Abstract
Pulmonary toxicity of cancer immunotherapies has emerged as an important clinical event that requires prompt identification and management. Although often referred to as pneumonitis, pulmonary toxicity associated with immunotherapy covers a broad and overlapping spectrum of pulmonary manifestations, and, once suspected, the range of differential diagnoses of infectious and neoplastic processes might make the diagnostic process challenging for physicians. Optimal care can require multidisciplinary effort by pulmonologists, medical oncologists, and radiologists, and awareness of the possibility of treatment-induced pulmonary toxicity by emergency department and primary care physicians. This Viewpoint gives an overview of the diagnosis and management of pulmonary toxicity arising from cancer immunotherapy, including widely used treatments, such as immune checkpoint inhibitors, and emerging therapies, such as chimeric antigen receptor T cells.
Pulmonary toxicities of cancer therapies
For decades, until the introduction of immunotherapy a few years ago, medical oncologists could treat patients with cancer with little concern for the effects of treatment (as opposed to disease effects) on pulmonary function. Chemotherapy frequently causes adverse events such as alopecia, gastrointestinal effects, neuropathy, and myelosuppression; yet, only rarely used cytotoxic chemotherapeutics, such as bleomycin, mitomycin C, and busulfan, are associated with clinically significant pulmonary toxicity.1 These events are often predictable and avoidable as long as the total lifetime dose of chemotherapy is below a specified threshold and pulmonary function is monitored. Similarly, thoracic radiation therapy has predictable effects on pulmonary function, reflecting anatomical location related to treatment ports.2 Although these toxicities might increase when radiation therapy is combined with chemotherapy, advancements in treatment planning and delivery have resulted in decreased radiation-related pulmonary toxicities.3
It is now clear that we need to understand and deal with the respiratory effects of a range of cancer treatments.4 Although receptor tyrosine kinase inhibitors and other molecularly targeted therapies might, in rare cases, be associated with adverse effects on pulmonary function, nowhere is the risk of pulmonary toxicity more pronounced and less clearly understood than with cancer immunotherapy. Immune checkpoint inhibitors have shown remarkable beneficial effects in several cancer types, leading to approvals for lung cancer, kidney cancer, melanoma, head-and-neck cancer, bladder cancer, lymphoma, Merkel cell cancer, and any tumour characterised by microsatellite instability (ie, a predisposition to mutations). Currently approved drugs in this class include ipilimumab, pembrolizumab, atezolizumab, nivolumab, durvalumab, and avelumab. Chimeric antigen receptor (CAR) T cells have also emerged as a promising therapy for refractory haematological malignancies.
This Viewpoint gives an overview of the approach to diagnosis and management of immune-related pulmonary toxicities.
Immune checkpoint inhibitors
Immune checkpoint inhibitors are monoclonal antibodies that target natural immune checkpoints such as cytotoxic T-lymphocyte antigen 4, programmed death 1 (PD1), and PD ligand 1 (PDL1). Such checkpoints exist to prevent the development of antihost immune effects, which would otherwise result in clinical autoimmune disease. By taking the brakes off host immune function, checkpoint inhibitors might lead to enhanced antitumour immunity. However, as a bystander effect, these drugs might also heighten immune activity against self-antigens, resulting in immune-related adverse events in a substantial pro portion of patients, which, in some cases, might be severe and possibly permanent. These autoimmune toxicities can affect almost every organ, including skin, brain, pituitary, eye, thyroid, liver, adrenal, kidney, pancreas, colon, and—the focus of this Viewpoint—lung.
Chimeric antigen receptor T cells
T cells that have been genetically modified to express chimeric antigen receptors, or CAR T cells, targeting the B-cell antigen CD19 have started a revolution in the treatment of haematological malignancies, including acute lymphoblastic leukaemia,5 chronic lymphocytic leukaemia,6 and non-Hodgkin lymphoma.7 This treatment has also shown early signs of efficacy in solid tumours.8–10
CAR T cells, which can be autologous or allogeneic, feature recombinant receptors that function in both antigen binding and T-cell activation. The most common acute and serious toxicity of CAR T cells is cytokine release syndrome. In severe cases, this immune-mediated release of massive amounts of cytokines, including interleukin 6, interferon-gamma, tumour necrosis factor, interleukin 2, interleukin 8, and interleukin 10,5,11,12 might lead to capillary leakage, renal and hepatic dysfunction, and cardiopulmonary compromise.13–16
Epidemiology
Large meta-analyses of randomised trials and retro spective studies using anti-PD1 and PDL1 antibodies across melanoma, lung cancer, and renal cancer found an overall incidence of pulmonary toxicity of 2·7–3·5%.17,18 Men and former or current smokers are at a higher risk of developing pulmonary toxicity than women and non-smokers, with a median age of 59 years.17 Risk of immune-related pulmonary toxicity might vary according to tumour type, with incidence particularly low among individuals with melanoma and highest among patients with lung cancer.18 Individuals with lung cancer are also at higher risk of developing high-grade and even fatal toxicities than those with other types of cancer.17 Whether this reflects juxtaposition of healthy tissue with tumour, a lower pretreatment pulmonary reserve than patients without lung cancer or lung abnormalities, or previous exposures (eg, tobacco smoke or ionising radiation) that could enhance inflammatory effects is not clear. The median time to onset of pulmonary toxicity after initiation of immunotherapy is 2·3 months, and tends to occur earlier in lung cancer (2·1 months) than melanoma (5·2 months).17 However, it is now apparent that immune-related adverse events, including pulmonary toxicities, might occur at any point during treatment, and who will develop these toxicities or how long they will last is unclear.
Over the past 5–10 years, the collective experience of researchers and clinicians has resulted in reduced rates of severe autoimmune pulmonary toxicity in clinical trials. In early immunotherapy clinical trials, fatal pulmonary toxicity was a documented risk. Early recognition and treatment have rendered such events far less common. At the same time, researchers, clinicians, sponsors, and regulators have expanded the use of immunotherapy to potentially riskier clinical scenarios. A phase 3 trial using the anti-PDL1 antibody durvalumab after completion of concurrent chemoradiation for locally advanced, stage III non-small-cell lung cancer showed a statistically and clinically meaningful improvement in progression-free survival.19 However, the addition of radiation to immunotherapy increased the risk of pulmonary toxicity when compared with historical incidence of pulmonary toxicity with immunotherapy alone.19 Risk also increases with the use of combinations of immune checkpoint inhibitors compared with monotherapy regimens.18
Pulmonary manifestations of cytokine release syndrome associated with CAR T-cell therapy can occur in up to 15% of patients.20 These include pulmonary oedema, hypoxia, dyspnoea, and interstitial lung disease.7,21,22 The severity of these manifestations can range from mild to severe forms requiring mechanical ventilation.
Studies of combination therapy with checkpoint inhibitors and oncoprotein-targeted agents have revealed unanticipated rates of pulmonary toxicity. In a phase 1 trial combining durvalumab and the epidermal growth factor receptor inhibitor osimertinib (each of which has <5% risk of pulmonary toxicity as monotherapy), more than 60% of patients developed interstitial lung disease, leading to early study discontinuation.23
Immunotherapy is now under investigation as adjuvant (postoperative) treatment for early-stage lung cancer and other early-stage malignancies. Depending on the specific disease stage, a reasonable proportion of these patients are cured with standard therapy alone. Large meta-analyses have suggested that treatment-naive patients with lung cancer might have a higher incidence of checkpoint inhibitor-related pulmonary toxicity than patients who received previous treatments.24 Thus, the possibility of life-threatening or permanent pulmonary toxicity, or the need for long-term corticosteroid requirements to manage these events, must be carefully considered before instigating such trials and treatments.
Clinical manifestations
Some immune-related adverse events are clearly defined and characterised; for example, thyroid, pituitary, or adrenal effects manifest as changes in discrete, quantifiable laboratory values, such as thyroid-stimulating hormone, free thyroxine, adrenocorticotropin, and cortisol. By contrast, immune-related pulmonary toxicities often present with relatively non-specific features. Symptoms might include dyspnoea, chest discomfort, cough, or, less commonly, fever (figure 1). Hypoxia might develop and, in some cases, progress rapidly.25 Rarely, the clinical presentation mimics asthma or allergic bronchopulmonary aspergillosis. Clinical manifestations might be quite difficult to distinguish from disease-related complaints or other treatment-related complications such as infection or anaemia.26
Figure 1: Clinical manifestations of immune checkpoint related pulmonary toxicity.
Symptoms might include dyspnoea, chest discomfort, cough, hypoxia, or, less commonly, fever. Differential diagnosis includes disease -related complaints or other treatment-related complications such as infection or anaemia.
Diagnostic evaluation
Imaging
Cross-sectional imaging with CT, particularly high-resolution CT,27,28 is the preferred diagnostic study when immune-related pulmonary toxicity is suspected, because chest radiography fails to detect up to one-quarter of these cases.29 On CT, radiographic findings might be variable, with reported patterns including cryptogenic organising pneumonia, non-specific interstitial pneumonia, hypersensitivity pneumonitis, and bronchiolitis (figure 2).17,30–33 More severe forms of pulmonary toxicity, such as acute interstitial pneumonia leading to acute respiratory distress syndrome, have also been reported.25,34 The most commonly reported radiographic findings are ground glass opacities, consolidations, bronchiectasis, inter-lobular septal thickening, and pleural effusions. In some cases, organising pneumonia might appear as pulmonary nodules, which presents a diagnostic dilemma because such nodules can be confused with new or worsening metastatic disease. Additionally, thoracic (including hilar and mediastinal) adenopathy or granulomatosis might develop in the setting of auto immune pulmonary toxicity, and these radiographic changes might also be confused with cancer progression.35 The reversed halo sign, which indicates central alveolar septal inflammation and cellular debris surrounded by granulomatous tissue within distal airways, might also be seen with immune-related pulmonary toxicity. This sign appears as a central ground glass opacity surrounded by a denser region of consolidation, and is distinguished from the halo sign indicating pulmonary haemorrhage typically seen in angioinvasive aspergillosis.
Figure 2: Selected case examples of checkpoint inhibitor associated pneumonitis.
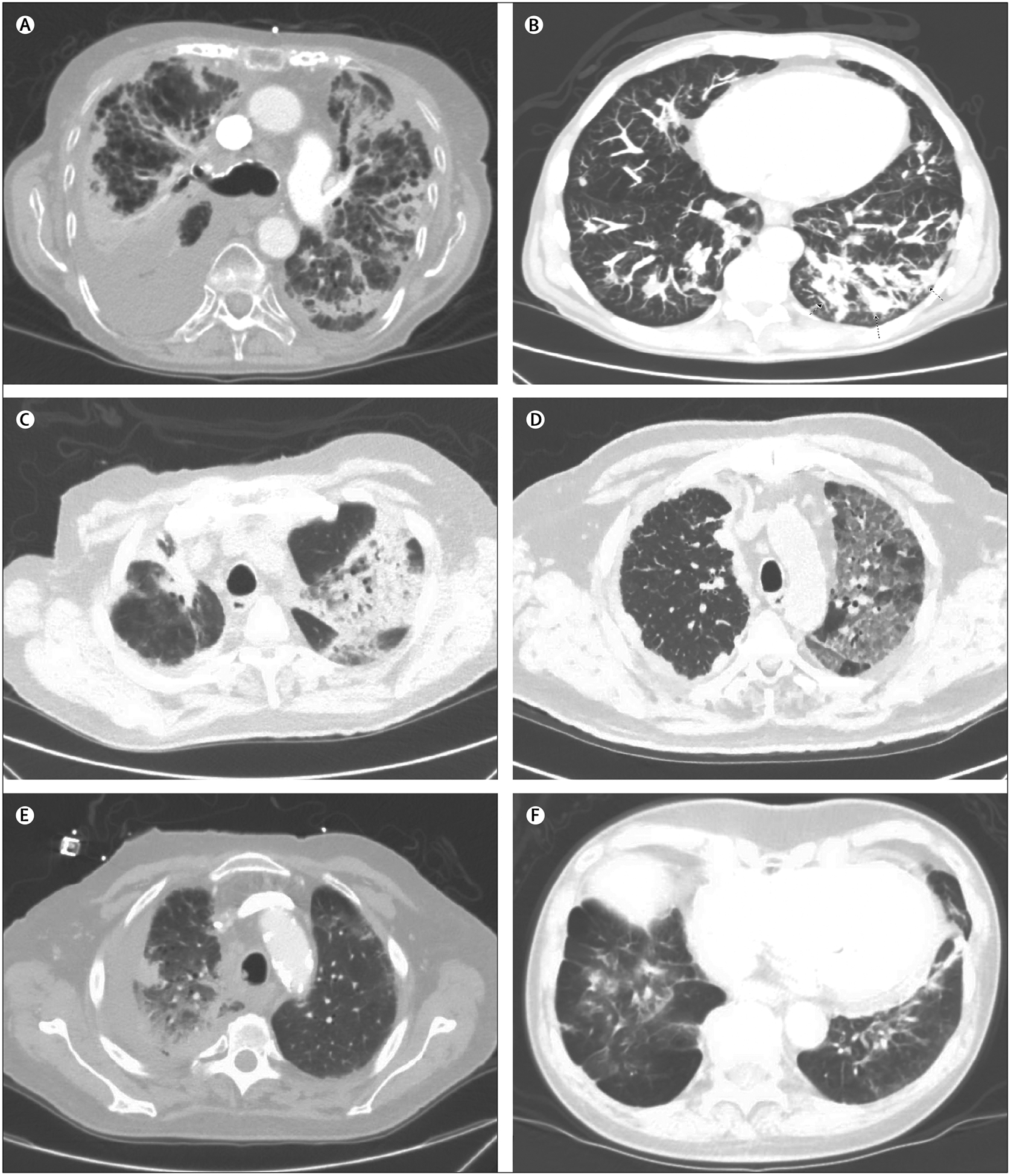
toxicity Case listings include: demographics, cancer type, immunotherapy type, pneumonitis onset, and radiographic appearance. (A) 87-year-old man, non-small-cell lung cancer (NSCLC), anti-programmed cell death ligand 1 (PDL1), 9 weeks, reticulonodular opacities, and septal thickening. (B) 60-year-old man, renal cell carcinoma, antiprogrammed cell death protein 1 (PD1), 5 months, nodular opacities. (C) 77-year-old man, melanoma, combination anti-PD1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, 6 weeks, patchy ground glass and nodular consolidation, cryptogenic organising pneumonia. (D) 71-year-old man, NSCLC, anti-PD1, 2 weeks, mosaic attenuation of ground glass opacities. (E) 70-year-old man, NSCLC, anti-PD1, 1 month, consolidation. (F) 78-year-old man, NSCLC, anti-PDL1, 4 months, consolidations and ground glass densities in the bases.
Pulmonary function testing
Regardless of the specific radiographic features, immune-mediated pulmonary toxicities most commonly show a restrictive pattern, featuring reduced diffusion capacity, in pulmonary function tests.30,36,37 Some studies suggest routine pulmonary function testing, including carbon monoxide diffusing capacity measurement, might be helpful in screening for immune-related pulmonary toxicity during treatment with checkpoint inhibitors.38 Furthermore, for patients undergoing treatment of immune-related pulmonary toxicity, pulmonary function tests including pulse oximetry, spirometry, and carbon monoxide diffusing capacity, at rest and exertion, might be useful in monitoring response to therapy.39
Cytological and pathological features
In a 2017 practice guideline on the management of immune checkpoint inhibitor toxicities,40 pathological assessment was considered a recommended, but not required, component of autoimmune pulmonary toxicity diagnosis. In some cases, cytological and pathological evaluation by bronchoscopy might discriminate infection and lymphangitic spread from the inflammatory changes characteristic of drug-induced pneumonitis. Bronchoalveolar lavage fluid might show inflammatory and lymphocytic infiltration.17,41 In a 2017 series,17 80% of cases had T-lymphocytic alveolitis.
If bronchoscopic biopsy is done, histopathological findings can include cellular interstitial pneumonitis (36%), organising pneumonia (27%), and diffuse alveolar damage (9%).29 In more than one-quarter of cases, no pathological abnormalities are identified. Sarcoid-like granulomatous reactions and interstitial fibrosis have also been reported.42
Management
Occasionally, patients might develop radiographic features suggestive of immune-related pneumonitis with no corresponding clinical features. In these cases (ie, grade 1 pulmonary adverse reaction), immune checkpoint inhibitor treatment might be continued. Clinical monitoring every 2 or 3 days and repeat imaging at least every 3 weeks is advised.
In cases of suspected clinically apparent immune-related pulmonary toxicity, management should be started immediately. When clinical suspicion is high, treatment should be started without waiting for pathological evaluation. The initial step in management is withholding immune checkpoint inhibitor treatment. For patients with asymptomatic pneumonitis, this might be sufficient.29 In contrast with conventional chemotherapy and targeted therapies, dose reduction has no role. Generally, patients with symptomatic pneumonitis are promptly started on corticosteroids (commonly the equivalent of prednisone 1 mg/kg daily).29,40 Tapering (often by 10 mg/week) begins after improvement to baseline of symptoms (or mild symptoms only) has occurred. With this approach, steroid courses might last months rather than weeks. As a result of the high doses and prolonged duration of steroid therapy, opportunistic infections might occur; in this case, pneumocystis prophylaxis might be warranted. For patients who experience recurrence of symptoms while receiving treatment with steroids or immunosuppressive agents, an infectious cause should be considered. Repeat endoscopy with bronchoalveolar lavage might be indicated in this situation.
The prolonged courses of corticosteroids (and associated toxicities) that result from this approach have come under scrutiny.43 Whether shorter courses of steroids might be adequate in some cases, or whether steroid-sparing treatments should be offered instead, is not known.
In cases refractory to steroids, additional immunosuppressive agents might be considered, including infliximab, cyclophosphamide, or mycophenolate mofetil. Although biological agents such as tumour necrosis factor inhibitors (eg, infliximab) have been associated with risk of malignancy and invasive chronic infections,44,45 in the clinical scenario of refractory and progressive immune-related pulmonary toxicity, it is generally agreed that the benefits of such treatments outweigh the risk.29
Rechallenge with immune checkpoint inhibitors in patients with pneumonitis is an area of debate. Most clinicians agree that for severe (grade 3–4) pneumonitis—which typically translates to intensive care unit-level care—permanent discontinuation of therapy is reasonable.46 For low-grade events, some physicians reinitiate treatment once clinical improvement has occurred and steroid doses are 10 mg/kg or less.29,40,47 Finally, retrospective data suggest that some patients with beneficial responses to immunotherapy who then discontinue treatment because of immune-related adverse events might maintain these responses long term without re-exposure to treatment.48
Treatment of cytokine release syndrome and related toxicities from CAR T-cell therapy differs somewhat from the management of immune checkpoint inhibitor-related adverse events. Although steroids are the mainstay treatment of checkpoint inhibitor-related pulmonary toxicity, they are generally reserved for second-line treatment of cytokine release syndrome.5,21 Instead, tocilizumab, an interleukin 6 receptor antagonist typically used to treat rheumatological disorders, has been successfully used as initial therapy.14,16,49
Future directions
How can future research advance the understanding and management of immunotherapy-related pulmonary toxicity (panel)? An early step is the development of a reliable means of diagnosis, such as a clear serological correlate. Before the advent of immune checkpoint inhibitors, patients with lung cancer had frequent and substantial thoracic symptoms. Thus, to distinguish between these disease-related processes and therapy-induced autoimmune toxicities is difficult. Blood-based biomarkers, similar to indicators of thyroid or adrenal dysfunction, would greatly aid in the management of these patients. Although the use of serum markers, such as surfactant protein, transforming growth factor β1, tumour necrosis factor α, interleukin 1β, and interleukin 6, for the prediction of radiation-induced pneumonitis has been studied extensively, for immuno-therapy-induced pulmonary toxicity, this area is a work in progress.50–53 Single-nucleotide polymorphisms of the PD1 gene might predict some immune-related adverse events,53 but are not used clinically.
The ability to predict risk of pneumonitis and other immune-related adverse events would allow clinicians to incorporate these factors into treatment decisions; for example, choosing single-agent, rather than combination, immunotherapy, or choosing chemo therapy, rather than immunotherapy, in patients expected to have similar therapeutic benefit but greater toxicity. To date, proposed risk factors include combination immunotherapy, coadministration of radiation therapy, impaired baseline pulmonary function, previous heavy smoking or continued smoking, and previous high-dose chemotherapy.54 However, these clinical factors are unlikely to sufficiently discriminate among risk groups.
Finally, medical oncologists and other clinicians are in need of evidence-based guidance on the management of pulmonary toxicity. If shorter courses of steroids would give similar outcomes to the weeks-long tapers currently used, patients would experience less steroid-related toxicity and might also be able to resume treatment sooner than if they had been on longer-term steroid treatment. This point has particular relevance, because it has been suggested that patients who develop immune-related adverse events are also the most likely to derive therapeutic benefit from these promising therapies.55
Key messages.
Improvements in survival and quality of life mean that immunotherapy is now considered standard of care for multiple cancers, including lung cancer, kidney cancer, melanoma, head-and-neck cancer, bladder cancer, lymphoma, Merkel cell cancer, and any tumour characterised by microsatellite instability
Immune-related adverse events might occur in more than 25% of patients with cancer treated with immunotherapy; these toxicities occur when host immune cells attack normal tissues
Immune-related adverse events are unpredictable, potentially severe, and can affect almost every organ system
The establishment of a diagnosis of immune-related pulmonary toxicity is challenging—the approach might require radiographic and bronchoscopic evaluation, and therefore might benefit from multidisciplinary collaboration between pulmonary medicine physicians and medical oncologists
The mainstay of management of pulmonary toxicity and other immune-related adverse events is to withhold immunotherapy and administer glucocorticoids
Panel: Unanswered questions.
How can we predict which patients considered for cancer immunotherapy will develop pulmonary toxicity or other immune-related adverse events?
What clinical interventions could decrease the risk of pulmonary toxicity and other immune-related adverse events in patients receiving cancer immunotherapy?
What is the optimal means to establish a diagnosis of immune-related pulmonary toxicity?
What is the optimal management of immune-related pulmonary toxicity?
Search strategy and selection criteria.
We searched PubMed with the terms “pulmonary toxicity”, “immunotherapy”, “checkpoint inhibitors”, “pneumonitis”, “radiographic patterns”, “pathogenesis”, “management”, “treatment”, “incidence”, “pulmonary function tests”, “CAR T cell”, and “bronchoalveolar lavage” for articles in English, published between Jan 1, 1990, and June 30, 2017. We prioritised first reports, meta-analyses, and original studies published in the past 7 years.
Acknowledgments
Supported, in part, by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG), the NCI University of Texas SPORE in Lung Cancer (P50CA70907), and the Harold C Simmons Comprehensive Cancer Center Biomarker Research Core (which is supported, in part, by NCI Cancer Center Support Grant 1P30 CA142543-01).
Footnotes
Declaration of interests
DEG has received grants or consultancy, or both, paid to his institution from ArQule, AstraZeneca, Boehringer Ingelheim, BerGenBio, Bristol-Myers Squibb, ImmunoGen, Karyopharm, Peregrine, and Synta. He has received payment for consultancy work from Guardant Medicine and Samsung Bioepis. JDM has received a grant paid to the University of Texas Southwestern Medical Center from Pfizer. SR declares no competing interests.
References
- 1.De Sanctis A, Taillade L, Vignot S, et al. Pulmonary toxicity related to systemic treatment of nonsmall cell lung cancer. Cancer 2011; 117: 3069–80. [DOI] [PubMed] [Google Scholar]
- 2.Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med 2017; 38: 201–08. [DOI] [PubMed] [Google Scholar]
- 3.Deng G, Liang N, Xie J, et al. Pulmonary toxicity generated from radiotherapeutic treatment of thoracic malignancies. Oncol Lett 2017; 14: 501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harri C, Sander CR. Late respiratory effects of cancer treatment. Curr Opin Support Palliat Care 2017; 11: 197–204. [DOI] [PubMed] [Google Scholar]
- 5.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33: 540–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014; 2: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Brawley VS, Hedge M, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 2015; 33: 1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015; 7: 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brentjens RJ, Rivière I, Park HJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124: 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Feldman MA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7: 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017; 50: 1700050. [DOI] [PubMed] [Google Scholar]
- 18.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016; 2: 1607–16. [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
- 20.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy–assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 2016; 34: 1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn MJ, Yang J, Yu H, et al. 136O: osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol 2016; 11: S115. [Google Scholar]
- 24.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 2017; 152: 271–81. [DOI] [PubMed] [Google Scholar]
- 25.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016; 2: 1346–53. [DOI] [PubMed] [Google Scholar]
- 26.Picchi H, Mateus C, Chouaid C, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect 2017; 24: 216–18. [DOI] [PubMed] [Google Scholar]
- 27.Lynch DA, Rose CS, Way D, King TE Jr, et al. Hypersensitivity pneumonitis: sensitivity of high-resolution CT in a population-based study. AJR Am J Roentgenol 1992; 159: 469–72. [DOI] [PubMed] [Google Scholar]
- 28.Rival G, Manzoni P, Lacasse Y, et al. High-resolution CT predictors of hypersensitivity pneumonitis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 117–23. [PubMed] [Google Scholar]
- 29.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35: 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol 2012; 30: e156–59. [DOI] [PubMed] [Google Scholar]
- 31.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016; 22: 6051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato T, Masuda N, Nakanishi Y, et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 2017; 104: 111–18. [DOI] [PubMed] [Google Scholar]
- 33.Diederich S Chest CT for suspected pulmonary complications of oncologic therapies: how I review and report. Cancer Imaging 2016; 16: 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015; 373: 288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirumani SH, Ramaiya NH, Keraliya, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015; 3: 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remy-Jardin M, Remy J, Wallaert B, et al. Subacute and chronic bird breeder hypersensitivity pneumonitis: sequential evaluation with CT and correlation with lung function tests and bronchoalveolar lavage. Radiology 1993; 189: 111–18. [DOI] [PubMed] [Google Scholar]
- 37.King TE Jr, Mortenson RL. Cryptogenic organizing pneumonitis. The North American experience. Chest 1992; 102: 8S–13S. [PubMed] [Google Scholar]
- 38.Franzen D, Schaz K, Kowalski B, et al. Ipilimumab and early signs of pulmonary toxicity in patients with metastastic melanoma: a prospective observational study. Cancer Immunol Immunother 2018; 67: 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist 2017; 22: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv119–42. [DOI] [PubMed] [Google Scholar]
- 41.Barjaktarevic IZ, Qadir N, Suri A, Santamauro JT, Stover D. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013; 143: 858–61. [DOI] [PubMed] [Google Scholar]
- 42.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer 2016; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–68. [DOI] [PubMed] [Google Scholar]
- 44.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295: 2275–85. [DOI] [PubMed] [Google Scholar]
- 45.Keane J TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology 2005; 44: 714–20. [DOI] [PubMed] [Google Scholar]
- 46.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–68. [DOI] [PubMed] [Google Scholar]
- 47.Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018; 29: 250–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 2017; 35: 3807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rübe CE, Palm J, Erren M, et al. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS One 2008; 3: e2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stenmark MH, Cai XW, Shedden K, et al. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2012; 84: e217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi H, Imai Y, Fujishima T, et al. Diagnostic significance of surfactant proteins A and D in sera from patients with radiation pneumonitis. Eur Respir J 2001; 17: 481–87. [DOI] [PubMed] [Google Scholar]
- 53.Prokunina L, Padyukov L, Bennet A, et al. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum 2004; 50: 1770–73. [DOI] [PubMed] [Google Scholar]
- 54.Nishino M, Ramaiya NH, Hatabu H, Hodi FS, Armand PF. PD-1 inhibitor-related pneumonitis in lymphoma patients treated with single-agent pembrolizumab therapy. Br J Haematol 2018; 180: 752–55. [DOI] [PubMed] [Google Scholar]
- 55.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


