Abstract
Behavior phenotypes are a powerful means of uncovering subtle xenobiotic chemical impacts on vertebrate nervous system development. Rodents manifest complex and informative behavior phenotypes but are generally not practical models in which to screen large numbers of chemicals. Zebrafish recapitulate much of the behavioral complexity of higher vertebrates, develop externally and are amenable to assay automation. Short duration automated assays can be leveraged to screen large numbers of chemicals or comprehensive dose-response for fewer chemicals. Here we describe a series of mostly automated assays including larval photomotor response, strobe light response, blue color avoidance, shoaling and mirror stimulus-response performed on the ZebraBox (ViewPoint Behavior Technologies) instrument platform. To explore the sensitivity and uniqueness of each assay endpoint, larval cohorts from 5 – 28 days post fertilization were acutely exposed to several chemicals broadly understood to impact different neuro-activities. We highlight the throughput advantages of using the same instrument platform for multiple assays and the ability of different assays to detect unique phenotypes among different chemicals.
Keywords: Behavioral paradigm, Zebrafish, Neurobehavioral, Rapid throughput screening
1. Introduction
Xenobiotic chemicals can affect human brain development and cause lasting neurobehavioral deficits that manifest as hyperactivity, anxiety, agitation, anti-social related schizophrenia, attention deficit hyperactive disorder and autism spectrum disorders [1–3]. Rodents have been the gold standard for modeling these effects in open field, T-maze and marble-burying tests [4, 5]. However, behavioral phenotyping in adult rodents is slow when considering the myriad chemicals for which no such hazard data exists, and studying impacts on behavior in developmental stage rodents is generally impractical.
The developmental model organism zebrafish offers sensitivity and throughput advantages unique among vertebrate models. It has genetic homology and brain anatomy similar to mammals [6–9]. Adult zebrafish have been used to study anxiety, learning, and social behaviors. The first automated behavioral tests were developed for adult zebrafish learning and memory in a self-contained shuttlebox [10, 11]. Higher throughput phenotyping of more rudimentary behaviors in larval zebrafish is practical in a 96 well plate format [12]. Zebrafish larvae are free-swimming by 5 days post fertilization (dpf) and exhibit hunting, escape and avoidance behaviors during development [13, 14]. Zebrafish exhibit a predictable photomotor response to rapid light/dark transition [15–17] and exhibit possible schizophrenia-like and anxiety-like behavior after strobe light induction at 5 dpf [18, 19]. These behaviors can be captured from 96 animals at once with an automated imaging and motion tracking system. At 15 dpf, zebrafish larvae exhibit shoaling behavior [20, 21] and at 21 dpf, they display active avoidance [22]. Later developmental stage behaviors clearly have a social component and can be captured from 8 or 24-well plates using the same automated imaging and motion tracking platforms used for earlier photomotor behaviors.
Larval zebrafish is more amendable to rapid-throughput testing than adults and the developing nervous system is more sensitive to chemical insults than that of adults. The tradeoff is less complex behaviors in larvae, relative to adults. Still, we hypothesized that larval zebrafish would display fundamental motor and social behaviors in a variety of assays sensitive enough to detect chemical perturbation of the nervous system. Specifically, we developed a suite of novel testing apparati that fit within the standard microtiter plate footprint of commercial tracking systems to determine the potential of larvae to exhibit aspects of social behavior in median throughput assays.
2. Materials and methods
2.1. Zebrafish husbandry
Wildtype Tropical 5D zebrafish were housed at Sinnhuber Aquatic Research laboratory (SARL), at Oregon State University (Corvallis, OR, US) according to Institutional Animal Care and Use Committee (IACUC) protocols. The fish were raised in 50 or 100-gallon tanks at a density of 500 or 1000 respectively, under 14:10h light/dark cycle with 28 °C recirculating fish water. The fish water consists of reverse osmosis water supplemented with a commercially available salt (Instant Ocean) and kept at a salinity of 600 micro siemens, with a pH of 7.4. The fish were fed twice daily with size-appropriate GEMMA Micro (Skretting, Fontaine Les Vervins, France). Spawning funnels were placed into tanks the night prior and embryos were collected between 7 to 10 AM the next day morning and staged as described by Kimmel et al [23].
For larval rearing, embryos at 6 hours post-fertilization (hpf) were sanitized with 5% sodium hypochlorite for 5 min, rinsed in 500 mL of 0.5mg/L methylene blue-treated embryo medium (EM) containing 0.35g sodium thiosulfate, and stored in a 10 cm petri dish until 5 dpf at a density of 1 embryo per mL with a maximum of 50 embryos per dish. The EM (pH = 7.1~7.5) consisted of 15 mM NaCl, 0.5 mM KCl, 1.3 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, and 0.7 mM NaHCO3 according to the zebrafish book [24]. At 5 dpf, abnormal larvae were removed, and the rest were transferred to 2.8 L polycarbonate tanks (~50 larvae per tank) for continuous rearing to 28 dpf. The larvae were fed twice daily with size appropriate GEMMA food (Skretting).
2.2. Chemicals
Validation chemicals include GABA receptor antagonist picrotoxin (CAS 124-87-8), NMDA antagonist eliprodil (CAS 119431-25-3), ketamine (CAS 1867-66-9), nicotine (CAS 54-11-5), and selective serotonin reuptake inhibitor fluoxetine (CAS 56296-78-7). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of picrotoxin (100 mM) and eliprodil (5 mM) were prepared in dimethyl sulfoxide (DMSO) with a final DMSO test concentration of 0.1%. The stock solution of ketamine (218.7 μM) and fluoxetine (280 μM) was made in fish water. The stock solution of nicotine (246 μM) was prepared on the day of assay.
2.3. Larval photomotor response (5 dpf)
A 96-well round-bottom plate (Falcon, #353227) with singulated 5 dpf fish in 200 μL was used for this assay (Fig. 1). For exposure, 2X working solutions of 25 μM picrotoxin or 2 μM eliprodil were made in EM and 100 μL was added to the wells with 5 dpf larvae in 100 μL EM (N = 24 fish per treatment). Vehicle controls (0.1% DMSO) and treatment groups were included on the same plate to control for plate effects. After an acute exposure of 50 min, the plate was placed directly into the ZebraBox (ViewPoint Behavior Technology, Lyon, FR), an automated observation and tracking system for zebrafish behavior analysis, and assayed for larval photomotor response, which consisted of one cycle of 1 min dark and 1 min constant light (1000 ± 8 Lux). Total distance (mm) traveled for every 6 s was tabulated for both the dark and light periods. Freeze index was calculated by subtracting total distance traveled in 1 min in the dark from total distance traveled in the light for each individual fish according to an earlier study [19]. A freeze index < 0 indicates freeze response, a freeze index > 0 indicates escape response, and a freeze index = 0 indicates no response. Boh embryo medium plates and exposure plates were repeated 3 times to confirm behavior response.
Figure 1. An overview of zebrafish larval behavior assays.
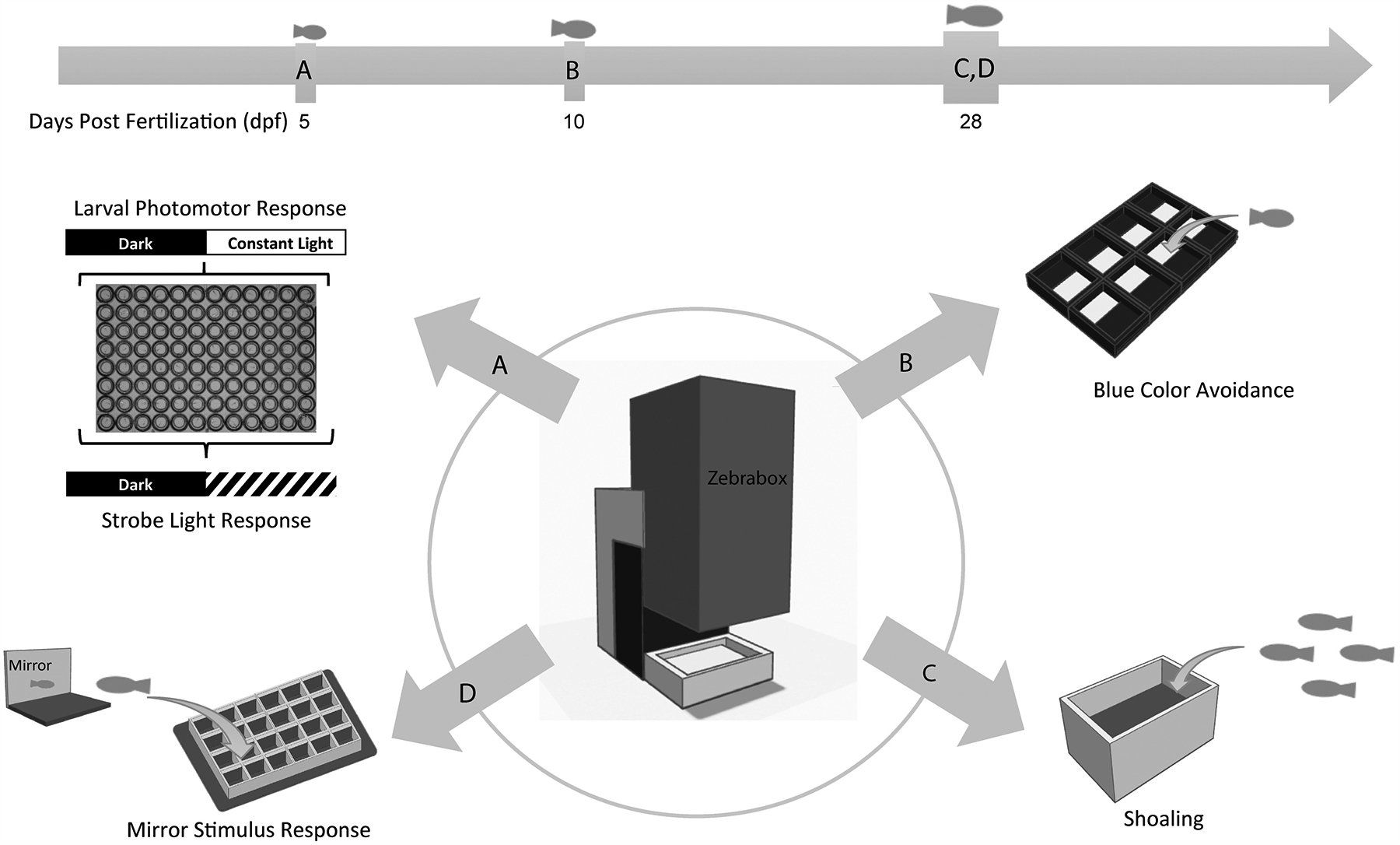
(A) Larval photomotor response and strobe light response were assessed at 5 days post fertilization (dpf) in a 96-well plate. (B) Blue color avoidance test the at 10 dpf in a customized apparatus. (C, D) Shoaling and mirror stimulus response were assessed at 28 dpf in customized apparatus.
2.4. Strobe light response (5 dpf)
Immediately after the larval photomotor response, the same plate was incubated in the dark for 10 min, and followed by a 2 min strobe light response assay. This assay was modified from Rennekamp [19] to create a medium intensity (1000 Lux) strobe-effect at 10 Hz. The assay consisted of one cycle of 1 min dark and 1 min strobe light. The total distance and freeze index was calculated as described for the larval photomotor response.
2.5. Blue color avoidance (10 dpf)
The apparatus used for the blue color avoidance test is an 8-chambered microtiter plate (84 mm × 124 mm; Fig. 1). It was custom-designed (Fusion 360; Tinkercad; https://www.tinkercad.com) and 3D printed (Prusa i3 MK3S; Prusa Research) with a food-grade polymer polylactic acid or PLA (Hatchbox, US; eSun, China). Each chamber is rectangular with a dimension of 31 mm (L) × 42 mm (W) × 18 mm (H). The chamber array was joined to a clear 2-mm thick polycarbonate base with aquarium silicone. Half of the open field in each chamber was designated as blue zone and the other half was designed as white zone with visible light. The blue zone was created with a blue photographic film (IR- transparent Congo blue filter, Roscolux #384 Midnight Blue Gel Filter), which blocked most of the visible component of the ZebraBox lighting but little of the infrared light (IR) component used for tracking. A control plate without a blue film was used to assess the background spatial preference of treated larvae in the open field.
For exposure, larvae at 10 dpf were placed into a mesh insert (34 mm in diameter) at a density of 4 fish/strainer, that was inserted into an individual well of a standard 6-well plate prefilled with 15 mL fish water containing 25 μM picrotoxin or 24.6 μM nicotine. Larvae were exposed to picrotoxin for 20 min and nicotine for 30 s (N = 32 per treatment). Control groups include 0.1% DMSO for picrotoxin and freshwater for nicotine. These exposure concentrations were selected based on earlier findings that picrotoxin at 25 μM for more than 30 min, or nicotine at 24.6 μM for 3 min, affected zebrafish locomotion [25, 26]. Exposure durations were optimized with preliminary testing to ensure no gross malformation or behavioral anomalies such as twitching or sedation. Following the exposure, the mesh inserts were transferred to a 6-well wash plate filled with fish water for 10 s. Immediately following the wash, larvae were transferred into the customized 8-chamber apparatus at 1 fish per well in 5 mL of fish water for open field or blue color open-field testing. The plate was immediately placed into the ZebraBox and larvae were acclimated in the dark for 5 min followed by 5 min free swimming in the light with both the visual identification system and IR lights on. Percent time of larvae swimming in the blue vs. white zones was calculated to assess the tendency of blue color avoidance. Each mesh insert with 4 fish was considered a biological replicate, and for each treatment, there were 3 biological replicates.
2.6. Shoaling behavior assay (28 dpf)
The apparatus used for the shoaling behavior assay is a single chamber with dimensions of 128 mm (L) × 86 mm (W) × 43 mm (H) (Fig. 1). The four walls of the chamber were 3D printed with white PLA to minimize reflection and the bottom of the chamber was 3D printed with translucent PLA to allow IR light passing for motion tracking in the ZebraBox. Larvae at 28 dpf were exposed to 24.6 μM nicotine for 30 s or 72.9 μM ketamine for 6 min as well as their respective vehicle controls. The exposure procedure was sam e as those described for the blue color avoidance test. Immediately after exposure and wash, four fish (N = 10 replicate mesh insert with 4 fish; per treatment) were transferred into the testing chamber containing 50 mL fish water, and their swim activities in the light were recorded for 5 min. The nearest neighbor distance (NND) and inter-individual distance (IID) were calculated to assess the shoaling behavior. The swim speed was recorded to assess motor function.
2.7. Mirror stimulus response (28 dpf)
The apparatus used for mirror stimulus-response was a custom 24-chamber array (102 mm (L) × 68 mm (W); Fig.1) adhered to a 2-mm thick clear acrylic base with a standard microtiter plate footprint. The individual wells were 17 mm squares (Fig. 1), 3D printed from white PLA. A slot (2 mm width) was incorporated on each side of the chamber well to allow easy insertion and removal of the 2 mm thick acrylic mirrors. Flipping the mirror produced a no-mirror control well. Removability of the mirror allowed random allocation of mirror vs. control wells in different columns and rows, minimizing possible column or row effect. Larvae at 14, 21, and 28 dpf were tested to assess which stage exhibited the least inter-individual variation in mirror response. Each treatment was run 6 per plate, with 4 replicate runs for a total of N = 24.
For chemical testing, larvae at 28 dpf were exposed to 72.9 μM ketamine for 6 min or 2.8 μM fluoxetine for 20 min, as well as their respective vehicle controls (N = 24 for each treatment). The exposure procedure was the same as described for the blue color avoidance test. Immediately after exposure and wash, larvae were transferred into the custom 24-chamber apparatus, at 1 fish per well, in 2 mL of fish water for mirror response assessment. The plate was placed into the ZebraBox and larvae were acclimated in the dark for 5 min followed by 5 min swim activity in the light with both the visible and IR lights on. The mirror zone was defined as a rectangular area extending 3 mm out from the mirror and running the length of the mirror. Percent time and total distance (mm) traveled in the mirror zone were calculated by subtraction from the assay totals as a measure of conspecific preference or social tendency. Each plate included 12 mirror and 12 control wells. The assay was replicated twice per chemical treatment (Fig.7A) and the data from the two plates were averaged.
Figure 7. Validation of mirror stimulus response with acute exposure to ketamine.
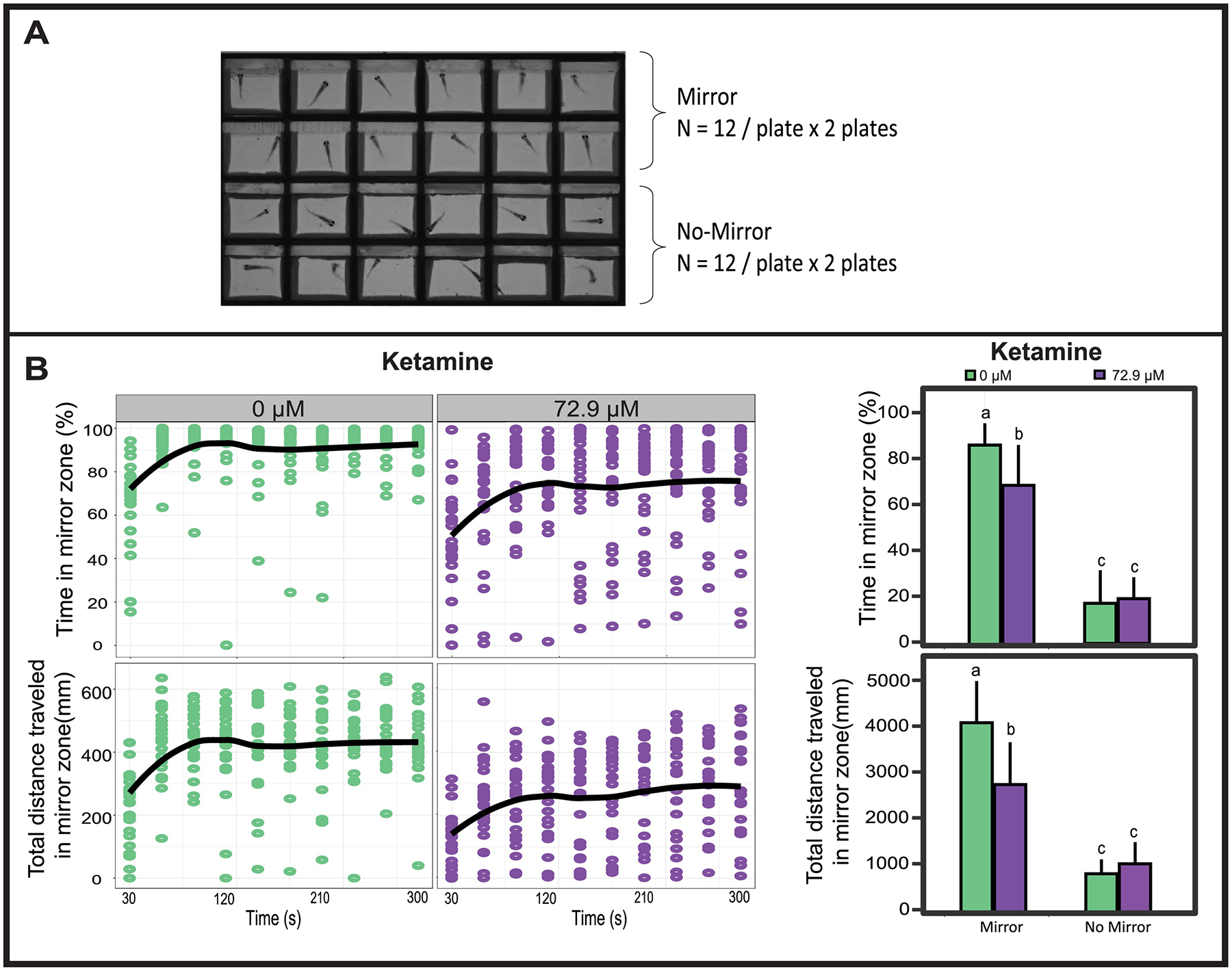
(A) Mirror stimulus response was assessed in a 24-well apparatus (one fish per well). Wells in the top two rows had mirrors and the wells in bottom two rows had no mirrors (mirror was flipped to expose the non-reflective side). (B) Zebrafish at 28 dpf were exposed to vehicle control (0 μM) or 72.9 μM ketamine for 6 min, and data were plotted as % time/total distance traveled in mirror zone over time (dot/line graph) or mean ± SD (bar graph) over the entire testing period of 5 min. Mirror zone was defined as a rectangle zone 3 mm away from the mirror. Dots represent data points from individual larvae and lines represent mean value per min from N = 24. Bars sharing same lower case letter indicate no statistical significance at P > 0.05 (Two-way ANOVA followed by Tukey’s posthoc comparisons).
2.8. Statistical analyses
All data meet the assumption of normality and homogeneity of variance validated by the Shapiro-Wilk normality test and Levene’s test. Custom R scripts [27] for T-tests were used for pairwise comparisons of two groups, and one or two-way ANOVAs followed by Tukey’s posthoc comparisons were used for comparisons of more than two treatment groups. The confidence level of P<0.05 was used for significance.
3. Results and discussion
3.1. Larval photomotor response and strobe light response
Zebrafish was previously used to model aspects of schizophrenic anxiety and seizure-like behavior, manifest as heightened escape behavior and a mixture of circular, erratic and rapid swimming movements[28–30]. The larval photomotor response was one of the assays used to assess motor function and escape behavior. Measurements of chemical impact on the zebrafish larval photomotor response are by no means standardized, but they typically employ a lights-on versus lights-off metric of swimming activity [12, 16, 17, 31]. Recently, strobe light (10 Hz) stimulation was shown to elicit freeze responses and high cortisol levels in zebrafish larvae [19]. We performed the larval photomotor response and the strobe light assays on the same platform with simple ZebraBox software configurations that involved no additional instrumentation or animal handling (Fig. 2). Control larvae at 5 dpf typically displayed high activity in the dark and low activity in the light (constant or strobe light) as well as negative freeze indices (Fig. 2).
Figure 2. Larval photomotor response and strobe light response (5 dpf).
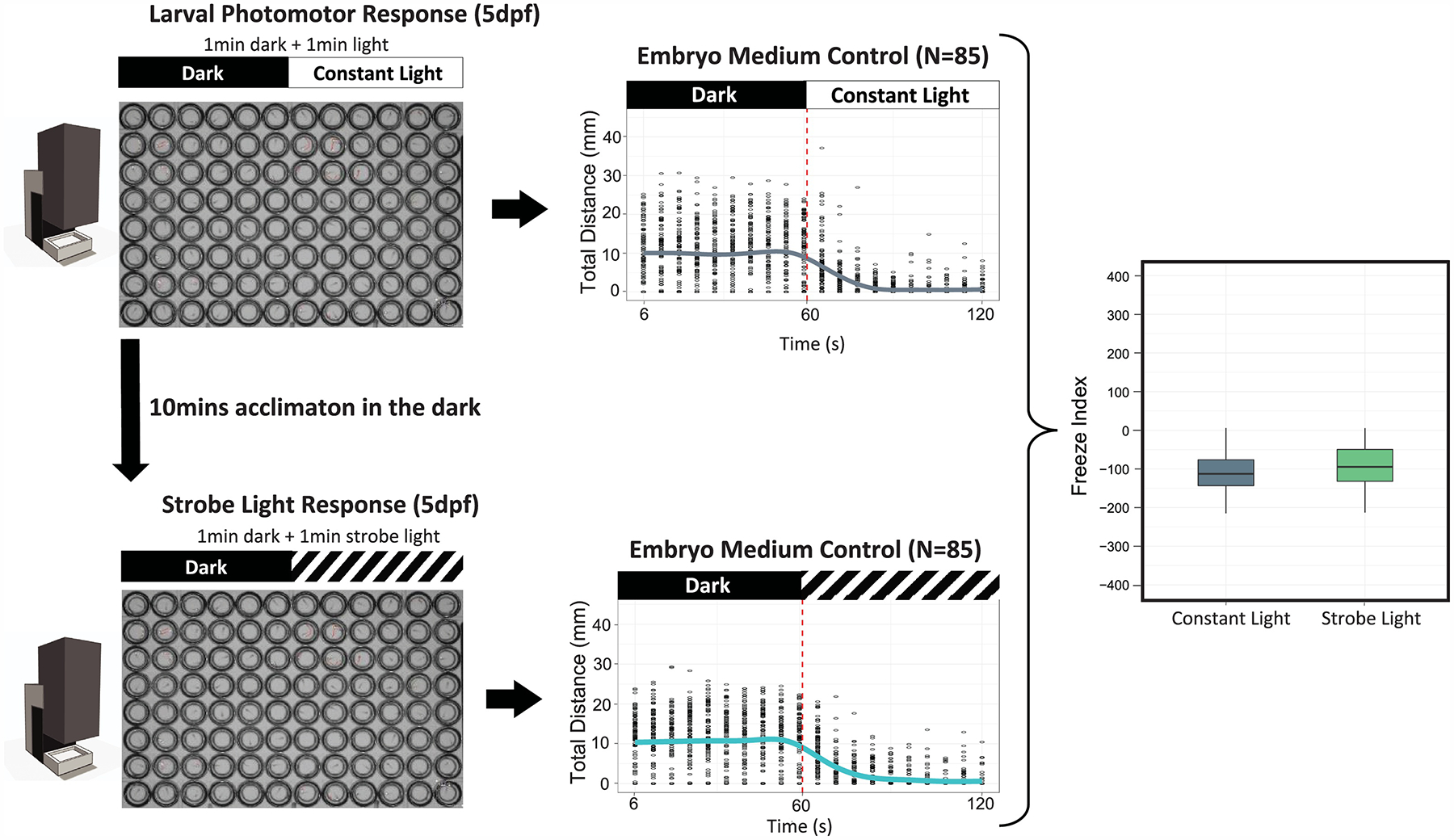
Zebrafish larvae at 5 dpf were transferred into a 96-well plate (one fish/well) and the plate was placed in the Viewpoint Zebrabox. Larvae were first exposed to 1 min dark/1 min constant light phase (larval phtomotor response), and then acclimated in the dark for 10 min, and followed by 1 min dark/1 min strobe light phase (strobe light response). Total distance (mean value per 6 s) traveled was plotted in the 2-min period for both assays (dots represent individual larvae; lines represent the mean value of all tested larvae). Freeze index was calculated by subtracting total distance traveled in 1 min in the dark from total distance traveled in the light. Freeze indices that were negative, positive, or zero indicated freeze response, escape response, and no response, respectively. Representative control data from one 96-well plate are shown in the graph (N = 85 after removal of dead or malformed larvae). Control larvae at 5 dpf typically displayed high activity in the dark and low activity in the light (constant or strobe light), and thus resulted negative freeze indices. No significant difference was found in freeze index between constant and strobe light (P > 0.05, T-test).
Picrotoxin and eliprodil have been shown to cause seizure or schizophrenia-like responses in zebrafish and rats [25, 28, 32]. In the present study, picrotoxin exposed larvae exhibited abnormal hyperactivity in the light phase with a more pronounced effect found in strobe light than constant light, while eliprodil exposed larvae exhibited hypoactivity in the dark and almost no response to light stimulation in both assays (Fig. 3A). While control larvae displayed a typical freeze response (freeze index < 0), picrotoxin exposed larvae displayed escape activity (freeze index > 0) in the strobe light and eliprodil exposed larvae behaved similar to the control animals in the dark (freeze index ~0) in both constant and strobe light regimens (Fig. 3B). A comparison of the strobe to constant photomotor response suggested that, while the strobe better detected picrotoxin-specific escape activity, the constant photomotor response assay also detected constant light phase hyperactivity more analogous to an escape response. We cannot conclude that the strobe assay detected bioactivity that was not also indicated by the photomotor response assay, however, the strobe light did elicit larger changes in the freeze index for both picrotoxin and eliprodil than did constant light. We only sampled 2 chemicals in this study, so it is likely that a larger query of chemicals with strobe versus constant light would uncover bioactivity specific to either assay. Performing the constant and strobe light photomotor assays on the same plate was an obvious throughput and probable data quality advantage as no additional animal handling was required. The 96-well platform in our study also provided 4x higher throughput than the 24-well platform for the larval photomotor response [33].
Figure 3. Validation of larval constant light photomotor response and strobe light response with acute exposure to picrotoxin or eliprodil.
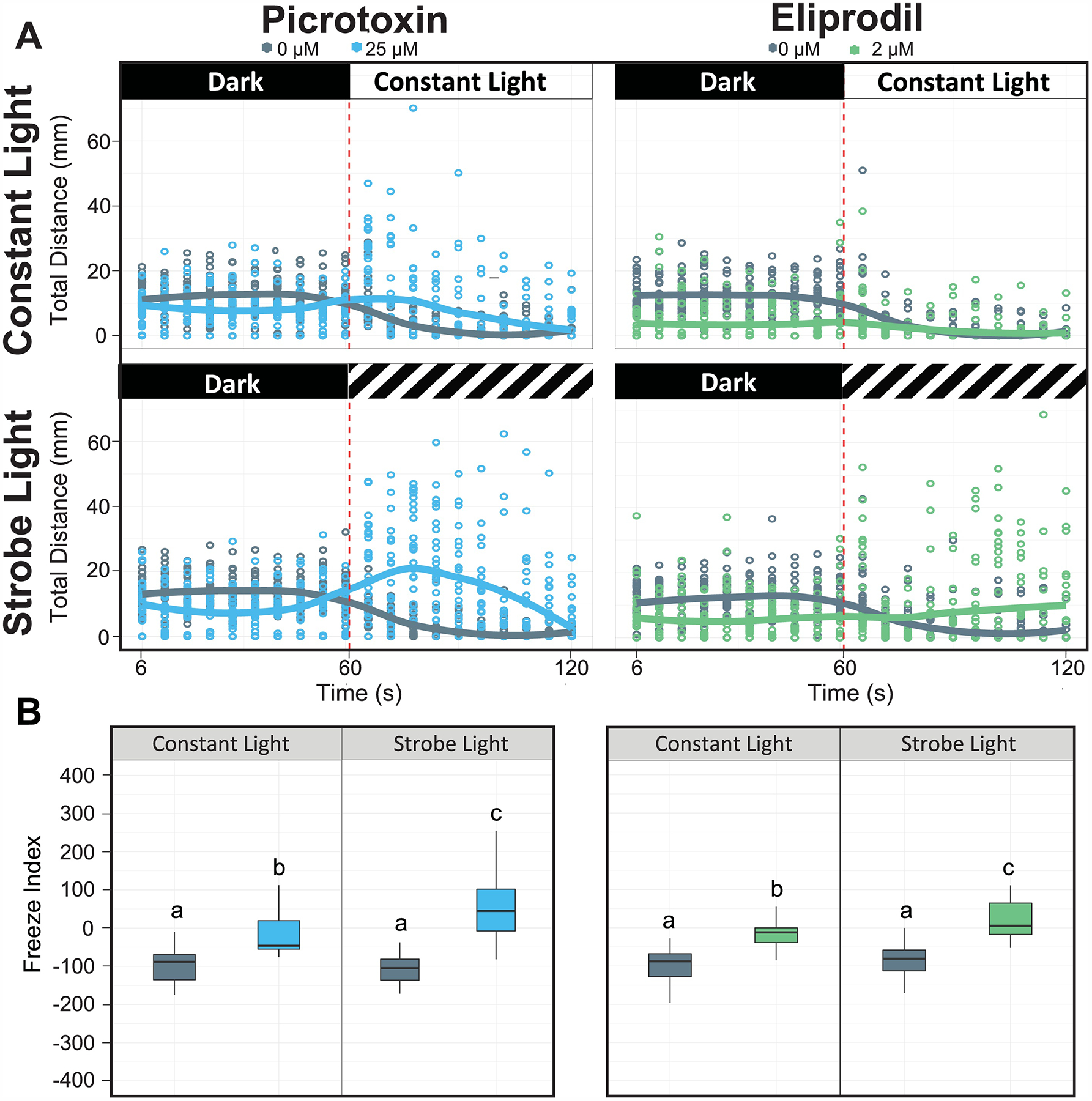
Zebrafish larvae at 5dpf were exposed to 25 μM picrotoxin (blue line) or 2 μM eliprodil (green line) for 50 min as well as their corresponding vehicle controls (0 μM) in the 96-well plates. Data were plotted as total distance traveled (mm) over time (A: larval photomotor response and strobe light response) or freeze index (B). Dots represent data points from individual larvae and lines represent the mean value of distance travelled from N = 24. Bars sharing the same lower case letter indicate no significant difference from the control group at P > 0.05 (Two-way ANOVA followed by Tukey’s posthoc comparisons).
3.2. Blue color avoidance
Previous studies demonstrated that larval zebrafish discriminate colors, with clear preferences, by 6 dpf [34–37]. Larval zebrafish most strongly avoided blue colored zones in a previous study [34]. We sought to use color discrimination as a test of chemical bioactivity during development. In the open field (white) test under visible light, 10 dpf control larvae spent approximately equal amounts of time (48% vs. 52%) in each half of the testing chamber, showing no spatial preference (Fig. 4A). But in the blue color field test, 10 dpf control larvae spent 77% of their time in the white zone (visible light) and only 23% of their time in the blue zone (Supplementary Video 1), confirming the previous report of blue avoidance by larvae [34] (Fig. 4B). Findings in this blue color avoidance test are similar to those found in light/dark preference test where zebrafish larvae exhibited a natural aversion to shaded areas (dark zone) in a novel environment [18].
Figure 4. Blue color avoidance test (10 dpf) and validation in acute exposure to picrotoxin or nicotine.
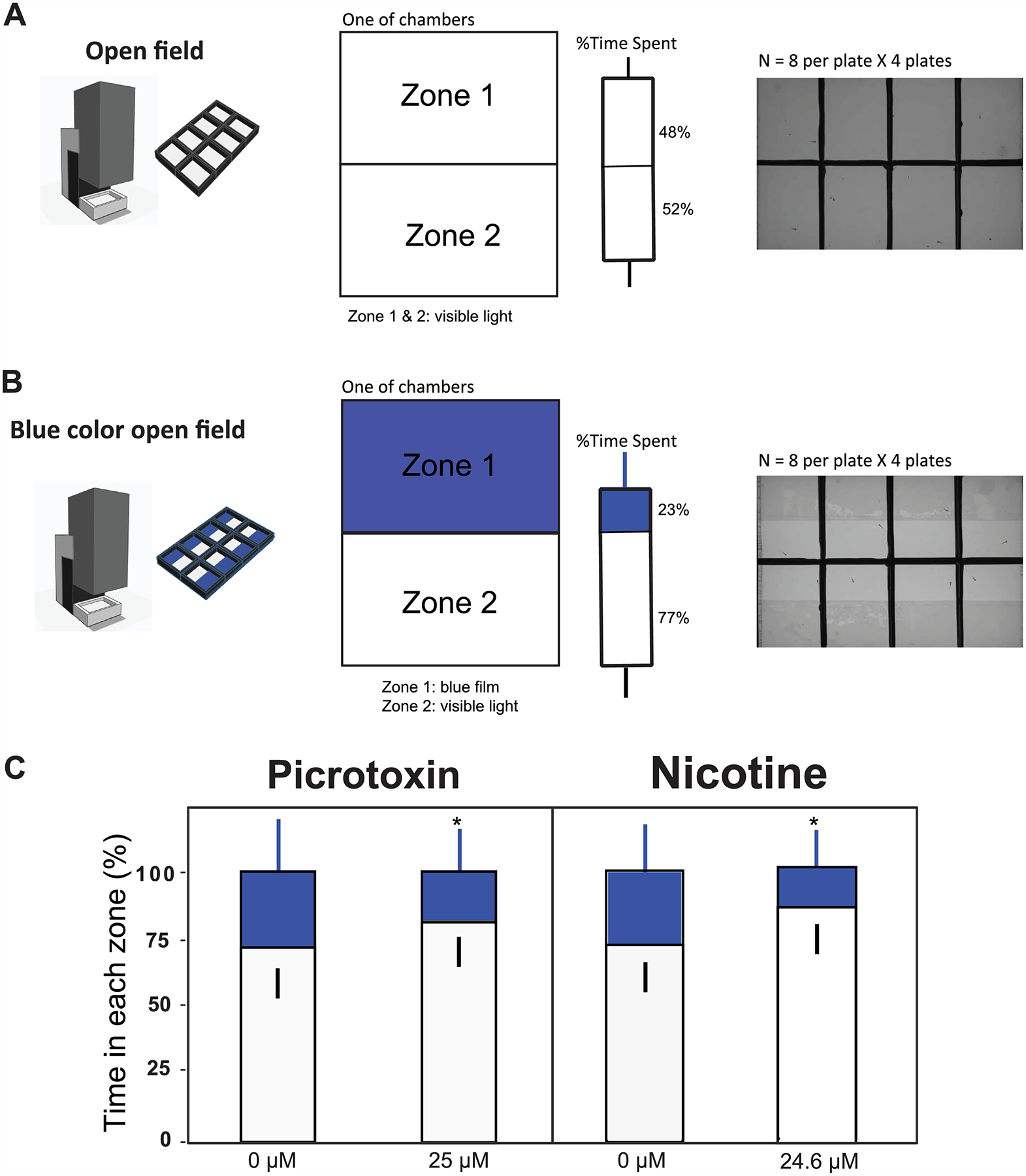
(A) Diagram of open field test split into two zones under visible light in a customized 8-well chamber (84 mm × 124 mm). The test was run for 5 min after 5-min acclimation in the dark, and percent time spend in each half of the open field was calculated. Bars showing equal preference of zone 1 and zone 2 in control larvae (N = 32). (B) Diagram of blue color field test with zone 1 covered by blue film that allowed only blue light to penetrate (blue color) and zone 2 with visible light. Bars showing preference of white light (blue color avoidance) in control larvae (N = 32). (C) Zebrafish at 10 dpf were exposed to 25 μM picrotoxin for 20 min or 24.6 μM nicotine for 30 s as well as their corresponding vehicle controls (0 μM). Values plotted are mean ± SD (N = 28). The asterisk indicates statistical significance at P < 0.05 (T-test).
Picrotoxin and nicotine have been widely shown to modulate animal locomotor activities in the open field test. A prior report that treated larval zebrafish with 125 μM or 625 μM picrotoxin demonstrated that the animals (ave. length 5 mm) spent less time in the center of 22 mm diameter wells of 24 well plate [25]. Elsewhere, acute exposure of adult zebrafish to 62 μM nicotine was associated with a reduced tendency to dive to the bottom and display thigmotaxis upon introduction to a novel tank environment [38]. In another study, acute exposure of adult zebrafish to 6.2 μM nicotine was associated with increased average duration in the lighted area of a light/dark preference test [36]. In adult rats, acute exposure to 0.06 mg/kg nicotine was associated with an increased duration of open-field center entries [39]. In our tests, acute exposure of 10 dpf larvae to 25 μM picrotoxin or 24.6 μM nicotine enhanced blue color avoidance compared to their respective vehicle control animals (Fig. 4C). The results suggested that the blue color field test was sufficiently sensitive to detect drug modulation of locomotor activity in zebrafish larvae. Our customized well plate permitted simultaneous assessment of 8 fish per test, with a side-by-side comparison of different treatment groups and more rapid screening than a traditional one chamber apparatus [18, 40].
3.3. Shoaling behavior
Zebrafish shoaling behavior, in which a group of fish remains in close proximity to each other for social reasons, has been used to model autism-like features in humans [41–44]. The two basic metrics indicating shoaling dynamics are the nearest-neighbor distance (NND) and inter-individual distance (IID) where NND refers to the mean distance between an individual and nearest fish in the group and IID refers to the mean distance between an individual and all other members of the shoal (Fig. 5A). Social behavior phenotypes are robust in adult zebrafish, but if we could assay them at the larval stage (up to 28 dpf), the throughput and sensitivity advantages of developmental zebrafish would greatly accelerate our ability to query chemical space for modulators of social behavior. In this study, we demonstrated that shoaling behavior in larval zebrafish was robust enough to be a readout for chemical perturbation of social behavior (Supplementary Video 2).
Figure 5. Shoaling behavior and validation with acute exposure to nicotine or ketamine.
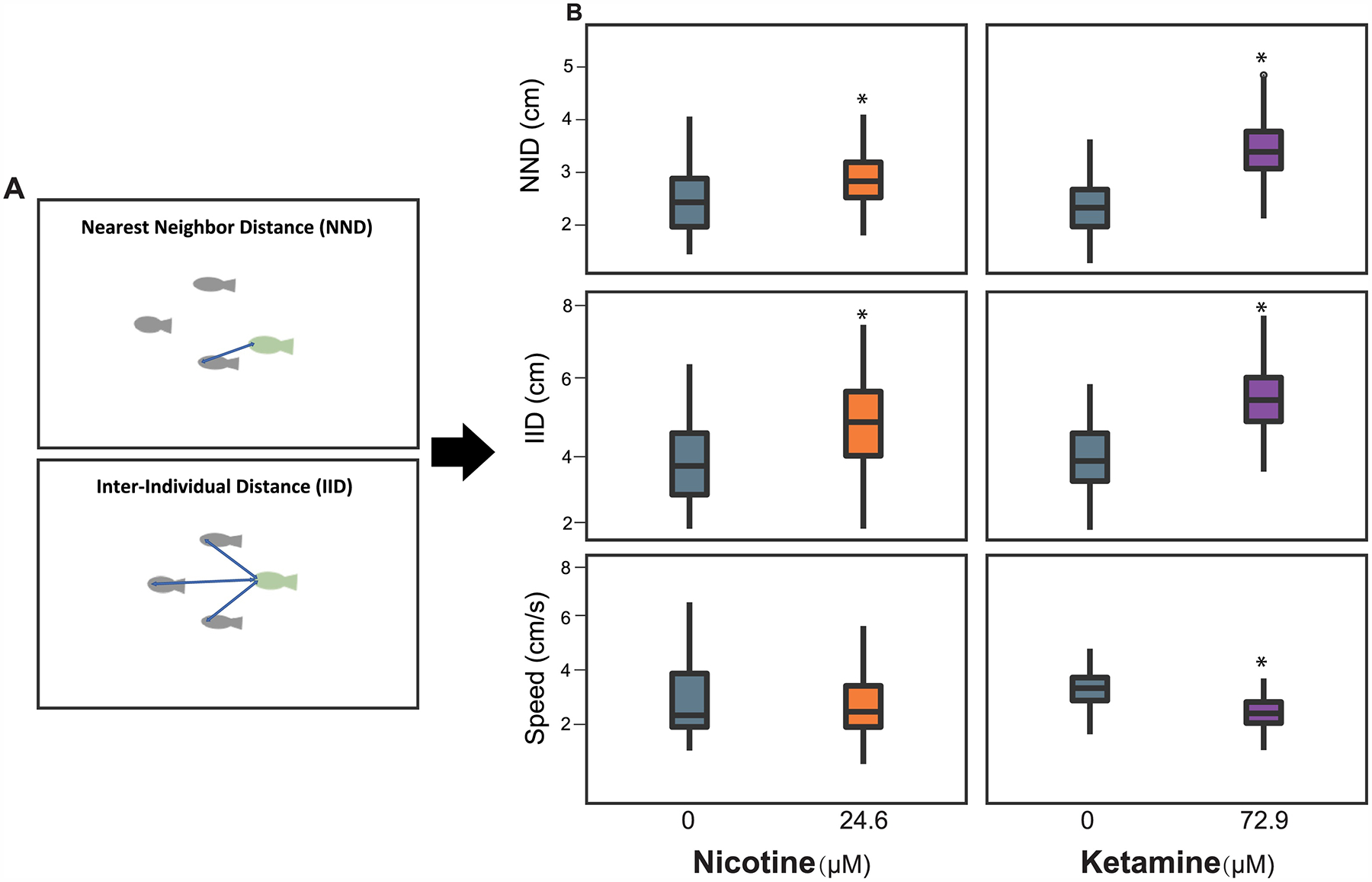
(A) Shoaling was assessed with a school of 4 fish in a tank containing 50 ml fish water. Endpoints included nearest neighbor distance (NND), inter-individual distance (IID), and swimming speed (cm/s). (B) Zebrafish at 28dpf were exposed to 24.6 μM nicotine for 30 s or 72.9 μM ketamine for 6 min as well as their corresponding vehicle controls (0 μM). Values plotted are mean ± SD (N = 10 tests with each test includes 4 fish). Asterisk indicates statistical significance at P < 0.05 (T-test).
Nicotine and ketamine are known to impair social behavior in rodents. For example, Sprague-Dawley rats exposed to 0.1 – 0.6 mg/kg nicotine for 3 weeks displayed reduced social interaction [45, 46]. Acute low dose ketamine (7 mg/kg) decreased the amount of time that adult male Wistar rats spent in social interaction [47]. Short-term exposure of juvenile mice to 20 mg/kg ketamine for 14 days or male Wistar rats to 25 mg/kg ketamine for 7 days severely reduced social interaction [48, 49]. In our assay, acute exposure of 28 dpf zebrafish larvae to 24.6 μM nicotine or 72.9 μM ketamine increased NND and IID (Fig. 5B), indicating reduced shoaling behavior. Average swim speed was depressed by exposure to ketamine, but not nicotine (Fig. 5B). These findings are consistent with previous zebrafish research where nicotine and ketamine exposure was also associated with larger NND and IID [26, 50]. While both drugs were associated with less cohesive shoaling, their mechanisms may be different. Nicotine is clearly anxiolytic in adult zebrafish and may act similarly in larvae [38]. A modest reduction in anxiety likely increases the desire of zebrafish to explore their environment and forego the perceived safety of a tighter group. Ketamine is also anxiolytic in zebrafish, but as a glutamatergic antagonist, it has a hallucinogenic and deeply sedative effect in humans [51, 52]. In adult zebrafish ketamine exposure has been associated with circling swim behavior and disregard for social interaction [50, 53]. The modest anxiolytic effects in our study were also predictably associated with a decreased average swim speed of the test group (Fig. 5B). Reliable shoaling behavior detected at 28 dpf (Supplementary Video 2) permitted early assessment of social behavior in juvenile stages rather than 3 months later as adults [26, 54].
3.4. Mirror stimulus response
In contrast to shoaling, a potentially simpler form of zebrafish social behavior can be assayed as an individual’s response to its reflection in a mirror[55–57]. Adult zebrafish typically respond to their own reflection with frequent approaches to the mirror, especially in a novel environment [42, 55, 57]. In our mirror stimulus-response, swim distance and percent time spent within a 3 mm wide arena along the mirror were tracked and recorded. The narrow arena, though not a standardized metric, bounded what we and others have regarded as proximity indicative of engagement with the reflection [55]. We conducted this assay for larvae at 14, 21, and 28 dpf. The average percent time and swim distance in the mirror zone increased with larval age (Fig. 6). The majority of 14 dpf zebrafish larvae did not unambiguously engage their reflection, whereas 21 and 28 dpf larvae spent 75% and 90% of their time, respectively, in the mirror zone over the first 90 s of the assay (Fig. 6). The variation in average percent time in the mirror zone was highest for 14 dpf larvae, revealing that some individuals at this age did not engage their reflection. The variation in average swim distance in the mirror zone was highest for the 21 dpf larvae, which might indicate conspecific preference attenuated by a less developed attention span[58]. The 28 dpf larvae were most appropriate for this assay, demonstrating the most consistent engagement behavior in the mirror arena (Fig. 6 and Supplementary Video 3).
Figure 6. Mirror stimulus response at different zebrafish larval ages.
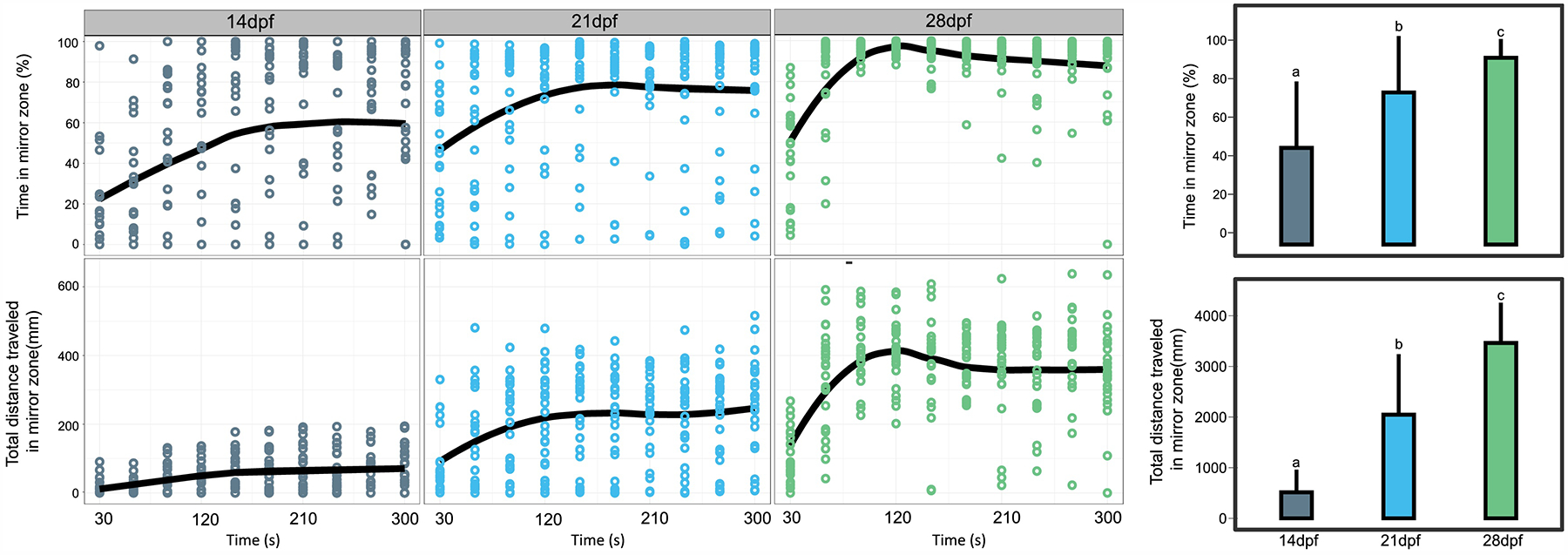
Zebrafish at 14, 21, and 28 dpf were tested in the mirror stimulus response. Percent time and total distance traveled in the mirror zone was recorded every 30 s for a total of 5 mins. Dots represent data points from individual larvae and lines represent mean value per min from N = 24. Bar graphs show average percent time/total distance (mean ± SD) traveled in mirror zone over the entire testing period of 5 min (N = 24). Bars sharing same lower case letter indicate no significant (P > 0.05) difference from the control group (One-way ANOVA followed by Tukey’s posthoc comparisons).
Acute ketamine or fluoxetine exposure has been found to depress social behavior in adult zebrafish [59, 60]. In rodents, peri-adolescent male wild-type rats treated with 10 mg/kg fluoxetine exhibited social behavior deficits in pining, pouncing and following [61]. Fluoxetine treatment (10 mg/kg) also reduced the aggressive behavior of dominant naked mole-rats when they interacted with unfamiliar animals [62]. In our study, 28 dpf larvae displayed distinct engagement responses in wells with a mirror compared wells with no-mirror (Fig. 7A). Acute exposure to 72.9 μM ketamine reduced mirror engagement, manifested as decreased percent time and distance traveled in the mirror zone, though the preference for the mirror arena persisted (Fig. 7B). This finding agrees with the attenuated social behavior found in shoaling for ketamine treated larvae. Acute exposure to 2.8 μM fluoxetine exhibited reduced engagement similar to that of ketamine (data not shown). Our results suggested that this simple and automated mirror response assay is sufficiently robust at the larval stage to detect chemical impacts on social behavior. Compared to previous studies using 1, 2 or 6 chambers [55–57], the 24-chamber array used in our study facilitated rapid comparisons among multiple treatment groups. Although less throughput than motor behavior assays based on 96-well plates [63], our system is a medium-throughput, and scalable social behavior assay.
4. Conclusion
We have presented several automated assays for behavior phenotyping. The approach leveraged throughput and sensitivity advantages of the developmental zebrafish and queried multiple, potentially distinct modalities of neurobehavior. With minimal fabrication techniques, other than low complexity 3D printing, we were able to perform some of our assays in the same plate. More technical approaches could accommodate nearly all of these assays in a single plate format, increasing throughput dramatically and obviating the need to transfer and stress animals. For example, the mirror could be upgraded to an electro-mirror that can be turned on and off by the computer. The same plate could be placed over a computer-controlled video screen to create a light and dark zone in that assay. The same video screen could display a shoal of zebrafish to assay shoaling preference. Fewer, larger wells could be employed at the cost of some throughput to accommodate all the readouts. The important point is that multiple short assays could be done without transferring fish. Pharmacological validation of the sensitivity and modality uniqueness of the behaviors suggested they could be low-dose, non-teratogenic replacements for mortality and malformation endpoints in chemical screens. This would have the dual benefit of reduced animal harm and reduced test chemical consumption.
Supplementary Material
Table 1.
Summary of behavior assays and endpoints.
| Life Stage (dpf) | Behavior Assay | Behavior Readout | Potential for behavior assay in the same plate |
|---|---|---|---|
| 5 | Larval photomotor response | Travel distance in light Travel distance in the dark Freeze index* |
Strobe light response |
| 5 | Strobe light response | Travel distance in light Travel distance in the dark Freeze index* |
Larval photomotor response |
| 10 | Blue color avoidance | % Time in blue color zone % Time in visible light zone |
Open field test; Environmental complexity test (multiple colors) |
| 28 | Shoaling behavior | Nearest neighbor distance
(NND) Inter-individual animal (IID) Speed |
Conspecific preference test |
| 28 | Mirror stimulus response | % Time in mirror zone Travel distance in mirror zone |
Visual test; Electro-mirror |
Freeze index= total travel distance in one min in the light – total travel distance in one min in the dark
Highlights.
Larval zebrafish manifest complex behaviors
Larval behavior assays are amenable to existing automation
Behavior assays can be multiplexed in the same platform (ZebraBox)
Acknowledgments
The authors would like to thank the staff at Sinnhuber Aquatic Research Laboratory for their help and guidance in the zebrafish husbandry, and Wayne Wood from Oregon State University Roots IT Support for his assistance in 3D design and printing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21477089). This research reported in this publication was also supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES016465. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of financial interests
None
References
- [1].Heyer DB, Meredith RM, Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders, Neurotoxicology 58 (2017) 23–41. [DOI] [PubMed] [Google Scholar]
- [2].Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, Kim S, Kim SY, Cho G, Kim YD, Suh E, Kim SK, Kim S, Kim GH, Moon HB, Park J, Kim S, Choi K, Eun SH, Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age-CHECK cohort study, Sci Total Environ 624 (2018) 377–384. [DOI] [PubMed] [Google Scholar]
- [3].Tuomainen U, Candolin U, Behavioural responses to human-induced environmental change, Biol Rev Camb Philos Soc 86(3) (2011) 640–57. [DOI] [PubMed] [Google Scholar]
- [4].Nelson LH, Lenz KM, Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats, Behav Brain Res 316 (2017) 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chang YC, Cole TB, Costa LG, Behavioral Phenotyping for Autism Spectrum Disorders in Mice, Curr Protoc Toxicol 72 (2017) 11 22 1–11 22 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miklosi A, Andrew RJ, The zebrafish as a model for behavioral studies, Zebrafish 3(2) (2006) 227–34. [DOI] [PubMed] [Google Scholar]
- [7].Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV, Zebrafish models for translational neuroscience research: from tank to bedside, Trends Neurosci 37(5) (2014) 264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Becker TS, Rinkwitz S, Zebrafish as a genomics model for human neurological and polygenic disorders, Dev Neurobiol 72(3) (2012) 415–28. [DOI] [PubMed] [Google Scholar]
- [9].Kalueff AV, Stewart AM, Gerlai R, Zebrafish as an emerging model for studying complex brain disorders, Trends Pharmacol Sci 35(2) (2014) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pather S, Gerlai R, Shuttle box learning in zebrafish (Danio rerio), Behav Brain Res 196(2) (2009) 323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Truong L, Mandrell D, Mandrell R, Simonich M, Tanguay RL, A rapid throughput approach identifies cognitive deficits in adult zebrafish from developmental exposure to polybrominated flame retardants, Neurotoxicology 43 (2014) 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL, Multidimensional in vivo hazard assessment using zebrafish, Toxicol Sci 137(1) (2014) 212–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colwill RM, Creton R, Imaging escape and avoidance behavior in zebrafish larvae, Rev Neurosci 22(1) (2011) 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Semmelhack JL, Donovan JC, Thiele TR, Kuehn E, Laurell E, Baier H, A dedicated visual pathway for prey detection in larval zebrafish, Elife 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL, Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles, Comp Biochem Physiol C Toxicol Pharmacol 155(2) (2012) 269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL, Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants, Toxicol Sci 145(1) (2015) 177–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knecht AL, Truong L, Marvel SW, Reif DM, Garcia A, Lu C, Simonich MT, Teeguarden JG, Tanguay RL, Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish, Toxicol Appl Pharmacol 329 (2017) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Steenbergen PJ, Richardson MK, Champagne DL, Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study, Behav Brain Res 222(1) (2011) 15–25. [DOI] [PubMed] [Google Scholar]
- [19].Rennekamp AJ, Huang XP, Wang Y, Patel S, Lorello PJ, Cade L, Gonzales AP, Yeh JR, Caldarone BJ, Roth BL, Kokel D, Peterson RT, sigma1 receptor ligands control a switch between passive and active threat responses, Nat Chem Biol 12(7) (2016) 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hinz RC, de Polavieja GG, Ontogeny of collective behavior reveals a simple attraction rule, Proc Natl Acad Sci U S A 114(9) (2017) 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Buske C, Gerlai R, Shoaling develops with age in Zebrafish (Danio rerio), Prog Neuropsychopharmacol Biol Psychiatry 35(6) (2011) 1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valente A, Huang KH, Portugues R, Engert F, Ontogeny of classical and operant learning behaviors in zebrafish, Learn Mem 19(4) (2012) 170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, Stages of embryonic development of the zebrafish, Dev Dyn 203(3) (1995) 253–310. [DOI] [PubMed] [Google Scholar]
- [24].Westerfield M, The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. [Google Scholar]
- [25].Yang X, Lin J, Peng X, Zhang Q, Zhang Y, Guo N, Zhou S, Li Q, Effects of picrotoxin on zebrafish larvae behaviors: A comparison study with PTZ, Epilepsy Behav 70(Pt A) (2017) 224–231. [DOI] [PubMed] [Google Scholar]
- [26].Miller N, Greene K, Dydinski A, Gerlai R, Effects of nicotine and alcohol on zebrafish (Danio rerio) shoaling, Behav Brain Res 240 (2013) 192–6. [DOI] [PubMed] [Google Scholar]
- [27].R.C. Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2017. [Google Scholar]
- [28].Wong K, Stewart A, Gilder T, Wu N, Frank K, Gaikwad S, Suciu C, Dileo J, Utterback E, Chang K, Grossman L, Cachat J, Kalueff AV, Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish, Brain Res 1348 (2010) 209–15. [DOI] [PubMed] [Google Scholar]
- [29].Mussulini BH, Leite CE, Zenki KC, Moro L, Baggio S, Rico EP, Rosemberg DB, Dias RD, Souza TM, Calcagnotto ME, Campos MM, Battastini AM, de Oliveira DL, Seizures induced by pentylenetetrazole in the adult zebrafish: a detailed behavioral characterization, PLoS One 8(1) (2013) e54515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H, Zebrafish Neuroscience Research C, Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond, Zebrafish 10(1) (2013) 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Padilla S, Hunter DL, Padnos B, Frady S, MacPhail RC, Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables, Neurotoxicol Teratol 33(6) (2011) 624–30. [DOI] [PubMed] [Google Scholar]
- [32].Schwartz-Giblin S, Korotzer A, Pfaff DW, Steroid hormone effects on picrotoxin-induced seizures in female and male rats, Brain Res 476(2) (1989) 240–7. [DOI] [PubMed] [Google Scholar]
- [33].Vignet C, Begout ML, Pean S, Lyphout L, Leguay D, Cousin X, Systematic screening of behavioral responses in two zebrafish strains, Zebrafish 10(3) (2013) 365–75. [DOI] [PubMed] [Google Scholar]
- [34].Ahmad F, Richardson MK, Exploratory behaviour in the open field test adapted for larval zebrafish: impact of environmental complexity, Behav Processes 92 (2013) 88–98. [DOI] [PubMed] [Google Scholar]
- [35].Fleisch VC, Neuhauss SC, Visual behavior in zebrafish, Zebrafish 3(2) (2006) 191–201. [DOI] [PubMed] [Google Scholar]
- [36].Duarte T, Fontana BD, Muller TE, Bertoncello KT, Canzian J, Rosemberg DB, Nicotine prevents anxiety-like behavioral responses in zebrafish, Prog Neuropsychopharmacol Biol Psychiatry 94 (2019) 109655. [DOI] [PubMed] [Google Scholar]
- [37].Easter SS Jr., Nicola GN, The development of vision in the zebrafish (Danio rerio), Dev Biol 180(2) (1996) 646–63. [DOI] [PubMed] [Google Scholar]
- [38].Levin ED, Bencan Z, Cerutti DT, Anxiolytic effects of nicotine in zebrafish, Physiol Behav 90(1) (2007) 54–8. [DOI] [PubMed] [Google Scholar]
- [39].Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A, Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict, Psychopharmacology (Berl) 204(3) (2009) 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kedikian X, Faillace MP, Bernabeu R, Behavioral and molecular analysis of nicotine-conditioned place preference in zebrafish, PLoS One 8(7) (2013) e69453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stewart AM, Nguyen M, Wong K, Poudel MK, Kalueff AV, Developing zebrafish models of autism spectrum disorder (ASD), Prog Neuropsychopharmacol Biol Psychiatry 50 (2014) 27–36. [DOI] [PubMed] [Google Scholar]
- [42].Chen J, Lei L, Tian L, Hou F, Roper C, Ge X, Zhao Y, Chen Y, Dong Q, Tanguay RL, Huang C, Developmental and behavioral alterations in zebrafish embryonically exposed to valproic acid (VPA): An aquatic model for autism, Neurotoxicol Teratol 66 (2018) 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li F, Lin J, Liu X, Li W, Ding Y, Zhang Y, Zhou S, Guo N, Li Q, Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs, Ann Transl Med 6(10) (2018) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maaswinkel H, Le X, He L, Zhu L, Weng W, Dissociating the effects of habituation, black walls, buspirone and ethanol on anxiety-like behavioral responses in shoaling zebrafish. A 3D approach to social behavior, Pharmacol Biochem Behav 108 (2013) 16–27. [DOI] [PubMed] [Google Scholar]
- [45].Aydin C, Oztan O, Isgor C, Long-term effects of juvenile nicotine exposure on abstinence-related social anxiety-like behavior and amygdalar cannabinoid receptor 1 (CB1R) mRNA expression in the novelty-seeking phenotype, Behav Brain Res 228(1) (2012) 236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung TH, Deviche P, Adams M, Alba J, Neisewander JL, Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats, Pharmacol Biochem Behav 100(1) (2011) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Silvestre JS, Nadal R, Pallares M, Ferre N, Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats, Depress Anxiety 5(1) (1997) 29–33. [PubMed] [Google Scholar]
- [48].Nagy LR, Featherstone RE, Hahn CG, Siegel SJ, Delayed emergence of behavioral and electrophysiological effects following juvenile ketamine exposure in mice, Transl Psychiatry 5 (2015) e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gama CS, Canever L, Panizzutti B, Gubert C, Stertz L, Massuda R, Pedrini M, de Lucena DF, Luca RD, Fraga DB, Heylmann AS, Deroza PF, Zugno AI, Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia, Schizophr Res 141(2–3) (2012) 162–7. [DOI] [PubMed] [Google Scholar]
- [50].Riehl R, Kyzar E, Allain A, Green J, Hook M, Monnig L, Rhymes K, Roth A, Pham M, Razavi R, Dileo J, Gaikwad S, Hart P, Kalueff AV, Behavioral and physiological effects of acute ketamine exposure in adult zebrafish, Neurotoxicol Teratol 33(6) (2011) 658–67. [DOI] [PubMed] [Google Scholar]
- [51].Sherwood AM, Prisinzano TE, Novel psychotherapeutics - a cautiously optimistic focus on Hallucinogens, Expert Rev Clin Pharmacol 11(1) (2018) 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Salloum NC, Fava M, Freeman MP, Flynn M, Hoeppner B, Hock RS, Cusin C, Iosifescu DV, Trivedi MH, Sanacora G, Mathew SJ, Debattista C, Ionescu DF, Papakostas GI, Efficacy of intravenous ketamine treatment in anxious versus nonanxious unipolar treatment-resistant depression, Depress Anxiety 36(3) (2019) 235–243. [DOI] [PubMed] [Google Scholar]
- [53].Zakhary SM, Ayubcha D, Ansari F, Kamran K, Karim M, Leheste JR, Horowitz JM, Torres G, A behavioral and molecular analysis of ketamine in zebrafish, Synapse 65(2) (2011) 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shams S, Rihel J, Ortiz JG, Gerlai R, The zebrafish as a promising tool for modeling human brain disorders: A review based upon an IBNS Symposium, Neurosci Biobehav Rev 85 (2018) 176–190. [DOI] [PubMed] [Google Scholar]
- [55].Carreno Gutierrez H, Vacca I, Pons AI, Norton WHJ, Automatic quantification of juvenile zebrafish aggression, J Neurosci Methods 296 (2018) 23–31. [DOI] [PubMed] [Google Scholar]
- [56].Chen J, Tanguay RL, Simonich M, Nie S, Zhao Y, Li L, Bai C, Dong Q, Huang C, Lin K, TBBPA chronic exposure produces sex-specific neurobehavioral and social interaction changes in adult zebrafish, Neurotoxicol Teratol 56 (2016) 9–15. [DOI] [PubMed] [Google Scholar]
- [57].Weber DN, Hoffmann RG, Hoke ES, Tanguay RL, Bisphenol A exposure during early development induces sex-specific changes in adult zebrafish social interactions, J Toxicol Environ Health A 78(1) (2015) 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dreosti E, Lopes G, Kampff AR, Wilson SW, Development of social behavior in young zebrafish, Front Neural Circuits 9 (2015) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Michelotti P, Quadros VA, Pereira ME, Rosemberg DB, Ketamine modulates aggressive behavior in adult zebrafish, Neurosci Lett 684 (2018) 164–168. [DOI] [PubMed] [Google Scholar]
- [60].Giacomini A, Abreu MS, Giacomini LV, Siebel AM, Zimerman FF, Rambo CL, Mocelin R, Bonan CD, Piato AL, Barcellos LJG, Fluoxetine and diazepam acutely modulate stress induced-behavior, Behav Brain Res 296 (2016) 301–310. [DOI] [PubMed] [Google Scholar]
- [61].Homberg JR, Pattij T, Janssen MC, Ronken E, De Boer SF, Schoffelmeer AN, Cuppen E, Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility, Eur J Neurosci 26(7) (2007) 2066–73. [DOI] [PubMed] [Google Scholar]
- [62].Mongillo DL, Kosyachkova EA, Nguyen TM, Holmes MM, Differential effects of chronic fluoxetine on the behavior of dominant and subordinate naked mole-rats, Behav Brain Res 258 (2014) 119–26. [DOI] [PubMed] [Google Scholar]
- [63].Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E, Taylor J, Lakhani P, Lensen D, Evron T, Lorello PJ, Huang XP, Kolczewski S, Carey G, Caldarone BJ, Prinssen E, Roth BL, Keiser MJ, Peterson RT, Kokel D, Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds, Nat Chem Biol 12(7) (2016) 559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


