Abstract
Background
Antibiotic-inappropriate prescribing for acute respiratory tract infections (ARTI) is 45% among urgent care centers (UCCs) in the United States. Locally in our UCCs, antibiotic-inappropriate prescribing for ARTI is higher—over 70%.
Methods
We used a quasi-experimental design to implement 3 behavioral interventions targeting antibiotic-inappropriate/non-guideline-concordant prescribing for ARTI at 3 high-volume rural UCCs and analyzed prescribing rates pre- and post-intervention. The 3 interventions were (1) staff/patient education, (2) public commitment, and (3) peer comparison. For peer comparison, providers were sent feedback emails with their prescribing data during the intervention period and a blinded ranking email comparing them with their peers. Providers were categorized as “low prescribers” (ie, ≤23% antibiotic-inappropriate prescriptions based off the US National Action Plan for Combating Antibiotic Resistant Bacteria 2020 goal) or “high prescribers” (ie, ≥45%—the national average of antibiotic-inappropriate prescribing for ARTI). An interrupted time series (ITS) analysis compared prescribing for ARTI (the primary outcome) over a 16-month period before the intervention and during the 6-month intervention period, for a total of 22 months, across the 3 UCCs.
Results
Fewer antibiotic-inappropriate prescriptions were written during the intervention period (57.7%) compared with the pre-intervention period (72.6%) in the 3 UCCs, resulting in a 14.9% absolute decrease in percentage of antibiotic-inappropriate prescriptions. The ITS analysis revealed that the rate of antibiotic-inappropriate prescribing was statistically significantly different pre-intervention compared with the intervention period (95% confidence interval, –4.59 to –0.59; P = .014).
Conclusions
In this sample of rural UCCs, we reduced antibiotic-inappropriate prescribing for ARTI using 3 behavioral interventions.
Keywords: antibiotic stewardship, antibiotic prescribing, behavioral interventions
Antibiotic resistance (AR) is an urgent public health threat in the United States and globally, affecting all countries regardless of socioeconomic status. One of the primary drivers contributing to the emergence and ongoing persistence of this threat is antibiotic misuse or overuse [1]. Outpatient settings are of particular concern—at least 47 million antibiotic prescriptions each year are unnecessary in the outpatient setting, with the majority being prescribed for respiratory conditions commonly caused by viruses [2].
The US Centers for Disease Control and Prevention (CDC) estimates that 30% of antibiotic prescribing in the outpatient setting is inappropriate, with acute respiratory tract infections (ARTIs) accounting for an even higher percentage (~50%) of nonindicated prescribing among all ages [3]. Palms and colleagues recently published a study comparing antibiotic prescribing in retail clinics, urgent care centers (UCCs), emergency departments (EDs), and office settings in the United States [4]. Their findings underscore the importance of antibiotic stewardship interventions in all ambulatory care settings [5], but specifically, they found that efforts targeting UCCs are urgently needed, as this area had the highest percentage (~45%) of non-guideline-concordant prescribing for ARTI. Other literature also supports this finding [6−8].
The ED and UCC environments are unique settings with high-volume episodic acute care visits, frequent turnover, and the need to make quick clinical decisions with limited information in the face of concerns about patient satisfaction [9]. Use of behavioral economics can improve clinical decision-making by engineering choices. These interventions have many advantages. They are practical and scalable, preserve clinician autonomy by never restricting choice options, and act to improve the quality of medical decisions [10]. Evidence published by Meeker and colleagues demonstrates that behavior-based interventions can reduce inappropriate prescribing for acute respiratory infection by 20% in primary care practices [11]. Preliminary work from the MITIGATE study (A Multifaceted Intervention To Improve PrescribinG for Acute RespiraTory Infection for Adults and Children in Emergency Department and Urgent Care Settings), including assessment of a CDC-funded cluster randomized comparative effectiveness trial, suggests that these interventions can be successfully tailored and adapted to academic and community ED and UCC settings with the potential to reduce inappropriate prescribing. The MITIGATE study found that stewardship interventions tailored to the local site and setting reduced inappropriate prescribing for ARTI by one-third in academic-based settings with low percentages of inappropriate prescribing [12]. However, implementation of acute care stewardship interventions in a broad array of settings, including rural community-based sites, is critical to ensure successful adoption in the real world. MITIGATE consists of 6 specific components: (1) provider education, (2) patient education, (3) provider commitment-enhanced patient education, (4) program champion, (5) department feedback, and (6) personalized feedback. For this study, we implemented provider and patient education, provider commitment, and personalized feedback [13].
This study focuses on the effect of these MITIGATE interventions implemented in 3 high-volume, rural UCCs. This is within the context of a large rural community hospital setting with a predominately elderly population, as compared with the MITIGATE study, which was conducted in academic-based settings. We examined the percentage of outpatient antibiotic prescribing for ARTIs pre-intervention and during the intervention period and discuss lessons learned and future directions.
METHODS
Intervention Design
We utilized a number of published literature sources to develop our intervention, specifically Meeker and colleagues and the MITIGATE toolkit [11, 12]. The Meeker and colleagues study tested 3 behavioral interventions in a randomized clinical trial—suggested alternatives, accountable justification, and peer comparison. They found that accountable justification and peer comparison resulted in lower percentages of antibiotic-inappropriate prescribing for ARTI; therefore, we first focused on utilizing the latter peer comparison intervention for this study. The MITIGATE toolkit provided detailed guidance for implementing the peer comparison and public commitment behavioral nudges that helped inform the logistics of our intervention (eg, diagnosis codes for ARTI and sample language for the peer comparison and public commitment). In addition, we included a blinded ranking comparison email that ranked UCC providers among their peers so they could see where they rank without being identified.
Physician and Patient Education
Presentations about the importance of antibiotic stewardship and appropriate, guideline-concordant prescribing were made at a several physician staff meetings throughout September and October 2018. In early November 2018, the Medical Director of Urgent Care sent out an email to all UCC providers notifying them that they would be receiving feedback emails on their percentages of non-guideline-concordant prescribing for ARTI, along with frequently asked questions about the peer comparison ranking and how they could improve their prescribing. The email also included information about US Antibiotics Awareness Week (November 12–18, 2018).
During US Antibiotics Awareness Week, we educated the broader community about antibiotic resistance and antibiotic overuse/misuse on a local CBS 2 News spot on November 10, 2018. The interview and other public education materials were shared on the health system’s social media Web pages. We educated physicians and clinical staff on our internal Web page by displaying a clickable infographic for US Antibiotics Awareness Week that included information about antimicrobial stewardship. Two lectures on antimicrobial stewardship were also given during this week by 2 infectious disease (ID) physicians to family medicine and internal medicine residents and to primary care and UCC physicians at their staff meeting.
Patient education materials were distributed to the 3 UCCs during that week. We selected 4 different print materials from the CDC’s “Be Antibiotics Aware” campaign and included our health system’s logo on them as they are public domain. UCC leadership was instructed to place them throughout waiting rooms and patient rooms. The materials were distributed between November and early December 2018.
Public Commitment
Using the MITIGATE toolkit [13], for public commitment, we used CDC’s “Commitment Letter to Our Patients” template and had the Medical Director of Urgent Care sign it for each of the respective locations. These were placed simultaneously with the education materials.
Peer Comparison
Individual feedback emails were sent to UCC providers with their prescribing data 3 times during the 2018–2019 flu season (October through March). We used the MITIGATE toolkit email templates [13]. The first round of emails, sent January 2019, included individual prescribing data for ARTI from completed encounter visits during October–December 2018. The second round of emails was sent March 2019, with prescribing data from January–February 2019, and the third round of emails was sent April 2019 with prescribing data for the month of March 2019. The emails were sent during these times based on data availability (eg, lag time in data validation and analysis).
Individual feedback emails were coupled with a blinded ranking email sent to all UCC providers immediately after the individual feedback emails, so they could see where they ranked compared with their peers. For the blinded ranking, we created stratified ranking categories based on individual percentages of non-guideline-concordant prescribing for ARTI among providers. We used the National Action Plan for Combating Antibiotic-Resistant Bacteria goal to reduce antibiotic-inappropriate prescribing for ARTI by 50% by 2020 [14]. Given that the current national rate of antibiotic-inappropriate prescribing for ARTI among UCCs in the United States is 45% [4], we calculated that a 50% decrease would result in a percentage below 23%. This was the cutoff used to define “low prescribers” in our study. “High prescribers” were providers who had a percentage higher than the national average of 45% among UCCs. We used the national prescribing rate as our benchmark to improve prescribing, as there is currently no local prescribing rate published. If 2 or more providers had the same percentage, the provider with the higher number of encounters for ARTI visits would be ranked higher (ie, a better ranking) than the clinician with fewer encounters. Lastly, for the second and subsequent feedback emails, we included a blinded list of clinicians with the greatest percent change in prescribing since the last review period to identify those with the greatest improvements and those who regressed from their previous rate.
Data Collected
The following data elements were collected: visit date, primary and secondary diagnoses, antibiotic prescriptions prescribed at the visit, provider name, and clinic location. If a patient received more than 1 antibiotic during the visit (eg, 2 or more antibiotics), it was counted once in our calculations. We did this to avoid any inflation of the numbers and produce more conservative rates.
Inclusion Criteria
Any patient with a completed visit from October 1, 2017, to April 30, 2018, and October 1, 2018, to April 30, 2019 (April was included to evaluate 1 month post–flu season), with an International Classification of Disease, 10th Revision (ICD-10), diagnosis code for nonspecific upper respiratory infection, acute bronchitis, influenza, common cold, ARTI, and/or bronchitis was included in the analysis (Supplementary Data) [15]. Structured query language (SQL) was used to generate a report with these codes for each month, by provider and location, using the inclusion and exclusion criteria. We used the MITIGATE toolkit as a guide for the selection of ICD-10 codes [13].
Exclusion Criteria
We excluded any concomitant conditions that warrant antibiotic use, such as bacterial infections (eg, pneumonia, skin and soft tissue infection, urinary tract infection, streptococcal pharyngitis, acute otitis media, acute bacterial sinusitis, and pertussis), comorbidities that would warrant antibiotics in the setting of bronchitis, such as immunosuppression or other specific indications for antibiotic therapy (eg, cystic fibrosis exacerbation, acute exacerbation of chronic obstructive pulmonary disease, including chronic bronchitis and emphysema), other underlying lung diseases other than asthma, and/or fever in patients with sickle cell disease or neutropenia.
For the intervention peer comparison emails, providers with <10 ARTI patient encounters during the review period were excluded from receiving an email with their prescribing rates.
Data Validation Chart Reviews
Data validation was conducted by a pharmacist and physician before data analysis. They reviewed a random sample of 10 patient charts from the 3 UCCs for a total of 30 patient charts to ensure that the SQL query was working properly. Specifically, they examined if viral upper respiratory infections (ICD-10 codes: J00 and J06.9) were listed as the primary diagnosis for the visit, or secondary diagnosis if the first listed diagnosis was a general medical exam; and if acute bronchitis (ICD-10 code: J20.X) was listed as the primary diagnosis for the medical visit, or secondary diagnosis if the first listed diagnosis was a general medical exam. There were no discrepancies found during the validation process.
Data Analysis
Encounter counts for ARTI, number of prescriptions written, percentage of prescriptions written, location, and provider degree (ie, doctor of medicine [MD], doctor of osteopathic medicine [DO], physician assistant [PA], or nurse practitioner [NP]) were analyzed. We used this information to calculate the primary outcome in this study—the proportion of antibiotic-inappropriate ARTI diagnosis visits that received an antibiotic. To assess changes month-to-month between the 2 flu seasons, we calculated the absolute percent change. Antibiotic-inappropriate prescriptions averted was calculated by applying the baseline percentage to the number of ARTI encounters during the intervention period. Descriptive statistics were generated using Power BI software, version 2.69.5467.1751 (64-bit, May 2019; Microsoft, Redmond, WA, USA).
Interrupted Time Series Analysis
In addition to each of the 6-month flu season periods (2017–2018 and 2018–2019) included in the descriptive analysis, we extended this time frame beyond the flu seasons for the interrupted time series (ITS). To determine if there was a significant difference between prescribing percentages before and after the intervention, we conducted the ITS analysis over a period of 22 months (July 1, 2017, to April 30, 2019) using SAS 9.4 statistical software in SAS studio (SAS Institute, Inc., Cary, NC, USA). Specifically, there were 16 months of pre-intervention time compared with the 6-month intervention period. We did this for a few reasons: (1) to increase the sample size of time points to improve power; (2) to examine seasonality given prior knowledge that our hospital volumes peak during winter months due to an influx of seasonal visitors and residents to the area; and (3) the start date (July 1, 2017) was chosen because this was the earliest date we could retrospectively pull records due to a switch to a new electronic health record system.
To account for seasonality, the ARIMA and REG procedures in SAS were used. Normality plots illustrated that the data were normally distributed; thus no transformations were performed. The Durbin-Watson statistic was used to measure autocorrelation. Trends of the percentage of antibiotic prescriptions written and parameter estimates were calculated.
The study was submitted to the local institutional review board and received an exemption determination as a quality improvement project according to the Code of Federal Regulations, 45 CFR 46.102 (l).
RESULTS
Over the course of the study period from October 2017 to April 2019, there were 11 837 antibiotic prescriptions written and 17 947 encounters for ARTI (Figure 1). During the 2017–2018 influenza season (6-month pre-intervention period), there were a total of 7712 ARTI patient visits, with 5633 antibiotic prescriptions written by 27 UCC providers (16 = MD, 1 = DO, 6 = NP, 4 = PA). Comparing this baseline data with the 2018–2019 influenza season (6-month intervention period), there were a total of 6751 ARTI patient visits with 3947 antibiotics written by 28 UCC providers (1 DO joined the group). The average age of UCC providers across the 3 locations was 54 years (median, 55 years).
Figure 1.
Trends of acute respiratory tract infection (ARTI) encounters, antibiotic prescriptions written for ARTI, and rate of antibiotic-inappropriate prescribing for ARTI in 3 urgent care centers, by month and year from October 2017 to April 2019 (n = 11 837 antibiotic prescriptions written/n = 17 947 ARTI encounters). Green bars = number of prescriptions written for ARTI (“Is_RxWritten”). Blue bars = number of ARTI encounters (“Encounter_Count”). Blue line = percentage of antibiotics written for ARTI over the number of ARTI encounters for each month (“Percent Rx Written”).
For the primary outcome, there was a lower percentage of antibiotic-inappropriate prescribing during the 6-month intervention period (57.7%) than the 6-month pre-intervention period (72.6%), resulting in an absolute 14.9% decrease in percentage of inappropriate prescribing or about 981 inappropriate prescriptions averted. The largest difference in percentage of antibiotic-inappropriate prescribing was comparing April 2018 (71.8%) vs April 2019 (46.1%), 1 month post–flu season, after the second round of peer comparison emails were distributed, resulting in an absolute difference of –25.7% (Table 1). The mean difference in antibiotic-inappropriate prescriptions in the UCCs after the 3 interventions were implemented was statistically significant (mean difference, –14.6%; 95% confidence interval [CI], –20.30% to –9.05%; t, 5.44; P < .0001).
Table 1.
Rates of Non-Guideline-Concordant Prescribing for Acute Respiratory Tract Infections During 6-Month Pre-intervention and 6-Month Intervention Flu Seasons, by Month, 2017–2019
| 6-Month Pre-intervention Period (Prescribing Percentage)a | 6-Month Intervention Period (Prescribing Percentage)a | Absolute Percent Change |
|---|---|---|
| November 2017 (68.6) | November 2018 (67.3) | –1.3 |
| December 2017 (73.9) | December 2018 (56.3) | –17.6 |
| January 2018 (72.3) | January 2019 (59.8) | –12.5 |
| February 2018 (73.5) | February 2019 (58.3) | –15.2 |
| March 2018 (74.2) | March 2019 (53.6) | –20.6 |
| April 2018 (71.8) | April 2019 (46.1) | –25.7 |
aPercentages were calculated by dividing the number of antibiotic-inappropriate prescriptions written for acute respiratory tract infection (ARTI) by the number of ARTI encounters.
ITS Results
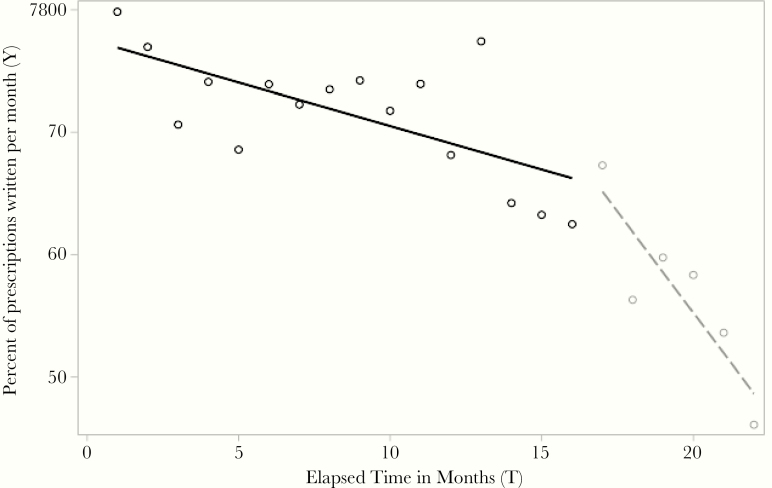
Beyond the 2 flu season comparisons, during the 22-month period included in the ITS analysis, the overall percentage of antibiotic-inappropriate prescribing for ARTI was 67.6%, with the mean percentage during the 16-month pre-intervention period at 70.3%, vs a mean percentage of 56.9% during the 6-month intervention period (Figure 2). Based on the segmented regression model, the mean percentage of antibiotic-inappropriate prescribing for ARTI was 79.8% (95% CI, 69.42% to 90.23%) when all independent variables were held constant. The estimated mean percentage of antibiotic-inappropriate prescribing for ARTI decreased by –0.71% (95% CI, –1.15% to –0.27%; P = .003) per month during the pre-intervention period, vs a mean percentage of –2.2% (95% CI, –10.8% to 6.3%; P = .59) per month during the intervention period and an additional decrease of –2.6% (95% CI, –4.59% to –0.59%; P = .014) after the intervention was completed.
Figure 2.
Percentage of antibiotic prescriptions written per month during the 16-month pre-intervention (solid line) and 6-month intervention (dashed line) periods, July 2017–April 2019. Percentage of prescriptions written (Y) is the proportion of antibiotic-inappropriate acute respiratory tract infection diagnosis visits that received an antibiotic.
DISCUSSION
In our rural community setting, with a baseline prescribing percentage of 72.6% during the 2017–2018 flu season, we observed an absolute 14.9% decrease in antibiotic-inappropriate prescribing, demonstrating that peer comparison data, when added to public commitment and education, can be effective in changing provider practice. These results parallel other peer comparison studies examining provider behavior change [8, 11, 16]. Meeker and colleagues found that 2 socially motivated interventions (ie, accountable justification and peer comparison) resulted in reductions in inappropriate antibiotic prescribing, whereas suggested alternatives that lacked a social component had no statistically significant effect. In addition to the social component, peer comparison performed favorably in comparison with traditional audit-and-feedback methods due to its public accountability factor [11]. This is why we chose to focus on implementing the peer comparison intervention rather than the audit-and-feedback method.
Also, although providers in our intervention were sent blinded emails that lacked identifying information (eg, names), they were aware they were being compared with peers within their specialty. Thus, there may have been an inherent competitiveness given this close network of urgent care providers (n = 27 providers during the 2017–2018 flu season and n = 28 providers during the 2018–2019 flu season). This contrasts with other peer comparison studies with larger networks of providers, which may have less inherent competitiveness given greater anonymity [11].
Another important difference between the Meeker study and this study is that in Meeker et al. clinicians were ranked in deciles from highest to lowest within each region using EHR data, whereas we used a national benchmark (ie, CARB 2020 goal) to determine whether providers were high or low prescribers. This was based on calculating a 50% reduction in inappropriate prescribing from the national average prescribing rate of 45% [4, 7]. We used the national benchmark for a few reasons: (1) there is currently no local or regional prescribing rate published that we could use to compare our 3 UCCs’ baseline prescribing percentages and (2) given the high baseline percentage of inappropriate prescribing across the 3 UCCs before the intervention, establishing a “low” group would not truly reflect low prescribing rates. For example, in the Meeker study, the mean antibiotic prescribing rate at baseline was 20% and declined to 4% at intervention month 18 (absolute difference, –16%). In our rural community setting, across the 3 UCCs, our rate at baseline was 72.6% and declined to 46.1% at intervention month 6 (April 2019), which still reflects a high percentage of inappropriate prescribing.
Other differences from past peer comparison studies include geographical location and setting attributes (eg, urban academic vs rural community settings). The MITIGATE study, which included sites in urban academic settings, found a statistically significant decrease in antibiotic prescribing between 2 influenza seasons (from 2.6% to 1.4%) but was unable to show a difference in the behaviorally enhanced vs adapted interventions [12]. This was likely due to very low rates of prescribing in this study. When comparing with this rural community setting with high rates of prescribing, other factors may be influencing prescribing behaviors that may not be apparent in urban academic settings. For example, in 1 study comparing rural practitioners with nonrural practitioners, rural practitioners stated that they felt it was more challenging to withhold antibiotics from a patient because of low patient volumes and concern for patient satisfaction [17]. Albeit, other evidence has shown that physicians with higher practice volumes are more likely to inappropriately prescribe than those with lower practice volumes, potentially due to decision fatigue [18–20]. Also, in our rural community setting, there is a large number of older adults with multiple comorbidities that may potentially be driving prescribing practices, as our population may be older relative to other peer comparison study populations. The second phase of this study aims to examine provider and population characteristics in greater detail to enumerate the potential facilitators and barriers to the long-term sustainability of this intervention.
Limitations
There were several limitations in this study. First, although we used several ICD-10 codes for ARTIs that did not warrant antibiotics, these may still have inaccurately reflected clinical decision-making, making it difficult to assess the appropriateness of the antibiotics prescribed for the diagnosis codes used. Although randomized chart auditing was conducted during the pre-intervention period, no chart validation process was conducted during the peer comparison phase. Second, navigating approval processes with leadership before intervention rollout was challenging and delayed the time of when the first peer comparison intervention emails were sent out. There was also a 2-month lag period to see changes after the intervention took place, and there was a 1-month lag period in collecting the data. Lastly, this study used a quasi-experimental design, which limited our ability to compare the 2 intervention arms given that bias may have played a role in the results. We also did not assess specific factors that might have influenced prescribing, including patient demographics, provider characteristics, or time of day or week effects [18, 19].
Future Directions
Given our results, we aim to continue this intervention in UCCs and expand to the emergency department and primary care locations in this health system. We also plan to examine provider and patient population characteristics that may contribute to identifying facilitators and barriers to the long-term sustainability of this intervention in a rural community context. An ongoing chart validation process will be conducted on a quarterly basis to ensure the validity of data collection. A secondary, long-term outcome includes utilizing existing tracking methods for both community- and hospital-associated Clostridioides difficile rates (eg, through the National Healthcare Safety Network). Lastly, evaluation of the types of antibiotics prescribed (eg, by drug class and location) will assist antimicrobial stewardship efforts to reduce percentages of inappropriate prescribing and contribute toward reaching the CARB 2020 goals.
CONCLUSIONS
The observed decrease in antibiotic-inappropriate prescribing for ARTI in this study suggests that utilizing a behavioral science approach may improve judicious use of antibiotics in community UCCs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Trenton Smith for creating the SQL database, and Dr. Erin Epson and Erin Garcia of the California Department of Public Health’s Healthcare-Associated Infections Program for their support of this work through California’s Emergency Department Antibiotic Stewardship Collaborative. We would also like to thank the Health Services Advisory Group (HSAG) for providing technical assistance on the initial data query pull, specifically Keith Chartier and Matthew Lincoln.
Potential conflicts ofinterest. All authors: no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. P.L.C., R.A., L.S.M., H.G., and M.D. conceived the idea and designed the intervention. P.L.C., R.A., R.G., and H.G. implemented the intervention. R.A. and R.G. performed data validation. P.L.C. and J.S. analyzed the data. R.G. and M.D. provided technical expertise and supervised the project. All authors contributed to writing the manuscript and reviewed, edited, and provided critical feedback on the final draft.
Availability of data. Data not publicly available.
References
- 1. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014;5:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Antibiotic prescribing and use in the US, antibiotic use in outpatient settings 2017. Available at: https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html. Accessed 20 March 2020.
- 4. Palms DL, Hicks LA, Bartoces M, et al. Comparison of antibiotic prescribing in retail clinics, urgent care centers, emergency departments, and traditional ambulatory care settings in the United States. JAMA Intern Med 2018; 178:1267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 6. Fay LN, Wolf LM, Brandt KL, et al. The urgent need for urgent care antimicrobial stewardship: evaluating prescribing and patient outcomes associated with a pharmacist-led stewardship program. Open Forum Infect Dis 2018; 5:S87. [Google Scholar]
- 7. Palms D, Hicks L, Hersh AL, et al. Variation in antibiotic prescribing among emergency departments, urgent care centers, and retail health clinics in the United States, 2014. Open Forum Infect Dis 2017; 4(Suppl 1):1267–9. [Google Scholar]
- 8. Yadav K, Meeker D, Mistry R, et al. A multifaceted intervention to improve prescribing for acute respiratory infection in adults and children in emergency department and urgent care settings (MITIGATE trial). Open Forum Infect Dis 2018; 5(Suppl 1):S43. [Google Scholar]
- 9. Incze MA, Redberg RF, Katz MH. Overprescription in urgent care clinics—the fast and the spurious. JAMA Intern Med 2018;178:1269–70. [DOI] [PubMed] [Google Scholar]
- 10. Meeker D, Doctor JN. Applications of behavioral economics to clinical quality improvement. In: Hanoch Y, Barnes A, Rice T. Behavioral Economics and Healthy Behaviors: Key Concepts and Current Research. London: Routledge, 2017:175–92. [Google Scholar]
- 11. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yadav K, Meeker D, Mistry RD, et al. A multifaceted intervention improves prescribing for acute respiratory infection for adults and children in emergency department and urgent care settings. Acad Emerg Med 2019; 26:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. May L, Yadav K, Gaona SD, et al. MITIGATE antimicrobial stewardship toolkit: a guide for practical implementation in adult and pediatric emergency department and urgent care settings 2018. Available at: http://shea-online.org/images/priority-topics/MITIGATE_TOOLKIT_final.pdf. Accessed 8 April 2020.
- 14. The White House. National action plan for combating antibiotic-resistant bacteria. 2015. Available at: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 20 March 2020. [Google Scholar]
- 15. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification Of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) Atlanta: Centers for Disease Control and Prevention; 2018–2019.
- 16. Persell SD, Doctor JN, Friedberg MW, et al. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016; 16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chamany S, Schulkin J, Rose CE Jr, et al. Knowledge, attitudes, and reported practices among obstetrician-gynecologists in the USA regarding antibiotic prescribing for upper respiratory tract infections. Infect Dis Obstet Gynecol 2005; 13:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadieux G, Tamblyn R, Dauphinee D, Libman M. Predictors of inappropriate antibiotic prescribing among primary care physicians. CMAJ 2007; 177:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linder JA, Doctor JN, Friedberg MW, et al. Time of day and the decision to prescribe antibiotics. JAMA Intern Med 2014; 174:2029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. May L, Gudger G, Armstrong P, et al. Multisite exploration of clinical decision making for antibiotic use by emergency medicine providers using quantitative and qualitative methods. Infect Control Hosp Epidemiol 2014; 35:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.