderate dense forests, area of open forests, area of scrub, area of water/riverine patches, area of non-forest, length of roads, length of river, nightlight, p < 0.05) differed significantly between the two study sites (Supplementary Table S5). Only distance to protected areas between the two study sites did not exhibit a statistically significant difference (p > 0.05).
Variable thresholds
The results of the conditional inference trees revealed significant thresholds for four variables regarding predation on livestock, i.e. distance from protected areas, altitude, area of open and very dense forests for North Bengal landscape. For Pauri Garhwal landscape, area of non-forested regions (i.e. human settlements) and area of open forests were identified within a landscape scale of 25 km2. Multivariate trees were found with a first split indicating highest predation probability (> 0.85) when distance from protected areas (≤ 6,385 m), altitude (≤ 193 m) and intermediate probability (0.80) when area of open forests (i.e. tea plantations) was greater than 7,430,000 sq. meters i.e. 30% for North Bengal. For Pauri Garhwal, predation risk was highest in areas (> 0.86) when area of non-forests was less than 6,820,000 m2 i.e. 27% and area of open forests was greater than 3,630,000 i.e. 15%. These results indicate a first priority for foothills, a distance of 6.3 km from protected areas, dense forests (dense vegetation cover) and open forests (tea plantations) for predation risk by leopards in North Bengal. For Pauri Garhwal landscape, risk of predation was highest in areas within a matrix of non-forests (human settlements) and open forests (low vegetation cover).
Discussion
Our results suggest that landscape features are major predictors of livestock predation by leopards in IHR. Our study also highlights that there is significant spatial clustering of livestock kills by leopards within multi-use landscapes of IHR. At a coarser spatial scale, livestock depredation risk was higher within both closed and open habitats in North Bengal (very dense forests, open forests, non-forests, scrub). In Pauri Garhwal risk was higher within a matrix of moderate and open habitats (moderate dense forests, open forests, scrub and non-forests). Generally, predation risk probabilities by large carnivores are reported to increase with dense vegetation cover16, 24, 41 but our results suggest that risk of livestock predation by leopards are higher within a multi-use matrix of open and closed habitats. Our results also indicate that the hunting behavior of leopards and choice of livestock kill sites are different compared to the other large carnivores who rely on dense vegetation cover. Such hunting strategies are probably an artifact of the adaptive nature of leopards, their ability to survive within close proximity of human settlements and consume a wide range of prey30, 32, 42. Human–carnivore conflicts have been generally reported along the periphery of protected areas8, 10, and probability of livestock killing by leopards increased with proximity to protected areas in North Bengal and decreased with increasing distance from such reserves in Pauri Garhwal. Through this study we also mapped human–leopard conflict hotspots within the IHR which should be prioritized by protected area managers, district administration, local communities and multi-interest groups.
Large carnivore species e.g., hyenas43, brown bears44, lions45 and tigers29 use protective vegetation cover to hunt both wild and domestic prey. In contrast, leopards are reported to kill wild prey within moderate vegetation cover such as open mixed woodlands21 in Africa whereas in India availability of water was a crucial spatial driver of leopard kills46. Our result is similar to a study conducted in the Himalayan region of Bhutan which documented that livestock predation by leopards was higher within a matrix of forest and agriculture47. Leopards are stalk and ambush predators frequenting the edge of protected reserves30, 48 and heterogeneous landscapes (settlements, grazing lands, interspersed with moderate forests) might offer better prey catchability compared to dense closed habitats. Vegetation cover is an essential requirement for large carnivores and open forests and scrubland interspersed within forested areas in North Bengal provide ideal refuges for leopards. Such vegetation cover is similar to sugarcane, coffee plantations and agricultural fields which have been identified as major drivers of human–leopard conflicts within fragmented habitats of South Asia35, 36. As reported by an earlier study, leopard attacks on humans in Pauri Garhwal were negatively associated with the presence of dense forests36 but more influenced by a matrix of agriculture lands, shrub cover and medium dense forest patches. Within a fine scale, availability of water has often been identified as a major driver of human–carnivore conflicts in arid ecosystems of Africa25, 41, 49 but within productive ecosystems of South Asia availability of water is probably not a limiting factor.
The threshold value of 6.3 km adjoining protected areas in North Bengal is similar to previous studies which documented livestock predation risk to be high within 5–12 km of the park boundary in Nepal and Cameroon43, 44. In South Africa risk of a leopard killing livestock was highest within the periphery of protected reserves50. Though the probability of livestock killing increased at the edge of protected reserves, we document contrasting results from the two landscapes. Such results could be due to interspecific competition between two large cats25, 39 within the same landscape i.e. tiger and leopard. Pauri Garhwal has two protected areas in the foothill region of the district with a sizeable population of Bengal tigers51 and due to competitive exclusion leopards probably are more widely distributed in the hills away from the reserve boundaries and hence conflicts with leopards are higher as we move farther away from the reserves. North Bengal region has no other apex predators to compete with leopards (due to recent extinction of tigers) and thus human–leopard conflicts are confined to the edge of protected areas and decrease with increasing distance from such reserves. Thus, patterns of conflicts with the species show a different spatial pattern between the two sites.
Leopards are reported to kill livestock in rugged areas of Bhutan47 but our results suggest that livestock kills occurred within flat low lying and mid elevated zones. In North Bengal the average elevation of sites where livestock were killed was low i.e. 270 m probably as a consequence of abundant livestock availability in the foothills compared to the hills. In Pauri Garhwal, the average elevation of livestock kill sites was 1,200 m indicating higher livestock availability in the middle Himalayas compared to the foothills52.
There was a strong effect of seasonality on the number of attacks with the majority occurring in the dry season (winter and summer). These results are in accordance with studies conducted in the Terai region of Nepal53 where leopard attacks on livestock were also reported to be higher in the dry season (winter and summer months). Our results are contrary to studies conducted in East Africa where predation on livestock by lions, hyenas and leopards were much higher in the wet season compared to the dry seasons54, 55. In Africa, wild prey availability is low during the wet season55 whereas in South Asia there is probably no significant association of wild prey and seasonality. The seasonal pattern of predation events further coincides with harvesting and planting of major agricultural crops such as maize, wheat and paddy. During these dry months, local community members are often not available to guard livestock. Livestock grazing is higher during winter within agricultural fields, tea plantations and scrubland due to unavailability of forage within the forest interiors.
Livestock kills within both sites were both diurnal and nocturnal which differs with similar studies conducted in the Himalayan region i.e. Pakistan56 and Bhutan57 where leopard attacks were nocturnal in nature. Though leopards have been reported to be nocturnal in areas with and without humans48, 58 our results suggest diurnal activity peaks within human dominated landscapes of the Himalayan region. Similar to leopards, cheetahs also exhibit diurnal activity peaks within human-dominated landscapes of eastern Africa59. Big cats have generally been reported to kill livestock at night as with jaguars60 in the Pantanal, lions, leopards in Africa61 and tigers in India62. Leopards probably hunt wild prey at night but livestock killing is more pronounced during the day due to availability and ease of prey catchability.
Leopards in general are reported to kill cattle, goats and sheep when wild prey biomass falls below 540 kg/km263. Limited studies on leopard diet within anthropogenic landscapes of India highlight the major contribution of livestock32, 33. Prey abundance and availability is a major driver of human–carnivore conflicts63 but landscape level data on prey abundance was not available for the study sites to confirm such a relationship. However, our results indicate that wild prey biomass could be low in the Indian Himalayan region leading to increased attacks on livestock. Cattle loss to leopard attacks was higher in Pauri Garhwal whereas predation on goats were higher in North Bengal. Cattle are largely left unsupervised due to the low economic benefits compared to goats in Pauri Garhwal and hence predation on cows were higher. In North Bengal, cattle provide greater economic benefits and hence are generally supervised by an experienced herder. Cattle density (indigenous and cross breed) was also high i.e. 66 animals per km2 whereas goat density was 27 animals per km2 in Pauri Garhwal (Livestock Census 2012). Cattle density in North Bengal was 92 animals per km2 whereas goat density was 80 animals per km2 (Livestock Census 2012). Leopards show preference for wild prey species with an estimated prey weight range (10–40) kg11, 54, 55, and hence exhibit similar size preference when preying on livestock. Thus, goats were killed in higher abundance in North Bengal compared to Pauri Garhwal.
Previous studies in the mountainous regions of South Asia have documented leopard attacks on livestock to be geographically widespread without much significant association with human presence57, 64. In Bhutan livestock predation probability at a fine scale by leopards and tigers were positively associated with the density of human settlements47. Our results suggest that at a wider landscape scale, human presence (indicated by night light) is negatively and positively related to probability of conflicts in North Bengal and Pauri Garhwal, respectively. North Bengal is a densely populated region with human densities ranging from 200–700 individuals per km2 whereas in Pauri Garhwal, human density is much lower i.e. 110 individuals per km2. Leopards probably are adapted to co-occur close to humans but there exists a threshold beyond which there is an underlying avoidance and could be a result of carnivore’s perception of fear (persecution risk) within multi-use landscapes65. Our results are similar to space use by lions and cheetahs who avoided humans at a landscape scale due to the fear of persecution59, 66.
Output of the risk models is generally used globally to focus interventions and plan implementation of mitigation measures for minimizing damage caused by human–carnivore conflicts15, 16. The conflict risk maps generated through this study will be helpful to prioritize mitigation measures and reduce livestock depredation by leopards within IHR. Such measures will help reduce the present extent of retaliation by local communities and ensure survival of leopards outside of protected reserves. Policy makers will be able to better allocate resources to compensate livestock losses, forest and wildlife administration will be able to concentrate mitigation measures within specific sites and local communities will be able to avoid grazing of livestock within high risk zones. Livestock depredation can be reduced by improving existing animal husbandry practices such as using trained livestock guardian dogs and professional herders while grazing. To reduce economic damage to leopard attacks, agro-pastoralist societies of the IHR should be provided with monetary incentives through community based nature tourism initiatives. Awareness programs should be organized to educate livestock owners about biology of leopards, high risk areas and patterns of human–leopard conflicts. Radio-telemetry studies should be initiated to understand fine scale habitat utilization by leopards within anthropogenic landscapes. Finally, collaborations between managers, conservationists, local communities and understanding cultural complexities of human–leopard relations will be crucial to ensure future coexistence within heterogeneous mountainous landscapes of South Asia.
Methods
Study area
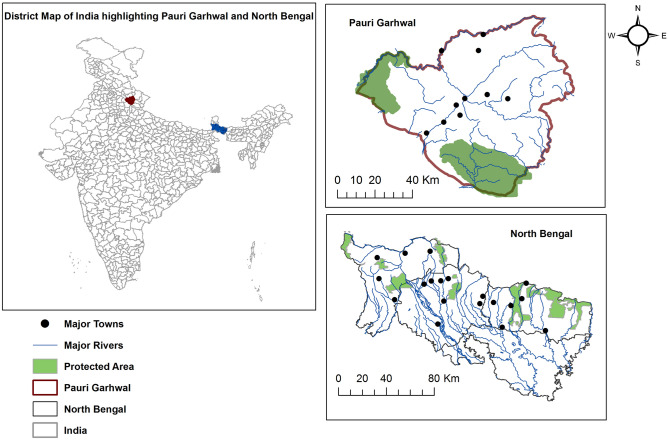
The study was conducted across two landscapes (North Bengal and Pauri Garhwal) located in eastern and western Himalaya.
The North Bengal landscape is located in the north-eastern part of India (Fig. 3) with a forest cover of 46%67. Predominant forest types are northern tropical semi-evergreen, moist deciduous forest and the landscape is a matrix of tea gardens, protected areas, agricultural lands and urban settlements59. Altitudinal variation is between 50 – 3,500 m with an annual rainfall range of 1,200–3,200 mm. Human density varies between 200 and 700 persons per km2 (Census 2011, https://www.census2011.co.in/census/district/1-darjiling.html. Accessed October 2019, https://www.census2011.co.in/census/district/2-jalpaiguri.html. Accessed October 2019, https://www.census2011.co.in/census/district/3-koch-bihar.html. Accessed October 2019) with the primary occupation being livestock farming, agriculture and tea industry68. Livestock density is about 340 per km233. Major wild prey is sambar (Rusa unicolor), chital (Axis axis), rhesus macaque (Macaca mulatta), and barking deer (Muntiacus muntjak). There has been the recent extinction of Bengal tiger (Panthera tigris tigris) from this region with leopard (Panthera pardus fusca) being the apex predator and only large carnivore present69.
Figure 3.
Location of North Bengal and Pauri Garhwal within India along with protected areas and major rivers prepared using Arc GIS 10.3 (https://enterprise.arcgis.com/en/portal/10.3/use/link-to-items.htm).
Pauri Garhwal district, part of the lesser, middle Himalaya and located in the north-western part of India (Fig. 3) has an altitudinal range of (200–3,200 m) with a forest cover of 64%67. Predominant forest types are moderate dense forests, open forest and scrubland58. Annual rainfall range is between 1,000 and 2,500 mm. Human density is 110 persons per km2 (Census 2011, data accessed on July 2019) and the local inhabitants are mainly agrarian with livestock farming, horticulture and cottage industries as major professions64. Livestock density of this region is 58 per km2 (Livestock Census, 19th All India Livestock Census Report 2012). This district has two protected areas in the foothill region with a sizeable population of Bengal tiger (Panther tigris tigris)51. Other than tigers, common leopards are the second most dominant apex predator in this region with presence of other mammals such as barking deer (Muntiacus muntjak), goral (Naemorhedus goral), sambar (Rusa unicolor), wild pig (Sus scrofa), rhesus macaques (Macaca mulatta) and common langur (Semnopithecus entellus).
Data collection
We used a two-step technique to collect data on leopard predation on livestock. First, we searched newspapers and West Bengal, Uttarakhand state forest department compensation registers for reports of incidents of leopard attacks on livestock in North Bengal and Pauri Garhwal between 2015 and 2018. Our primary aim was to avoid strong spatial bias in data and hence we checked for incidents which were spatially spread out and not confined to specific localities or regions. The compensation registers contained information such as date of incident, carnivore species involved, number and type of livestock killed and name of village/locality. Second, we conducted structured interviews across the regions and asked the owners and community members about age and species of livestock killed by leopards (survey protocol, see Supplementary Information Appendix 1). The livestock owner and forestry officials who were present during the attack or had verified the authenticity of the incident escorted the research team during such field visits. If there was ambiguity in response for a particular incident, we refereed only to the compensation register detail. We also crosschecked every detail of the incident with the report submitted by forestry officials after the initial investigation. The forestry officials were responsible for evaluation of wildlife attacks which is the basis for payment of compensation payments. To avoid exaggeration of livestock losses, we informed the community members that there will be no incentive or compensation provided as a part of the survey and the results will be solely used to help demarcate high and low conflict risk zones. We also recorded season and a time to livestock killed by leopards and asked owners about how often shepherds or community members used to accompany the animals during grazing (Supplementary Information Appendix 1). Based on the information gathered, we visited 857 sites in eastern Himalaya (North Bengal) and 375 sites in western Himalaya (Pauri Garhwal) where leopards had killed livestock. All field visits were made by the research team between November 2017 and November 2019. The forestry department officials accompanied the research team on all field visits and interviews. Ethical approval was obtained from the Wildlife Institute of India and office of the Chief Wildlife Wardens of Uttarakhand and West Bengal for conducting interviews. All methods and experiments (non-medical) were carried out in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. Informed consent was obtained from all subjects or their parents, legal guardians if they were below the age of 18 years. The locations visited were later mapped with a spatial accuracy of 20–50 m. To better understand the circumstances of attacks, we recorded all major habitat types and number of houses present within a radius of 50 m of each site.
Data analysis
Since precise timing for livestock predation was not known we used coarser 8 h intervals to assign correct time of the depredation events. Each 24-h period was subdivided into 8-h intervals (12AM–4AM, 4AM–8AM, 8AM–12PM, 12PM–4PM, 4PM–8PM, 8PM–12AM). We were interested in examining broader seasonal patterns of depredation (summer, monsoon and winter) and not just for individual months, hence each year was divided into 3 seasons of 4 months each (winter—November–February, summer i.e. February–June, monsoon i.e. June–November). We examined seasonal and temporal variation of attacks and difference in habitat types within the vicinity of predation sites using the chi-square test in R version 3.4.0. Statistical significance was p ≤ 0.05 for all analyses. All spatial analyses were performed with Arc GIS 10.3.3 and R 3.4.0.
The study areas were stratified into a scale of 5*5 i.e. 25 km2 which resulted to a total of 601 cells for North Bengal and 254 for Pauri Garhwal respectively. The cell size was selected based on ecological considerations to reduce chances of autocorrelation and identify landscape drivers of human–leopard conflicts. We generated a count statistic for the exact number of predation events for each cell. Cells where there were no attacks were assigned 0. To model the spatial spread and extent of livestock depredation we prepared both binary (presence-1 cells with at least one or multiple attacks, absence-0-no attacks) and count data (presence—exact number of attacks recorded and absence 0-no attacks). The number of cells on which leopard predation on livestock was recorded was 127 and 72 for North Bengal and Pauri Garhwal, respectively.
Data preparation for spatial risk analysis
We identified a total of 5 major landscape features (Habitat, Water, Human presence and infrastructure, Distance to Protected Reserves and Altitude) for North Bengal and Pauri Garhwal (Table 3) based on their ecological importance to model predation risk.
Habitat: We hypothesized that predation risk by leopard will be higher in areas with forests, presence of riverine patches, water bodies. Area of land use types: We calculated landscape variables i.e. area under different land-use types and water sources such as area of riverine patches, water bodies from Forest type map of India (2014)70.
Water: Large predators are reported to kill prey in areas with availability of water15, 20, thus we hypothesized that availability of water will be a major driver of leopard predation on livestock. We calculated the length of rivers and area of river bodies from the Roads and Drainage layers obtained from Digital Chart of the World and Forest type map of India70.
Human presence and infrastructure: We hypothesized that leopards would avoid killing livestock in areas with increased human presence71. We extracted night light values using the 1,000-m spatial resolution night-time visible light data of India72. We calculated length of roads using the Roads and Drainage layers obtained from Digital Chart of the World.
Distance to Protected Reserves: We hypothesized that the probability of leopard killing livestock will be higher in areas closer to protected areas17. We calculated distance from protected areas (Protected Area Network of India) using the Euclidean distance tool for each cell.
Altitude: Considering that carnivores prefer to kill livestock in areas with gradient in altitude47, we hypothesized that predation risk by leopards will be higher in elevated regions. Hence, we generated the mean altitude value for each cell based on 90-m spatial resolution digital elevation maps 74.
Table 3.
Major predictor variables considered for spatial analysis in the two study sites (25 km2).
| Type of variable | Predictor variable | Unit | Range (North Bengal) | Range (Pauri Garhwal) | Resolution | Source |
|---|---|---|---|---|---|---|
| Habitat (landscape variables) | Area of non-forests | m2 | 0–2.3 × 107 | 0–2.1 × 107 | 30 m | FSI 2014 |
| Area of scrubland | m2 | 0–4.9 × 106 | 0–5.4 × 106 | 30 m | FSI 2014 | |
| Area of moderate dense forests | m2 | 0–1.3 × 107 | 0–2.2 × 107 | 30 m | FSI 2014 | |
| Area of very dense forests | m2 | 0–2.3 × 107 | 0–1.5 × 107 | 30 m | FSI 2014 | |
| Area of open forest | m2 | 0–9.7 × 105 | 0–2.2 × 107 | 30 m | FSI 2014 | |
| Water | Area of river/water bodies | m2 | 0–5.6 × 105 | 0–2.2 × 107 | 30 m | Landsat 8TM |
| Length of rivers | km | 0–1.9 | 0.9.07 | 25 km2 | Digital chart of the world | |
| Human presence and infrastructure | Night light | Radiance | 0–60 | 0–49 | 25 km2 | Census India 2011 |
| Length of roads | km | 0–4.75 | 0–8.92 | 25 km2 | Digital chart of the world | |
| Distance to protected reserves | Distance from protected areas | M | 0–60,758 | 0–67,324 | 25 km2 | Moef and CC, Govt. of India |
| Altitude | DEM | M | 0–1,018 | 0–2,750 | 90 m | DEM |
Once the cell files were finalized and compiled, we clipped all ecological variables to 25 km2 cells. We omitted redundant correlated variables ≥ 0.7074 based on Pearson correlation coefficient values calculated using R version 3.4.0. Area of moderate dense forests was positively correlated with the area of very dense forests in North Bengal (value 0.76) and hence it was excluded from the analysis. Length of river was also positively correlated with length of roads (value 0.8) and hence it was also removed from the analysis. Altitude was positively correlated with distance to protected areas in Pauri Garhwal (value 0.76) and hence it was excluded from the analysis.
We used 4 analytical approaches to model probability of livestock depredation by leopard. In the 1st step we evaluated spatial autocorrelation among livestock kills within the cells using function moran.test (Moran’s I) in package (spdep)75 in R 3.4.0. In the 2nd and 3rd approach we used generalized linear models (GLMs) with binomial and poisson structures to quantify the effect of landscape features (area of habitat types, availability of water, human presence and infrastructure, distance to protected areas and altitude) on livestock predation. All the predictor variables considered for the analysis were continuous in nature. We used a priori candidate models and ranked them based on AIC, AICc values76. Models with the lowest AICc values were considered the best or dominant model and the output (coefficients and estimates) explained the probability of livestock predation by leopards within IHR. Based on the results of the dominant model or the model averaged coefficients, we generated conflict hotspots for both the study sites. We used coefficients of the best model (binomial structure) or averaged all candidate models (GLM with binomial structure) to estimate probability of livestock depredation for each cell (25 km2) using the equation p (x) = exp (z)/ (1 + exp (z)) and generated human–leopard conflict hotspots in Arc GIS 10.377. We generated ROC curve and AUC values to predict reliability of the dominant models using package ROCR78 in R 3.4.0. Since predictor variables between the two study sites were not normally distributed, we compared the identical landscape features using nonparametric Wilcoxon Signed-Rank Test in R 3.4.0.
In the 4th step, we used the predictor variables of the dominant models to calculate conditional inference (CTREEs), as prescribed in the R-package “partykit”79. This method was adopted to obtain threshold values for the significant variables for conflict mitigation recommendations. Trees based on maximally selected rank statistics were fitted using the Bonferroni correction for multiple testing and a minimum sum of weights. In addition, univariate trees were fitted for variables with a significant split in the multivariate tree. The results of our two analytical approaches (regression and ctree) are similar and provide an overall understanding of landscape features prone to livestock predation in accordance with the behavior of common leopards. Both analytical methods are based on a maximum likelihood approach and when interpreted together provide meaningful results. The GLM models computes probabilities of an event based on a logistic regression framework while the CTREE uses a machine learning classification approach and assigns values to predefined categories. The decision tree approach is a non-parametric approach which helps simplify complex relationships between dependent and predictor variables.
Supplementary information
Acknowledgements
This study was funded by was funded by the Ministry of Environment, Forest and Climate Change, Government of India under the National Mission for Himalayan Studies (NMHS). The grant number is NMHS/LG/2016/009. We thank the Principal Chief Conservator of Forests and Chief Wildlife Warden (Govt. of West Bengal and Uttarakhand) for providing us permissions, logistic support to conduct field work.
Author contributions
Conceptualization: D.N., M.H., Methodology: D.N., M.H., Data collection: S.K.D., A.C., G.S., P.C., Formal analysis and investigation: D.N., Writing—original draft preparation: D.N.; Writing—review and editing: D.N., M.H., S.S., Funding acquisition: SS, GSR, Supervision: M.H.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67980-w.
References
- 1.Sergio F, et al. Top predators as conservation tools: Ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 2008;39:1–19. [Google Scholar]
- 2.Estes JA, et al. Trophic downgrading of planet earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 3.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 4.Ugarte CS, Moreira-Arce D, Simonetti JA. Ecological attributes of carnivore-livestock conflict. Front. Ecol. Evol. 2019;7:1–9. [Google Scholar]
- 5.Thirgood S, Woodroffe R, Rabinowitz A. The impact of human–wildlife conflict on human lives and livelihoods. Conserv. Biol. Ser. Camb. 2005;9:13. [Google Scholar]
- 6.Dickman AJ, Macdonald EA, Macdonald DW. A review of financial instruments to pay for predator conservation and encourage human–carnivore coexistence. Proc. Natl. Acad. Sci. USA. 2011;108:13937–13944. doi: 10.1073/pnas.1012972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyhus PJ. Human–wildlife conflict and coexistence. Annu. Rev. Environ. Resour. 2016;41:143–171. [Google Scholar]
- 8.Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- 9.Carter NH, Linnell JDC. Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol. 2016;31:575–578. doi: 10.1016/j.tree.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Inskip C, Zimmermann A. Human-felid conflict: A review of patterns and priorities worldwide. Oryx. 2009;43:18–34. [Google Scholar]
- 11.Kissui BM. Livestock predation by lions, leopards, spotted hyenas, and their vulnerability to retaliatory killing in the Maasai steppe, Tanzania. Anim. Conserv. 2008;11:422–432. [Google Scholar]
- 12.Ikanda D, Packer C. Ritual vs. retaliatory killing of African lions in the Ngorongoro Conservation Area. Tanzania. Endanger. Species Res. 2008;6:67–74. [Google Scholar]
- 13.Hazzah L, Bath A, Dolrenry S, Dickman A, Frank L. From attitudes to actions: Predictors of lion killing by Maasai warriors. PLoS ONE. 2017;12:1–13. doi: 10.1371/journal.pone.0170796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogada DL. The power of poison: Pesticide poisoning of Africa’s wildlife. Ann. N. Y. Acad. Sci. 2014;1322:1–20. doi: 10.1111/nyas.12405. [DOI] [PubMed] [Google Scholar]
- 15.Treves A, Martin KA, Wydeven AP, Wiedenhoeft JE. Forecasting environmental hazards and the application of risk maps to predator attacks on livestock. Bioscience. 2011;61:451–458. [Google Scholar]
- 16.Miller JRB, Jhala YV, Jena J, Schmitz OJ. Landscape-scale accessibility of livestock to tigers: Implications of spatial grain for modeling predation risk to mitigate human–carnivore conflict. Ecol. Evol. 2015;5:1354–1367. doi: 10.1002/ece3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broekhuis F, Cushman SA, Elliot NB. Identification of human–carnivore conflict hotspots to prioritize mitigation efforts. Ecol. Evol. 2017;7:10630–10639. doi: 10.1002/ece3.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukeka JM, Ogutu JO, Kanga E, Røskaft E. Human–wildlife conflicts and their correlates in Narok County Kenya. Glob. Ecol. Conserv. 2019;18:e00620. [Google Scholar]
- 19.Laundre JW, Calderas JMM, Hernandez L. Foraging in the landscape of fear, the predator’s dilemma: Where should I hunt?! Open Ecol. J. 2009;2:1–6. [Google Scholar]
- 20.Grant J, Hopcraft C, Sinclair ARE, Packer C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005;74:559–566. [Google Scholar]
- 21.Balme G, Hunter L, Slotow R. Feeding habitat selection by hunting leopards (Panthera pardus) in a woodland savanna: Prey catchability versus abundance. Anim. Behav. 2007;74:589–598. [Google Scholar]
- 22.Gorini L, et al. Habitat heterogeneity and mammalian predator–prey interactions. Mamm. Rev. 2012;42:55–77. [Google Scholar]
- 23.Davie HS, Murdoch JD, Lhagvasuren A, Reading RP. Measuring and mapping the influence of landscape factors on livestock predation by wolves in Mongolia. J. Arid Environ. 2014;103:85–91. [Google Scholar]
- 24.Kolowski JM, Holekamp KE. Spatial, temporal, and physical characteristics of livestock depredations by large carnivores along a Kenyan reserve border. Biol. Conserv. 2006;128:529–541. [Google Scholar]
- 25.Abade L, Macdonald DW, Dickman AJ. Assessing the relative importance of landscape and husbandry factors in determining large carnivore depredation risk in Tanzania’s Ruaha landscape. Biol. Conserv. 2014;180:241–248. [Google Scholar]
- 26.Daniel Kissling W, Fernández N, Paruelo JM. Spatial risk assessment of livestock exposure to pumas in Patagonia, Argentina. Ecography. 2009;32:807–817. [Google Scholar]
- 27.Karanth KK, Gopalaswamy AM, DeFries R, Ballal N. Assessing patterns of human–wildlife conflicts and compensation around a Central Indian protected area. PLoS ONE. 2012;7:e50433. doi: 10.1371/journal.pone.0050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarco-González MM, Monroy-Vilchis O, Alaníz J. Spatial model of livestock predation by jaguar and puma in Mexico: Conservation planning. Biol. Conserv. 2013;159:80–87. [Google Scholar]
- 29.Soh YH, et al. Spatial correlates of livestock depredation by Amur tigers in Hunchun, China: Relevance of prey density and implications for protected area management. Biol. Conserv. 2014;169:117–127. [Google Scholar]
- 30.Jacobson AP, et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ. 2016;4:1–28. doi: 10.7717/peerj.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Athreya V, Odden M, Linnell JDC, Karanth KU. Translocation as a tool for mitigating conflict with leopards in human-dominated landscapes of India. Conserv. Biol. 2011;25:133–141. doi: 10.1111/j.1523-1739.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 32.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth KU. A cat among the dogs: Leopard (Panthera pardus) diet in a human-dominated landscape in western Maharashtra, India. Oryx. 2016;50:156–162. [Google Scholar]
- 33.Kshettry A, Vaidyanathan S, Athreya V. Diet selection of leopards (Panthera pardus) in a human-use landscape in North-Eastern India. Trop. Conserv. Sci. 2018;11:194008291876463. [Google Scholar]
- 34.Kshettry A, Vaidyanathan S, Athreya V. Leopard in a tea-cup: A study of leopard habitat-use and human–leopard interactions in north-eastern India. PLoS ONE. 2017;12:1–15. doi: 10.1371/journal.pone.0177013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidhu S, Raghunathan G, Mudappa D, Raman TRS. Conflict to coexistence: Human–leopard interactions in a plantation landscape in Anamalai Hills, India. Conserv. Soc. 2017;15:474–482. [Google Scholar]
- 36.Naha D, Sathyakumar S, Rawat GS. Understanding drivers of human–leopard conflicts in the Indian Himalayan region: Spatio-temporal patterns of conflicts and perception of local communities towards conserving large carnivores. PLoS ONE. 2018;13:1–19. doi: 10.1371/journal.pone.0204528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athreya V, et al. Spotted in the news: Using media reports to examine leopard distribution, depredation, and management practices outside protected areas in southern India. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0142647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar, D. Study of Leopard Menace, Food Habits and Habitat Parameters in Mandi District, Himachal Pradesh. PhD thesis. Saurashtra University (2011).
- 39.Miller JRB, Jhala YV, Jena J. Livestock losses and hotspots of attack from tigers and leopards in Kanha Tiger Reserve, Central India. Reg. Environ. Change. 2016;16:17–29. [Google Scholar]
- 40.Lamichhane BR, et al. Spatio-temporal patterns of attacks on human and economic losses from wildlife in Chitwan National Park, Nepal. PLoS ONE. 2018;13:e0195373. doi: 10.1371/journal.pone.0195373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beattie K, Olson ER, Kissui B, Kirschbaum A, Kiffner C. Predicting livestock depredation risk by African lions (Panthera leo) in a multi-use area of northern Tanzania. Eur. J. Wildl. Res. 2020;66:11. [Google Scholar]
- 42.Hayward MW, et al. Prey preferences of the leopard (Panthera pardus) J. Zool. 2006;270:298–313. [Google Scholar]
- 43.Boydston EE, Kapheim KM, Watts HE, Szykman M, Holekamp KE. Altered behaviour in spotted hyenas associated with increased human activity. Anim. Conserv. 2003;6:207–219. [Google Scholar]
- 44.Ordiz A, Støen OG, Delibes M, Swenson JE. Predators or prey? Spatio-temporal discrimination of human-derived risk by brown bears. Oecologia. 2011;166:59–67. doi: 10.1007/s00442-011-1920-5. [DOI] [PubMed] [Google Scholar]
- 45.Suraci JP, et al. Behavior-specific habitat selection by African lions may promote their persistence in a human-dominated landscape. Ecology. 2019;100:1–11. doi: 10.1002/ecy.2644. [DOI] [PubMed] [Google Scholar]
- 46.Karanth KK, Gopalaswamy AM, Prasad PK, Dasgupta S. Patterns of human–wildlife conflicts and compensation: Insights from Western Ghats protected areas. Biol. Conserv. 2013;166:175–185. [Google Scholar]
- 47.Rostro-García S, et al. Scale dependence of felid predation risk: Identifying predictors of livestock kills by tiger and leopard in Bhutan. Landsc. Ecol. 2016;31:1277–1298. [Google Scholar]
- 48.Odden M, Athreya V, Rattan S, Linnell JDC. Adaptable neighbours: Movement patterns of GPS-collared leopards in human dominated landscapes in India. PLoS ONE. 2014;9:e112044. doi: 10.1371/journal.pone.0112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Boer WF, et al. Spatial distribution of lion kills determined by the water dependency of prey species. J. Mammal. 2010;91:1280–1286. [Google Scholar]
- 50.Constant, N. A Socio-Ecological Approach Towards Understanding Conflict Between Leopards (Panthera pardus) and Humans in South Africa: Implications for Leopard Conservation and Farming Livelihoods. PhD thesis, Durham University (2014).
- 51.Bisht S, Banerjee S, Qureshi Q, Jhala Y. Demography of a high-density tiger population and its implications for tiger recovery. J. Appl. Ecol. 2019;56:1725–1740. [Google Scholar]
- 52.Kotru R. Participatory forest management and sustainable development outcomes in the subtropical Himalayas: A sequel of environment, economy and equity through social empowerment. In: Günter S, Weber M, Stimm B, Mosandl R, editors. Silviculture in the Tropics. Berlin: Springer; 2011. pp. 35–42. [Google Scholar]
- 53.Tamang B, Baral N. Livestock depredation by large cats in Bardia National Park, Nepal: Implications for improving park-people relations. Int. J. Biodivers. Sci. Manag. 2008;4:44–53. [Google Scholar]
- 54.Kuiper TR, et al. Seasonal herding practices influence predation on domestic stock by African lions along a protected area boundary. Biol. Conserv. 2015;191:546–554. [Google Scholar]
- 55.Kissui BM, Kiffner C, König HJ, Montgomery RA. Patterns of livestock depredation and cost-effectiveness of fortified livestock enclosures in northern Tanzania. Ecol. Evol. 2019;9:11420–11433. doi: 10.1002/ece3.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qamar ZQ, Dar NI, Ali U, Minhas RA, Ayub J, Anwar M. Human–leopard conflict: An emerging issue of common leopard conservation in Machiara National Park, Azad Jammu and Kashmir, Pakistan. Pakistan J. Wildl. 2010;1:50–56. [Google Scholar]
- 57.Sangay T, Vernes K. Human–wildlife conflict in the Kingdom of Bhutan: Patterns of livestock predation by large mammalian carnivores. Biol. Conserv. 2008;141:1272–1282. [Google Scholar]
- 58.Odden M, Wegge P. Spacing and activity patterns of leopards (Panthera pardus) in the Royal Bardia National Park, Nepal. Wildl. Biol. 2005;11:145–152. [Google Scholar]
- 59.Broekhuis F, Grünewälder S, McNutt JW, Macdonald DW. Optimal hunting conditions drive circalunar behavior of a diurnal carnivore. Behav. Ecol. 2014;25:1268–1275. [Google Scholar]
- 60.Soto-Shoender JR, Giuliano WM. Predation on livestock by large carnivores in the tropical lowlands of Guatemala. ORYX. 2011;45:561–568. [Google Scholar]
- 61.Loveridge AJ, et al. Bells, bomas and beefsteak: Complex patterns of human–predator conflict at the wildlife–agropastoral interface in Zimbabwe. PeerJ. 2017;5:e2898. doi: 10.7717/peerj.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borah J, et al. Livestock depredation by Bengal tigers in fringe areas of Kaziranga Tiger Reserve, Assam, India: Implications for large carnivore conservation. Human-Wildl. Interact. 2018;12:186–197. [Google Scholar]
- 63.Khorozyan I, Ghoddousi A, Soofi M, Waltert M. Big cats kill more livestock when wild prey reaches a minimum threshold. Biol. Conserv. 2015;192:268–275. [Google Scholar]
- 64.Goyal, S. P., Chauhan, D. S. & Yumnam, B. Status and Ecology of Leopard in Pauri Garhwal: Ranging patterns and reproductive biology of leopard (Panthera pardus) in Pauri Garhwal Himalaya. Final Report, Wildlife Institute of India (2007).
- 65.Ciuti S, et al. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE. 2012;7:e50611. doi: 10.1371/journal.pone.0050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oriol-Cotterill A, Macdonald DW, Valeix M, Ekwanga S, Frank LG. Spatiotemporal patterns of lion space use in a human-dominated landscape. Anim. Behav. 2015;101:27–39. [Google Scholar]
- 67.India State of Forest Report . Forest and Tree Resources in States and Union Territories. Dehradun: Forest Survey of India; 2017. [Google Scholar]
- 68.Chatterjee PA. Time for Tea: Women, Labor, and Post/Colonial Politics on an Indian Plantation. Durham: Duke University Press; 2001. [Google Scholar]
- 69.Bhattacharjee A, Parthasarathy N. Coexisting With large carnivores: A case study from western Duars, India. Hum. Dimens. Wildl. 2013;18:20–31. [Google Scholar]
- 70.Forest Type Map of India . Forest Survey of India Report. Dehradun: Forest Survey of India; 2014. [Google Scholar]
- 71.Loveridge AJ, Valeix M, Elliot NB, Macdonald DW, Lyon CB. The landscape of anthropogenic mortality: How African lions respond to spatial variation in risk. J. Appl. Ecol. 2017;54:815–825. [Google Scholar]
- 72.Gaba, K. M., Min, B., Thakker, A. & Elvidge, C. Nightlights.io: Twenty Years of India Lights (2016).
- 73.Rodríguez E, Morris CS, Belz JE. A global assessment of the SRTM performance. Photogramm. Eng. Remote Sens. 2006;72:249–260. [Google Scholar]
- 74.Dormann FC, et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography. 2007;30:609–628. [Google Scholar]
- 75.Bivand RS, Pebesma E, Gomez-Rubio V. Applied Spatial Data Analysis with R. 2. Berlin: Springer; 2013. [Google Scholar]
- 76.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 77.Environmental Systems Research Institute Inc. ArcGIS 10.3. Redlands, California, USA (2014).
- 78.Sing T, Sander O, Beerenwinkel N, Lengauer TROCR. Visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 79.Hothorn, T., Hornik, K., Strobl, C. & Zeileis, A. Party A Laboratory for Recursive Partitioning. R Package Version1.2-4 (accessed 6 July 2019); https://cran.r-project.org/web/packages/party/vignettes/party.pdf (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.