Abstract
Escherichia coli is the dominant bacterial cause of UTI among the uropathogens in both developed and developing countries. This study is to investigate the effect of Acacia nilotica aqueous extract on the survival and biofilm of isolated pathogens to reduce UTIs diseases. A total of 170 urine samples were collected from Luxor general hospital and private medical analysis laboratories in Luxor providence, Egypt. Samples were screened for the incidence of uropathogens by biochemical tests, antibiotics susceptibility, detection of virulence, and antibiotic-resistant genes by multiplex PCR, biofilm formation, and time-killing assay. Escherichia coli is by far the most prevalent causative agent with the percentage of 73.7% followed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeuroginosa, and Acinetobacter baumanii. Isolates were multidrug-resistant containing blaTEM, blaSHV, blaCTX, qnrs, and aac(3)-Ia resistant genes. All isolates were sensitive to 15–16.7 mg ml−1 of Acacia nilotica aqueous extract. Time killing assay confirmed the bactericidal effect of the extract over time (20–24 h). A high percentage of 3-Cyclohexane-1-Carboxaldehyde, 2,6,6-trimethyl (23.5%); á-Selinene (15.12%); Oleic Acid (14.52%); Globulol (11.35%) were detected among 19 bioactive phytochemical compounds in the aqueous extract of A. nilotica over the GC-mass spectra analysis. The plant extract reduced significantly the biofilm activity of E. coli, K. pneumoniae, P. mirabilis, and P. aeuroginosa by 62.6, 59. 03, 48.9 and 39.2%, respectively. The challenge to improve the production of A. nilotica phytochemicals is considered a very low price for the return.
Subject terms: Microbiology, Molecular biology
Introduction
Urinary tract infections (UTIS) are one of the most prevalent and predominant nosocomial human infections. It infects patients of all ages and both gender with the greatest occurrence in females1. Signs and symptoms may include fever, chills, dysuria, urinary urgency, frequency, and cloudy or malodorous urine2. UTIs are caused by a variety of bacteria such as E. coli, K. pneumoniae, P. mirabilis, Pseudomonas sp., S. aureus, Enterococcus faecalis, Streptococcus sp., and Citrobacter sp.3. Each organism has its virulence genes that contribute to its invasion and toxicity. The increasing prevalence of UTI and antibiotic-resistant bacteria have made empirical antibiotic treatment more and more difficult4. A urine culture and antibiotic susceptibility tests are important for diagnosing the disease, recommending suitable antibiotics, and reducing the number of antibiotic-resistant uropathogens2. On the other hand, the genus Acacia belongs to the Leguminosae family. It contains more than 1,350 species, distributed throughout tropical and warm areas5. Several species of Acacia have been proven as significant antibacterial and antifungal agents6,7. It also has a great effect against multidrug-resistant strains of bacteria initiating nosocomial and community-acquired infections8. The plant is a tree with yellow mimosa-like flowers and long grey pods9. The use of plants and herbs extract in the therapy of human disease is very ancient traditions and scientists in Africa and other developing countries are carrying research on local plants numerous in the continent for use in conventional medicine10. The current study was performed to determine the resistant patterns of uropathogens and to highlight the efficacy of phytochemical compounds in Acacia nilotica aqueous extract against the survival and biofilm of these pathogens to reduce urinary tract infection diseases.
Results
The incidence of uropathogenic bacteria among examined urine samples
The prevalence of the isolated uropathogens in urine samples were illustrated in Table 1. Among the 170 urine samples, only 133 (78.2%) sample was positive for urine culture. Positive samples comprise 32 (53.3%) samples from males and 101 (91.8%) from females. The most common prevalent organism (from each corresponding positive samples) was E. coli which isolated from 98 patient with a percentage of 73.7%, followed by K. pneumoniae 13.5% (18), P. mirabilis 6.7% (9), P. aeruginosa 4.5% (6) and A. baumannii 1.5% (2). The total bacterial count of all samples was ranged from 1.88 to 215 × 107 CFU/ml.
Table 1.
Incidence of isolated uropathogenic bacteria in urine samples.
| Parameters | No. of positive isolatesb | (%)c | CFU/mld |
|---|---|---|---|
| Isolatesa | |||
| Escherichia coli | 98 | 73.7 | 215 × 107 |
| Klebsiella pneumoniae | 18 | 13.5 | 16.8 × 107 |
| Proteus mirabilis | 9 | 6.7 | 111 × 107 |
| Pseudomonas aeuroginosa | 6 | 4.5 | 1.88 × 107 |
| Acinetobacter baumanii | 2 | 1.5 | 202 × 107 |
| Total | 133 | ||
Total number of examined urine samples was 170 sample, they were 110 sample from females and 60 samples from male. Number of positive samples was 133 samples comprised from 101 sample from females and 32 sample from males.
aDifferent isolated uropathogens from urine samples.
bNumber of positive isolates from total number of positive samples.
cPercentage of positive isolates from total number of positive samples.
dColony forming unit per ml for each isolated bacterium.
Antimicrobial susceptibility testing
The rate of resistance to all isolated uropathogenic bacteria to a panel of antibiotics with different potency was illustrated in Table 2, the resistance rate of ampicillin-sulbactam, ampicillin, gentamicin, nalidixic acid, amikacin, ceftazidime, ciprofloxacin, piperacillin, and cefepime was observed in all isolated uropathogens. While resistance level of piperacillin-tazobactam was noticed in all isolated pathogens except P. aeruginosa that was sensitive for this antibiotic. Interestingly, sensitivity level imipenem and meropenem were observed only against E. coli and P. mirabilis. So, antimicrobial susceptibility demonstrated that K. pneumoniae and A. baumannii were resistant to all tested antibiotics (100%). Followed by P. aeruginosa that was resistant to 91.6% of all tested antibiotics. Finally, E. coli and P. mirabilis were resistant to 83.3% of antibiotics.
Table 2.
Antimicrobial susceptibility and antibiotic resistant genes of multidrug resistant uropathogens isolated from urine.
| Isolatesa | E. coli | K. pneumoniae | P. mirabilis | P. aeruginosa | A. baumannii |
|---|---|---|---|---|---|
| Antibioticsb | |||||
| SAM | R | R | R | R | R |
| AM | R | R | R | R | R |
| GM | R | R | R | R | R |
| NA | R | R | R | R | R |
| AMK | R | R | R | R | R |
| CAZ | R | R | R | R | R |
| CIP | R | R | R | R | R |
| PIP | R | R | R | R | R |
| PTZ | R | R | R | S | R |
| CPE | R | R | R | R | R |
| MEM | S | R | S | R | R |
| IPM | S | R | S | R | R |
| Antibiotic resistant genesc | |||||
| blaTEM | + | – | + | – | + |
| blaSHV | + | + | + | + | + |
| blaCTX | + | – | – | + | + |
| qnrS | + | + | + | + | + |
| aac(3)-Ia | + | + | + | + | + |
| mexRd | — | — | — | + | — |
aEscherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeuroginosa, Acinetobacter baumanii.
bSAM (Ampicillin-sulbactam), AM (Ampicillin), GM (Gentamicin), NA (Nalidixic acid), AMK (Amikacin), CAZ (Ceftazidime), CIP (Ciprofloxacin), PIP (Piperacillin), PTZ (Piperacillin-tazobactam), CPE (Cefepime), MEM (Meropenem), IPM (Imipenem). R = Resistant; S = Sensitive.
cAntibiotic resistant genes,+: present; –: absent.
dmexR gene: it is specific gene for Pseudomonas aeuroginosa only.
Detection of virulence and antibiotic-resistant genes
The multiplex PCR screening for virulence and antibiotic-resistant genes showed that hly, papC, and fimH virulence genes were present E. coli while eaeA was absent. For K. pneumoniae, aerobactin gene was present while, rmpA, TraT, and fimH were absent. For the P. mirabilis atpD gene was a present while, the fimH gene was absent. For P. aeruginosa, the pslA gene was present while, lasB, toxA, and fliC were absent. Finally, A. baumannii doesn't contain any of the detected genes (cnf1, cvaC, iutA, and fimH) (Table 3; Fig. 1). On the other hand, the antibiotic-resistant genes, blaSHV, qnrS, and aac(3)-Ia were present in all uropathogenic isolates (100%). blaTEM was present among E. coli, P. mirabilis, and A. baumannii with a percentage of 100% and absent in other isolates. BlaCTX gene was detected among E. coli, P. aeruginosa and A. baumannii while they were absent in other isolates. Finally, mexR was positive among P. aeruginosa isolates (Table 2; Fig. 2).
Table 3.
The relation between virulence genes and antibacterial activity of Acacia nilotica extract for isolated uropathogens.
| Isolatesa | Virulence genesb | Antibacterial efficacy | |||
|---|---|---|---|---|---|
| MIC/MBCc (mg ml−1) | |||||
| E. coli | hly | fimH | eaeA | papC | 11.7/13.3 |
| + | + | – | + | ||
| K. pneumoniae | rmpA | aerobactin | fimH | TraT | 11.7/13.3 |
| – | + | – | – | ||
| P. mirabilis | fimH | atpD | 15/16.7 | ||
| – | + | ||||
| P. aeruginosa | lasB | toxA | pslA | fliC | 11.7/15 |
| – | – | + | – | ||
| A. baumanii | cnf1 | cvaC | iutA | fimH | 11.7/15 |
| – | – | – | – | ||
aDifferent isolated bacteria from urine samples; Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Acinetobacter baumannii.
bPresent ( +), absent (–).
cMinimal inhibitory concentration/ minimal bactericidal concentration of the extract against isolated uropathogens represented in mg ml−1.
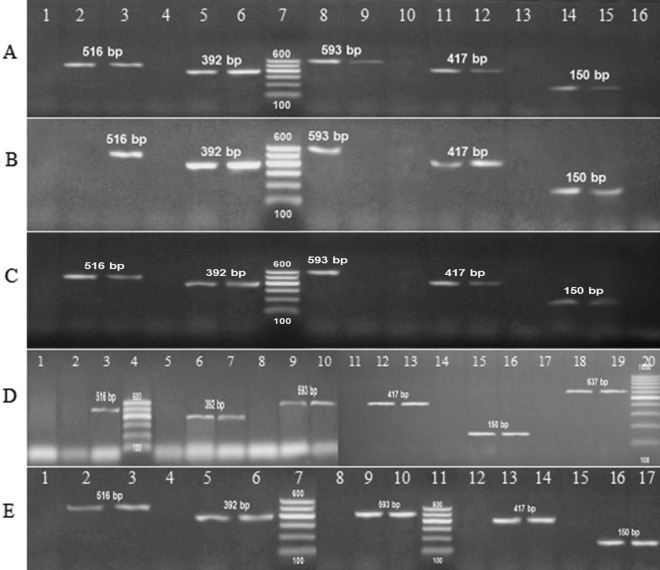
Figure 1.
1.5% agarose gel electrophoresis of multiplex PCR of virulence genes characterized for the isolated uropathogens. (A) E. coli; Lane 1, 4, 10, 13: negative control for detected genes; Lane 3, 6, 8, 11 positive control of DNA confirmed by reference laboratory for quality control; Lane 2 hly (1,177 bp), Lane 5 fimH (508 bp), Lane 9 negative eaeA (248), Lane 12 papC (501 bp); Lane 7 Gel Pilot 100 bp plus ladder (cat. no. 239045) supplied from QIAGEN (USA). (B) Klebsiella pneumoniae; Lane 1, 4, 10, 13: negative control for detected genes; Lane 3, 6, 8, 11 positive control of DNA; Lane 2 negative rmpA (535 bp), Lane 5 negative TraT (556 bp), Lane 9 negative fimH (508), Lane 12 aerobactin (307 bp); Lane 7, 100 bp ladder as molecular size DNA marker (cat. no. 239035) supplied from QIAGEN (USA). (C) Pseudomonas aeruginosa; Lane 1, 4, 8, 11: negative control for detected genes; Lane 3, 6, 10 and 13: positive control of DNA; Lane 2 negative lasB (1,220 bp); Lane 5 negative toxA (396); Lane 9 pslA (656 bp); Lane 12 negative fliC (180 bp); Lane 7 Gel Pilot 100 bp plus ladder (cat. no. 239045) supplied from QIAGEN (USA). (D) Acinetobacter baumanii; Lane 1, 4, 7, 10: negative control for detected genes; Lane 3, 6, 9 and 12: positive control of DNA; Lane 2 negative cnf1 (620 bp); Lane 5 negative cvaC (760); Lane 8 negative iutA (300 bp); Lane 11 negative fimH (508 bp); Lane 13 Gene ruler 100 bp DNA ladder (cat. no. SM0243) supplied from Fermentas. (E) Proteus mirabilis; Lane 1 and 4: negative control for detected genes; Lane 3 and 6: positive control of DNA; Lane 2 negative fimH (508 bp); Lane 5 atpD (595 bp); Lane 7, 100 bp ladder as molecular size DNA marker (cat. no. 239035) supplied from QIAGEN (USA).
Figure 2.
1.5% agarose gel electrophoresis of multiplex PCR of antibiotic resistant genes for the isolated uropathogens. (A) E. coli; (B) Klebsiella pneumoniae; (C) Proteus mirabilis; Lane 1, 4, 10, 13 and 16: Negative control for detected genes; Lane 3, 6, 8, 11 and 14: Positive control of DNA confirmed by reference laboratory for quality control; Lane 7: 100 bp ladder as molecular size DNA marker (cat. no. 239035) supplied from QIAGEN (USA). Lane 2 blaTEM (516 bp); Lane 5 blaSHV (392 bp); Lane 9 blaCTX (593 bp); Lane 12 qnrS (417); Lane 15 aac(3)-Ia (150 bp). (D) Pseudomonas aeuroginosa; Lane, 1, 5, 8, 11, 14 and 17: Negative control for detected genes; Lane 3, 7, 10, 13, 16 and 19: Positive control of DNA; Lane 2 blaTEM; Lane 6 blaSHV; Lane 9 blaCTX; Lane 12 qnrS; Lane 15 aac(3)-Ia; Lane 18 mexR (637 bp). Lane 4: 100 bp ladder as molecular size DNA marker (cat. no. 239035) supplied from QIAGEN (USA). Lane 20: Gene ruler 100 bp DNA ladder (cat. no. SM0243) supplied from Fermentas. (E) Acinetobacter baumanii; Lane 1, 4, 8, 12, 15: Negative control for detected genes; Lane 3, 6, 10, 14 and 17: Positive control of DNA; Lane 2 blaTEM; Lane 5 blaSHV; Lane 9 blaCTX; Lane 13 qnrS; Lane 16 aac(3)-Ia. Lane 7 and 11: 100 bp ladder as molecular size DNA marker (cat. no. 239035) supplied from QIAGEN (USA).
GC–MS analysis
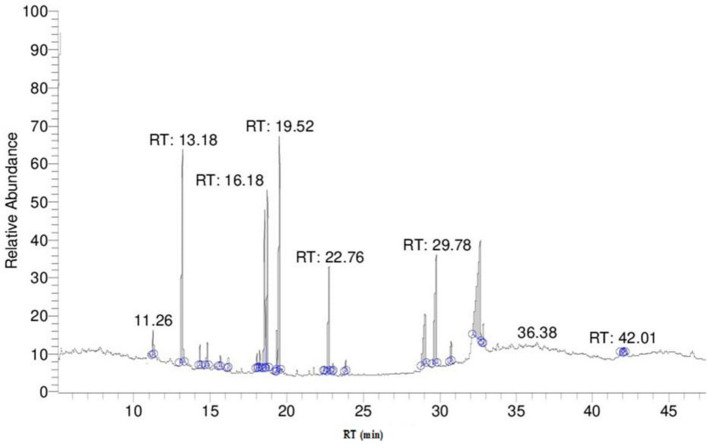
The analysis and extraction of plant material play a significant role in the progress, reconstruction, and quality control of herbal formulations. Hence one of the important aims in the present study was to find out the bioactive compounds present in the aqueous extract of A. nilotica by using Gas chromatography-Mass spectroscopy. This shows the presence of 19 bioactive phytochemical compounds in the aqueous extract of A. nilotica. The highest percentage content of the compounds are as follows: 3-Cyclohexane-1-Carboxaldehyde, 2,6,6-trimethyl (23.5%); á-Selinene (CAS) (15.12%); Oleic Acid (14.52%); Globulol (11.35%). Other active compounds with their peak number, concentration (peak area%), and retention time (RT) are presented in (Table 4; Fig. 3).
Table 4.
GC-mass spectra of Acacia nilotica showing different active compounds.
| S. no. | RT (min) | Compound | Area (%) | M. formula | M. wt |
|---|---|---|---|---|---|
| 1 | 11.26 | Carane, 4,5-epoxy-, (E)- | 0.86 | C10H16O | 152 |
| 2 | 13.18 | 3-Cyclohexane-1-Carboxaldehyde, 2,6,6-trimethyl- | 23.5 | C10H16O | 152 |
| 3 | 14.33 | Cycloundecene, 1-methyl- | 0.76 | C12H22 | 166 |
| 4 | 14.80 | 2,4-Decadienal, (E, E)-(CAS) | 1.05 | C10H16O | 152 |
| 5 | 15.63 | 1-Decanol, 2-methyl- | 0.47 | C11H24O | 172 |
| 6 | 16.18 | Cyclohexane,1-ethyl-1-methyl-2,4-bis(1-methyl)-(CAS) | 0.72 | C15H24 | 204 |
| 7 | 18.06 | á-Chamigrene | 0.52 | C15H24 | 204 |
| 8 | 18.21 | á-Guaiene | 0.59 | C15H24 | 204 |
| 9 | 18.57 | á-Selinene (CAS) | 15.12 | C15H24 | 204 |
| 10 | 19.32 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | 1.05 | C11H12O3 | 192 |
| 11 | 19.52 | Globulol | 11.35 | C15H26O | 222 |
| 12 | 22.76 | 1,3-Benzodioxole, 4,7-dimethoxy-5-(2-propeny1)-(CAS) | 5.54 | C12H14O4 | 222 |
| 13 | 23.02 | a-acorenol | 0.18 | C15H26O | 222 |
| 14 | 23.86 | 1,2-Epoxycyclooct-3-ene, 5,5-dimethyl-8-methylene- | 0.32 | C11H16O | 164 |
| 15 | 29.09 | Hexadecanoic acid (CAS) | 4.59 | C16H32O2 | 256 |
| 16 | 29.78 | 26,8a-(Dimethoxy)-3,5,7-trion tetracyclo [7.2.1.0(4,11).0(6,10)] dodecane | 8.51 | C11H16O5 | 228 |
| 17 | 30.75 | Isobergapten | 1.09 | C12H80O | 216 |
| 18 | 32.66 | Oleic acid | 14.52 | C18H34O2 | 282 |
| 19 | 32.86 | Isochiapin B | 1.18 | C19H26O6 | 346 |
RT: Retention time per minute; active compounds detected by GC mass; area (%): percentage of compound; M. formula: molecular formula; M. wt: molecular weight of the compound.
Figure 3.
Gas chromatography spectra of biologically active compounds of Acacia nilotica.
Efficacy of A. nilotica aqueous extract as an antimicrobial agent
Antibacterial activity of A. nilotica aqueous extract against the isolated uropathogens was analyzed by minimal inhibitory concentrations (MIC) by determining the bacterial viability using a colorimetric INT-formazan assay. Thus, we additionally determined the minimal bactericidal concentrations (MBC) which confirmed the killing of the isolated uropathogens over time. The results showed a reproducible and effective antibacterial effect against all isolated uropathogens (Preventing INT color change). Where, the concentration of 11.7 mg ml−1 was enough as MIC for all tested organisms except P. mirabilis that required a higher concentration of 15 mg ml−1. Generally, the efficacy of the extract as bactericidal (MBC) natural product against E. coli and K. pneumoniae was 13.3 mg ml−1. Pseudomonas aeruginosa and A. baumanii recorded MBC value of 15 mg ml−1. Interestingly, P. mirablis verified the highest MBC value of 16.7 mg ml−1 as shown in Table 3.
Static biofilm assay
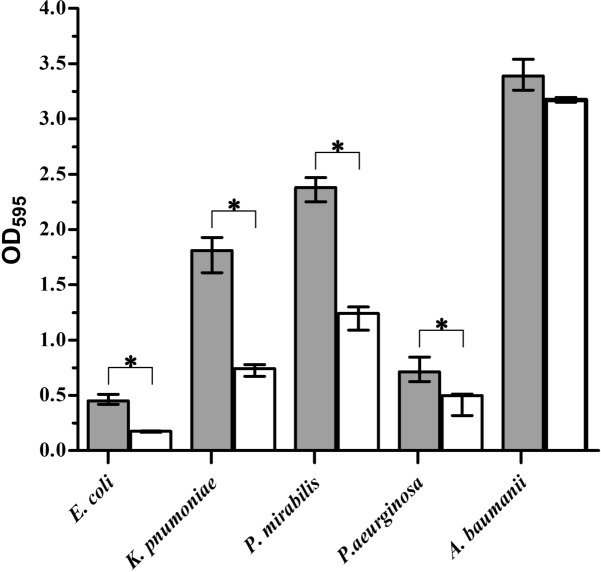
Quantitative determination of biofilm amount (OD595) of the isolated uropathogens as control and after treatment with A. nilotica extract (Fig. 4). According to mean values of OD595 nm, the results of control (isolated uropathogens) were interpreted as low, moderate, and high bacterial biofilm former when OD595 nm was < 1; 1–2.9 and > 2.9 respectively. Accordingly, A. baumannii was a high biofilm former while, K. pneumoniae and P. mirabilis had a moderate ability. On the other hand, E. coli and P. aeruginosa were low biofilm former. Interestingly, after treatment with the MBC-values of A. nilotica aqueous extract, E. coli, K. pneumoniae, P. mirabilis, and P. aeruginosa significantly reduce biofilm by 62.6; 59; 49 and 39.2%, respectively (Fig. 4).
Figure 4.
Impact of Acacia nilotica aqueous extract on biofilm of some uropathogenic isolates. Quantitative determination of biofilm amount of the isolated uropathogens as control (gray bars) and after treatment with Acacia nilotica aqueous extract (open bars). After an additional 24 h biofilm formation was quantified by crystal violet staining and subsequent determination of the OD595. Shown are the medians from at least three independent measurements. The error bars indicate the interquartile range. Significant differences between the data sets are marked by asterisks (P < 0.05; Kruskal–Wallis test and post hoc Dunn’s multiple comparisons).
Survival curve of the isolated uropathogens in the presence of Acacia nilotica aqueous extract
The killing dynamic of A. nilotica aqueous extract against log-phase cultures of the isolated uropathogens was determined to compare with a positive control for each uropathogens (Fig. 5). The MBC-values for the A. nilotica aqueous extract were affected by different isolates' growth. The results revealed that after 8 h there is a continuous decrease of all uropathogens cultures OD595 with no detectable growth after 20 h exposition for E. coli and K. pneumoniae (Fig. 5a, b). In the case of P. mirabilis, P. aeruginosa and A. baumannii killing curve were also recorded a steady decrease of OD595 over time starting after 8 h exposition with no viable microorganism in the initial inoculums could be observed after 24 h. Significant reduction starting at 16 h of treatment for all isolates. Positive control had a continuous increase to 24 h (Fig. 5c–e). Based on these results, Time-kill kinetic profile indicates that A. nilotica extracts exhibited bactericidal actions against all uropathogenic isolates (Fig. 5).
Figure 5.
Survival curve of some uropathogenic isolates in the presence of Acacia nilotica aqueous extract. Shown are the median OD595 values of Escherichia coli (a); Klebsiella pneumoniae (b); Proteus mirabilis (c); Pseudomonas aeuroginosa (d); and Acinetobacter baumanii (e) in TSB media (solid line) or supplemented with MBC value of Acacia nilotica aqueous extract (dashed line) for each isolate at 37 °C. Shown are the medians from at least three independent measurements. The error bars indicate the interquartile range. Significant differences between the data sets are marked by asterisks (P < 0.05; Kruskal–Wallis test and post hoc Dunn’s multiple comparisons).
Discussion
UTIs are considered one of the most common groups on infections in humans worldwide that upset kidney, pyelonephritis, bladder, and cystitis11. As stated by the CDC, UTIs are the greatest common bacterial infection demanding medical care, resulting in 8.6 million ambulatory care visits in 200712. It is an infection of the urinary tract with a pathogen causing inflammation and occasionally life-threatening13. In the current study, although all patients were showing some or all of UTIs symptoms like burning feeling during urination, frequent urge for urination, cloudy appearance of urine, and pain in the back or the lower abdomen14. Only about 78.2% of 170 patients had UTIs in this study (Table 1). This is possible because UTI symptoms are not a dependable indicator of disease. So, urine culture is necessary for the diagnosis of UTI for confirming the presence of bacteriuria14. The study also verified a lower UTI rate of 7.2% in males comparing with 92.8% in females In agreement with15, who stated that UTIs are common in women than men with a ratio of 8:1. A low rate of infection in males may be due to the occurrence of antimicrobial substances in prostatic fluid. Also, maybe a long urethra (20 cm) that provides a distance barrier that eliminates microorganisms from the bladder16.
The principal step for effective treatment of UTIs is to classify the type of infection, such as acute uncomplicated cystitis or pyelonephritis, acute complicated cystitis or pyelonephritis, catheter associated-UTI, asymptomatic bacteriuria (ASB), or prostatitis depending on identification of causing organism for describing defective antibiotic17,18. About 95% of uncomplicated UTIs are mono-bacterial and E. coli is the major causing agent of uncomplicated UTI, which accounts for up to 75–90% of cases19,20. This study is in agreement with our study where E. coli isolated with a percentage of 73.7% from all isolated uropathogens. Klebsiella pneumoniae was the second isolated organism with a percentage of 13.5%, in agreement with20,21. Followed by P. mirabilis (6.7%), P. aeruginosa (4.5%), and A. baumanii (1.5%) (Table 1). Also in agreement with22, who revealed that uropathogenic E. coli (UPEC) is the most common causative agent for both complicated and uncomplicated UTIs, other causative agents are involved like K. pneumoniae, P. mirabilis, P. aeruginosa and other types. Uropathogenic E. coli (UPEC) contains several virulence factors that facilitate its colonization and invasion of host cells23,24. Surface virulence factors (adhesions) are among the most important virulence factors25,26. As the main attachment factor. P fimbriae are particularly associated with pyelonephritis and cystitis which encoded by pap genes27,28. Other important virulence factors in UPEC are toxins (secretory virulence factors)26. The most important toxin is a-hemolysin (HlyA), which encoded by hly gene that has been detected among pyelonephritis and cystitis28. eaeA (intimin or E. coli attaching and effacing gene)29. Most bacteria regulate a multitude of fimbrial adhesions such as fimbriae 1 type which encoded by fimH gene that was first recognized in E. coli 30. Our results confirmed the presence of papC, hly and fimH genes among E. coli isolates while, the eaeA gene was absent among E. coli isolates (Table 3; Fig. 1). Several factors contribute to the virulence of K. pneumoniae such as the capsular serotype, lipopolysaccharides, iron-scavenging system, and adhesions31. These genes include those encoding for regulators of mucoid phenotype A (rmpA) which is detected among local urinary isolates32,33. Other Klebsiella virulence genes such as type 1 (fimH), type 3 adhesions (mrkD), aerobactin (hydroxamate siderophore which is produced by some enterobacterial strains and TraT gene34–38. Our results confirmed the presence of TraT gene and the absence of aerobactin, fimH, and rmpA genes among K. pneumoniae isolates (Table 3; Fig. 1). Proteus mirabilis contains several virulence genes that contribute to its pathogenicity such as an atpD gene (ATP synthase beta chain)39. This gene detected with the percentage of 100% among P. mirabilis isolates in our study (Table 3; Fig. 1). Virulence genes in P. aeruginosa such as Pilli, exoenzyme S, endotoxin A, and phospholipase C are important for the acute phase of disease while, siderophores and pseudo-capsule of alginate are essential for chronic phase of infections40. Elastase (LasB gene), phospholipase C, toxin A (toxA), and exoenzyme S was assessed in P. aeruginosa isolates from UTI41 (Table 3; Fig. 1). Our study confirmed the presence of pslA gene and absence of lasB, toxA, and fliC genes. Some of the most significant virulence genes of A. baumannii are colicin V production, curi fibers (csg), siderophores like aerobactin (iutA), and cytotoxic necrotizing factor (cnf)42,43. Acinetobacter baumannii in our study was free from these genes. However, there is always the possibility of mutation at the level of the corresponding gene, leading to the lack of its detection. Consequently, a positive PCR shows the occurrence of the virulence gene, but a negative PCR does not point to its absence44–46 (Table 3).
Biofilm is an accumulation of bacteria reserved within a microbial-derived matrix, which assists their persistence47. It contains water passages for transporting oxygen and essential nutrients for growth. Microcolony is the main structural unit of the biofilm it may be composed of 10–25% cells and 75–90% exopolysaccharide (EPS) matrix depending on the species complex48. They characterized by a high degree of resistance to antibiotics and host immune defense response substances4,49. It also plays an essential role in the pathogenicity of several chronic human infections50. In our study, all isolated uropathogens were biofilm former (Fig. 4). Interestingly, the detection of latent virulence genes in the clinical urine isolates also the ability for biofilm formation confirmers the pathogenicity of these isolated uropathogens in the current study. Also has some great epidemiological outcomes to control the dissemination of infectious disease caused by these pathogens. Increasing rates of antibiotic resistance and high repetition rates impend to greatly enhance the problem that these common infections place on society22. In a study by51, they revealed that antibiotics such as ciprofloxacin and ampicillin are the most commonly recommended therapeutics for UTIs. Interestingly, our study confirmed a great resistance for all isolated uropathogens to ampicillin and ciprofloxacin. In another study by Abuhandan et al.52, they reported that all of the isolated uropathogens were resistant to ampicillin-sulbactam, with, high resistance rates recorded for E. coli (64.1%) They also stated that the most effective antimicrobial agents were determined to be imipenem, quinolone, and aminoglycosides. It is worth saying that our study showed 100% resistance to ampicillin-sulbactam, 60% resistance to imipenem, 100% resistance to two members of aminoglycosides (Gentamicin and Amikacin) also 100% resistance observed for one member of quinolones (ciprofloxacin) Table 2. This confirms the seriousness of the wrong use of antibiotics over the years. In the current study, the presence of multidrug-resistant genes was determinant such as blaSHV, blaCTX, blaTEM as it was determined earlier by53,54. These genes were detected in our isolates with a percentage of 100, 60, and 60%, respectively (Table 2; Fig. 2). Quinolone resistance is usually resulting from mutations in genes coding for chromosomally-encoded type II topoisomerases, efflux pumps, or porn-related proteins, it also can be plasmid-mediated55,56. The plasmid resistance determinants are qnrA, qnrB, and qnrS56,57. The qnrS gene was detected with percentages of 100% among all isolated uropathogens (Table 2; Fig. 2). Multidrug resistance in P. aeruginosa can be caused by regulatory mutations nalB (mexR), nfxB or nfxC (mexT) leading to overexpression of three separate RND efflux systems which causing multiple antibiotic resistance profiles58. In our study mexR gene was detected with the percentage of 100% among P. aeruginosa isolates (Table 2; Fig. 2). Aminoglycosides resistant genes such as aac and aad59. An example of Gm resistance (Gmr) genes was aac(3)-Ia60. Our results confirmed a 100% resistance to aminoglycosides through the detection of aac(3)-Ia gene (Table 2; Fig. 2).
Antibiotic resistance is one of the biggest problems that face the world. Scientists have begun to search for new safe antibiotic alternatives. Medicinal plants are a good substitute for antibiotics61–66. The pods of A. nilotica extract was good antibacterial agent against different bacterial pathogens64. Our study confirmed the greatest efficacy of Acacia nilotica extract against all isolated uropathogens with MBC of 15–16.7 mg ml−1 with the greatest MBC value obtained by P. mirabilis (Table 3). The analysis of time killing data confirmed that A. nilotica aqueous extract kills E. coli and K. pneumoniae (within 20 h) faster than other uropathogens (Fig. 5). Acacia nilotica extract also reduces the biofilm of the tested pathogens (Fig. 4). This is could be due to the presence of some active phytochemicals such as 3-Cyclohexane-1-Carboxaldehyde, 2,6,6-trimethyl; á-Selinene; Oleic Acid; Globulol and Isochiapin that were detected in the GC–MS analysis (Table 4; Fig. 3). The antibacterial activity of crude extracts and different fractions could be largely due to the effect of the phytochemicals detected67. In study by68, the phytochemical analysis of A. nilotica pod extracts by LCMS, HPLC/DAD, and FTIR was confirmed as antibacterial agents against antibiotic-resistant strains of E. coli and Salmonella sp. Cyclohexane, for example, is considered the most potent antibacterial agent that had a reduced ability to inhibit solute transport in comparison with other active analogs69. The oleic acid produced by marine spp. also could be valuable as a biocontrol against gram-negative bacteria including Vibrio parahaemolyticus and might denote an influence in the clinical use70. Other important phytochemical components detected with a high percentage in A. nilotica aqueous extract such as á-Selinene; Globulol and Isochiapin were also recorded for their antibacterial activities71–73.
In conclusion, a new preventive measure against multidrug-resistant isolated uropathogens which confirmed by multiplex PCR, consists of the use of A. nilotica aqueous extract. Acacia nilotica considered a natural antimicrobial agent to prevent bacterial growth, biofilm formation, and decreases the dissemination of these multidrug-resistant strains. However, optimizing the production of the active organic products of A. nilotica extract is a challenge that must be considered to use this compound to contrast the pathogenic action of UTIs. Also, it is recommended to make purification of A. nilotica extract to test one or more of the larger concentration of some compounds like 3-Cyclohexane-1-Carboxaldehyde, 2,6,6-trimethyl; á-Selinene (CAS); Oleic Acid; Globulol in the composition of the extract against uropathogens and performing in vivo experiments.
Materials and methods
Ethical approval and informed consent
The study protocol was approved by the local Medical Ethics Committees of the Medical University of Assiut, Egypt, which has been approved by the Egyptian Ministry of Higher Education and Scientific Research on 11/2009. General hospital and private medical analysis laboratories in Luxor province, Egypt ethically approved urine sampling and informed consent was obtained from all participants during the study work. The methods were carried out in accordance with the relevant guidelines and regulations, and the subjects gave written informed consent.
Sampling; isolation and identification of uropathogens
A total of one hundred and seventy urine samples were collected between January to June (2019) from the general hospital and private medical analysis laboratories in Luxor province, Egypt. Patients were between 8 and 86 years old, they were 110 females and 60 males. Urine samples were collected by clean catch mid-stream urine collection method into the sterile container from patients who had not received antimicrobials within the previous one week. Guidelines for proper specimen collection were given to all patients on a printed card74. All samples were subjected to COMISCREEN 10 SL urine test strips for a rapid- semi-quantitative determination of leucocyte (pyuria) using a leucocyte esterase test (LET) and nitrite to detect bacteriuria. Samples were examined using a light microscope/high-power (HPF) (LEICA DMLF2, China) for the presence of 10 or more white blood cells75. For isolation of uropathogens, samples were streaked onto MacConkey (OXOID), Eosin methylene blue (BIOWORLD, USA), and Tryptic soy agar (OXOID) plates then incubated at 37°C for 24h. Isolates were picked up and identified by standard biochemical methods76–78. CFU/ml (colony forming units) were also determined.
Antimicrobial sensitivity testing
The antibiograms for all the recovered isolates were determined as described earlier according to the Kirby Bauer disk diffusion method79. The susceptibility of all isolates was tested for 12 antibiotics from different groups (BIOANALYZE). The used antibiotics were Imipenem (10 μg), Meropenem (10 μg), Ciprofloxacin (5 μg), Ceftazidime (30 μg), Amikacin (30 μg), Nalidixic acid (30 μg), Gentamicin (10 μg), Ampicillin (10 μg), Ampicillin-sulbactam (10/10 μg), Piperacillin (100 μg), Piperacillin-tazobactam (100/10 μg) and Cefepime (30 μg). Interpretation of the results was performed according to clinical and laboratory standard institute guidelines80 to determine if the isolate is resistant, intermediate, or susceptible to the tested antibiotics.
Detection of virulence and antibiotic-resistant genes of isolated uropathogens
Molecular characterization of the recovered uropathogens was carried out by multiplex PCR. The detected enterotoxins genes for E. coli were (fimH, papC, hly, and eaeA). For K. pneumonia (fimH, rmpA, TraT and aerobactin), for P. mirbilis (fimH and atpD), for P. aeruginosa (toxA, lasB, fliC, and pslA), for A. baumannii (fimH, Cnf1, iutA and cvaC). While the detected antibiotic-resistant genes for all isolates were blaTEM, blaSHV, blaCTX, aac(3)-Ia, and qnrs. Besides, the mexR gene was performed for P. aeruginosa only. The encoding enterotoxins and antibiotic-resistant genes (twenty-one) were performed using (forty-two) primers sets including forward and reverse. All primer sequences with corresponding references are listed in Table 532,81–97.
Table 5.
Primers sequences, target genes, amplicon sizes and cycling conditions.
| Target gene | Sequence | Amplified segment (bp) | Primary denaturation | Amplification (35 cycles) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secondary denaturation | Annealing | Extension | Final extension | |||||||||
| Virulence genes used for E. coli isolates | ||||||||||||
| fimH |
TGCAGAACGGATAAGCCGTGG GCAGTCACCTGCCCTCCGGTA |
508 | 94 °C/5 min | 94 °C/30 s | 50 °C/40 s | 72 °C/45 s | 72 °C/10 min | 81 | ||||
| hly |
AACAAGGATAAGCACTGTTCTGGCT ACCATATAAGCGGTCATTCCCGTCA |
1,177 | 94 °C/5 min | 94 °C/30 s | 60 °C/40 s | 72 °C/1 min | 72 °C/12 min | 82 | ||||
| papC |
TGATATCACGCAGTCAGTAGC CCGGCCATATTCACATAA |
501 | 94 °C/5 min | 94 °C/30 s | 58 °C/40 s | 72 °C/40 s | 72 °C/10 min | 83 | ||||
| eaeA |
ATGCTTAGTGCTGGTTTAGG GCCTTCATCATTTCGCTTTC |
248 | 94 °C/5 min | 94 °C/30 s | 51 °C/30 s | 72 °C/30 s | 72 °C/7 min | 84 | ||||
| Virulence genes used for Klebsiella pneumonia isolatesa | ||||||||||||
| rmpA |
ACTGGGCTACCTCTGCTTCA CTTGCATGAGCCATCTTTCA |
535 | 94 °C/5 min | 94 °C/30 s | 50 °C/40 s | 72 °C/40 s | 72 °C/10 min | 85 | ||||
| TraT |
GATGGCTGAACCGTGGTTATG CACACGGGTCTGGTATTTATGC |
307 | 94 °C/5 min | 94 °C/30 s | 55 °C/30 s | 72 °C/30 s | 72 °C/7 min | 86 | ||||
| aerobactin |
GCATAGGCGGATACGAACAT CACAGGGCAATTGCTTACCT |
556 | 94 °C/5 min | 94 °C/30 s | 50 °C/40 s | 72 °C/45 s | 72 °C/10 min | 32 | ||||
| Virulence genes used for Proteus mirabilis isolatesa | ||||||||||||
| atpD |
GTATCATGAACGTTCTGGGTAC TGAAGTGATACGCTCTTGCAG |
595 | 94 °C/5 min | 94 °C/30 s | 58 °C/40 s | 72 °C/45 s | 72 °C/10 min | 87 | ||||
| Virulence genes used for pseudomonas aeruginosa isolates | ||||||||||||
| toxA |
GACAACGCCCTCAGCATCACCAGC CGCTGGCCCATTCGCTCCAGCGCT |
396 | 94 °C/5 min | 94 °C/30 s | 55 °C/40 s | 72 °C/40 s | 72 °C/10 min | 88 | ||||
| lasB |
ACAGGTAGAACGCACGGTTG GATCGACGTGTCCAAACTCC |
1,220 | 94 °C/5 min | 94 °C/30 s | 54 °C/40 s | 72 °C/1 min | 72 °C/10 min | 89 | ||||
| pslA |
TCCCTACCTCAGCAGCAAGC TGTTGTAGCCGTAGCGTTTCTG |
656 | 94 °C/5 min | 94 °C/30 s | 60 °C/40 s | 72 °C/45 s | 72 °C/10 min | 90 | ||||
| fliC |
TGAACGTGGCTACCAAGAACG TCTGCAGTTGCTTCACTTCGC |
180 | 94 °C/5 min | 94 °C/30 s | 56.2 °C/30 s | 72 °C/30 s | 72 °C/7 min | |||||
| Virulence genes used for acinetobacter baumannii isolatesa | ||||||||||||
| cnf1 |
TATATAGTCGTCAAGATGGA CACTAAGCTTTACAATATTGAC |
620 | 94 °C/5 min | 94 °C/30 s | 63 °C/40 s | 72 °C/30 s | 72 °C/10 min | 91 | ||||
| iutA |
GGCTGGACATGGGAACTGG CGTCGGGAACGGGTAGAATCG |
300 | 94 °C/5 min | 94 °C/30 s | 63 °C/30 s | 72 °C/45 s | 72 °C/7 min | 92 | ||||
| cvaC |
CACACACAAACGGGAGCTGTT CTTCCCGCAGCATAGTTCCAT |
760 | 94 °C/5 min | 94 °C/30 s | 63 °C/40 s | 72 °C/ 45 s | 72 °C/10 min | |||||
| Antibiotics resistance genes for all isolates including Pseudomonas aeruginosa | ||||||||||||
| blaTEM |
ATCAGCAATAAACCAGC CCCCGAAGAACGTTTTC |
516 | 94 °C/5 min | 94 °C/30 s | 54 °C/40 s | 72 °C/45 s | 72 °C/10 min | 93 | ||||
| blaSHV |
AGGATTGACTGCCTTTTTG ATTTGCTGATTTCGCTCG |
392 | 94 °C/5 min | 94 °C/30 s | 54 °C/40 s | 72 °C/45 s | 72 °C/10 min | 82 | ||||
| blaCTX |
ATGTGCAGYACCAGTAARGTK ATGGC TGGGTRAARTARGTSACCAGA AYCAGCGG |
593 | 94 °C/5 min | 94 °C/30 s | 54 °C/40 s | 72 °C/45 s | 72 °C/10 min | 94 | ||||
| qnrs |
ACGACATTCGTCAACTGCAA TAAATTGGCACCCTGTAGGC |
417 | 94 °C/5 min | 94 °C/30 s | 55 °C/40 s | 72 °C/45 s | 72 °C/10 min | 95 | ||||
| aac(3)-Ia |
TTGATCTTTTCGGTCGTGAGT TAAGCCGCGAGAGCGCCAACA |
150 | 94 °C/5 min | 94 °C/30 s | 55 °C/30 s | 72 °C/30 s | 72 °C/7 min | 96 | ||||
| Antibiotics resistance gene for Pseudomonas aeruginosa isolate | ||||||||||||
| mexR |
GCGCCATGGCCCATATTCAG GGCATTCGCCAGTAAGCGG |
637 | 94 °C/5 min | 94 °C/30 s | 55 °C/30 s | 72 °C/30 s | 72 °C/7 min | 97 | ||||
The specific sequences that were amplified for each of the used primers (Metabion, Germany).
afimH gene was also detected for these isolates.
DNA amplification for the selected virulence and antibiotic resistance genes of isolates
The extraction of DNA was carried out according to QIAamp DNA mini kit instructions (QIAGEN, Germany, GmbH) as described earlier by Bisi-Johnson21 with modification from the manufacturer's recommendations. Briefly, 200 μl of the sample suspension was inoculated with 10 μl of proteinase K and 200 μl of lysis buffer at 56 °C for 10 min. After incubation, 200 μl of 100% ethanol was added to the lysate. The sample was then washed and centrifuged following the manufacturer's recommendations. Nucleic acid was eluted with 100 μl of elution buffer provided in the kit. PCR amplification was performed using oligonucleotide primer (METABION, Germany) that were utilized in a 25 μl reaction containing 12.5 μl of EMERALDAMP Max PCR Master Mix (TAKERA, Japan), 1 μl of each primer of 20 pmol concentration, 5.5 μl of dist. water and 6 μl of DNA template. The reaction was performed in an applied biosystem 2,720 thermal cycler. All primers amplicon sizes and cycling conditions are summarized in Table 532,81–97. The products of PCR were separated by electrophoresis on 1.5% agarose gel (APPLICHEM, Germany, GmbH) in 1xTBE buffer at room temperature using gradients of 5 V/cm. For gel analysis, 15 μl of the products were loaded in each gel slot. Gelpilot 100 bp and 100 bp plus ladders (QIAGEN, Germany, GmbH) and GeneRuler 100 bp ladder (FERMENTAS, THERMO) was used as a marker for electrophoresis to determine the fragment sizes. The gel was photographed by a gel documentation system (ALPHA INNOTECH, BIOMETRA) and the data was analyzed through computer software (AUTOMATIC IMAGE CAPTURE, USA).
Gas chromatography-mass spectrometer (GC–MS) analysis
GC–MS technique was used in this study as described earlier by Sadiq et al.68, to identify the Phyto-components present in the plant extract. For preparing A. nilotica aqueous extract, 20 gm of dry pods were ground into fine powder by using an electric grinder (SOGO, China). The powder was soaked in 100 ml of hot distilled water and then cooled down with continuous stirring at room temperature by using bigger bill shaker, USA, for extraction of active ingredients98. The mixture was filtered then sterilized using a syringe filter equipped with a 45μ membrane filter; then kept at 4 °C. Acacia nilotica material was subjected to gas chromatography-mass spectrometer technique (GC–MS) (THERMO SCIENTIFIC TECHNOLOGIES, TRACE 1,310) with capillary column TG-5 (30 m × 250 μm × 0.25 μm) system were used. The mass detector used in split mode and helium gas with a flow rate of 1.5 ml/min was used as a carrier. The injector was operated at 230 °C and the oven temperature for the initial setup was 60 °C for 2 min. ramp 10/min. to 300 °C for 8 min. Mass spectra were taken at 70 eV, total GC running time was 35 min.
Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by INT reduction assay
The determination of MIC and MBC were assayed as described by99. Where the freshly prepared culture of isolated uropathogens was adjusted to OD595 of 0.01. 100 μl of each bacterial fresh culture was put into sterilized 96-well plates. Then 20 μl of the original extract was added (serial dilutions of 10−1–10−10 were used, 8 replicates were made for each dilution into complete raw of the 96-well plate). The plates incubated at 37 °C for 24 h. MIC was determined by the addition of 40 μl of p-iodonitrotetrazolium violet chloride (INT) (0.2 mg/ml, SIGMA-ALDRICH) to the plates and re-incubated at 37 °C for 30 min., the lowest concentration which banned color change is the MIC100,101. MBC was determined according to to99,102.
Static biofilm assay
The recovered uropathogenic isolates were assessed for their biofilm activity in a microtiter plate according to to103 after modifications by104 as follows: Isolates were grown on TSA for 24 h at 37 °C, suspended in TSB, adjusted to an OD595 of 0.02. Then, 130 μl from each isolate culture were plated into a 96-well microtiter plate (U BOTTOM, STERILIN) for 24 h at 37 °C. Then for studying the antibiofilm activity of the extract, 30 µl of the A. nilotica aqueous extract—(MBC) value for each isolate—was added After 24 h. The addition of 30 µl of sterilized H2O to the original biofilm of the isolated uropathogens served as control. Wells were consequently rinsed with H2O and the biofilm was stained with 0.1% crystal violet, solubilized in 96% ethanol, and the OD595 was measured using INFINITE F50 ROBOTIC (Ostrich) Microplate Reader to quantify the amount of biofilm. Each treatment was added to three wells i.e. three replicates.
Survival curve of the isolated uropathogens in the presence of A. nilotica aqueous extract
An increase, both in total cell mass and cell number can readily be estimated by measuring the turbidity of a cell suspension using an instrument such as a spectrophotometer105. So, the microbial population at the initial and completion of the experiment isolates were grown overnight on TSA plates, suspended in TSB to an OD595 of 0.01 then incubated with the MBC value of A. nilotica aqueous extract for each isolate at 37 °C. Adjusted culture from each isolated uropathogens at OD595 of 0.01 served as the positive control. Approximately, 1 ml aliquot was tested from the culture medium over time (0, 4, 8, 12, 16, 20, and 24 h) for monitoring the optical density of all bacterial treatments at OD595 nm using the ‘ ‘SPECTRONIC GENESYS 2PC” Spectronic Instruments, USA. Readings were taken three times. Results were confirmed by taking 50 µl of each treatment at OD595 of 0.0 (complete killing) onto fresh TSA and incubation at 37 °C for 24 h (Three plates were used for each isolate).
Statistical data analysis
Data were analyzed using the Mann–Whitney U test or a Kruskal–Wallis test followed by post hoc Dunn’s multiple comparisons. Differences were considered significant at P values of ≤ 0.05. For all statistical analyses, GraphPad Prism version 5 was used.
Author contributions
W.S. supervised the whole research. R.E. performed practical work, analyzed the data, and wrote the manuscript. F.A. gave some feedbacks about this research. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ. Med. J. 2013;13:359. doi: 10.12816/0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiot (Basel, Switzerland). 2014;3:174–92. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed MA, Alnour TMS, Shakurfo OM, Aburass MM. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac. J. Trop. Med. 2016;9:771–776. doi: 10.1016/j.apjtm.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues WF, Miguel CB, Nogueira APO, Ueira-Vieira C, Paulino TDP, Soares SDC, De Resende EAMR, Lazo-Chica JE, Araújo MC, Oliveira CJ. Antibiotic resistance of bacteria involved in urinary infections in Brazil: a cross-sectional and retrospective study. Int. J. Environ. Res. Public Health. 2016;13:918. doi: 10.3390/ijerph13090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslin BR, Miller JT, Seigler DS. Overview of the generic status of Acacia (Leguminosae: Mimosoideae) Aust. Syst. Bot. 2003;16:1–18. [Google Scholar]
- 6.Mahmood A, Qureshi RA. Antimicrobial activities of three species of family mimosaceae. Pak. J. Pharm. Sci. 2012;25:203–206. [PubMed] [Google Scholar]
- 7.Sharma AK, Kumar A, Yadav SK, Rahal A. Studies on antimicrobial and immunomodulatory effects of hot aqueous extract of Acacia nilotica L. leaves against common veterinary pathogens. Vet. Med. Int. 2014;2014:1–9. doi: 10.1155/2014/747042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan R, Islam B, Akram M, Shakil S, Ahmad AA, Ali SM, Siddiqui M, Khan A. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14:586–597. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orchard AE, Maslin BR. Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved type. Taxon. 2003;52:362–363. [Google Scholar]
- 10.Wagate CG, Mbaria JM, Gakuya DW, Nanyingi MO, Kareru PG, Njuguna A, Gitahi N, Macharia JK, Njonge FK. Screening of some Kenyan medicinal plants for antibacterial activity. Phyther. Res. 2010;24:150–153. doi: 10.1002/ptr.2866. [DOI] [PubMed] [Google Scholar]
- 11.Thomson C, Armitage A. Urinary tract infection. In: Warrell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. Oxford: Oxford University Press; 2010. pp. 4103–4122. [Google Scholar]
- 12.CDC. Vital and Health Statistics. Ambulatory Medical Care Utilization Estimates for 2007. Series 13, Number 159. Hyattsville, MD (2011). [PubMed]
- 13.Rashid, T. & Ebringer, A. Rheumatoid arthritis is caused by asymptomatic Proteus urinary tract infections. In Clinical Management of Complicated Urinary Tract Infection. (InTech, 2011).
- 14.Hannan TJ, et al. Host–pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh B, Tilak R, Srivastava RK, Katiyar D. Urinary tract infection and its risk factors in women: an appraisal. J. Pure Appl. Microbial. 2014;8(5):Proof. [Google Scholar]
- 16.Ouno GA, Korir SC, Cheruiyot J, Ratemo OD, Mabeya BM, Mauti GO, Mauti EM, Kiprono SJ. Isolation, identification and characterization of urinary tract infectious bacteria and the effect of different antibiotics. J. Nat. Sci. Res. 2013;3:150–158. [Google Scholar]
- 17.Angoti G, Goudarzi H, Hajizadeh M, Tabatabaii Z. Bacteria isolated from urinary tract infection among patients and determination of the antibiotic susceptibility patterns of the gram negative bacteria in Iran. Nov. Biomed. 2016;4:1–4. [Google Scholar]
- 18.Cortes-Penfield NW, Trautner BW, Jump RLP. Urinary tract infection and asymptomatic bacteriuria in older adults. Infect. Dis. Clin. N. Am. 2017;31:673–688. doi: 10.1016/j.idc.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am. J. Med. 2002;113:14–19. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 20.Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, editors. Principles and Practice of Infectious Diseases. 8. Philadelphia: Elsevier Saunders; 2014. pp. 886–913. [Google Scholar]
- 21.Prakash D, Saxena RS. Antimicrobial susceptibility pattern of human pathogenic bacteria related to Enterobacteriaceae family causing urinary tract infection. Adv. Appl. Sci. Res. 2013;4:98–104. [Google Scholar]
- 22.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejrnæs K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan. Med. Bull. 2011;58:4187. [PubMed] [Google Scholar]
- 24.Kudinha T, Kong F, Johnson JR, Andrew SD, Anderson P, Gilbert GL. Multiplex PCR-based reverse line blot assay for simultaneous detection of 22 virulence genes in uropathogenic Escherichia coli. Appl. Environ. Microbiol. 2012;78:1198–1202. doi: 10.1128/AEM.06921-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. N. Am. 2008;35:1–12. doi: 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Bien, J., Sokolova, O. & Bozko, P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int. J. Nephrol. (2012). [DOI] [PMC free article] [PubMed]
- 27.Jadhav S, Hussain A, Devi S, Kumar A, Parveen S, Gandham N, Wieler LH, Ewers C, Ahmed N. Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from a semi urban locality in India. PLoS ONE. 2011;6:e18063. doi: 10.1371/journal.pone.0018063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firoozeh F, Saffari M, Neamati F, Zibaei M. Detection of virulence genes in Escherichia coli isolated from patients with cystitis and pyelonephritis. Int. J. Infect. Dis. 2014;29:219–222. doi: 10.1016/j.ijid.2014.03.1393. [DOI] [PubMed] [Google Scholar]
- 29.Franck SM, Bosworth BT, Moon HW. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J. Clin. Microbiol. 1998;36:1795–1797. doi: 10.1128/jcm.36.6.1795-1797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouin, S. G., Roos, G. & Bouckaert, J. Discovery and application of FimH antagonists. In Carbohydrates as Drugs. 123–168 (2014).
- 31.Fuursted K, Schøler L, Hansen F, Dam K, Bojer MS, Hammerum AM, Dagnæs-Hansen F, Olsen A, Jasemian Y, Struve C. Virulence of a Klebsiella pneumoniae strain carrying the New Delhi metallo-beta-lactamase-1 (NDM-1) Microbes Infect. 2012;14:155–158. doi: 10.1016/j.micinf.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Siu, L. K., Fung, C. P., Chang, F. Y., Lee, N., Yeh, M., Koh, T. H. & Ip, M. Molecular typing and virulence analysis among serotype K1 Klebsiella pneumoniae isolated from liver abscess patients and stool carriage from non-infectious subjects in Hong Kong, Singapore and Taiwan. J. Clin. Microbiol. 00977 (2011). [DOI] [PMC free article] [PubMed]
- 33.Nahavandinejad M, Asadpour L. Mucoviscosity determination and detection of magA and rmpA genes in clinical isolates of Klebsiella pneumoniae in Northern Iran. Crescent J. Med. Biol. Sci. 2017;4:104–107. [Google Scholar]
- 34.Perry RD, Balbo PG, Jones HA, Fetherston JD, DeMoll E. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology. 1999;145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 35.Podschun R, Fischer A, Ullmann U. Characterization of Klebsiella terrigena strains from humans: haemagglutinins, serum resistance, siderophore synthesis, and serotypes. Epidemiol. Infect. 2000;125:71–78. doi: 10.1017/s0950268899004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 37.El Fertas-Aissani R, Messai Y, Alouache S, Bakour R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol. Biol. 2013;61:209–216. doi: 10.1016/j.patbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Al-Muhanna AS, Al-Rediany RS, Alzuhairi MA. Molecular characterization of aerobactin gene among Klebsiella isolated from wound and burn infections. Int. J. Curr. Microbiol. App. Sci. 2014;3:26–31. [Google Scholar]
- 39.Pearson MM, Yep A, Smith SN, Mobley HLT. Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect. Immun. 2011;49:IAI-05152. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalifa ABH, Moissenet D, Thien HV, Khedher M. Les facteurs de virulence de Pseudomonas aeruginosa: mécanismes et modes de regulations. Ann. Biol. Clin. (Paris) 2011;69:393–403. doi: 10.1684/abc.2011.0589. [DOI] [PubMed] [Google Scholar]
- 41.Hamood AN, Griswold JA, Duhan CM. Production of extracellular virulence factors by Pseudomonas aeruginosa isolates obtained from tracheal, urinary tract, and wound infections. J. Surg. Res. 1996;61:425–432. doi: 10.1006/jsre.1996.0140. [DOI] [PubMed] [Google Scholar]
- 42.Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT, Brown MH. Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics. 2014;15:1020. doi: 10.1186/1471-2164-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erac B, Yilmaz FF, Hosgor Limoncu M, Ozturk I, Aydemir S. Investigation of the virulence factors of multidrug-resistant Acinetobacter baumannii isolates. Mikrobiyol. Bul. 2014;48:70–81. [PubMed] [Google Scholar]
- 44.Le Bouguenec C, Archambaud M, Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 1992;30:1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Licznar P, Eychenne I, Azéma C, Decramer S, Bouissou F, Fayet O, Prère MF. Revised prevalence of afa+ Escherichia coli strains in acute pyelonephritis of children. Pathol. Biol. 2003;51:512–515. doi: 10.1016/s0369-8114(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 46.Usein C, Damian M, Tatu-Chitoiu D, Capusa C, Fagaras R, Tudorache D, Nica M, Le Bouguénec C. Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell Mol. Med. 2001;5:303–310. doi: 10.1111/j.1582-4934.2001.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabin N, et al. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- 48.Denstedt JD, Reid G, Sofer M. Advances in ureteral stent technology. World J. Urol. 2000;18(4):237–242. doi: 10.1007/pl00007074. [DOI] [PubMed] [Google Scholar]
- 49.Vieira HLA, Freire P, Arraiano CM. Effect of Escherichia coli morphogene bolA on biofilms. Appl. Environ. Microbiol. 2004;70:5682–5684. doi: 10.1128/AEM.70.9.5682-5684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003;57:677–770. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 51.Foxman B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 52.Abuhandan M, Güzel B, Oymak Y, Çiftçi H. Antibiotic sensitivity and resistance in children with urinary tract infection in Sanliurfa. Turk. J. Urol. 2013;39(2):106–110. doi: 10.5152/tud.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemente L, Manageiro V, Ferreira E, Jones-Dias D, Correia I, Themudo P, Albuquerque T, Caniça M. Occurrence of extended-spectrum β-lactamases among isolates of Salmonella enterica subsp. enterica from food-producing animals and food products in Portugal. Int. J. Food Microbiol. 2013;167:221–228. doi: 10.1016/j.ijfoodmicro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Liebana E, Carattoli A, Coque TM, Hasman H, Magiorakos AP, Mevius D, Peixe L, Poirel L, Schuepbach-Regula G, Torneke K, Torren-Edo J, Torres C, Threlfall J. Public health risks of enterobacterial isolates producing extended-spectrum-lactamases or AmpC-lactamases in food and food-producing animals: an EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin. Infect. Dis. 2013;56:1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 55.Martínez-Martínez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai. China. Antimicrob. Agents Chemother. 2003;47:2242–2248. doi: 10.1128/AAC.47.7.2242-2248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hata M, Suzuki M, Matsumoto M, Takahashi M, Sato K, Ibe S, Sakae K. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 2005;49:801–803. doi: 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Germ M, Yoshihara E, Yoneyama H, Nakae T. Interplay between the efflux pump and the outer membrane permeability barrier in fluorescent dye accumulation in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 1999;261:452–455. doi: 10.1006/bbrc.1999.1045. [DOI] [PubMed] [Google Scholar]
- 59.Jia X, Zhang J, Sun W, He W, Jiang H, Chen D, Murchie AIH. Riboswitch control of aminoglycoside antibiotic resistance. Cell. 2013;152:68–81. doi: 10.1016/j.cell.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 60.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006;34:3–10. doi: 10.1016/j.ajic.2006.05.219. [DOI] [PubMed] [Google Scholar]
- 61.Abdallah EM. Plants: an alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011;1:16–20. [Google Scholar]
- 62.Salem W, Leitner DR, Zingl FG, Schratter G, Prassl R, Goessler W, Reidl J, Schild S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015;305:85–95. doi: 10.1016/j.ijmm.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sayed WF, Salem WMA, Haridy MAM, Hassan NH. Efficacy of Caltropis procera and Ficus sycomorus extracts in treating MRSA (Methicillin-resistant Staphylococcus aureus)-keratitis in rabbit. EXCLI J. 2015;14:747–757. doi: 10.17179/excli2015-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdallah EM. Antibacterial efficacy of Acacia nilotica (L.) pods growing in Sudan against some bacterial pathogens. Int. J. Curr. Res. Biosci. Plant Biol. 2016;3:6–11. [Google Scholar]
- 65.Saber H, Alwaleed EA, Ebnalwaled KA, Sayed A, Salem W. Efficacy of silver nanoparticles mediated by Jania rubens and Sargassum dentifolium macroalgae; Characterization and biomedical applications. Egypt. J. Basic Appl. Sci. 2017;4:249–255. [Google Scholar]
- 66.Elamary RB, Shibat El-Hamed DMW, Sayed WF, Salem WM. Molecular studies on the effect of some Antibiotics and Allium sativum extract on some Escherichia coli serovars isolated from chickens. SVU Int. J. Vet. Sci. 2018;1:33–49. [Google Scholar]
- 67.Singh R. Phytochemical analysis and antibacterial activity of Acacia nilotica (L.) leaves against pathogenic bacteria. Int. J. Green Pharm. 2016;10:104–110. [Google Scholar]
- 68.Sadiq MB, Hanpithakpong W, Tarning J, Anal AK. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind. Crops Prod. 2015;77:873–882. [Google Scholar]
- 69.Lloyd WJ, Broadhurst AV, Hall MJ, Andrews KJ, Barber WE, Wong-Kai P. Cyclohexane triones, novel membrane-active antibacterial agents. Antimicrob. Agents Chemother. 1988;32:814–818. doi: 10.1128/aac.32.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leyton Y, Borquez J, Darias J, Cueto M, Díaz-Marrero AR, Riquelme C. Oleic acid produced by a marine Vibrio spp. acts as an anti-Vibrio parahaemolyticus agent. Mar. Drugs. 2011;92:155–2163. doi: 10.3390/md9102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan M, Zhou L, Huang Y, Wang Y, Hao X, Wang J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008;22:569–575. doi: 10.1080/14786410701592745. [DOI] [PubMed] [Google Scholar]
- 72.Vijayaram S, Kannan S, Saravanan KM, Vasantharaj S, Sathiyavimal S, Palanisamy SP. Preliminary phytochemical screening, antibacterial potential and GCMS analysis of two medicinal plant extracts. Pak. J. Pharm. Sci. 2016;29:819–822. [PubMed] [Google Scholar]
- 73.Khayat S, Al-Zahrani SH, Basudan N, Al-Zahrani NH, Subahi JA. Chemical composition and in vitro antibacterial activities of traditional medicinal plant: Olea sp. Biomed. Res. 2018;29:1037–1047. [Google Scholar]
- 74.Forbes BA, Sahm DF, Weissfeld AS, Baron EJ. Bailey & Scott’s diagnostic microbiology. St. Louis: Mosby; 2007. pp. 10–840. [Google Scholar]
- 75.Naber KG. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU): EAU guidelines for the management of urinary and male genital tract infections. Eur. Urol. 2001;40:576–588. doi: 10.1159/000049840. [DOI] [PubMed] [Google Scholar]
- 76.Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A. Laboratory strategy in the diagnosis of infective syndromes, Mackie McCartney. Pract. Med. Microbiol. 1996;14:53–94. [Google Scholar]
- 77.Quinn PJ, Markey BK, Carter ME, Donnelly WJC, Leonard FC. Veterinary Microbiology and Microbial Diseases. 1. Hoboken: Iowa State University Press, Blackwell Science; 2002. [Google Scholar]
- 78.Begum K, Reza TA, Haque M, Hossain A, Hassan FMK, Hasan SN, Akter N, Ahmed A, Barua U. Isolation, identification and antibiotic resistance pattern of Salmonella spp. from chicken eggs, intestines and environmental samples. Bangladesh Pharm. J. 2010;13:23–27. [Google Scholar]
- 79.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 80.Clinical and Laboratory Standards Institute (CLSI) Document M100: Performance standards for susceptibility testing. Clin. Lab. Stand. Inst. 2017;37(1):282. [Google Scholar]
- 81.Ghanbarpour R, Salehi M. Determination of adhesin encoding genes in Escherichia coli isolates from omphalitis of chicks. Am. J. Anim. Vet. Sci. 2010;5:91–96. [Google Scholar]
- 82.Piva IC, Pereira AL, Ferraz LR, Silva RSN, Vieira AC, Blanco JE, Blanco M, Blanco J, Giugliano LG. Virulence markers of enteroaggregative Escherichia coli isolated from children and adults with diarrhea in Brasilia. Braz. J. Clin. Microbiol. 2003;41:1827–1832. doi: 10.1128/JCM.41.5.1827-1832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin WJ, Zheng ZM, Zhang YZ, Qin AJ, Shao HX, Liu YL, Jiao W, Wang QQ. Distribution of virulence-associated genes of avian pathogenic Escherichia coli isolates in China. Agric. Sci. China. 2008;7:1511–1515. [Google Scholar]
- 84.Bisi-Johnson MA, Obi CL, Vasaikar SD, Baba KA, Hattori T. Molecular basis of virulence in clinical isolates of Escherichia coli and Salmonella species from a tertiary hospital in the Eastern Cape, South Africa. Gut Pathog. 2011;3:9. doi: 10.1186/1757-4749-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, Chen TL, Chang FY, Koh TH. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J. Clin. Microbiol. 2007;45:466–471. doi: 10.1128/JCM.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaipainen T, Pohjanvirta T, Shpigel NY, Shwimmer A, Pyörälä S, Pelkonen S. Virulence factors of Escherichia coli isolated from bovine clinical mastitis. Vet. Microbiol. 2002;85:37–46. doi: 10.1016/s0378-1135(01)00483-7. [DOI] [PubMed] [Google Scholar]
- 87.Bi S, Tang S, Wu X, Chen S. Quantitative detection of Proteus species by real-time polymerase chain reaction using SYBR Green I. Ann. Microbiol. 2013;63:1205–1208. [Google Scholar]
- 88.Matar GM, Ramlawi F, Hijazi N, Khneisser I, Abdelnoor AM. Transcription levels of Pseudomonas aeruginosa exotoxin A gene and severity of symptoms in patients with otitis externa. Curr. Microbiol. 2002;45:350–354. doi: 10.1007/s00284-002-3703-z. [DOI] [PubMed] [Google Scholar]
- 89.Finnan S, Morrissey JP, O’gara F, Boyd EF. Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J. Clin. Microbiol. 2004;42:57–83. doi: 10.1128/JCM.42.12.5783-5792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghadaksaz A, Fooladi AAI, Hosseini HM, Amin M. The prevalence of some Pseudomonas virulence genes related to biofilm formation and alginate production among clinical isolates. J. Appl. Biomed. 2015;13:61–68. [Google Scholar]
- 91.Kadhum HJ, Finlay D, Rowe MT, Wilson IG, Ball HJ. Occurrence and characteristics of cytotoxic necrotizing factors, cytolethal distending toxins and other virulence factors in Escherichia coli from human blood and fecal samples. Epidemiol. Infect. 2008;136:752–760. doi: 10.1017/S0950268807009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yaguchi K, Ogitani T, Osawa R, Kawano M, Kokumai N, Kaneshige T, Noro T, Masubuchi K, Shimizu Y. Virulence factors of avian pathogenic Escherichia coli strains isolated from chickens with coli septicemia in Japan. Avian Dis. 2007;51:656–662. doi: 10.1637/0005-2086(2007)51[656:VFOAPE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 93.Colom K, Pérez J, Alonso R, Fernández-Aranguiz A, Lariño E, Cisterna R. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA–1 genes in enterobacteriaceae. FEMS Microbiol. Lett. 2003;223:147–151. doi: 10.1016/S0378-1097(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 94.Archambault M, Petrov P, Hendriksen RS, Asseva G, Bangtrakulnonth A, Hasman H, Aarestrup FM. Molecular characterization and occurrence of extended-spectrum β-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb. Drug Resist. 2006;12:192–198. doi: 10.1089/mdr.2006.12.192. [DOI] [PubMed] [Google Scholar]
- 95.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 2006;12:83. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 96.Frana TS, Carlson SA, Griffith RW. Relative distribution and conservation of genes encoding aminoglycoside-modifying enzymes in Salmonella enterica serotype Typhimurium phage type DT104. Appl. Environ. Microbiol. 2001;67:445–448. doi: 10.1128/AEM.67.1.445-448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sánchez P, Linares JF, Ruiz-Díez B, Campanario E, Navas A, Baquero F, Martínez JL. Fitness of in vitro selected Pseudomonas aeruginosa nalB and nfxB multidrug resistant mutants. J. Antimicrob. Chemother. 2002;50:657–664. doi: 10.1093/jac/dkf185. [DOI] [PubMed] [Google Scholar]
- 98.Deen, S.A.R. Antibacterial effect of Acacia nilotica subspecies nilotica and Acacia nilotica subspecies adansonii (Sunt) pod extracts and their synergistic effect with antibiotics on selected bacteria, Ph.D. thesis university of Khartoum (2015).
- 99.Salem W, Shibat El-hamed D, Sayed W, Elamary R. Alterations in virulence and antibiotic-resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: the bactericidal efficacy of Allium sativum. Microb. Pathog. 2017;108:91–100. doi: 10.1016/j.micpath.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 100.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 101.Lall, N., Henley-Smith, C. J., De Canha, M. N., Oosthuizen, C. B. & Berrington, D. Viability reagent, presto blue, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int. J. Microbiol. (2013). [DOI] [PMC free article] [PubMed]
- 102.Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro. Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seper A, Fengler VHI, Roier S, Wolinski H, Kohlwein SD, Bishop AL, Camilli A, Reidl J, Schild S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011;82:1015–1037. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salem W, Schneditz G, Dornisch E, Schild S. In vitro effects on biofilm viability, cytotoxicity and antibacterial activities of green Ag and ZnO nanoparticles against Nontypeable Haemophilus influenzae strains. J. Exp. Appl. Anim. Sci. 2015;1:369–383. [Google Scholar]
- 105.Dalgaard P, Koutsoumanis K. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods. 2001;43:183–196. doi: 10.1016/s0167-7012(00)00219-0. [DOI] [PubMed] [Google Scholar]