Abstract
Several studies show that dietary nitrate enhances exercise performance, presumably by increasing muscle blood flow and improving oxygen utilization. These effects are likely mediated by nitrate metabolites, including nitrite and nitric oxide (NO). However, the mechanisms of nitrate production, storage, and metabolism to nitrite and NO in skeletal muscle cells are still unclear. We hypothesized that exogenous nitrate can be taken up and metabolized to nitrite/NO inside the skeletal muscle. We found rapid uptake of exogeneous nitrate in both myoblasts and myotubes, increasing nitrite levels in myotubes, but not myoblasts. During differentiation we found increased expression of molybdenum containing proteins, such as xanthine oxidoreductase (XOR) and the mitochondrial amidoxime-reducing component (MARC); nitrate and nitrite reductases. Sialin, a known nitrate transporter, was detected in myoblasts; nitrate uptake decreased after sialin knockdown. Inhibition of chloride channel 1 (CLC1) also led to significantly decreased uptake of nitrate. Addition of exogenous nitrite, which resulted in higher intracellular nitrite levels, increased intracellular cGMP levels in myotubes. In summary, our results demonstrate for the first time the presence of the nitrate / nitrite / NO pathway in skeletal muscle cells, namely the existence of strong uptake of exogenous nitrate into cells and conversion of intracellular nitrate to nitrite and NO. Our results further support our previously formulated hypothesis about the importance of the nitrate to nitrite to NO intrinsic reduction pathways in skeletal muscles, which likely contributes to improved exercise tolerance after nitrate ingestion.
Keywords: nitrate, nitrite, nitric oxide, skeletal muscle, myoblasts, myotubes, molybdenum-containing proteins
Introduction
In mammalian cells, NO is generated by conversion of arginine to citrulline by nitric oxide synthase (NOS) enzymes or, alternatively, by reduction of nitrite (and in certain situations, nitrate) to NO by various heme- or molybdenum-containing proteins [1–11]. The latter reactions are part of a reduction chain carried out by mammalian nitrate and nitrite reductases, which have not been well characterized during the past 20 years. The study of the physiological effects of nitric oxide (NO) was initiated due to its vasodilatory properties, with endothelium as a key player and blood as a distribution pathway. However, the role of specific organs in the synthesis and distribution of NO has started to be acknowledged, with liver first recognized and described by Zweier’s group as one of the major nitrite reducing organs [3, 4]. Similar results have also been reported for the heart [7, 8, 10, 12, 13].
So far, nitrite has been in the center stage, being the direct precursor of NO in just one step reduction, mostly by a variety of 5-coordinated heme proteins and Mo-containing proteins (XOR, AO, MARC-1 and MARC-2) [1, 5, 6, 9, 11, 14]. Nitrate to nitrite reduction was initially thought to be carried out only by the mammalian microbiome [15–17]. With the discovery of the mammalian nitrate reductase activity of some Mo-containing proteins [18], the ability of certain tissues and organs to supply NO on site, emerged. We recently described the existence of a large nitrate reservoir in skeletal muscle of mice and rats and showed that nitrate and nitrite levels in this reservoir respond to changes in dietary nitrate intake and exercise levels [19–21]. Similar values for human skeletal muscle were reported by Verdijk [22] and interesting work on fish showed that, in the state of deep hypoxia, skeletal muscle of crucian carp reduces nitrate [23].
In the current study, we hypothesized that human skeletal muscle cells could also utilize exogenous nitrate by its intrinsic machinery, based on our previous observations in rodents [19–21], which showed that skeletal muscle sequesters and stores large quantities of nitrate to generate nitrite and NO. To further explore the origins of this nitrate reservoir in cultured skeletal muscle cells, we concentrated on the mechanism of dietary nitrate fluxes and metabolism into the muscle cells, including the role of sialin, a known nitrate transporter [24]. We also tested an alternate possibility of nitrate translocation into cells involving chloride channels CLC1, which are partially permeable for anions other than chloride, including nitrate [25, 26].
Single type cell culture approaches allow us to estimate the contribution and importance of exogeneous nitrate sources into the overall nitrate flows and eliminates the contribution of endothelium and the bloodstream to nitrate fluxes that is unavoidable when whole tissues, organs or animals are studied. Using both myoblasts and myotubes (undifferentiated and differentiated skeletal muscle cells, respectively) we were also able to track significant changes in nitrate fluxes and its metabolism and in the expression of proteins related to the NO pathways during cell development and maturation.
Material and Methods
Cell culture
Primary human skeletal muscle cells (hSkMCs) were purchased from Lonza (CC-2561). Myoblasts (undifferentiated hSkMCs) were cultured in skeletal muscle cell growth medium (151-500; Cell Applications Inc.) in 75 cm2 culture flask in a tri-gas CO2 incubator at 5% O2. The medium was replaced daily. Cells were subcultured at 70-80% confluence with subculture reagent kit (CC-5034; Lonza). For differentiation, cultured myoblasts were plated in collagen-coated dishes with seeding density 20,000 cells/cm2. After overnight incubation, cells were differentiated using skeletal muscle cell differentiation medium (151D-250; Cell Applications Inc.) for 5 days. Expression of myosin heavy chain (MHC), a marker of fully differentiated myotubes, was detected at 5-days of differentiation (supporting Figure 1S).
Nitrate uptake experiments
Cultured cells were washed 2 times with nitrate/nitrite free PBS, pH 7.4. Then, human skeletal muscle cell basal medium (150-500; Cell Applications Inc.) with addition of nitrate in form of NaNO3 to final concentration of 10 -1000 μM was added to cells and incubated up to 4 hours at 37°C in a CO2 incubator. We chose the concentrations of nitrate based on the previous studies that showed the nitrate levels in plasma reached 500 to 1000 μM after the administration of nitrate-rich diet to humans [27, 28]. After incubation, cells were washed 2 times with nitrate/nitrite free PBS and lysed with lysis buffer (50 mM Tris, 0.5% NP-40 and 150 mM NaCl, pH. 7.4). Cell lysates were collected with scrappers and rapidly frozen in dry ice. Samples were stored at −80°C. Intracellular nitrate and nitrite levels in cell lysates were measured by chemiluminescence using the NO analyzer (NOA i280, GE, USA). In a separate set of experiments, nitrate at concentrations of 10, 100, 500 and 1000 μM was added to myotubes and incubated for 40 minutes at 37°C in a CO2 incubator. Nitrate and nitrite levels were measured in collected cell lysates.
Western blots
Cell lysates from undifferentiated and day-1, day-3 and day-5 differentiated HSkMCs were collected in lysis buffer with a proteinase inhibitor cocktail III (1:500, Calbiochem). Protein at 10-15 μg from cell lysates were separated by 10% or 4-12% SDS-PAGE and transferred to PVDF membrane. Membranes were blocked with 5% BSA for 1 hour at room temperature and incubated with anti-xanthine oxidase (ab109235, Abcam), antialdehyde oxidase (sc-365291, Santacruz biotechnology), anti-MARC-1 (ap-9754c, Abgent), anti-MARC-2 (HPA028702, Sigma-Aldrich) anti-NOS-1 (610309, BD Transduction Laboratories), anti-sialin (SIAL11-A, AlphaDiagnostics), anti-CLCN1 (PA5-37147, Invitrogen) and GAPDH (2118, Cell Signaling) antibodies at 4°C overnight. Goat-anti-mouse or goat-anti-rabbit antibodies conjugated with horseradish peroxidase (Jackson Immunoresearch) were used as secondary antibodies and followed by ECL detection (SuperSignal West Femto maximum sensitivity substrate, ThermoFisher Scientific). Band density was quantified using NIH Image J software.
cGMP measurements
Myotubes were washed 2 times with PBS and pre-incubated with 500 μM IBMX, a phosphodiesterase inhibitor (to prevent cGMP degradation) and/or 10 μM ODQ, a sGC inhibitor, at 37°C in CO2 incubator for 15 minutes. Human skeletal muscle cell basal medium (150-500; Cell applications Inc.) containing 1 mM NaNO2 was added and cells were incubated for 40 minutes at 37°C in CO2 incubator. Cells were washed 2 times with PBS and lysed with 0.1M HCl with 1% Triton X-100. Samples were immediately frozen and kept at −80°C. Intracellular cGMP and nitrite levels were measured by cGMP EIA kit (Enzo Lifescience) and chemiluminescence using a NO analyzer (Sievers NOA 280i, GE, USA), respectively.
Preparation of lentivirus particles
Human sialin shRNA plasmid kit (TL309394) and scramble control (TR30021) from Origene were transformed in competent E. Coli cells. Plasmid DNA was extracted by Purelink™ hiPure plasmid Midiprep (Thermo). For lentiviral production, HEK293T cells were plated in 100 mm dishes and incubated in a CO2 incubator for overnight. Sialin shRNA or scramble control (10 μg) were co-transfected with 4 μg VSV-G envelopes and 10 μg packaging plasmid psPAX2 T by Lipofectamine 2000. At 24 hours after transfection, skeletal muscle cell growth medium was added into transfected HEK293T cells. At 48 hours after transfection, skeletal muscle cell growth medium was collected and centrifuged at 1000 ×g for 10 minutes and then filtered with a syringe filter (0.45 micron). Lentiviral particles were kept at −80°C.
Lentiviral knockdown experiments
Myoblasts were plated in collagen coated 6-well plates with seeding density 20,000 cell/cm2. After overnight incubation, sh-sialin or scramble lentiviral particles were diluted 1:1 into skeletal muscle cell growth medium containing 8 μg/mL polybrene and added to myoblasts. The 6-well plates were centrifuged at 1000 ×g for 2 hours at 37 °C. Cells were incubated in tri-gas CO2 incubator (5% O2) for 48 hours before starting 0.5 μg/mL puromycin selection. Nitrate uptake assay was done after 3 days puromycin selection.
Anion transporter inhibitor experiments
Primary Human skeletal muscle cells (hSkMCs) were grown and differentiated into myotubes as described above. Myotubes were washed 2 times with PBS. Human skeletal muscle cell basal medium (150-500; Cell applications Inc.) containing 0.64 mM anion channel inhibitor, 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS, Sigma-Aldrich) was added to the myotubes and incubated for 30 minutes and then removed. Cells were immediately washed 2 times with PBS before addition of human skeletal muscle cell basal medium (150-500; Cell Applications Inc.) containing 1 mM NaNO3. Cells were incubated at 37°C in CO2 incubator for 10 minutes. After incubation, cells were washed 2 times with PBS and lysed with lysis buffer (50 mM Tris, 0.5% NP-40 and 150 mM NaCl, pH. 7.4). Cell lysates were collected with scrappers and rapidly frozen in dry ice. Samples were stored at −80°C. Intracellular nitrate and nitrite levels in cell lysates were measured by chemiluminescence using a NO analyzer (Sievers NOA 280i, GE, USA). All data are presented as mean ± SEM.
Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were done using GraphPad Prism® version 4 (GraphPad software Inc., San Diego, CA).
Results
Nitrate uptake and metabolism in myoblasts and myotubes.
Nitrate uptake from the medium containing 1 mM concentration of nitrate was rapid and levels reached a maximum at about 5 minutes in both myoblasts and the differentiated myotubes (Figure 1A, C). While the levels of nitrate in myotubes were maintained for 4 hours, the levels of nitrate in myoblasts decreased after 40 minutes following the nitrate addition. Intracellular nitrate levels in the myoblasts and myotubes reached similar values, 5.3 μM vs 6.6 μM, respectively. When normalized to protein content and expressed in nmol/mg protein, the 3-fold difference in nitrate uptake values between myoblasts and myotubes is due to the increased total protein levels of the myotubes. Interestingly, intracellular nitrite levels significantly increased only in myotubes, 40 to 60 minutes after addition of nitrate (with rough estimation of about 1% nitrate uptake being converted to nitrite), and no change in intracellular nitrite levels was detected in in myoblasts (Figure 1B, D). Nitrate uptake and its limited reduction to nitrite in dose-dependent manner after addition of exogeneous nitrate was clearly detectable in the myotubes (Figure 2), with no evident cytotoxicity.
Figure 1. Nitrate uptake and metabolism in myoblasts (A, B) and myotubes (C, D).
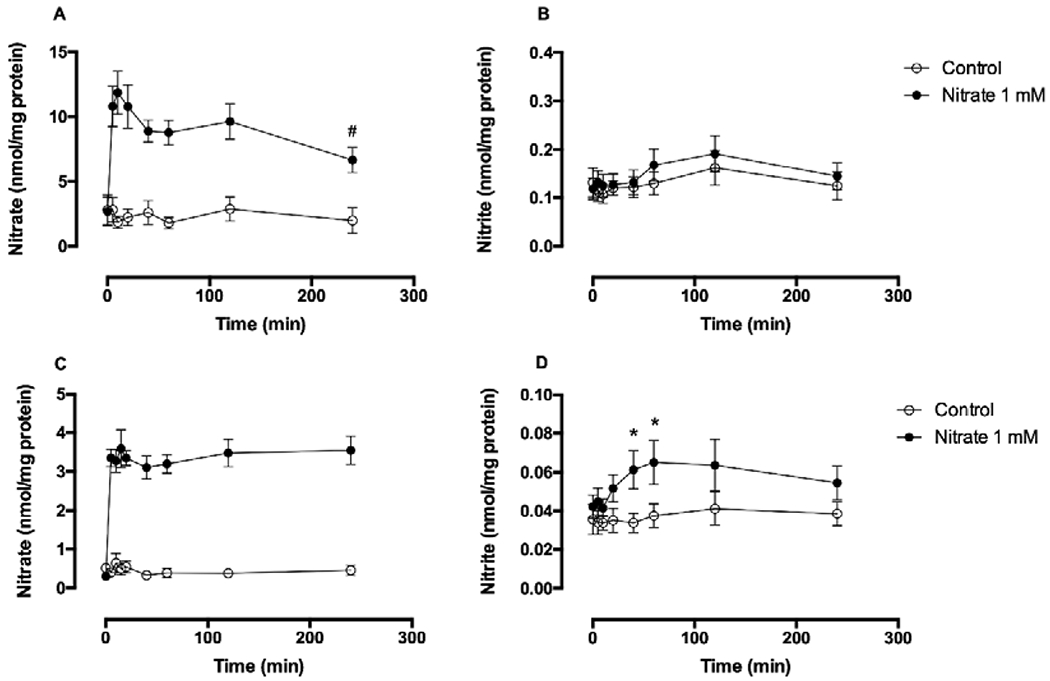
Primary human skeletal muscle cells (5 × 105 cells) were plated in a 60-mm dish. For myotube formation, human skeletal muscle cells were cultured in differentiation medium for 5 days. Nitrate (1 mM) was added to either myoblasts or myotubes and incubated at 37°C for 5, 10, 20, 40, 60, 120 and 240 minutes. After washings with PBS, cell lysates were collected into lysis buffer. Intracellular nitrate and nitrite levels were measured by chemiluminescence and normalized to protein concentration. Data are mean ± SEM (n=7). *P < 0.05 compared with control and tested by repeated measured ANOVA with Tukey’s multiple comparison. #P < 0.05 compared with 10-minute intracellular nitrate levels and tested by t-test.
Figure 2. Concentration-dependent nitrate uptake and metabolism in myotubes.
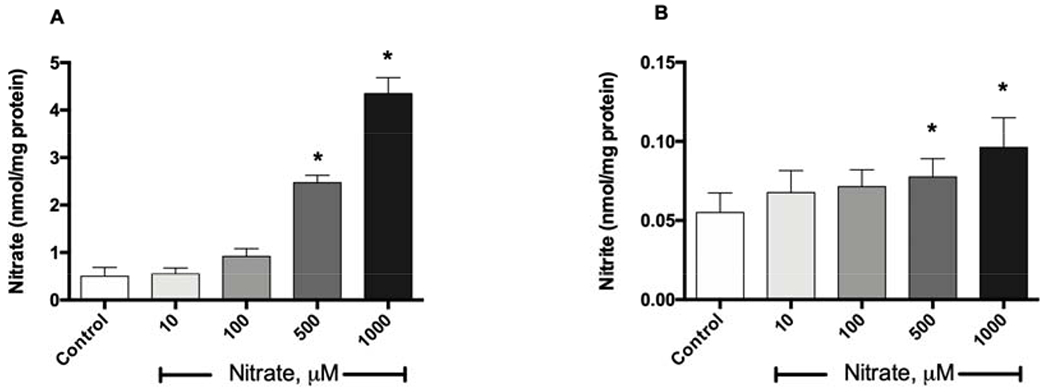
Primary human skeletal muscle cells (5 × 105 cells) were plated in a 60-mm dish and differentiated into myotubes in differentiation medium for 5 days. Myotubes were incubated with 10, 100, 500 and 1000 μM nitrate at 37°C for 40 minutes. After washing with PBS, cell lysates were collected in lysis buffer. Intracellular nitrate (A) and nitrite (B) levels were measured by chemiluminescence and normalized to protein concentration. Data are mean ± SEM (n=5). *P < 0.05 compared with control and tested by repeated measured ANOVA with Tukey’s multiple comparison.
Expression of molybdenum-containing proteins during myoblast differentiation.
Increased intracellular nitrite after addition of exogenous nitrate in myotubes suggested the presence of nitrate reductase enzymes in these cells. Since several molybdenum-containing proteins are among the known groups of proteins that can reduce nitrate to nitrite in mammalian cells, the expressions of xanthine oxidase, aldehyde oxidase, MARC-1 and MARC-2 were investigated during differentiation of myoblasts (Figure 3). Expression of xanthine oxidase significantly increased at day-1 after differentiation (Figure 3A) while MARC-1 and MARC-2 significantly increased at day-3 and day-5 after differentiation, respectively (Figure 3C, D) but expression of aldehyde oxidase did not change during differentiation (Figure 3B). NOS-1, the form of NOS previously reported in muscle cells, was expressed in myoblasts and its expression didn’t change significantly during differentiation (Figure 3E).
Figure 3. The expression of molybdenum-containing proteins and NOS1 during differentiation of myoblasts into myotubes.
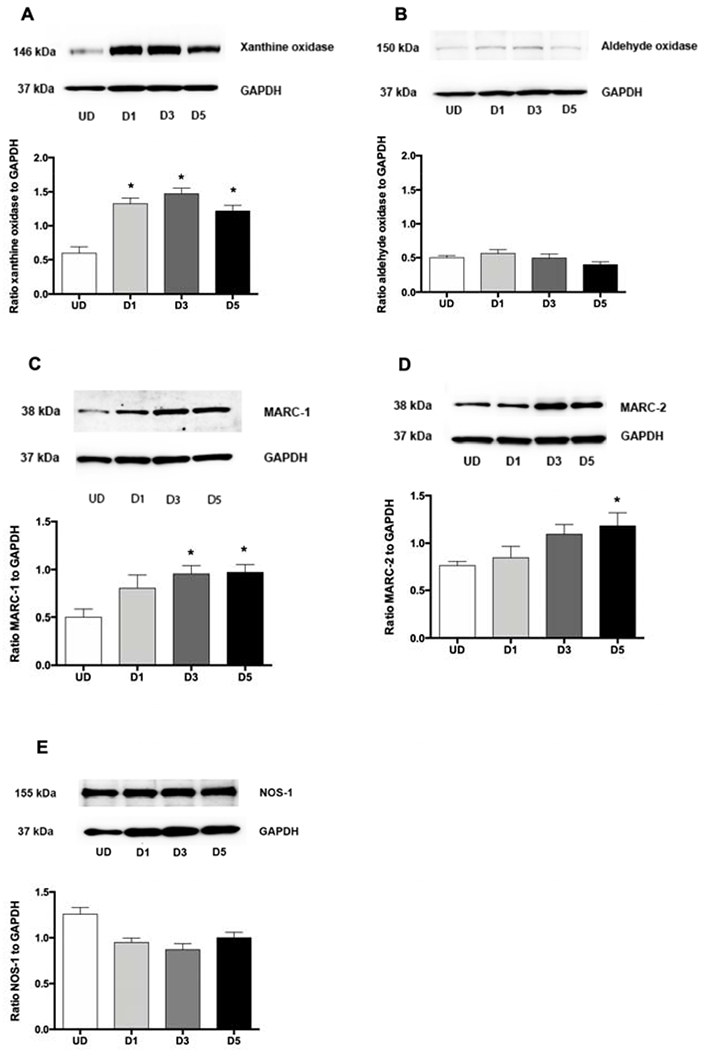
Primary human skeletal muscle cells (5 × 105 cells) were plated in a 60-mm dish. Cell lysate was collected before (undifferentiated myoblasts or UD) and 1 (D1), 3 (D3) and 5 (D5) days after differentiation. The expressions of xanthine oxidase (A), aldehyde oxidase (B), MARC-1 (C), MARC-2 (D) and NOS1 (E) were measured by western blots. Data are mean ± SEM (n = 4-6). *P < 0.05 compared with undifferentiated cells (UD) and tested by ANOVA with Tukey’s multiple comparison.
Nitrite uptake and increased cGMP levels in myotubes.
In addition to nitrate, nitrite itself was also rapidly taken up by the muscle cells. Intracellular nitrite increased from 0.03 ± 0.002 to 2.61 ± 0.13 μmol/mg protein after 10 minutes following the addition of nitrite to final concentration of 1mM, and these levels of intracellular nitrite were maintained through 40 minutes (Figure 4A). Increased intracellular nitrite significantly raised the levels of cGMP in myotubes compared with control with or without IBMX (Figure 4B). IBMX, a PDE inhibitor, alone did not increase cGMP levels significantly in myotubes. In addition, ODQ (a sGC inhibitor) treatment prevented nitrite-mediated elevation of cGMP completely. However, these effects were not measurable at the lower levels of nitrite that we accomplished with nitrate additions to the medium.
Fig. 4. Increases of nitrite (A) and cGMP (B) levels in myotubes.
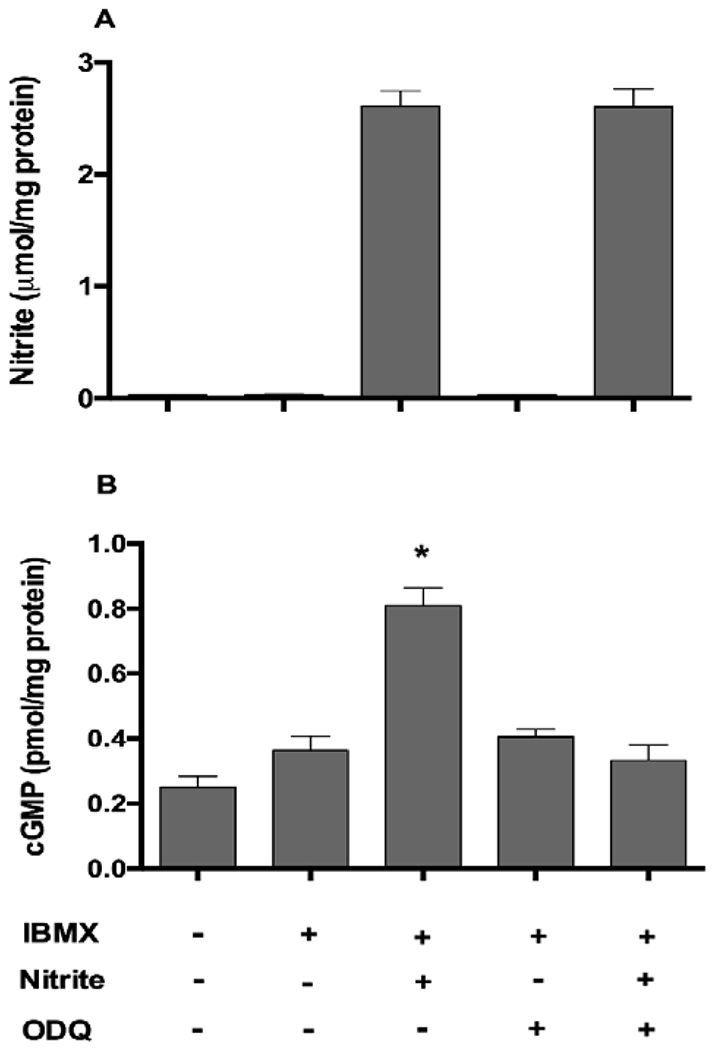
IBMX (500 μM) and/or ODQ (10 μM) were pre-incubated in myotubes for 15 min in CO2 incubator. Nitrite at a concentration of 1 mM was added to myotubes and incubated at 37 °C for 40 min. Cell lysates were collected in 0.1 M HCL containing 1% Triton X-100. Intracellular cGMP and nitrite levels were measured by ELISA kit and chemiluminescence. Data are mean ± SEM (n = 5). *P < 0.05 compared with other bar graphs and tested by ANOVA with Tukey’s multiple comparison.
Anion transporters-mediated nitrate uptake in skeletal muscle cells.
DIDS, an anion transporter inhibitor, was used to examine the role of the anion transporters for nitrate uptake in skeletal muscle cells. DIDS completely inhibited nitrate uptake into myotubes (Figure 5A). The levels of nitrate decreased from 1.67 ± 0.37 to 0.36 ± 0.06 nmol/mg protein which is in the range of baseline nitrate levels in myotubes. CLC1 and sialin, both previously identified as possible nitrate transporters, were upregulated during the differentiation of muscle cells (Figure 5B, C). Since there is no known selective sialin inhibitor, expression of sialin in myoblasts was knocked down by sh-sialin lentivector (Figure 6A). Nitrate uptake in myoblasts transduced with sh-sialin lentivectors decreased significantly, as compared to cells transduced with a scramble control (Figure 6B) suggesting a role of this protein in nitrate uptake in these cells. However, knockdown of sialin did not completely inhibit nitrate transport in myoblasts suggesting a possible role of CLC1 and other anion-transporters in nitrate transport as well. Due to technical difficulties we were not able to perform sialin knockdown in myotubes and CLC1 expression was low in myoblasts.
Figure 5. Anion-transporter mediated nitrate uptake in myotubes.
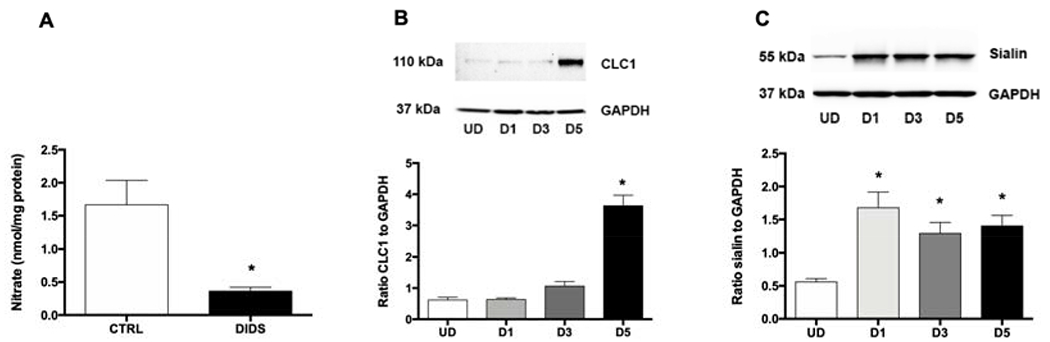
(A) DIDS inhibition of nitrate uptake into myotubes. Myotubes were first incubated with DIDS for 30 min, washed with PBS and then incubated with nitrate. Intracellular nitrate levels were measured in myotubes lysate after 10 minutes incubation with nitrate (1 mM). The expression of CLC1 (B) and sialin (C) during the differentiation of myotubes were confirmed by Western blot. Data are mean ± SEM (n=3). *P < 0.05 compared with control and tested by unpaired t-test.
Figure 6. Nitrate uptake in myoblasts transduced with sh-sialin lentivector.
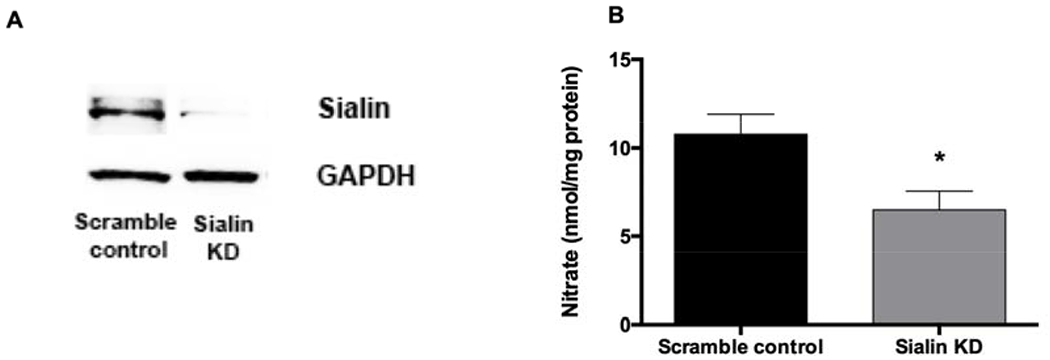
(A) Myoblasts were transduced with sh-sialin or scramble-sh lentiviral vectors and sialin expression was confirmed by Western blot. (B) Intracellular nitrate levels were measured in myoblasts lysate after 10 minutes incubation with nitrate (1 mM). Data are mean ± SEM (n=5). *P < 0.05 compared with scramble control and tested by unpaired t-test.
Discussion
In the present study we have investigated the possibility that skeletal muscle cells contain the full cellular machinery able to take nitrate into the cell and reduce it to nitrite and NO, which is then used either locally to support cellular metabolism and regulate blood flow or systemic processes. Our results demonstrate that exogenous nitrate is rapidly taken up by cultured skeletal muscle cells. The anion transporters including sialin and CLC1 play a role, at least in part, for nitrate transport. In differentiated skeletal muscle cells, nitrate is metabolized to nitrite/NO by molybdenum containing proteins expressed in these myotubes. However, approximately 1% of nitrate is metabolized to nitrite with slow rate of conversion. Our current view of nitrate/nitrite/NO pathways in differentiated skeletal muscle cells is shown in Figure 7. The nitrate uptake and intracellular conversion to nitrite and NO by skeletal muscle cells indicates the presence of nitrate/nitrite/NO conversion pathways in skeletal muscle and supports the importance of this pathway for the physiologic function of skeletal muscle, which may also associate with the improved exercise tolerance after nitrate ingestion.
Figure 7. Schematic representation of nitrate/nitrite/NO pathways in human skeletal muscle cells explored in this study.
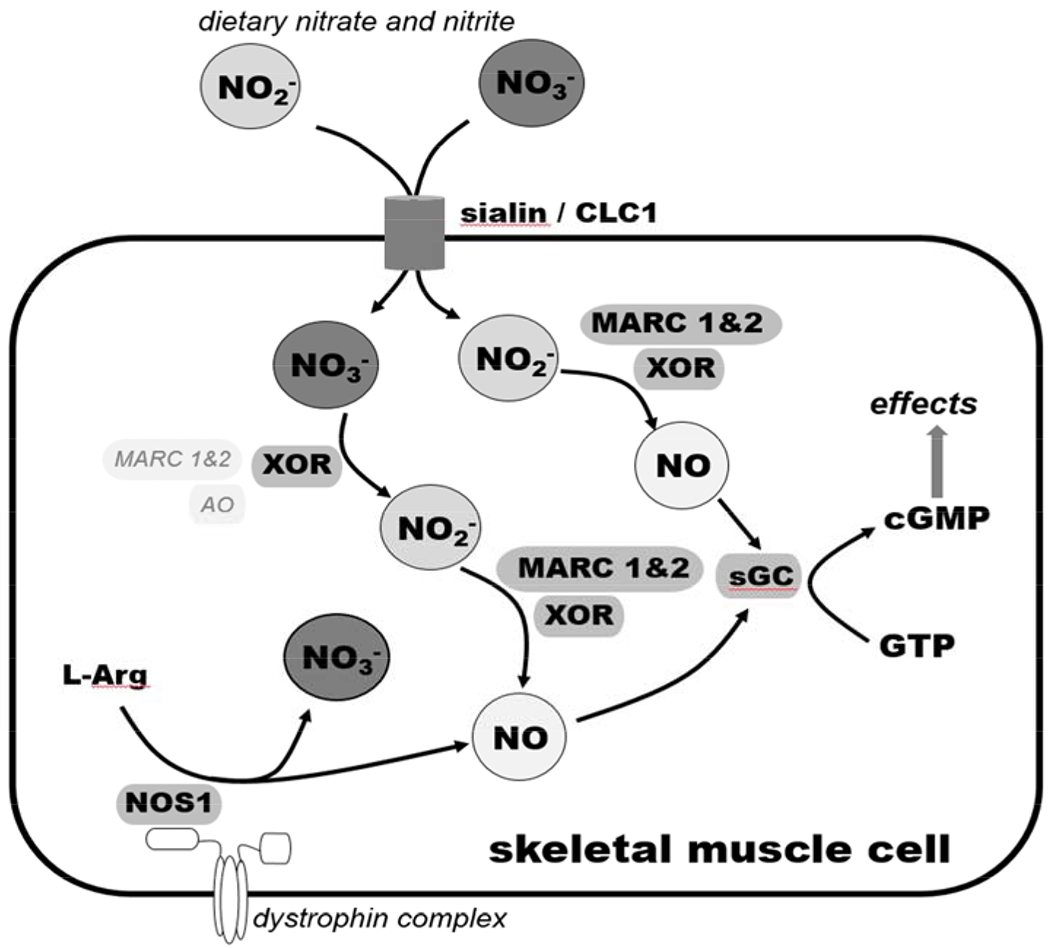
Dietary nitrate and, to a lesser extent, nitrite, are presumably transported into the human skeletal muscle cell by anion transporters, such as sialin and the chloride channel 1 (CLC1). Nitrate is also produced during the “futile” cycle of NOS1. Inside the cell, nitrate can be reduced into nitrite by xanthine oxidoreductase (XOR), which is a known mammalian nitrate reductase. Other molybdenum-containing proteins, mitochondrial amidoxime-reducing component (MARC-1 and MARC-2) and aldehyde oxidase (AO), are also expressed in these cells and might potentially play a role in reducing nitrate into nitrite and nitrite into NO. NO is also produced during productive cycle of NOS1. NO acts on soluble guanylyl cyclase (sGC), which produces cyclic GMP (cGMP), the downstream messenger of nitric oxide. This scheme shows only the part of the nitrate/nitrite/NO cycle directly connected with the present study. Other known pathways (not shown on the scheme), are the reduction of nitrite to NO by deoxymyoglobin and oxidation of nitrite and NO into nitrate by oxymyoglobin.
The importance of skeletal muscle tissue as the largest body nitrate reservoir and one of the major sites of nitrate and nitrite metabolism had been discovered only recently [19–21, 23]. The function seems to be widespread though the animal kingdom, as it now has been observed in several mammalian species – rats, mice [21] and humans [22]; and also, in fish [23]. Skeletal muscle, as an organ with the largest mass in such animals is well positioned for a reservoir function that could also first evolve from the need to rapidly initiate and sustain increased blood flow during exercise. We confirmed that locally produced/stored nitrate is, indeed, consumed during exercise in rats [20] and that decreased nitrate availability from diet leads to nitrate “hoarding” by skeletal muscle when dietary nitrate is made available again [19]. Both observations confirm the importance of this ion for the proper physiology of skeletal muscle and, perhaps, for whole-body physiology via nitrate fluxes out of the muscle and/or its metabolism directly in the tissue by the residing nitrate and nitrite reductases (Mo-containing proteins and 5-coordinated heme proteins).
In our previous work, we hypothesized and partially confirmed that nitrate is generated on site via the “futile” cycle of resident NOS1 (nNOS) as well as via oxidation of NOS1-produced NO by oxymyoglobin [21]. Diet is also an important source of nitrate in skeletal muscle, as was shown in our follow-up study [19]. The fact that, after regaining access to dietary nitrate, the resulting nitrate concentration in skeletal muscle exceeds several folds that in blood, led us to the hypothesis of the involvement of active (or starvation-activated) transport of nitrate ions from blood into muscle tissue, against the muscle-to-blood concentration gradient. So far, sialin is the only described nitrate transporter; it concentrates nitrate from blood into salivary glands [16]. Similar to the muscle/blood situation, nitrate in saliva is ~100 times more concentrated than in blood, which shows the high efficiency of sialin-mediated nitrate transport and the importance of the oral bacteria contribution in the entero-salivary pathway for nitrate metabolic pathways [15–17].
In the current study, we found that nitrate is indeed stored and, in part, also formed within the body of the skeletal muscle cell and not necessarily in the other parts of the muscle tissue – such as vascular walls, tendons or connective tissue. (This possibility was not completely ruled out based only on our previous studies using the whole muscle tissues). Both, cultured myoblasts and myotubes, contain measurable amount of nitrate and nitrite at baseline conditions before any treatment (Figure 1, time 0 and Figure 2, control). This is presumably the intrinsic nitrate and nitrite concentrations generated by NOS1 and the NO/oxymyoglobin systems, as there is no added nitrate/nitrite to the system at these points. We confirmed the expression levels of NOS1 protein in both myoblasts and myotubes (Figure 3) and showed that the addition of L-NAME, a NOS inhibitor, for 24 hours decreased basal nitrate levels significantly in myotubes (supplementary Figure 2S).
None-the-less, muscle cells are able to quickly transport (actively and/or passively) exogenously added nitrate inside the cells, with maximum levels achieved after only 5 minutes following the nitrate addition (Figure 1A, C). This suggests that there is a well-developed nitrate transport system. The nitrate uptake is also concentration-dependent (Figure 2A). After rapid nitrate intake into myoblasts and myotubes, part of the nitrate is reduced into nitrite by nitrate reductases in myotubes, with maximum nitrite levels achieved after ~40 minutes following increased nitrate availability (Figure 1B, D); the amount of nitrite produced depends on the amount of available nitrate (Figure 2B). In addition to nitrate reductase enzymes in oral bacteria, our study indicates the possibility of nitrate reduction to nitrite inside myotube. Arrays of proteins acting as nitrate and nitrite reductases (XOR, AO, MARC1 and MARC2) are also expressed in myoblasts with increased levels during cell differentiation into myotubes (Figure 3). We believe that the presence of all components needed for nitrate generation as well as its reduction into nitrite and NO confirms the importance of the nitrate/nitrite/NO reduction pathways for the normal physiology of muscle cells and shows that the formation of nitrite is not only happening within the muscle tissue itself, but that this process can be supplemented by the import of exogeneous, dietary nitrate from the bloodstream.
The amount of nitrite endogenously present in myotubes is quite low, and we were not able to directly detect nitrite reduction into NO, which is active in subnanomolar concentrations by activating soluble guanylyl cyclase that leads to formation of cGMP. However, even at the low “native” nitrite concentrations, we could detect the presence of cGMP (Figure 4). Due to rapid degradation of cGMP, a PDE inhibitor (IBMX) was used to preserve most of the synthetized cGMP [29]. Without exogenous nitrite, IBMX slightly increased intracellular cGMP levels indicating the presence of endogenous NO production in myotubes. When intracellular levels of nitrite were increased using exogeneous nitrite, we were able to clearly detect significant increase of cGMP production, which was inhibited by ODQ, a sGC inhibitor, demonstrating the conversion of nitrite into NO in the myotube (Figure 4B).
Due to negative charge of nitrate, anion transporters are proposed to mediate nitrate uptake into cells. Addition of DIDS caused robust 4.6-fold decrease of nitrate uptake into myotubes suggesting the role of anion transporters in transporting nitrate ions into skeletal muscle cells. Among anion transporters, sialin and CLC1 were previously identified as nitrate transporter. We hypothesized in our previous work that sialin, a nitrate transporter identified in salivary glands, is involved in transport of nitrate into skeletal muscle cells. Using Western blot technique, we confirmed significant sialin expression in myoblasts and increased sialin expression with differentiation into myotubes (Figure 5C), which suggested that sialin could be an important nitrate transporter not only in salivary glands but also in skeletal muscle cells. To our knowledge, this fact was not widely known. Using lentivirus to knockdown the expression of sialin in myoblasts and measuring the amount of nitrate transported into cells transfected with either scrambled or active virus, we confirmed that sialin is, indeed, significantly involved in the transport of nitrate into skeletal muscle cells (Figure 6). Due to technical difficulties, we were only able to knockdown sialin in myoblasts, but we believe that sialin knockdown would have the similar effect on myotubes. However, sialin knockdown did not completely block nitrate transport, which in part, was due to less than perfect transfection efficiency. In addition, the potential role of other nitrate transporters in muscle cells cannot be excluded. Another, more likely possibility is that the nitrate uptake remaining after sialin knockdown, is due to another anion transporter system, namely CLC1 (chloride anion transporter), which is exclusive to muscle. CLC1 is a member of the ubiquitous CLC family, members of which had been extensively studied for decades [30]. The permeability effect of CLC1 for anions other than chloride, including nitrate, has been described [25, 26]. In this study, increased CLC1 expression was detected in fully differentiated myotubes; however, the role of CLC1 in nitrate transport in muscle cells needs further investigation.
In summary, we have found that skeletal muscle cell contains the complete machinery for nitrate uptake and synthesis as well as for the nitrate-nitrite-NO reduction pathway. At the cultured cell level, the amount of endogenously synthesized nitrate is significantly lower as compared to the total amount that can be achieved by sequestering exogeneous nitrate. We hypothesize that muscle cells likely cannot ordinarily achieve endogenously levels high enough to satisfy all ongoing routine and emergency processes and rely heavily on nitrate transport from exogeneous sources to achieve necessary nitrate stores for sufficient conversion to nitrite and NO. We point out the important role of sialin and CLC1 as nitrate transporters. To further confirm our hypothesis, more research on cells as well as animal models is still needed.
Supplementary Material
Skeletal muscle is the largest nitrate reservoir in mammalian body
Muscle-to-liver nitrate gradient persists after dietary manipulation and exercise
Nitrate transporters are involved in maintaining this nitrate gradient
Sialin and CLC1 function as nitrate transporters in primary human muscle cell
Acknowledgments
Funding:
This work was funded by NIH intramural grant to Dr. Alan N. Schechter (NIH intramural project, DK025093 (2018), Nitric oxide metabolism and transport).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Alan N. Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. All other authors declare that they have no conflicts of interest.
References
- [1].Cosby K, Partovi KS, Crawford JH, et al. , Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 9 (2003) 1498–505. 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- [2].Forstermann U and Sessa WC, Nitric oxide synthases: regulation and function. Eur Heart J. 33 (2012) 829–37, 837a–837d. 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li H, Cui H, Kundu TK, Alzawahra W, and Zweier JL, Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 283 (2008) 17855–63. 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li H, Samouilov A, Liu X, and Zweier JL, Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 276 (2001) 24482–9. 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- [5].Maia LB and Moura JJG, Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes. Redox Biol. 19 (2018) 274–289. 10.1016/j.redox.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maia LB, Pereira V, Mira L, and Moura JJ, Nitrite reductase activity of rat and human xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase: evaluation of their contribution to NO formation in vivo. Biochemistry. 54 (2015) 685–710. 10.1021/bi500987w. [DOI] [PubMed] [Google Scholar]
- [7].Omar SA and Webb AJ, Nitrite reduction and cardiovascular protection. J Mol Cell Cardiol. 73 (2014) 57–69. 10.1016/j.yjmcc.2014.01.012. [DOI] [PubMed] [Google Scholar]
- [8].Pellegrino D and Parisella ML, Nitrite as a physiological source of nitric oxide and a signalling molecule in the regulation of the cardiovascular system in both mammalian and non-mammalian vertebrates. Recent Pat Cardiovasc Drug Discov. 5 (2010) 91–6. [DOI] [PubMed] [Google Scholar]
- [9].Shiva S, Huang Z, Grubina R, et al. , Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 100 (2007) 654–61. 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- [10].Tota B, Quintieri AM, and Angelone T, The emerging role of nitrite as an endogenous modulator and therapeutic agent of cardiovascular function. Curr Med Chem. 17 (2010) 1915–25. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Z, Naughton D, Winyard PG, et al. , Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 249 (1998) 767–72. 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- [12].McNulty PH, Scott S, Kehoe V, et al. , Nitrite consumption in ischemic rat heart catalyzed by distinct blood-borne and tissue factors. Am J Physiol Heart Circ Physiol. 295 (2008) H2143–8. 10.1152/ajpheart.00050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sandvik GK, Nilsson GE, and Jensen FB, Dramatic increase of nitrite levels in hearts of anoxia-exposed crucian carp supporting a role in cardioprotection. Am J Physiol Regul Integr Comp Physiol. 302 (2012) R468–77. 10.1152/ajpregu.00538.2011. [DOI] [PubMed] [Google Scholar]
- [14].Sparacino-Watkins CE, Tejero J, Sun B, et al. , Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J Biol Chem. 289 (2014) 10345–58. 10.1074/jbc.M114.555177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koch CD, Gladwin MT, Freeman BA, et al. , Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 105 (2017) 48–67. 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Limirio PH, Rocha FS, Batista JD, et al. , The Effect of Local Delivery Doxycycline and Alendronate on Bone Repair. AAPS PharmSciTech. 17 (2016) 872–7. 10.1208/s12249-015-0411-0. [DOI] [PubMed] [Google Scholar]
- [17].Vanhatalo A, Blackwell JR, L’Heureux JE, et al. , Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 124 (2018) 21–30. 10.1016/j.freeradbiomed.2018.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jansson EA, Huang L, Malkey R, et al. , A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 4 (2008) 411–7. 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- [19].Gilliard CN, Lam JK, Cassel KS, et al. , Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide. 75 (2018) 1–7. 10.1016/j.niox.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piknova B, Park JW, Kwan Jeff Lam K, and Schechter AN, Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 55-56 (2016) 54–61. 10.1016/j.niox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Piknova B, Park JW, Swanson KM, et al. , Skeletal muscle as an endogenous Nitrate reservoir. Nitric Oxide. 47 (2015) 10–16. 10.1016/j.niox.2015.02.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nyakayiru J, Kouw IWK, Cermak NM, et al. , Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J Appl Physiol (1985). 123 (2017) 637–644. 10.1152/japplphysiol.01036.2016. [DOI] [PubMed] [Google Scholar]
- [23].Hansen MN, Lundberg JO, Filice M, et al. , The roles of tissue nitrate reductase activity and myoglobin in securing nitric oxide availability in deeply hypoxic crucian carp. J Exp Biol. 219 (2016) 3875–3883. 10.1242/jeb.149195. [DOI] [PubMed] [Google Scholar]
- [24].Qin L, Liu X, Sun Q, et al. , Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A. 109 (2012) 13434–9. 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang T, Han W, Maduke M, and Tajkhorshid E, Molecular Basis for Differential Anion Binding and Proton Coupling in the Cl(−)/H(+) Exchanger ClC-ec1. J Am Chem Soc. 138 (2016) 3066–75. 10.1021/jacs.5b12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, and Bretag AH, Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J Gen Physiol. 111 (1998) 653–65. 10.1085/jgp.111.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Webb AJ, Patel N, Loukogeorgakis S, et al. , Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 51 (2008) 784–90. 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wylie LJ, Park JW, Vanhatalo A, et al. , Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol. (2019). 10.1113/JP278076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mo E, Amin H, Bianco IH, and Garthwaite J, Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. J Biol Chem. 279 (2004) 26149–58. 10.1074/jbc.M400916200. [DOI] [PubMed] [Google Scholar]
- [30].Jentsch TJ and Pusch M, CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol Rev. 98 (2018) 1493–1590. 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


