Abstract
The treatment of advanced thyroid cancer has undergone rapid evolution in the last decade, with multiple kinase inhibitor drug approvals for each subtype of thyroid cancer and a number of other commercially available drugs that have been studied for this indication. Although most of the US Food and Drug Administration (FDA)–approved drugs are antiangiogenic multikinase inhibitors—vandetanib, cabozantinib, sorafenib, lenvatinib—there are two FDA indications that are mutation specific—dabrafenib/trametinib for BRAF-mutated anaplastic thyroid cancer and larotrectinib for NTRK-fusion thyroid cancer. Furthermore, other mutation-specific drugs, immunotherapies, and novel strategies for advanced thyroid cancer are under investigation. Understanding the molecular basis of thyroid cancer, the drugs of interest for treatment of advanced thyroid cancer, and how these drugs can be administered safely and in the appropriate clinical scenario are the topics of this review.
Essential Points
A better understanding of the genetic landscape in thyroid cancer has led to major advances in treatment of advanced thyroid cancer
Antiangiogenic tyrosine kinase inhibitor drugs represent the major targets for advanced thyroid cancers
In addition to the two US Food and Drug Administration (FDA)–approved drugs for medullary thyroid cancer, new approaches with agents targeting the RET receptor and peptide receptor radionucleotide therapies are in the pipeline
For differentiated thyroid cancer, two FDA-approved drugs also exist, but more personalized agents that target specific genetic mutations are either recently approved or in clinical trials
A major breakthrough for BRAF-mutated anaplastic thyroid cancer led to FDA approval of a drug combination with a BRAF and MAPK kinase inhibitor
Prevention, early recognition, and aggressive treatment of adverse events will secure patient compliance and treatment outcomes
The incidence of thyroid cancer continues to rise, in large part due to the detection of incidental small papillary thyroid cancers (PTCs) using high-resolution imaging (1). The prognosis for thyroid cancer in most patients is excellent, with an overall disease-specific survival (DSS) rate >90% for at least 10 years (2).
Differentiated thyroid cancer
Therapeutic approaches for low-risk differentiated thyroid cancers (DTCs) have therefore evolved, with greater restraint being exercised now. Extent of surgery, thyroid hormone suppression, and use of radioactive iodine (RAI) therapy have become less aggressive in low-risk thyroid cancer, as adverse effects of these treatments may be greater than those of the disease itself.
In contrast, patients with locally invasive and/or distant metastatic DTCs have a much poorer overall survival (OS) rate. However, their clinical courses are often highly variable, with a spectrum of slowly progressive, asymptomatic disease to rapidly progressive, widespread metastatic disease. The mean OS duration for patients with advanced thyroid cancers is dependent on the histologic classification, ranging from shorter than 6 months to ∼5 years (3).
Histologically, DTCs are derived from thyroid follicular cells and represent most thyroid cancers (85%). DTCs are classified histologically as PTCs (75%), follicular thyroid cancers (FTCs; ∼15%), and Hürthle cell cancers (HTCs; <10%). Although HTC traditionally has been classified as a subtype of FTC, emerging genomic data have validated the long-held idea that HTCs are distinct from FTCs (4, 5). Poorly differentiated thyroid cancers (PDTCs) are heterogeneous, advanced thyroid cancers with a higher risk of relapse and higher mortality. These tumors fall under the DTC category. In one classification scheme, PDTCs are characterized by the presence of increased mitoses and/or necrosis (6). Alternatively, the Turin classification of PDTC is that of a solid, insular, or trabecular growth pattern lacking papillary nuclear features with mitoses and/or necrosis (7). Whichever classification is used, patients with PDTC experience high rates of disease relapse with frequent locally invasive disease in the trachea and/or esophagus as well as distant progression to the lungs, liver, bone and, in ∼10% of cases, brain (8, 9).
Some PDTC tumors are refractory to T4-mediated TSH suppression as well as RAI therapy, defined by a lack of iodine uptake in foci of disease and/or lack of response to iodine-131 (131I) therapy (10).
Survival in patients with DTC has, until recently, been most accurately estimated using the MACIS scoring system, which is specific for PTC (11). MACIS scores of <7 and ≥7 reliably reflect 10-year DSS rates of >90% and 45% to 75%, respectively. The algorithm in the MACIS system relies on the following well-validated prognostic parameters: status of distant metastases (M), age (A), completeness of surgical resection (C), presence of grossly invasive disease (I), and sex (S). The recently updated 8th edition of the American Joint Committee on Cancer TNM classification for the first time provides an accurate staging system for DTCs that appropriately reflects survival, with excellent prognoses for stage I and II DTCs (>75% to 95% 10-year DSS rate) but precipitously worse prognoses for stage III and IV disease (60% to <50% 10-year DSS rate) (12).
Medullary thyroid cancer
Medullary thyroid cancers (MTCs) represent <5% of all thyroid cancers and are distinguished from DTCs by their origination from parafollicular or C cells of the thyroid neuroendocrine-type cells, embryonically derived from neural crest cells. Unlike DTC and PDTC, up to 30% of MTCs are hereditary due to germline alterations of the RET proto-oncogene, which gives rise to multiple endocrine neoplasia (MEN) type 2 syndrome A and B (13). Patients with either sporadic or hereditary MTC confined to the thyroid and/or cervical lymph nodes and with low levels of postoperative tumor markers have excellent prognoses, with a >90% 10-year DSS rate (14–16). In patients with advanced MTC, progression most commonly involves the liver, lungs, and bone. Patients with distant micrometastases often experience prolonged latency periods of slowly progressive, asymptomatic disease that is often followed years later by more symptomatic, high-risk disease. In patients with advanced, structurally evident distant metastatic disease (whether at initial presentation or after a prolonged period of latency), tumor marker doubling times for calcitonin and/or carcinoembryonic antigen (CEA) levels of up to 12 months identify patients with high mortality rates within the ensuing 5 years (17). Overall, the DSS rate in patients with stage IV MTC at 10 years is <30%. Emerging novel therapeutics targeting the RET proto-oncogene in both advanced hereditary and sporadic MTCs are showing great promise, with durable control rates that may translate into improved DSS for high-risk patients.
Anaplastic thyroid cancer (undifferentiated thyroid carcinoma)
Anaplastic thyroid cancer (ATC), representing only ∼1% of all thyroid cancers, disproportionately contributes to thyroid cancer–related deaths because it is almost uniformly fatal (18). Patients presenting with ATC often describe a rapidly growing neck mass, on the order of days to several weeks, that is often associated with acute hoarseness, dysphagia, dyspnea, and/or neck pain, altogether constituting an endocrine emergency. ATCs are usually derived from DTCs or PDTCs; therefore, these entities often coexist within the same tumor and retain the mutations of the tumor from which they are derived. However, the ATC component is devoid of any of the morphologic features of follicular cells. Thus, ATCs are best described histologically as undifferentiated carcinomas. Microscopically, ATCs are described as high-grade cancers and are often composed of giant cells or cells with squamous-like features with high levels of mitosis and necrosis. The squamous morphology can lead to confusion regarding the diagnosis, as ATC is often mistaken for squamous cell carcinoma of the head and neck or lung. Retained immunohistochemical expression of the transcription factor TTF-1 and/or PAX-8 in tumor cells, when present, is helpful to confirm the thyroid origin of these cancers, particularly when tumor tissue is obtained outside the thyroid. Up to 50% of the ATC tumor volume is composed of infiltrating tumor-associated macrophages (19). Historically, median OS of patients with ATC is <3 to 6 months (20). Thus, ATC is the most aggressive of any human malignancy. Death due to asphyxiation caused by a rapidly progressing primary tumor may be prevented with emergent and aggressive combination multimodal therapies with or without primary tumor resection (21). Despite such approaches, most patients with ATC receiving aggressive treatment die of distant metastatic disease (in the lungs, distant soft tissue, bone, and/or brain) within 1 to 2 years of diagnosis (20). Such outcomes reflect the unique staging of ATC using the American Joint Committee on Cancer TNM classification (22), in which stage IV is the only disease stage. Stage IV is then subdivided into A, B, and C defined as ATC that is confined to the thyroid (IVA), confined to the neck but extending beyond the thyroid gland (IVB), and spread distantly (IVC). Current multimodal therapies for ATC in patients desiring aggressive treatment include potential R0 or R1 primary tumor resection, external beam radiation therapy combined with radiosensitizing chemotherapy, targeted kinase inhibitors based on tumor-derived molecular alterations (discussed below), and emerging immune-mediated combination therapies.
Molecular Basis of Thyroid Cancer
Next-generation, high-throughput deep sequencing genomic interrogations have generated comprehensive molecular fingerprints of the major thyroid cancer histotypes, validating previously published cohort studies and identifying novel pathways in the pathogenesis of advanced thyroid cancers.
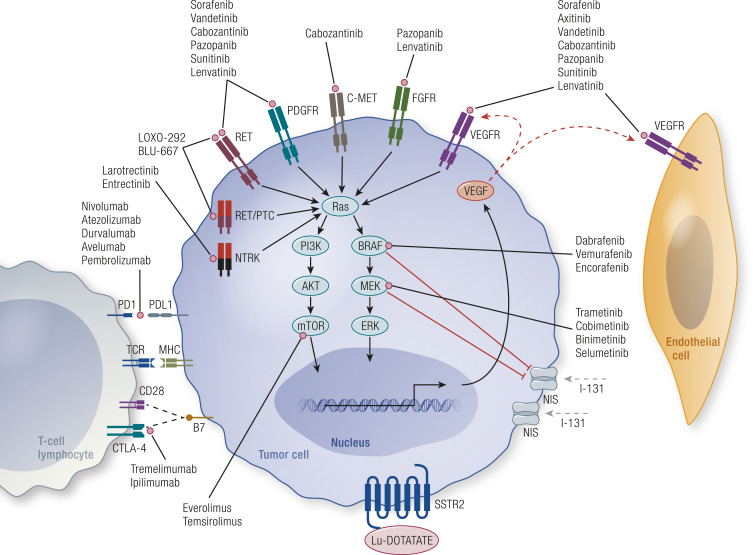
Investigators with The Cancer Genome Atlas (TCGA) comprehensively interrogated nearly 500 PTCs comprising mostly well-differentiated low- to intermediate-risk cases (23). Data from the TCGA confirmed the findings of published studies demonstrating that nonoverlapping alterations within the MAPK [MAPK kinase (MEK)/ERK] signaling pathway dominate, with mutant BRAF, RAS, and RET fusions contributing to ∼80% of the known alterations in PTCs. Thus, the MAPK pathway is central to the onset and progression of thyroid cancer (Fig. 1). Oncogenic BRAF is present in ∼60% of PTC cases, followed by HRAS and NRAS in ∼10% of cases and RET fusions in ∼5% of cases (23). In vivo functional studies have demonstrated that thyroid-specific activation of oncogenic BRAF induces potent downstream phosphorylation of MEK/ERK that results in thyroid tumorigenesis, closely recapitulating the development of human PTCs. Moreover, translational studies demonstrated that in addition to affecting tumor transformation and growth, reversible MEK/ERK signaling regulates thyroid-specific genes, which is particularly relevant to therapeutic targeting of the TSH receptor and sodium iodide symporter (NIS) (24). Thus, oncogenic activation of MEK/ERK is an important initiation event in PTC and impacts responses to therapy in patients with advanced disease.
Figure 1.
Upregulation of the MAPK pathway in tumor cells leads to proliferation and progression of thyroid cancer. Increased signaling along the MAPK pathway is caused by genetic mutations occurring in the pathway (such as those of BRAF and RAS), activation of receptor tyrosine kinases due to mutations (such as those of RET), overactivation of these kinases by growth factors, and fusion receptor tyrosine kinases (such as RET and NTRK fusions). Shown are several drugs capable of targeting upstream receptor tyrosine kinases, specific mutations, and genetic rearrangements that are either FDA approved for thyroid cancer or of interest in treating this disease. Furthermore, constitutively activated MEK (due to BRAF V600E or RAS mutations) leads to decreased expression of thyroid-specific genes, particularly NIS, resulting in RAI refractoriness. Inhibition of BRAF or MEK reverses this effect and restores RAI avidity. Furthermore, complex interplay between the immune system and cancer cells exists, including inhibitory and stimulatory interactions. Expression of inhibitory ligands such as programmed death-ligand 1 (PD-L1) on tumor cells effectively turns off the T cells, leading to evasion of the immune response. Blockade of such signals with checkpoint inhibitor drugs is depicted in the figure. Several immunotherapeutic drugs are approved for several solid tumors but not thyroid cancer. CTLA-4, cytotoxic T-lymphocyte–associated protein 4; FGFR, fibroblast growth factor receptor; MHC, major histocompatibility complex; NIS, sodium iodide symporter; PD-1, programmed cell death protein 1; PI3K, phosphoinositide 3-kinase; TCR, T-cell receptor.
In a minority of PTCs without MAPK pathway alternations, researchers identified mutant EIF1AX and fusions within PPAR-γ, NTRK1/3, and THADA as novel pathways associated with well-differentiated PTCs. NTRK1 and NTRK3 fusions, previously shown to be associated with pediatric thyroid cancers (25), and ALK (26) and ROS1 (27) fusions, although rare, are novel targetable genetic events in a subset of patients who experience progression to more advanced disease.
Recent next-generation sequencing (NGS) studies of advanced thyroid cancers (PDTCs and ATCs) have provided molecular clues underlying the progression of DTC to PDTC and ATC. Landa et al. (28) characterized 84 PDTCs and 33 ATCs using MSK-IMPACT NGS exclusively. Also, Pozdeyev et al. (29) combined genomic data from both MSK-IMPACT and FoundationOne platforms, altogether representing 568 adult “advanced” PTCs and 196 ATCs, with only a limited number of samples defined as PDTC. Similar to data from TCGA, alterations in the MAPK pathway induced by oncogenic BRAF, RAS, and RET fusions dominate the genomic landscape. However, promoter mutations within TERT, important for telomere elongation and cell survival, are highly enriched in patients with advanced thyroid cancers (∼9% TCGA study of PTC vs ∼60% PTC/PDTC). Multiple clinicopathologic studies have confirmed that the presence of TERT alterations is associated with advanced disease and reduced DSS rates in DTC (30–34).
Data from the MSK-IMPACT study identified hot-spot alterations within EIF1AX in 11% of PDTCs and ATCs, as well as this alteration that appeared to colocalize with oncogenic RAS, suggesting a novel pathway in thyroid cancer progression (28). EIF1AX alterations are associated with worse outcomes than those of EIF1AX wild-type advanced thyroid cancers. Recent functional studies of cell lines and mouse models demonstrated that mutant EIF1AX increases amino acid transporters via downstream ATF4 and c-MYC activation, with the latter further stimulated by oncogenic RAS (35). Thus, mutant EIF1AX and oncogenic RAS cooperate to promote increased ATF4- and c-MYC–dependent mammalian target of rapamycin (mTOR) activation, resulting in enhanced cellular protein synthesis and survival. Therapeutic targeting of MEK, c-MYC, and/or mTOR may thus be a novel strategy for patients with advanced thyroid cancers harboring these alterations.
Data from both Landa et al. (28) and Pozdeyev et al. (29) identified other pathways that likely mediate molecular and clinical progression of disease, including alterations in phosphoinositide 3-kinase/AKT/mTOR pathway signaling, alterations within the SWI/SNF complex (ARI1D1A/ARID1B/SMARCB1/PBRM1/ATRX), histone methyltransferase pathway mutations (KMT2A/KMT2C/KMT2D/SETD2), and deficiencies in genes of DNA repair (MSH2/MSH6/MLH1/BRCA1/BRCA2/ATM). Investigators have also identified alterations in the tumor suppressors TP53, NF1, NF2, and MEN1 in advanced PTCs and PDTCs, albeit at low frequencies.
Limited NGS has been performed for advanced FTCs, with 65 FTCs characterized by Pozdeyev et al. (29). RAS mutations were identified in 66% of advanced FTCs, with mutations in NRAS being the most frequent subtype. Similar to PTCs and PDTCs, ∼70% of FTC samples had TERT alterations. They also observed enrichment of PTEN (14%) and RB1 (9%) alterations. Of note, FTCs without known MAPK driver mutations harbored alterations in PTEN, TP53, RB1, and MEN1, demonstrating that these tumor suppressors are potential oncogenic drivers of thyroid tumorigenesis.
Traditionally classified as a “subset” of FTC, two large comprehensive data sets have for the first time clearly demonstrated that HTC is a distinct clinicopathologic subtype of thyroid cancer, a concept that has been long understood at the bedside, at least (4, 5). For example, HTCs do not harbor BRAF alterations, whereas a small subset does harbor NRAS alterations (10%), albeit at a lower frequency than in FTCs. Also, 22% of advanced or widely invasive HTCs contain TERT promoter mutations or, less commonly, associated alterations of the telomerase-related gene DAXX or ATRX. EIF1AX mutations are present in 11% of HTCs, similar to PDTCs, but they do not appear to be coexpressed with oncogenic RAS as in PDTCs. MADCAM1 alterations, involved in leukocyte trafficking as well as AKT phosphorylation and protein synthesis, is altered in 20% of advanced HTCs. Alterations within the phosphoinositide 3-kinase/AKT/mTOR pathway suggest a dominant role in HTC and, importantly, the pathway is a therapeutic target (Fig. 1). Indeed, in at least one clinical trial using a combination of kinase and mTOR inhibitors, the combination has shown promise in patients with advanced HTC (36). Importantly, alterations in mitochondrial genes along with duplications of chromosomes 5 and 7 and widespread loss of heterozygosity appear to be key genomic alterations involved in and unique to the pathogenesis of advanced HTC. The emerging molecular profile of HTCs may provide important tools that can reliably discriminate Hürthle cell adenomas from HTCs preoperatively.
Molecular alterations observed in well-differentiated DTCs and PDTCs overlap those in ATCs with high-risk alterations, such as those of TERT, observed with greater incidence in ATC (30, 31). Oncogenic BRAF is present in 35% to 40% of ATCs, followed by RAS in 18% to 27% of cases, which perhaps is not unexpected, as up to 50% of ATCs arise from preexisting, often occult DTCs. TERT and TP53 alterations, alternatively, are highly enriched in ATCs, present in 65% to 75% of cases. This suggests a molecular evolution of some ATCs in which BRAF- or RAS-driven DTCs progress with subclonal acquisition of mutant TERT and TP53 that drive tumor heterogeneity and dedifferentiation. Targeting BRAF in preclinical and clinical studies demonstrated that BRAF is an important oncogenic driver in ATCs because inhibition of mutant BRAF impedes disease progression, at least partially.
Additionally, alterations of the tumor suppressors ATM, RB1, NF2, and MEN1 are identified at higher frequencies in ATCs than in either advanced PTCs or PDTCs. Hierarchical clustering of genomic data sets by Pozdeyev et al. (29) further identified distinct molecular signatures of ATCs according to their histological and molecular evolution from either DTCs (PTC vs FTC vs HTC) compared with molecular signatures of non–DTC-derived ATCs. Determining whether a generalized chemoradiation approach for all ATCs is more effective than a more molecular-based tailored treatment approach will be important given the rapid progression of the disease. Moreover, data have clearly identified the presence of an immune-rich microenvironment as a striking component of ATC, with tumor-associated macrophages contributing to ∼50% of the tumor volume regardless of genetic makeup (19). Preclinical studies of BRAF-driven PDTCs demonstrated that tumor-associated macrophages facilitate tumor growth and promote dedifferentiation (37). Immunohistochemical studies further implicate the presence of an immunosuppressive environment with increased expression of programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), both important in dampening antitumor-specific cytotoxic T cells (38). Future therapies that are durable in patients with ATC will likely have to be combination approaches aimed at the tumor cells, as well as the recruitment of host-driven antitumor immunity.
Mutations of the RET proto-oncogene, which codes for the RET tyrosine kinase with resulting ligand-independent receptor dimerization and/or ligand-independent kinase activation, are present in >95% of hereditary MTC (13) and ∼40% of sporadic MTC (39) tumors. The RET proto-oncogene is composed of ∼26 exons with hot-spot–activating alterations most commonly identified within exons 10 and 11. Genotype–phenotype correlations characterize germline RET alterations and guide clinical care. Codon 634 point mutations give rise to MEN2A syndrome, with 100% penetrance of MTC that may begin as early as 5 years of age followed by a pheochromocytoma incidence rate of ∼50% and 10% to 30% risk of primary hyperparathyroidism (14, 15). Germline RET 918 point mutations seen in MEN2B represent a particularly virulent form of MTC, with recommendations for thyroidectomy by 1 year of age in affected patients. Patients with MEN2B have a high risk of developing pheochromocytoma and patients phenotypically having a Marfanoid habitus and mucosal neuromas. In sporadic MTCs, ∼40% of somatic alterations are due to RET mutations, followed by ∼15% due to RAS mutations (39). The RET 918 point mutation is the most common somatic alteration in sporadic MTC. Researchers have identified several other alterations within the RET proto-oncogene at lower frequencies, including amplifications and deletions in sporadic MTC.
RET represents an important therapeutic target in advanced, RET-mutated MTC, and it provided the rationale for the first clinical trials of the multikinase inhibitors vandetanib and cabozantinib for this disease. However, clinical efficacy of these inhibitors is most likely related to their antiangiogenesis effects via vascular endothelial growth factor receptor (VEGFR) pathway inhibition, as these drugs are rather weak RET kinase inhibitors. The emerging small molecule inhibitors LOXO-292 (selpercatinib) and BLU-667 (pralsetinib) are long-awaited potent RET-specific kinase inhibitors that have shown promise in phase 1 clinical trials in patients with advanced cancers driven by RET alterations.
Standard/Initial Treatment of DTC, ATC, and MTC
Initial management of DTC and MTC
The initial treatment of DTC and MTC almost always begins with surgical resection of the primary tumor and metastatic disease in the neck and upper mediastinum. However, surgical approaches for these two diseases differ slightly and are described in great detail in the American Thyroid Association guidelines (10, 13). In all patients with a preoperative diagnosis of thyroid cancer, a thorough evaluation for cervical lymphadenopathy must be performed prior to surgical intervention. Regarding ATC, surgery should be performed only in patients in whom complete resection can be achieved, as debulking has not been shown to be beneficial for these patients and will delay radiation and/or systemic therapy (40).
Among patients with DTC who undergo total thyroidectomy, those with intermediate to high risk of recurrent disease as well as those with distant metastatic disease should then undergo treatment with RAI and measurement of thyroglobulin (10). Patients with pulmonary micrometastases that take up RAI can have complete remissions with either one treatment or sequential doses given every 6 to 12 months (10). In patients with diffuse lung involvement, those receiving high doses of RAI have experienced pneumonitis and fibrosis. Other adverse effects that can occur soon after RAI are sialadenitis, dry mouth (leading to dental caries), and nasolacrimal duct obstruction (10). Long-term consequences of RAI include permanent salivary gland dysfunction and secondary malignancies. TSH suppression <0.1 mU/L in patients with a high risk of recurrence (including patients with distant metastatic disease) is recommended (10).
Management of DTC and MTC in patients with distant metastatic disease
For patients with DTC with distant metastatic disease that is RAI refractory as defined in Table 1 (10, 41, 42) and patients with MTC with distant metastatic disease, a period of observation, or “watchful waiting,” may be appropriate. Many patients with MTC and DTC have an indolent clinical outcome despite having metastases. These patients usually exhibit slow, minimal, or no progression over time and are asymptomatic. For these patients, observation with periodic long-term follow-up including clinical examination, cross-sectional radiographic studies, and serial measurement of tumor markers (calcitonin and CEA in MTC and thyroglobulin in patients with DTC) is usually sufficient. The rationale for not administering systemic therapy to all patients with MTC and DTC with distant metastatic disease is outlined in Table 2.
Table 1.
Characteristics of RAI-Refractory Thyroid Cancer
| Any one of the following meets the criteria for RAI-refractory DTC: |
| • Lack of RAI uptake in all tumors upon a diagnostic whole-body scan or on a posttreatment scan after a treatment dose of 131I in a patient with known structural disease |
| • Progression of disease within 6–12 mo after treatment with 131I |
| • Progression of disease in a patient who has received ≥600 mCi of 131I |
Table 2.
Rationale for Observation or Localized Therapies in Patients With DTC or MTC and Distant Metastatic Disease
| • Kinase inhibitor-based therapies are not curative. Continuous therapy is necessary to maintain a response. |
| • Side effects associated with kinase inhibitors may reduce the patient’s quality of life, and medications may have the potential to cause harm. |
| • The clinical benefits derived from these drugs (tumor size reduction, stabilization of disease, symptom improvement) last for a limited period of time. In fact, trials and clinical experience demonstrate that the disease will invariably progress over time. |
| • Despite prolonging progression-free survival, the benefits regarding OS have yet to be clearly established. |
Alternatively, some patients with advanced thyroid cancer exhibit disease progression that may place them at risk for complications and symptoms that alter their quality of life (QOL). These patients may be candidates for localized or systemic therapy. When feasible, localized therapy is often used first to delay the initiation of systemic therapy for a period of time. Palliative external beam radiation therapy is often used in patients with thyroid cancer with painful bony metastases, as are bone-modulating drugs such as zoledronic acid and denosumab (10, 43). Localized treatment of disease sites and metastasectomy also may be options for some patients with disease that is progressive or threatening limb or organ function (10, 43). Localized therapies include external beam radiation, thermal ablation, ethanol ablation, and chemoembolization.
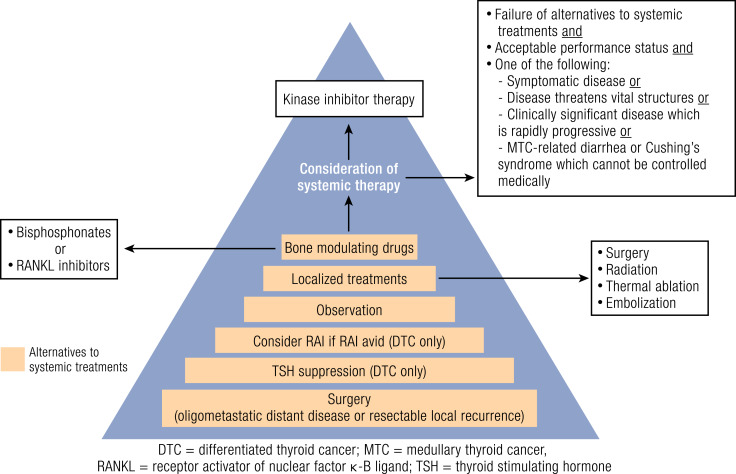
Systemic therapy is used in patients with MTC and DTC that has failed localized therapies or in whom these therapies are not feasible and who have disease progression that is expected to lead to morbidity or mortality in <6 months, symptomatic disease, or diffuse disease progression (10, 13, 43). Figure 2 shows the management of locally advanced or metastatic DTC and MTC. The tumor burden may also be an indication for treatment as suggested by the phase 3 lenvatinib trial (44). In this clinical trial, the median duration of response was similar in lenvatinib-treated subgroups according to age and sex. However, when analyzed according to baseline disease burden (<35 mm, 35 to 60 mm, >60 to 92 mm, and >92 mm), the median duration of response to lenvatinib decreased as the tumor burden increased (44.3, 27.5, 18.0, and 15.7 months, respectively). These data suggest that initiating treatment in patients earlier, when the tumor burden is smaller, may lead to more favorable outcomes.
Figure 2.
Management of locally advanced and/or metastatic differentiated cancer or MTC and the criteria for initiation of systemic therapy with kinase inhibitors. RANKL, receptor activator of nuclear factor-кB. [Reprinted by permission from Springer Nature, Hormones & Cancer; Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G. BRAF inhibitors: experience in thyroid cancer and general review of toxicity. Horm Cancer. 2015;6:21–36.]
Initial management of ATC
Treatment of ATC has changed dramatically during the last decade due to improved outcomes with the discovery of effective therapy for tumors harboring a BRAF V600E mutation. The initial management of ATC consists of (i) evaluation of the stability of the airway, (ii) extensive metastatic workup, (iii) evaluation of the resectability of the primary tumor, and (iv) a rapid test to determine whether the tumor harbors a BRAF V600E mutation (45). First and foremost, the airway should be assessed to determine whether immediate intervention is necessary to stabilize the airway. A metastatic disease evaluation must also be performed, as 50% of patients with ATC present with distant disease at diagnosis (46), which changes the initial approach to treatment. In the absence of distant disease, the patient should be evaluated for surgery to remove the primary tumor. Surgery should only be undertaken when the tumor can be fully resected, as debulking does not improve outcomes and only delays effective therapy. If the tumor is deemed resectable, surgery should be performed promptly, and the patient should receive chemoradiation ∼2 to 4 weeks after surgery. When the primary tumor is not resectable in a patient without distant metastases, definitive intensity-modulated radiation therapy (≥60 Gy) to the primary tumor, with adjuvant cytotoxic chemotherapy, represents the standard treatment of choice. When distant metastases are present at diagnosis, determination of whether the patient has small- or large-volume metastases will identify the best initial treatment. In patients with small-volume distant metastatic disease, a feasible approach may be to first administer intensity-modulated radiation therapy to gain locoregional control, which is more likely to cause morbidity and mortality. Definitive treatment with high-dose radiation (with adjuvant chemotherapy) may be possible in patients with small-volume distant metastatic disease that is not rapidly progressive. These patients should be monitored during radiation by imaging the areas of concern to determine whether the patient has rapidly progressive disease (PD) and needs to start systemic therapy instead. Systemic therapy should follow chemoradiation. In patients with large-volume metastases, palliative radiation therapy (a shortened, rapid course) can be attempted followed by systemic therapy vs upfront systemic therapy. Systemic therapy in patients with BRAF V600E mutations should begin with the FDA–approved combination of dabrafenib and trametinib, which is discussed at length below. Consideration should be given to patients participating in a clinical trial of a BRAF/MEK inhibitor in combination with other novel therapies/approaches, as resistance to BRAF/MEK inhibitors eventually ensues. In patients with ATC without a BRAF V600E mutation, the preference is for enrollment in a clinical trial given the high mortality rate in these patients or for standard cytotoxic chemotherapy with a taxane, a platinum-based drug, doxorubicin, or a combination of two of these drugs. Further information regarding cytotoxic chemotherapeutic regimens and combinations can be found in the American Thyroid Association guidelines for ATC (40). One caveat is the rare chance that the tumor harbors an NTRK fusion, as larotrectinib is approved by the FDA for all solid tumors with NTRK fusions (discussed below).
Rapid testing for BRAF V600E mutations in patients with thyroid cancer
Because ATC is rapidly fulminant, early determination of the BRAF status is imperative in the management of this tumor. The BRAF/MEK inhibitor combination of dabrafenib and trametinib is approved by the FDA for ATC, but this treatment is reserved only for patients whose tumors harbor a BRAF V600E mutation. Thus, tests to rapidly identify the existence of a BRAF V600E mutation are essential in the management of ATC. The gold standard for determination of BRAF status is tissue-based, direct mutation testing. This is often performed with NGS or single-point mutation testing with Sanger sequencing. Two rapid tests have been studied and are currently in use for ATC. The first test is the BRAF V600E immunohistochemistry (IHC) stain, which tests for protein expression. This test was developed for patients with melanoma and then studied in patients with thyroid cancer. Because most thyroid nodules and thyroid cancers are diagnosed using fine-needle aspiration (FNA), one group studied BRAF V600E by IHC on FNA material in thyroid cancer specimens. They evaluated FNA smears vs FNA cell blocks and compared this evaluation with the gold standard of NGS (47). The authors found that although this test was reliable using FNA cell blocks, it resulted in false-positives using FNA smears. The turnaround time for this test varies by institution; excluding sample preparation, slide cutting, and quality control by a pathologist, authors have reported the turnaround time to be ∼2 hours (48).
Another rapid test studied in patients with ATC is a liquid biopsy-based test. Liquid biopsy can detect circulating tumor cells and fragments of circulating tumor DNA shed into the bloodstream from a tumor. Researchers studied a commercial cell-free DNA (cfDNA) test using the Guardant360 platform in ATC cases and found it to have the highest concordance with molecular testing on tissue in untreated patients with ATC (49). In this series of 23 consecutive patients with ATC, 9 had BRAF V600E mutations. Patients were placed in three groups: group 1 contained treatment-naive patients with active disease, group 2 contained those who previously received treatment but had residual disease, and group 3 contained patients with no evidence of disease. Patients in group 1 had a 100% concordance rate for the BRAF V600E mutation between liquid biopsy and tumor molecular testing. The median time for reporting of results was 13 days for Guardant360 and 19 days for tissue-based molecular testing (P = 0.009) in this series.
A second study evaluating circulating BRAF V600E mutation by digital droplet PCR found that concordance between this test and tissue testing to be 93%, with a sensitivity of 85% and specificity of 100% (50). The investigators also found that this test could be used as a biomarker of response to treatment.
Investigators performed a systematic review of cfDNA in DTC in nine studies (51). The objectives were to understand the utility of cfDNA as a diagnostic tool, a marker of advanced disease, and a marker for monitoring disease progression. In seven studies, researchers specifically examined cfDNA for BRAF V600E mutation. In these studies, only 19% of patients with BRAF-mutated tumors had detectable BRAF V600E mutations on cfDNA. This low rate of detection was possibly due to inclusion of patients with early-stage disease. In these patients, shedding of tumor DNA into the circulation is less likely than in patients with advanced thyroid cancer. However, one study in this systematic review had a markedly higher BRAF V600E detection rate in cfDNA than the other studies. In this study, 22 patients with PTC had intact primary tumors, 12 of which were BRAF mutated according to PCR analysis. Eleven of the 12 patients with BRAF-mutated tumors had detectable BRAF in cfDNA. However, the investigators found three false-positive results among patients without BRAF-mutated disease (52). Based on these data, detection of BRAF mutations in cfDNA in PTCs is promising for rapid BRAF testing in patients with advanced disease, but it is far less reliable (and more expensive) than BRAF V600E using IHC. Further studies with more sensitive assays are warranted.
Systemic therapy for locally advanced and/or metastatic thyroid carcinoma
Five drugs or drug combinations have been approved for locally advanced and/or metastatic thyroid cancer during the last decade, and a number of other drugs have been studied for these diseases. Furthermore, several kinase inhibitors and other drugs have been studied in thyroid cancer and are detailed below. Figure 1 shows the two important molecular pathways in thyroid cancers (MAPK and phosphoinositide 3-kinase) that are activated by overexpression of receptor tyrosine kinases or mutations along the pathways, as well as the available drugs that target these pathways. Table 3 (53–80) lists the commercially available drugs that have been studied in thyroid cancer trials, their targets, and results of these trials. Many of these drugs, although not FDA approved for some types of thyroid cancers, are recommended by the National Comprehensive Cancer Network and are included in that organization’s guidelines for thyroid cancer (43).
Table 3.
Commercially Available Drugs Studied in Treatment of Thyroid Cancer
| Drug | Mechanism of Action: Target(s) | FDA-Approved Indication | Number of Patients With Thyroid Cancer Enrolleda | Results in Patients With Thyroid Cancer | Common Adverse Events | References |
|---|---|---|---|---|---|---|
| Axitinib | TKI: VEGFR1–3 | RCC | DTC: 45 | 30% PR (responses not broken down by histology) | HTN, HFSR, diarrhea, fatigue | Cohen et al. (53) |
| MTC: 11 | ||||||
| ATC: 2 | ||||||
| DTC: 34 | DTC: 2/34 CR, 8/34 PR | Capdevila et al. (54) | ||||
| MTC: 13 | MTC: 3/13 PR | |||||
| Cabozantinib | TKI: VEGFR2, MET, FLT3, RET, c-kit | MTC, RCC, HCC | MTC: 219 | MTC: 28% PR | HTN, HFSR, diarrhea, fatigue | Elisei et al. (55) |
| DTC (second or third line): 25 | DTC: 40% PR | Cabanillas et al. (56) | ||||
| Dabrafenib (single agent) | STKI: BRAF V600E | BRAF-mutated melanoma | DTC: 13 | DTC: 4/13 PR | Fever, diarrhea, skin rash and skin growths (including SCC), HFSR, fatigue | Falchook et al. (57) |
| Dabrafenib in combination with trametinib | STKI: dabrafenib, BRAF V600E | BRAF-mutated ATC, melanoma, non–small cell lung cancer | ATC: 16 | ATC: 63% PR (by central review) | Acneiform rash (trametinib); see “dabrafenib” for other AEs, but some AEs decreased with addition of trametinib | Subbiah et al. (58) |
| STKI: trametinib, MEK1/2 | ||||||
| Everolimus | STKI: mTOR | RCC, SEGA, TS | DTC: 85 | DTC: 2/85 PRb | Mucositis, myelosuppression, infection | Lim et al. (59) |
| MTC: 19 | MTC: 2/19 PR | Schneider et al. (60, 61) | ||||
| ATC: 13 | ATC: 1/13 PR | Hanna et al. (62) | ||||
| Lenvatinib | TKI: VEGFR1–3, FGFR1–4, PDGFR, RET, c-kit | DTC; approved for RCC in combination with everolimus | DTC: 261 | DTC: 65% CR + PR | HTN, HFSR, diarrhea, fatigue | Schlumberger et al. (63, 64) |
| MTC: 59 | MTC: 36% PR | Tahara et al. (65) | ||||
| ATC: 17 | ATC: 24% PR | |||||
| Larotrectinib | TrkI: NTRK fusions | Adult and pediatric patients with solid tumors and NTRK fusions (tumor agnostic) | NTRK-fusion thyroid carcinoma (histologies not reported): 5 | 5/5 PR | Fatigue, dizziness, nausea, LFT elevation | Drilon et al. (66) |
| Pazopanib | TKI: VEGFR1–3 PDGFR, FGFR1/2, c-kit | RCC | DTC: 37 | DTC: 49% PR | HTN, HFSR, diarrhea, fatigue, hypopigmentation | Bible et al. (67–69) |
| MTC: 35 | MTC: 14% PR | |||||
| ATC: 15 | ATC: 0% responses | |||||
| Sorafenib | TKI: VEGFR1–3, PDGFR, RET, c-kit, BRAF | DTC, HCC, RCC | DTC: 209 | DTC: 12% PR | HTN, HFSR, diarrhea, fatigue | Brose et al. (70) |
| MTC: 31b | MTC: 8/31 (25%) PR | Lam et al. (71) Capdevila et al. (72) | ||||
| ATC: 3 | ATC: 1/3 PR | |||||
| Sunitinib | TKI: VEGFR1–3, PDGFR, RET, c-kit, CSF-1R, Flt-3 | GIST, RCC, pNET | DTC: 23 | DTC: 26% PR | HTN, HFSR, diarrhea, fatigue, hypopigmentation | Bikas et al. (73) |
| MTC: 58 | MTC: 24% PR | Carr et al. (74) | ||||
| Ravaud et al. (75) | ||||||
| Vandetanib | TKI: VEGFR2/3, EGFR, RET | MTC | MTC: 231 | MTC: 45% PR | HTN, HFSR, diarrhea, photosensitivity, fatigue | Wells et al. (76) |
| DTC: 72 | DTC: 6/72 PR | Leboulleux et al. (77) | ||||
| Vemurafenib | STKI: BRAF V600E | BRAF-mutated melanoma | PTC: 51 | Treatment naive: 39% PR | Skin rash and skin growths (including SCC), photosensitivity, alopecia, fatigue | Brose et al. (78) |
| Previous VEGFR: 27% PR |
Note that only peer-reviewed published trials are included.
Abbreviations: AE, adverse event; CSF-1R, colony-stimulating factor 1 receptor; FGFR1–4, FGF receptors 1–4; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HFSR, hand-foot skin reaction; HTN, hypertension; pNET, pancreatic neuroendocrine tumor; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; SEGA, subependymal giant cell astrocytoma; STKI, serine-threonine kinase inhibitor; TrkI, tropomyosin receptor kinase inhibitor; TS, tuberous sclerosis.
Data from the highest-phase trials were used. When more than one trial of the same phase was available, their data were pooled.
Pooled data.
FDA-approved drugs for thyroid cancer
Vandetanib
Vandetanib is the first medication approved by the FDA for the treatment of advanced and progressive and/or symptomatic MTC. Vandetanib is a multi-tyrosine kinase inhibitor (TKI) that blocks epidermal growth factor (EGF), RET, VEGF2, and VEGF3 receptors. The actions of vandetanib may lead to downregulation and/or inhibition of several hallmarks of cancer, such as the sustaining proliferative signaling mediated by tyrosine kinase receptors (responsible for cell migration, growth, and differentiation), angiogenesis, and apoptosis.
The antitumor effects of vandetanib were demonstrated in two phase 2 clinical trials in adult patients with locally unresectable or metastatic hereditary or sporadic MTC (81, 82). The larger of these trials included 30 patients (81). These patients initially received vandetanib at a dose of 300 mg daily. Twenty percent of these patients had a partial response (PR), whereas 53% had stable disease (SD) for >24 weeks as their best radiographic response. The other trial included 19 patients given 100 mg of vandetanib daily (82). Of these patients, 16% had PRs and 53% had SD for at least 24 weeks. These two trials demonstrated that the toxicity of vandetanib was acceptable.
The results of these phase 2 clinical trials led to the development of a phase 3 clinical trial known as the ZETA trial (76). In this trial, researchers compared the antitumor effects and toxicity of vandetanib with those of a placebo in patients with locally advanced/unresectable or metastatic MTC. The primary endpoint was the median progression-free survival (PFS) and the secondary endpoints included the objective response rate (ORR) and safety. The investigators recruited 331 patients to the ZETA trial. Patients were blindly randomized to receive either vandetanib at 300 mg daily or a placebo at a randomization ratio of 2:1. A large majority of the patients (94%) had distant metastases (mainly to the liver, lungs, and/or bone). The trial included patients with sporadic disease as well as those with germline RET mutations (10%). In total, 38% of the patients had either a germline or somatic RET mutation. The RET mutation status in 41% of the patients was not known. The patients were not required to have disease progression before entering the study. Crossover from the placebo to the treatment arm because of tumor progression was allowed. The study met the primary endpoint. Patients given vandetanib had a significantly longer median PFS duration than did patients given the placebo [30.5 vs 19.3 months; hazard ratio (HR), 0.46; 95% CI, 0.31 to 0.69; P < 0.0001]. Additionally, 44% of the patients had a PR. Clinical benefits of vandetanib, irrespective of RET germline status, tumor burden and location, age, and sex were noted. Because of the ZETA trial’s crossover design, an OS benefit was difficult to demonstrate.
Fifty-five percent of the patients in the ZETA trial had adverse events, with 24% having at least grade 3 toxicities. Diarrhea was the most common adverse event (56%) followed by skin abnormalities (rash, acne, and folliculitis; ∼50%). Other common side effects were abdominal pain, fatigue, headaches, hypertension, decreased appetite, and nausea, which happened in 20% to 30% of the patients. Proteinuria was noted in 10% of the patients. The most concerning side effect was prolongation of the QT interval, which can predispose patients to torsade des pointes and sudden death. Fourteen percent of the patients given vandetanib in the ZETA trial had prolongation of the QT interval. Treatment-related lethal toxicity occurred in five patients (2%). However, their causes of death were not clearly related to prolongation of the QT interval. A substantial number of patients required adjustment of the vandetanib dose to improve side effects. Thirty-five percent of the patients needed dose reduction, and 12% discontinued treatment. Rare but serious adverse events included interstitial lung disease, Stevens–Johnson syndrome, cerebrovascular accidents, congestive heart failure, and reversible posterior leukoencephalopathy. Vandetanib has a half-life of 19 days; therefore, the adverse events associated with vandetanib may persist for some time despite dose reduction or discontinuation. However, evidence suggesting that the efficacy of vandetanib decreases with doses <300 mg daily is lacking. The FDA approved vandetanib in 2011. Because of a black box warning for QT interval prolongation, vandetanib can be prescribed in the United States only by clinicians who have completed the Risk Evaluation and Mitigation Strategies program.
Vandetanib has also been effective in children and adolescents with hereditary MTC and in a few patients with ectopic Cushing syndrome due to progressive MTC. A phase 2 trial in children and adolescents with advanced MTC in the context of MEN2B included 16 patients (83). The results of that trial revealed that 47% of these patients had a PR with acceptable toxicity. Occasional case reports have described that vandetanib may improve the overwhelming clinical manifestations and biochemical abnormalities observed in patients with advanced MTC complicated with ectopic Cushing syndrome (84, 85). However, unpublished but extensive clinical experience also demonstrates that controlling the manifestations of ectopic Cushing syndrome at the same time that a patient is given vandetanib can be challenging, as a prolonged QT interval may develop because of difficulty in controlling hypokalemia. Additionally, the medications that inhibit the synthesis of cortisol may not be easy to use, and their effectiveness may be limited (ketoconazole has a black box warning because of liver toxicity, and metyrapone and mifepristone are expensive and may worsen hypokalemia). Also important to remember is that not every patient with MTC responds to treatment with vandetanib. Therefore, a bilateral adrenalectomy should be considered in patients with Cushing syndrome due to MTC before any systemic therapy.
Preclinical studies suggest that MTC associated with the RET V804M mutation is resistant to vandetanib. These cases of RET V804M mutation with an aggressive clinical course are rare, and experience with systemic therapy in them is limited. Until proven otherwise, therapy with vandetanib may still be offered to patients with MTC associated with germline or somatic RET V804M mutations. However, newer and more selective inhibitors of RET (see below) were designed to inhibit RET V804M (86) and may be considered within the context of a clinical trial.
Vandetanib in DTC
Although vandetanib is not approved for DTC, it has been studied in a randomized, double-blinded, placebo-controlled phase 2 clinical trial for advanced RAI-refractory DTC (77). A total of 145 patients were enrolled: 72 to the vandetanib group and 73 to the placebo group. The median PFS in the vandetanib group was 11.1 months, and that in the placebo group was 5.9 months (HR, 0.63; 60% CI, 0.54 to 0.74; P = 0.008). PRs occurred in both groups (six in the vandetanib group and four in the placebo group). The median OS did not differ between the two groups.
Cabozantinib
Cabozantinib was the second drug approved by the FDA for the treatment of advanced and progressive and/or symptomatic MTC. It is also approved for renal cell carcinoma (in combination with everolimus) and hepatocellular carcinoma. Cabozantinib is a TKI that blocks the c-MET, RET, and VEGF2 receptors. Similar to vandetanib, the actions of cabozantinib may lead to downregulation and/or inhibition of hallmarks of cancer, such as sustained proliferative signaling, angiogenesis, and apoptosis. Additionally, downregulation of the c-MET pathway may prevent invasiveness and metastatic spread and the development of tumor resistance; the latter effect may lead to more prolonged clinical responses than those to other TKIs. Cabozantinib is also a more potent antiangiogenic drug than vandetanib.
The discovery of cabozantinib as an effective treatment of MTC derived from a phase 1 dose escalation trial that included 37 patients with progressive, mainly sporadic MTC (87). Almost 50% of these patients previously received a TKI. Twenty-nine percent of these patients had a PR, and 41% had SD for at least 24 weeks. The maximum tolerated dose of cabozantinib was 140 mg daily. Because of these impressive results, a randomized, double-blinded, placebo-controlled phase 3 clinical trial was proposed (55). This trial, known as the EXAM trial, included 330 patients. Unlike the ZETA trial, patients in the EXAM trial were required to have had PD for 14 months before recruitment. Eighty-six percent of the patients had sporadic tumors, and 21% had received prior therapy with a TKI. The primary endpoint was PFS, and secondary endpoints included ORR, safety, and OS. To assess OS, the trial did not allow crossover between treatment arms. Patients continued receiving treatment until disease progression or development of unacceptable toxicity. The trial achieved its primary endpoint. Patients given cabozantinib had a median PFS of 11.2 months, whereas patients given a placebo had a median PFS of 4 months (HR, 0.28; 95% CI, 0.19 to 0.40; P < 0.001). Prolonged PFS was observed across the trial irrespective of age, sex, tumor burden and location, progression rate, prior treatment with a TKI, and mutational status (RAS and RET). The PFS duration achieved with cabozantinib was much shorter than that observed with vandetanib. Two factors explain the difference in PFS between the ZETA and EXAM trials: (i) the ZETA trial included patients with indolent disease (minimal or no progression), and (ii) the EXAM trial included patients previously given other TKIs who likely had very aggressive disease. Overall, 28% of the patients given cabozantinib had a PR, with a median duration of response of 14.7 months. No patients given a placebo had a PR.
A survival subanalysis of the EXAM trial, based on tumor genotype, produced interesting results (80). Of the 330 patients enrolled in this trial, 215 were evaluable for RET mutation status. One hundred sixty-nine (51%) patients had either germline or somatic RET mutations, 46 patients did not have a RET mutation, and 115 patients had an unknown RET mutation status because of insufficient samples for analysis, assay failure, sample discrepancy, or discovery of novel RET mutations not listed in the American Thyroid Association guidelines. A significant difference in median OS was observed in patients with MTC with RET M918T mutations treated with cabozantinib when compared with the placebo group (44.3 vs 18.9 months; HR, 0.60; 95% CI, 0.38 to 0.94; P = 0.03). Similar results for median PFS were seen. For the RET M918T mutation–negative patients, there was no difference in median OS in cabozantinib vs placebo-treated groups. Sixteen patients (4.8%) had somatic RAS mutations. There was a trend toward improved OS in patients with RAS mutations treated with cabozantinib that was not statistically significant; however, the numbers of patients were very small.
The most common side effect reported in the EXAM trial was diarrhea (63%) followed by elevation of serum concentrations of TSH, hypocalcemia, stomatitis, hand-foot syndrome, weight loss, nausea, lack of appetite, and fatigue (40% to 50%). About 30% of the patients experienced hypertension. Very few patients tolerated the daily cabozantinib dose of 140 mg. Seventy-nine percent of the patients needed dose reduction, and 16% discontinued treatment. Sixty-nine percent of the patients had grade 3 or 4 adverse events, and 6% experienced a fatal adverse event. Rare but serious adverse events included gastrointestinal perforation, fistula formation, bleeding, thromboembolic phenomena, osteonecrosis of the jaw, poor wound healing, and reversible posterior leukoencephalopathy. Current clinical trials with cabozantinib use a starting dose of 60 mg daily instead of the 140 mg daily dose listed on the package insert.
Selection of the first-line systemic therapy (cabozantinib vs vandetanib) for MTC must be individualized (88). The patient’s performance status, age, comorbidities, social aspects, disease progression, physical examination, medication availability, and especially safety are important aspects to consider when deciding which medication to provide. Clinical experience suggests that vandetanib is easier to tolerate than cabozantinib; thus, vandetanib may be the better option for fragile and/or older individuals. However, recognizing that some patients given cabozantinib may experience minimal or no side effects is important. Alternatively, cabozantinib is a more potent antiangiogenic medication than vandetanib. Thus, cabozantinib may be the more effective medication in patients with MTC that is rapidly progressing. In fact, about one third of the patients who exhibited clinical benefits in the EXAM trial had rapid disease progression before recruitment. Table 4 shows aspects that are important to consider when making decisions regarding which of these two medications to use first for MTC treatment.
Table 4.
Information That Can Be Incorporated Into Decision-Making Regarding Which Drug to Initiate First in Patients With MTC Needing Systemic Therapy
| Cabozantinib is favored in patients with the following characteristics: | Vandetanib is favored in patients with the following characteristics: |
|---|---|
| • Inherited long QT syndrome, personal or family history of cardiac arrest, unexplained fainting or unexplained seizures | • Diverticulitis, Crohn disease, ulcerative colitis, history of gastrointestinal bleeding, fistulas of any kind |
| • Exposure to medications known to prolong the QT interval | • History of external beam radiation therapy to the neck and/or mediastinum |
| • Electrolyte abnormalities that are difficult to correct (e.g., persistent hypocalcemia, hypokalemia, hypomagnesemia, hypoparathyroidism) | • Tumor that invades the trachea, esophagus, or major vessels or encases major vessels |
| • Photosensitivity, inability to protect against sunlight exposure | • Low body mass index |
| • Treatment with a CYP3A4 inducera | • Treatment with a CYP3A4 inhibitora |
| • Rapidly progressive MTC | • Patients with jobs requiring use of the hands (e.g., musician, housekeeper, dishwasher) |
A list of CYP3A4 inhibitors and inducers can be found at www.medicine.iupui.edu/clinpharm/ddis/.
Cabozantinib in DTC.
Cabozantinib, although not currently approved for DTC, has been studied in patients with RAI-refractory DTC in two clinical trials. The first was a phase 1 trial in which 15 patients with DTC were enrolled (89). Most of the patients had received VEGF pathway inhibitors prior to enrollment. Eight patients (53%) had a PR to treatment with cabozantinib. Patients received the FDA-approved starting dose of 140 mg daily. The second trial, specifically focusing on second- or third-line treatment with cabozantinib, was then undertaken (56). In this phase 2 trial, patients had to have experienced disease progression on the previous VEGFR-targeted therapy. The starting dose was much lower—60 mg daily—than in the other trial, with the option to escalate the dose to 80 mg daily for patients who did not have a response. Twenty-five patients were enrolled, 10 (40%) of whom experienced a PR. The median PFS and OS were 12.7 and 34.7 months, respectively.
Sorafenib
Sorafenib inhibits the VEGF1–3, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), KIT, and RET receptors. It also weakly inhibits RAF kinases but its effect on tumor shrinkage is likely due to its antiangiogenic properties. Sorafenib is approved for the treatment of 131I-refractory DTC, renal cell carcinoma, and hepatocellular carcinoma. Two prospective phase 2 trials demonstrated that sorafenib was efficacious against DTC (90, 91), leading to design of the phase 3 DECISION trial (70). In this randomized, double-blinded, placebo-controlled study, patients with 131I-refractory DTC received a starting dose of sorafenib of 400 mg twice a day. A total of 417 patients were enrolled globally, of whom 207 were randomized to sorafenib and 209 were randomized to a placebo. All patients were kinase inhibitor naive. The median PFS was 10.8 months in the sorafenib group and 5.8 months in the placebo group (HR, 0.59; 95% CI, 0.45 to 0.76; P < 0.0001). Patient allocation was unblinded at progression, and patients were allowed to cross over from the placebo to the treatment arm. The response rates were 12.2% (24/196) in the sorafenib group and 0.5% (1/201) in the placebo group. The median OS did not differ between the two groups. The most common adverse events in this trial were hand-foot skin reaction (76%), diarrhea (69%), alopecia (67%), and rash and fatigue (50% each). Weight loss and hypertension were also seen in 47% and 41% of patients, respectively.
Sorafenib in MTC.
Two phase 2 clinical trials have evaluated the antineoplastic activity of sorafenib in patients with MTC. The first trial included 16 patients with sporadic MTC (10 with PD during 12 months) (71). The primary endpoint was ORR. Patients were given sorafenib at 400 mg twice every day. One patient (6%) had a PR, and 14 patients (88%) had SD after 6 months of therapy. The median PFS was 17.9 months. The second clinical trial included 15 patients with MTC, with ORR as the primary endpoint (72). The ORR was 25% and the median PFS was 12 months. Both trials described acceptable toxicity. Hypertension, hand-foot syndrome, rash, and diarrhea were the most common adverse events.
Lenvatinib
Lenvatinib is an antiangiogenic TKI that inhibits the VEGF1–3, FGF1–4, PDGF, KIT, and RET receptors. It is approved by the FDA for the treatment of advanced and progressive 131I- refractory thyroid cancers DTC, hepatocellular carcinoma, and, in combination with everolimus, for renal cell carcinoma. Lenvatinib was first studied in thyroid cancer in a phase 2 trial in both patients with DTC and patients with MTC (79). Favorable results in the group with DTC led to the design of the phase 3 SELECT trial in patients with RAI-refractory DTC (63). This trial was a multicenter, randomized, placebo-controlled study in which 392 patients were assigned to lenvatinib (n = 261) and placebo (n = 131) arms. Patients who had been given no more than one VEGF kinase inhibitor were eligible. The median PFS was 18.3 months in the lenvatinib group and 3.6 months in the placebo group (HR, 0.21; 99% CI, 0.14 to 0.31; P < 0.001). A statistically significant PFS advantage was also observed in patients who had previously been treated with a VEGF kinase inhibitor. The overall response rate was 64.8% in the lenvatinib group, which included four complete responses (CRs). No differences in median OS were reported. However, a subanalysis of patients stratified by age did demonstrate an OS advantage in older patients (>65 years of age) who initially received lenvatinib vs those given the placebo (not reached vs 18.4 months; HR, 0.53; 95% CI, 0.31 to 0.91; P = 0.02) despite crossover from the placebo to the treatment arm (92).
Lenvatinib in MTC.
Lenvatinib was evaluated in a phase 2 clinical trial in patients with either hereditary or sporadic MTC (64). The patients had unresectable, progressive tumors during a period no longer than 12 months. The study allowed participation of patients previously treated with a TKI such as cabozantinib, sorafenib, sunitinib, or vandetanib. The primary endpoint was ORR. Treatment with lenvatinib was provided at a dose of 24 mg daily. The investigators recruited 59 patients to the trial. The study demonstrated that 36% of the patients had a PR. Specifically, 36% of the patients previously given a TKI and 35% of TKI-naive patients had PRs. Forty-four percent of the patients had SD. The median PFS was 9 months. Antitumor responses did not correlate with RET status. All patients experienced side effects, most of which were grade 1 to 2. The most common adverse events were diarrhea (75%), fatigue (53%), hypertension (51%), weight loss (42%), nausea and vomiting (∼41%), and headaches (41%). Biochemical abnormalities such as proteinuria and elevation of liver enzymes were common. Grade 3 adverse events included diarrhea (15%), hypertension and decreased appetite (7%), fatigue, dysphagia, and liver enzyme elevation (5%). Almost 60% of the patients required dose reduction, 75% required treatment interruption, and 24% discontinued treatment.
NTRK inhibitors in NTRK-fusion DTC and ATC
NTRK fusions are rare and can be seen across a number of solid tumors, including PTC and PTC-derived thyroid cancers such as PDTC and ATC. Although this fusion, as well as other fusions, are rare in thyroid cancer, if another driver mutation (such as BRAF, RAS, NF1) is not found in the tumor, the presence of a fusion event will be more likely. These fusions are oncogenic drivers and are usually mutually exclusive of other oncogenic driver mutations.
Larotrectinib
Larotrectinib is a highly selective inhibitor of tropomyosin receptor kinase (TRK) A (TRKA), TRKB, and TRKC and is approved by the FDA for treatment of solid tumors harboring NTRK fusions in adult and pediatric patients. This is one of the few “tumor-agnostic” indications granted by the FDA. The drug is available in both capsule and suspension formulation. The approved dose for adult patients and pediatric patients with a body surface area >1 m2 is 100 mg twice per day. The approved dose for pediatric patients with a body surface are <1 m2 is 100 mg/m2 twice daily.
The drug was studied in two phase 1 trials (one in adults and one in children) and a phase 2 basket trial in adults and adolescents. The data on 55 enrolled patients in these three trials were reported together in the primary analysis (66) and later updated with an additional 67 supplemental patients (93). In the primary analysis 9% of the patients had thyroid cancer, but the supplemental analysis was comprised of 19% thyroid cancer patients. The thyroid cancer subtypes were not reported. Of the 109 evaluable patients (all solid tumors) in the primary and supplemental data sets, 63% had a PR (which included 10 patients with thyroid cancer) and 17% had a CR (which included 3 patients with thyroid cancer). Only two patients with thyroid cancer did not have a PR or CR. Although few data on activity of larotrectinib in the brain exist, one patient with lung cancer and brain metastases had a PR in the brain lesions. The most common adverse events (regardless of attribution) reported in the primary analysis were increased liver function tests (42%), fatigue (36%), vomiting (33%), dizziness (31%), and nausea (31%). Most adverse events were grade 1 to 2 with only a minority of patients having grade 3 adverse events. There were no grade 4 treatment-related adverse events.
Entrectinib is also a selective inhibitor of TRKA, TRKB, and TRKC but also inhibits ALK and ROS1 tyrosine kinases (94). The drug was designed to penetrate the blood–brain barrier, making it possible to target brain metastases or primary brain cancers harboring NTRK 1/2/3, ROS1, or ALK fusions. The drug received FDA approval in 2019. Results of two phase 1 trials in patients with NTRK-fusion solid tumors, including patients with metastasis to the brain, showed favorable responses. No patients in this study had thyroid cancer. One patient with lung cancer achieve a CR in the brain (95). A larger cohort of patients was presented at the European Society for Medical Oncology annual meeting in 2018 and included data from three trials on 54 adult patients with NTRK-fusion–positive solid tumors (94). The ORR was 57.4%, and four patients (7%) had a CR. Of these 54 patients, 4 reportedly had thyroid cancer, and 2 of them had PRs. Entrectinib adverse events included fatigue (46%), dysgeusia (42%), paresthesias (29%), nausea (28%), and myalgias (23%), which were mostly grade 1 to 2. Higher-grade adverse events were rare and included grade 3 fatigue (4%), weight increase (2%), diarrhea and arthralgia (both 1%), and grade 4 eosinophilic myocarditis, which resolved after discontinuation of the drug (95).
FDA-approved BRAF/MEK inhibitors for the treatment of ATC
Combination of dabrafenib and trametinib
Dabrafenib is a selective inhibitor of BRAF V600E kinase, and trametinib is an inhibitor of MEK1/2 kinase. The two drugs are approved by the FDA for use in combination for melanoma, ATC, and non–small cell lung cancer that harbor a BRAF V600E mutation. The approval for BRAF V600E–mutated ATC was based on the results of a phase 2 clinical trial (58). Eligibility criteria were strict, allowing only patients who were able to swallow oral medications and those with a good performance status (Eastern Cooperative Oncology Group score ≤2). All patients initially received treatment at the FDA-approved starting dabrafenib dose of 150 mg twice daily and starting trametinib dose of 2 mg daily. In the initial report, 16 patients recruited during 2.5 years from 47 centers worldwide were included. The overall response rate according to central radiology review was 63%. The responses were durable, although the median PFS and OS were not reached at the time of publication. At a median follow-up time of 12 months, 79% of the patients were continuing to have responses to the treatment, and 80% were alive. An updated report at the meeting of the European Society for Medical Oncology in 2018 included data on 28 patients (96). The median PFS duration was 60 weeks, and the median OS was 86 weeks. The most common adverse events in the ATC cohort were fatigue (44%), fever (31%), nausea (31%), hyperglycemia (31%), and chills (28%). The most common grade 3 to 4 adverse event was anemia (13%), followed by fatigue, hyperglycemia, and diarrhea (all 6%).
Other Kinase Inhibitors Studied in Patients With Thyroid Cancer
Anlotinib
Anlotinib is an antiangiogenic multikinase inhibitor that inhibits VEGFR2, PDGF receptor (PDGFR), and FGF receptor (FGFR)1. The results of a phase 1 clinical trial of anlotinib demonstrated antitumor activity in patients with non–small cell lung cancer and MTC (97). Also, results of a phase 2 clinical trial in Chinese patients with advanced MTC were recently reported (98). The primary endpoint of this study was PFS. Secondary endpoints included ORR and safety. Fifty-nine patients were treated with anlotinib at 12 mg by mouth daily 2 weeks on and 1 week off. This trial demonstrated that 57% of the patients had a PR, and the PFS rate was 85% at 48 weeks. Calcitonin and CEA levels decreased by >50% when compared with the baseline in 78% of the patients. The most common adverse events were hand-foot syndrome, hypertriglyceridemia, hypercholesterolemia, fatigue, and proteinuria. Although these results are impressive, whether all patients had disease progression prior to recruitment is unclear. Nevertheless, thus far, anlotinib is the only medication tested in clinical trials involving a Chinese population. Therefore, anlotinib may become the first medication approved for the treatment of MTC in China. Cabozantinib and vandetanib have not been tested in clinical trials among Chinese individuals.
Axitinib
Axitinib is a potent antiangiogenic inhibitor of VEGFR1–3. Axitinib is approved for the treatment of renal cell carcinoma. Because this medication does not inhibit other tyrosine kinase receptors, it may be less toxic than other multikinase inhibitors. Axitinib has been evaluated in two phase 2 clinical trials in patients with different types of thyroid cancer. Patients in these trials received axitinib at 5 mg twice daily. The first trial included 45 patients with DTC, 11 patients with MTC, and 2 patients with ATC (53). The overall response rate was 30%. However, disease progression was not required for trial entry, making interpretation of these results difficult. The second trial included 34 patients with DTC and 11 patients with MTC (54). In this study, disease progression at trial entry was required. Two patients with DTC had a CR, whereas eight of them had a PR. Three of the patients with MTC had a PR.
“The authors concluded that vemurafenib was a potential new treatment option for BRAF-mutated PTC, but the drug has yet to be studied in a phase 3 trial.”
Dovitinib
Dovitinib is an antiangiogenic oral TKI that inhibits VEGFR, FGFR, PDGFR, and the RET receptor. A phase 2 multicenter trial in patients with progressive RAI-refractory DTC and MTC in South Korea enrolled 28 patients with DTC and 12 patients with MTC (99). The dose of dovitinib was 500 mg given on a 5 days on/2 days off schedule. Six patients with DTC and two patients with MTC had a PR. However, the median PFS was surprisingly short (5.4, 6.3, 3.2, and 4.5 months for all patients, those with PTC, FTC, and MTC, respectively). Common adverse events were diarrhea, anorexia, nausea and vomiting, and fatigue.
Dabrafenib and vemurafenib: selective BRAF inhibitors for the treatment of DTC
Vemurafenib is a selective inhibitor of mutated BRAF kinase with no antiangiogenic properties. It is FDA approved for treatment of BRAF-mutated melanoma. Vemurafenib was the first BRAF inhibitor used to treat BRAF-mutated thyroid cancer (100). A nonrandomized, open-label, multi-institutional phase 2 trial of single-agent vemurafenib was conducted in patients with BRAF V600E–mutated PTC (78). The primary endpoint was the best overall response in patients who had never received a VEGFR-targeted multikinase inhibitor (cohort 1). Patients previously given a VEGFR-targeted multikinase inhibitor (cohort 2) were analyzed as part of the secondary endpoints. In cohort 1, 38.5% of the patients had a PR, 57.5% had SD, and 3.8% had PD as the best response. The median PFS duration in this group was 18.2 months (95% CI, 15.5 to 29.3 months); the median OS had yet to be reached. Response rates in cohort 2 were lower, with 27.3% having a PR, 63.6% having SD, and 4.5% having PD. The median PFS was 8.9 months [95% CI, 5.5 months to nonestimable (NE)], and the median OS was 14.4 months (95% CI, 8.2 to 29.5 months). Common adverse events were skin rash, fatigue, alopecia, arthralgia, diarrhea, and multiple skin changes, including hand-foot skin reaction, skin papilloma, keratoacanthomas, and squamous cell carcinomas of the skin. The authors concluded that vemurafenib was a potential new treatment option for BRAF-mutated PTC, but the drug has yet to be studied in a phase 3 trial. A retrospective trial using vemurafenib in the same patient population had similar results but also demonstrated that thyroglobulin levels had no correlation with change in tumor size and that several patients had rising thyroglobulin levels in the face of tumor size reduction (101). The authors concluded that an increasing thyroglobulin level may represent tumor redifferentiation, which could lead to an increase in RAI uptake (discussed below).
Vemurafenib has been studied in a very small number of patients with BRAF V600E–mutated ATC as part of a basket trial in patients with nonmelanomatous BRAF-mutated solid tumors (102). One of seven patients had a CR, one had a PR, and one had PD as the best response. PFS and OS were not reported.
Dabrafenib is a selective inhibitor of mutant BRAF kinase that is approved as a single agent for the treatment of melanoma. The drug was first studied in patients with thyroid cancer in a basket trial and found to be a promising treatment of this disease (57). Based on these favorable findings, a randomized, multicenter, open-label phase 2 trial was conducted in patients with BRAF V600E–mutated PTC (103). Patients were randomized to single-agent dabrafenib (arm A) or dabrafenib in combination with trametinib (arm B). Patients receiving single-agent dabrafenib were allowed to cross over to the combination treatment upon disease progression. Ten of 26 patients (38%) in arm A and 9 of 27 patients (33%) in arm B had a PR. The authors used “minor response” (defined as a 20% to 29% decrease in the sum of the target lesions) in their predefined definition of ORR, thus reporting response rates of 50% in arm A and 54% in arm B. This definition of objective response has not been used in other thyroid cancer clinical trials; therefore, the rate cannot be compared with the ORRs in those trials. The median PFS was 11.4 and 15.1 months in arms A and B, respectively. Common adverse events included anemia, diarrhea, fever, fatigue, nausea, skin disorders, and alopecia.
Everolimus
Everolimus is a serine-threonine kinase inhibitor of mTOR. It is FDA approved for treatment of renal cell carcinoma, subependymal giant cell astrocytoma, and tuberous sclerosis. Everolimus has been studied in several trials for treatment of thyroid cancer. In all of these trials, the starting dose was 10 mg given by mouth daily. All trials required disease progression for trial entry.
The first reported trial was a multicenter, open-label, single-arm phase 2 study in South Korea that enrolled patients with all thyroid cancer histologies (59). Forty patients were enrolled, 38 of whom were evaluable for response (25 had DTC, 6 had ATC, and 9 had MTC). Two patients (one with PTC and one with FTC) had a PR. One patient with ATC had a marked reduction in tumor of 21%. The median PFS across histological groups was 47 weeks. The PFS according to histology was 43 weeks in DTC, 10 weeks in ATC, 46 weeks in PDTC, and not reached in MTC.
Another similar trial of patients with all thyroid cancer histologies in the Netherlands enrolled 28 patients with DTC, 7 patients with ATC, and 7 patients with MTC (60, 61). None of the patients had a PR. The median PFS and OS were 33 and 30 weeks, respectively, in the MTC group and 9 and 18 months, respectively, in the DTC group. Details on the ATC cohort were not given, but the investigators deemed the results to be disappointing. Of note, changes in tumor markers in patients with MTC and DTC did not correlate with clinical outcomes.
The most recent reported trial of everolimus was a multicenter phase 2 trial in patients with locally advanced or metastatic thyroid cancer of all histologic subtypes that enrolled 33 patients with DTC, 10 patients with MTC, and 7 patients with ATC (62). In this trial, patients with DTC and MTC were required to have disease progression within 6 months of enrollment to qualify for trial entry. The primary objective was PFS in patients with DTC. The median PFS in this group was 12.9 months, and the median OS was not reached. In patients with MTC, the median PFS and OS were 13.1 and 21.4 months, respectively. The researchers observed one PR in each histologic groups, including nearly complete remission in a patient with ATC with a TSC2 mutation. This case was described in detail previously (104).
Common adverse events associated with everolimus use are mucositis, rash, anorexia, anemia, and liver function test abnormalities. Less common but notable events are hyperglycemia, hypercholesterolemia, pneumonitis, and infection.
The combination of everolimus and sorafenib was studied in patients with DTC and MTC in another trial (105). Forty-one patients were enrolled in this trial, 15 of whom had undergone VEGF-targeted therapy. Of the patients with DTC, 17 (61%) had a PR. Of note, seven of nine patients with HTC had a PR. Also, 4 of 10 patients with MTC had a PR. Based on these favorable results, a phase 2 clinical trial in HTC was initiated (NCT02143726).
Pazopanib
Pazopanib is an antiangiogenic TKI that inhibits VEGF1–3, FGF1/2, PDGF, KIT, and RET receptors. This medication is FDA approved for the treatment of renal cell carcinoma and soft tissue sarcomas. Pazopanib was evaluated in a phase 2 clinical trial in patients with DTC, MTC, and ATC.
The DTC trial included 37 evaluable patients with progressive, RAI-refractory DTC (67). Patients received pazopanib at 800 mg daily and the primary endpoint was ORR. Eighteen patients had a PR (49%). Of note, the highest response rates were seen in patients with FTC and HTC, although they were not statistically different from the rates in patients with PTC.
The MTC trial included 35 patients with PD during a period of 6 months (69). Patients received pazopanib at 800 mg daily and the primary endpoint was ORR. The ORR was 14.3%, and 57% of the patients had SD (most with some degree of regression). One patient had a PR, and six patients with SD previously received cabozantinib, sunitinib, or vandetanib. The median PFS was 9.4 months. Toxicity was acceptable. The most common side effects were hypertension (33%), fatigue (14%), diarrhea (9%), and elevation of liver enzymes (6%). Nine percent of the patients discontinued treatment because of adverse events. One death was likely related to pazopanib (due to colitis and sepsis).
Pazopanib was also studied in a multi-institutional phase 2 trial for ATC (68). Again, patients received pazopanib at 800 mg daily and the primary endpoint was ORR. Fifteen patients were treated in the study, but the trial was stopped early due to futility. Although some disease regression was observed, no patients had a PR, and the median OS was 111 days. The researchers concluded that the drug had minimal activity in patients with ATC. Enrollment has completed for a randomized, multi-institutional, placebo-controlled phase 2 trial combining pazopanib or a placebo with cytotoxic chemotherapy as adjuvant treatment of ATC during irradiation, but results have yet to be reported (NCT01236547).
Sunitinib
Sunitinib is an antiangiogenic TKI that inhibits the VEGF1–3, PDGF, KIT, and RET receptors. Sunitinib is approved for the treatment of renal cell carcinoma and pancreatic neuroendocrine carcinomas. Three phase 2 clinical trials of sunitinib have been reported. A phase 2 study of patients with thyroid cancer included 29 evaluable patients with DTC and 6 evaluable patients with MTC (74). They received sunitinib at 37.5 mg daily. Eight patients with DTC and three patients with MTC had a response. Of the patients with DTC, one had a CR. The second trial included 41 patients with DTC, 4 patients with ATC, and 26 patients with MTC with progressive, advanced disease (75). Patients received sunitinib at 50 mg daily 4 weeks on and 2 weeks off. All patients were kinase inhibitor-naïve. Nine patients with DTC had a response (22%), including one CR. Ten patients with MTC had a PR (38%), and no patients with ATC had responses. The third study included only patients with DTC and demonstrated a response rate of 26% (6/23) (73). Patients received sunitinib at 37.5 mg daily.
The most common adverse events observed in these trials were fatigue, diarrhea, hand-foot syndrome, neutropenia, and hypertension.
Novel Therapies and Approaches for Thyroid Cancer
Selective RET inhibitors
LOXO-292 (selpercatinib)
LOXO-292 is a potent and selective RET receptor inhibitor. The drug targets both RET mutations (seen in MTC) and RET fusions (seen in PTC, PDTC, and ATC). The drug was designed to target the gatekeeper mutation, RET V804, which is associated with resistance to RET-targeted kinase inhibitors. LOXO-292 also crosses the blood–brain barrier and reaches therapeutic concentrations in the central nervous system, making it a potential treatment of brain metastases with RET mutation or fusion. Preliminary results of the LIBRETTO-001 phase 1 dose escalation/expansion trial were presented at the American Society of Clinical Oncology annual meeting in 2018 (106). The primary endpoints of this study are determination of the maximum tolerated dose, recommended phase 2 dose, and the safety and tolerability of the drug. Secondary endpoints include ORR and duration of response. This study allows participation of patients as young as 12 years of age with locally advanced or metastatic solid tumors that are refractory to standard therapies or who are intolerant of them. In the phase 1 portion of the trial, the maximum tolerated dose of LOXO-292 was not reached. Preliminary results for 22 patients with RET-mutant MTC [most with RET M918T mutations (62%)] demonstrated a 45% ORR. Forty-one percent of the patients had SD as the best radiographic response, and all of them had some degree of tumor regression. One patient had a CR. This patient had a germline RET V804M mutation, painful hepatomegaly, and diarrhea and previously received cabozantinib, lenvatinib, and vandetanib. The patient’s symptoms resolved while on LOXO-292. This case demonstrated that tumors with RET V804M mutations can respond to treatment with selective RET inhibitors such as LOXO-292.
Patients with RET-fusion solid tumors were also enrolled in this trial, including seven patients with DTC. All seven of these patients had a PR. Of all the patients with RET-fusion solid tumors, three had brain metastases that were considered target lesions and all three patients responded in the brain lesions.
“Although researchers have studied several…drugs for redifferentiation in DTC in the past without success…use of MEK and BRAF inhibitors has revived this field.”
The medication was very well tolerated. The most common adverse events were grade 1 to 2 fatigue, diarrhea, constipation, dry mouth, nausea, and dyspnea (10% to 20%). Only two grade 3–related adverse events have been reported. These were an asymptomatic elevation of alanine aminotransferase level and a case of tumor lysis syndrome. The maximum tolerated dose was not reached. The LIBRETTO-001 trial is now in the expansion phase, which includes more patients with RET-mutated MTC and RET-fusion cancers, including PTC, PDTC, and ATC.
BLU-667 (pralsetinib)
BLU-667 is also a highly selective and potent RET inhibitor. Similar to LOXO-292, it also targets RET fusions and RET mutations, including the RET V804 mutation. A phase 1 escalation/expansion clinical trial of BLU-667 is ongoing, and results were presented at the American Association for Cancer Research meeting in 2018 (107). The endpoints of this study include determination of the maximum tolerated dose, safety, pharmacokinetics, and preliminary antitumor activity. Twenty-five evaluable patients with MTC with RET mutation and one evaluable patient with PTC with RET fusion were enrolled in the phase 1 escalation portion of the trial. Of the 25 patients with MTC, the ORR was 40% (1 had a CR, 9 had a PR, and 15 had SD as their best response). The single patient with PTC achieved a PR with tumor shrinkage of ∼70%. Toxicity was minimal, which included constipation, increased alanine aminotransferase and aspartate amintransferase, hypertension, leukopenia, headache, insomnia, and fatigue.
Redifferentiation Therapy for DTC
Patients with advanced DTC often have RAI-refractory disease, likely due to decreased NIS expression, and are thought to have less differentiated and consequently more aggressive disease than do patients with RAI-avid disease. Redifferentiation therapy refers to treatment designed to increase or restore RAI uptake in tumors, enabling treatment with RAI. Although researchers have studied several different drugs for redifferentiation in DTC in the past without success, successful use of MEK and BRAF inhibitors has revived this field.
The BRAF V600E mutation has been implicated to play a role in loss of NIS, leading to RAI refractoriness. A series of experiments demonstrated loss of expression of several genes important for RAI uptake in an animal model of BRAF V600E–mutated thyroid cancer (24). Inhibition of the MAPK pathway by MEK or BRAF inhibitors partially restored expression of these genes and RAI uptake in the animal model. These findings led to a small proof-of-concept human trial of the MEK1/2 inhibitor selumetinib (75 mg daily) (108). Twenty patients with RAI-refractory DTC received selumetinib, 12 of whom had increased uptake of RAI upon a diagnostic whole-body scan. Eight patients reached the dosimetry threshold for RAI therapy and received RAI. Five of the eight patients had a PR to RAI. Of note, four of these patients had an NRAS mutation, and one had a BRAF V600E mutation. None of three patients with RET/PTC fusions had increased RAI uptake after treatment with selumetinib. Common adverse events included fatigue, rash, and elevated liver transaminase levels. One patient who received RAI was diagnosed with myelodysplastic syndrome that progressed to acute leukemia. A phase 2 trial with a similar design is under way (NCT02393690). Also, a randomized, placebo-controlled phase 3 study evaluating the CR rate for selumetinib in the setting of adjuvant treatment with RAI was recently reported (109). The patients included those the authors defined as having a high risk of treatment failure: primary tumor >4 cm, gross extrathyroidal extension (T4 disease), N1a or N1b disease with at least one lymph node measuring at least 1 cm, or N1a or N1b disease involving at least five lymph nodes. Patients with known distant metastases were excluded. The primary endpoint was to improve the CR rate by 20% in the treatment group over that of the placebo group. Of 400 enrolled patients, 233 were randomized to receive selumetinib plus RAI (n = 155) or a placebo plus RAI (n = 78). At 18 months, the CR rate was not significantly higher in the selumetinib plus RAI group than in the control group (OR, 1.07; 95% CI, 0.61 to 1.87; P = 0.8205). Subgroup analysis of mutation status (NRAS or BRAF) did not demonstrate any significant statistical differences in CR rate. The authors concluded that selumetinib combined with RAI did not improve the CR rate in patients with a high risk of primary treatment failure. The authors also concluded that the design of the trial may have been flawed, as the CR rate in the placebo group was substantially higher than they had predicted (39% vs the predicted 30%), and that the expected difference between the placebo and selumetinib group may have been too ambitious.
Another prospective trial designed to restore RAI uptake in patients with BRAF V600E–mutated, RAI-refractory PTC using the selective BRAF inhibitor dabrafenib enrolled 10 patients (110). Patients received 150 mg of dabrafenib twice a day for 25 days before undergoing a diagnostic whole-body scan. Patients who had RAI uptake continued receiving dabrafenib for another 17 days before receiving 150 mCi of 131I. Six patients had restoration of 131I uptake upon the diagnostic whole-body scan and received high-dose 131I. Of these six patients, two had a PR at restaging after 3 months. The expected adverse events with dabrafenib use were seen, including squamous cell carcinoma of the skin in one patient.
Long-term adverse events in patients given high-dose 131I after redifferentiation therapy have yet to be reported. However, a report of a retrospective clinical trial has described the experience with the use of this approach and the long-term adverse events (111). Thirteen patients who received a BRAF inhibitor (dabrafenib or vemurafenib), a MEK inhibitor (trametinib or an investigational MEK inhibitor), or a combination of BRAF and MEK inhibitors followed by a whole-body diagnostic scan were included in this report. Ten patients had PTC, one had FTC, and two had PDTC. Nine patients had BRAF V600E–mutated thyroid cancer, three had RAS-mutated thyroid cancer, and one did not have either mutation. Of the 13 patients, 8 had significant uptake upon a diagnostic whole-body scan and received a treatment dose with 131I. One patient who did not have increased uptake received empiric treatment with 131I. The median follow-up time for patients who received therapeutic 131I was 14.3 months (range, 4.5 to 20.6 months). All three patients with an RAS mutation, five patients with a BRAF mutation, and one patient with a wild-type BRAF/RAS mutation had significant uptake of 131I. Three of nine patients given RAI had a PR after high-dose treatment with 131I. All nine patients stopped receiving systemic therapy after RAI therapy and remained off systemic therapy for a median duration of 14.3 months (range, 45.0 to 20.6 months). Adverse events related to high-dose treatment with 131I after redifferentiation therapy included pneumonitis (n = 2) and bilateral sialadenitis with multifocal parotid ductal steonosis (n = 1). Symptoms resolved in these three patients within 3 months of onset of symptoms.
Whether inhibition of the MAPK pathway by RET or NTRK inhibitors restores RAI uptake in patients with RET- or NTRK-fusion PTC has yet to be determined.
Neoadjuvant Kinase Inhibitor–Based Therapy for DTC and ATC
Neoadjuvant chemotherapy refers to the use of systemic therapy prior to surgical resection of tumor. The goal of neoadjuvant chemotherapy is to shrink a tumor, improving surgical resection of it. Most kinase inhibitors used for thyroid cancer treatment are antiangiogenic (targeting VEGFR) and thus may lead to poor wound healing and fistula formation (112). VEGF is one of the most important factors that promote wound healing. Because most therapies for thyroid cancer inhibit VEGFR, the drugs must be discontinued several weeks prior to surgery to allow for proper postoperative wound healing. However, the selective BRAF, RET, and NTRK inhibitors have little to no antiangiogenic properties and therefore can be considered in the neoadjuvant setting. Use of neoadjuvant larotrectinib has already been described in children with NTRK-fusion sarcoma (113). No postoperative complications were seen.
A clinical trial of vemurafenib, a selective BRAF inhibitor, in patients with BRAF-mutated PTC in the neoadjuvant setting has been reported (114). The primary endpoint was a translational one, and the secondary endpoints were the safety and efficacy of this approach. Patients received 960 mg of vemurafenib twice daily for 56 days before undergoing surgical resection. Vemurafenib was taken until the day of surgery and then discontinued. Seventeen patients were enrolled with the intent to operate after 56 days. Of these patients, 11 underwent surgery. Six patients did not complete the 56 days of treatment with vemurafenib due to toxicity (n = 3) or disease progression (n = 2), and one patient refused surgery. Prior to surgery, three patients had a PR, and nine had SD with regression. Of the 11 patients who had surgery, 8 underwent either a complete resection (R0) or resection leaving microscopic residual disease (R1), and 3 underwent incomplete resection (R2). Two complications of surgery occurred: one patient had a pectoralis donor hematoma, and the other had fatal hemorrhage 2 weeks after surgery. The latter had tumor with carotid involvement. Adverse effects of vemurafenib were as expected.
A case report of neoadjuvant treatment with dabrafenib/trametinib/pembrolizumab (DTP) in a patient with BRAF V600E–mutated ATC was recently published (115). The patient was deemed to have unresectable disease prior to receiving DTP due to 360° carotid artery encasement and prevertebral fascial, laryngotracheal, and superior mediastinal involvement. After 3 months of DTP use, the patient had a remarkable response and underwent a total thyroidectomy, bilateral central compartment dissection, and bilateral lateral neck dissection following neoadjuvant therapy. The patient did not need laryngectomy or tracheostomy and had preserved ability to swallow. The patient resumed taking DTP after completing chemoradiation due to a local recurrence. This highlighted the fact that patients with ATC will likely have to continue DTP-based treatment after completion of surgery and chemoradiation.
“...MIBG is currently the only medication approved by the FDA for the treatment of metastatic or unresectable pheochromocytoma and paraganglioma.”
Radiopharmaceutical Medications for MTC
Radiopharmaceutical medications are molecules labeled with a radionuclide and having affinity for different cellular receptors. Interaction with these receptors allows for the intake of these drugs, releasing lethal radiation to the target cells. Because of their neuroendocrine origin, MTC cells express several receptors that are targetable by radiopharmaceutical agents. These receptors include those for somatostatin and the catecholamine transporter. Somatostatin is a peptidic hormone that regulates hormone synthesis and secretion and cell proliferation. The somatostatin receptors are members of the G-coupled receptor superfamily, and they are located in the membrane of MTC cells in some patients (116). The catecholamine transporter is a monoamine transporter responsible for the sodium chloride–dependent reuptake of extracellular noradrenaline. This transporter may also be located in the membrane of MTC cells (117). The catecholamine transporter allows for reuptake of noradrenaline to facilitate its storage and synthesis. Interest in these agents has increased substantially during the last year thanks to the FDA approval of lutetium-177 (177Lu)-DOTATATE for treatment of gastrointestinal and pancreatic neuroendocrine tumors and of high-specific-activity 131I meta-iodobenzylguanidine (MIBG) for the treatment of metastatic/unresectable pheochromocytoma and paraganglioma (118, 119). 177Lu-DOTATATE targets somatostatin receptor 2 (SSTR2), and high-specific-activity 131I MIBG targets the catecholamine transporter.
177Lu-DOTATATE and yttrium-90–DOTATOC
MTC cells produce somatostatin and express its receptors. SSTR2 is expressed by MTC cells (120). Cold (not labeled with radioactivity), short-acting, and long-acting octreotide have been used for the treatment of MTC. Although these medications may improve the hormonal manifestations of the disease (diarrhea, flushing), objective tumor responses are rarely reported (121). These observations demonstrated that cold somatostatin analogs have minimal or no antiproliferative activity in patients with MTC.
However, molecules labeled with radioactivity (hot molecules) may have a positive impact on these patients. In addition to palliation of hormonal manifestations of the disease, the drugs may be translocated to the cell interior, releasing a high dose of lethal radioactivity. Furthermore, toxicity in normal tissues may be minimal or absent, as most normal cells do not express SSTR2. Positron emission tomography with gallium-68–DOTATATE or other peptides (e.g., DOTATOC, DOTANOC) is necessary to confirm the expression of SSTR2 in MTC cells. Based on the intensity and distribution of the tracer, therapy with yttrium-90 (90Y)-DOTATOC or 177Lu-DOTATATE may be planned. Results of phase 2 clinical trials are encouraging. In a phase 2 clinical trial of 90Y-DOTATOC, researchers investigated survivorship and safety in 31 patients with advanced and progressive MTC (122). Responders had a significantly longer median OS than did nonresponders (108.8 vs 80.4 months; P < 0.0001). Another study of 90Y-DOTATOC in patients with thyroid cancer included 12 patients with progressive MTC among those with DTC and ATC (123). Forty-two percent of the patients with MTC and 29% of patients with DTC had SD (no patient with ATC had SD) as the best tumor response. None of them had PRs or CRs. The median time to progression of SD was 8 months (range, 3 to 14 months). No grade 3, 4, or 5 adverse events occurred. A third study of 90Y-DOTATOC included 21 patients with either unresectable or metastatic MTC (124). Clinical benefit was noted in 67% of the patients. The responses were mainly SD, and no single PRs were noted. However, two patients had a CR. Responses lasted from 3 to 40 months. Also, no grade 3, 4, or 5 adverse events were noted. Patients with smaller tumors had better responses.
A phase 2 clinical trial of 177Lu-DOTATATE given at 200 mCi four times every 6 to 10 weeks in seven patients with progressive MTC revealed that three patients had a PR and three patients had SD (125). One patient did not have a response to the therapy. All responders exhibited improvement in QOL and pain whenever present. No grade 3, 4, or 5 adverse events were reported. Of note, not all patients with MTC had high expression of SSTR2 at the time of screening. In fact, 16 patients were consented but 9 were excluded because of weak expression of SSTR2 according to indium-111–DTPA-octreotide scintigraphy.
Results of these studies demonstrated that a substantial number of patients with MTC, but not all, have expression of the SSTR2 receptor. Therefore, these are the patients who may benefit from treatment with peptide receptor radionucleotide therapy. Important facts to keep in mind are that expression of SSTR2 is heterogeneous and the detection rate of uptake on gallium-68–DOTATATE positron emission tomography is ∼50% (116). Therefore, a substantial number of patients with MTC may not benefit from this treatment. A phase 2 clinical trial of 177Lu-DOTATATE given at 200 mCi four times every 8 weeks in patients with progressive MTC is under development. The primary endpoint is ORR. This proposal allows for the participation of systemic therapy-naive patients as well as patients who were previously treated with other medications. Patients must demonstrate expression of SSTR2 in screening gallium-68–DOTATATE positron emission tomography.
High-specific-activity MIBG
High-specific-activity MIBG is currently the only medication approved by the FDA for the treatment of metastatic or unresectable pheochromocytoma and paraganglioma (118). High-specific-activity MIBG is manufactured from a solid-phase precursor (ultratrace). Unlike low-specific-activity MIBG (manufactured via simple isotope exchange), high-specific-activity MIBG releases a much higher dose of radiation to the tumor. This medication was approved for patients with metastatic pheochromocytoma and paraganglioma based on the impressive results of a pivotal phase 2 clinical trial (118). The intervention in that study was 500 mCi of high-specific-activity MIBG given twice ∼3 months apart (8 mCi/kg per dose for patients weighing <62.5 kg). Twenty-five percent of the patients met the primary endpoint, and 92% had a PR or SD. Many patients who failed to meet the primary endpoint exhibited antitumor responses, and their symptoms improved. Of note, antitumor responses became more obvious over time. Also, the number of PRs quadrupled from 6% at 3 months to 24% at 12 months, suggesting a prolonged antitumor effect. Toxicity was acceptable. Bone marrow suppression was noted in ∼50% of the patients. All patients recovered from bone marrow suppression over time (118).
Case series have demonstrated that patients with MTC may have tumors avid for 131I- or 123I-MIBG and that some of these patients had PRs after treatment with low-specific-activity MIBG (126). The sensitivity rate for 131I-MIBG has been reported to be 53% for familial/MEN-associated MTC and 87% for sporadic MTC (117). Some investigators have special interest in studying high-specific-activity MIBG in patients with MTC given the impressive long-term antitumor responses noted in patients with metastatic pheochromocytoma.
Immunotherapy-Based Trials
Only a few immunotherapy trials in patients with thyroid cancer have been published to date, but several trials are ongoing. A clinical trial of single-agent pembrolizumab (an anti–PD-1 antibody) in patients with DTC, KEYNOTE-028, was completed (127). In this trial, only patients with DTC with at least 1% PD-L1 expression in the tumor were included. Despite this, response rates were low, with only 2 of 22 (9%) patients having a PR. Most patients had SD [12/22 (55%)], and 36% had PD as their best response. The median PFS was 6.8 months (95% CI, 1.9 to 14.1 months), and the median OS was not reached (95% CI, 18.6 months to not reached). The 6- and 12-month OS rates were 100% and 89.9%, respectively. However, progression was not a requirement for trial entry. Diarrhea and fatigue were the most common adverse events. The low response rates may be explained by the fact that some of these patients may have had indolent disease capable of evading the immune system. Furthermore, tumor mutation burden is known to correlate with response to immunotherapy, but thyroid cancer is a low-mutation-burden malignancy (128).
Spartalizumab, an anti–PD-1 drug, has been studied as a single agent in ATC (129). Thirty patients were enrolled but only five patients (17%) achieved a PR and no OS data were reported. A strategy to improve responses of cancers that do not typically respond to single-agent immunotherapy is to combine this therapy with either a targeted therapy or with radiation. A clinical trial combining lenvatinib and pembrolizumab in patients with DTC is underway (NCT02973997). Also, a clinical trial using a backbone of atezolizumab (an anti–PD-L1 antibody) plus targeted therapy (vemurafenib/cobimetinib for tumors with BRAF mutations, cobimetinib for those with RAS or NF1 mutations, and bevacizumab for those with other mutations) in patients with ATC and PDTC is underway (NCT03181100). Data from a study using a mouse model of ATC suggest that anti–PD-1/PD-L1 treatment potentiates the effects of BRAF inhibitors on tumors (130).
Several clinical trials investigating the use of radiation to potentiate the effects of immunotherapy (abscopal effect) on thyroid cancer are also underway. Two of these trials use external beam radiation with immunotherapy for ATC (NCT03122496 and NCT02239900). Another trial uses RAI with immunotherapy for DTC (NCT03215095).
Data supporting the use of immunotherapy for MTC are limited. Two preclinical studies characterized the expression of immune markers in specimens from patients with MTC (131, 132). Both studies demonstrated that the MTC microenvironment is not immunogenic, with low PD-L1 expression and a low frequency of tumor-infiltrating lymphocytes. A clinical trial of pembrolizumab in patients with MTC is underway (NCT03072160). This study has two groups: patients who received previous treatment with an immune-stimulating vaccine and those who did not.
“For patients with life-threatening hypertension (grade 4), treatment with TKIs should be permanently discontinued.”
Management of Adverse Effects of Kinase Inhibitors
Management of side effects is critical for patients to be able to stay on their systemic therapy as long as possible. Several side effects in different organs and systems have been described. Also, their clinical presentations are variable. Some patients may discontinue treatment early because of severe toxicity, whereas others experience minimal or no side effects. Why some patients tolerate medications better than others is unclear. What is clear is that higher doses are usually associated with more frequent and intense side effects. Side effects also may differ according to patients’ genetic makeup, as adverse events tend to be more severe in Japanese individuals, which is the topic of a subanalysis of patients in the SELECT trial (133). An overview of how these side effects are best managed is provided below.
Cardiovascular toxicity
All TKIs currently approved for the treatment of DTC and MTC are multikinase inhibitors with antiangiogenic properties. Inhibition of VEGFRs decreases activation of endothelial nitric oxide synthase with activation of the endothelin system (134). A reduction in endothelial nitric oxide concentrations leads to increased vascular tone and hypertension (135, 136). Patients given antiangiogenic medications may experience severe hypertension, predisposing them to acute cardiovascular complications such as myocardial infarction and stroke. Blood pressure should be very well controlled prior to initiation of antiangiogenic TKIs. Specifically, blood pressure should be maintained at <140/90 mm Hg (137) and should be monitored several times weekly upon initiation of treatment with these agents. Evaluation of blood pressure should continue for 4 to 6 weeks and then monthly. In patients with hypertension (blood pressure >140/90 mm Hg), antihypertensive medications are indicated (137). Patients may be started on angiotensin-converting enzyme inhibitors (lisinopril, enalapril), angiotensin receptor blockers (irbesartan, losartan), beta-blockers (propranolol, atenolol, metoprolol), or calcium channel blockers (amlodipine, verapamil, nifedipine) (137). Doses vary depending on the patient’s blood pressure response and tolerability of the antihypertensives’ side effects (137). TKIs have interactions with many drugs; some antihypertensives may interact with TKIs, predisposing patients to toxicity (138). For instance, the combination of sunitinib and diltiazem may predispose patients to cardiac arrhythmias (138). In patients in whom the systolic blood pressure remains >160 mm Hg or the diastolic pressure remains >100 mm Hg (grade 3 hypertension) despite receiving optimal antihypertensive treatment, treatment with TKIs should be discontinued until the blood pressure normalizes (137). The TKI may then be restarted at a lower dose. For patients with life-threatening hypertension (grade 4), treatment with TKIs should be permanently discontinued (137, 139). For these patients, a different TKI or another type of systemic therapy should be considered. Grade 3 to 4 hypertension has been described in 10% of patients given sorafenib or sunitinib, 7% given pazopanib, 8% given cabozantinib, 9% given vandetanib, 20% given axitinib, and up to 44% given lenvatinib (usually with lenvatinib at doses ≥20 mg daily) (64, 67, 76, 140). Although caution must be applied with every patient given a TKI, treatment with lenvatinib requires very close blood pressure follow-up (64).
Inhibition of PDGFRβ adds to the cardiovascular toxicity mediated by VEGFR inhibition. PDGF inhibition leads to pericyte disappearance and severe deregulation of the microcirculation. These abnormalities may predispose patients to heart failure and coronary artery spasm (141). Periodic echocardiograms then may be recommended. In patients who experience congestive heart failure and/or ischemia, TKI-based therapy must be discontinued (137).
As discussed previously, QT interval prolongation and predisposition to torsade des pointes and sudden death have been described in patients with MTC treated with vandetanib (76). These abnormalities may also occur with treatment with other TKIs because of direct endothelial and cardiac toxicity (137). Thus, baseline and follow-up ECGs are required for patients while receiving vandetanib. After initiation of vandetanib, ECGs must be performed every week to every 2 weeks for 1 month and then once a month for 3 months. Therapy with vandetanib should be interrupted in a patient with QT interval prolongation >500 ms. Once the corrected QT interval improves to no more than 450 ms, therapy can be resumed at a lower dose with close ECG follow-up (137, 139). An important point to remember is that vandetanib has a long half-life of 19 days, and resolution of a prolonged QT interval may take a while (142). Also, electrolyte levels must be evaluated and abnormalities corrected at baseline and during follow-up (142). Patients with MTC may have diarrhea secondary to excessive production of calcitonin. Additionally, TKIs may cause diarrhea in any patient with thyroid cancer (143). Patients with diarrhea may experience hypokalemia, hypomagnesemia, and hypocalcemia. In patients in whom electrolyte abnormalities are difficult to control, an alternative kinase inhibitor associated with a lower risk of QT interval prolongation is preferred (e.g., cabozantinib may be preferred over vandetanib in patients with MTC and hypomagnesemia that is difficult to correct) (142).
Skin and mucosa side effects
Kinase inhibitors are frequently associated with mucocutaneous side effects (144–147). These side effects are caused by the inhibition of VEGFR, PDGFRβ , and/or EGF receptor (EGFR) (148, 149). Side effects may include rash, monomorphic acne (associated with vandetanib due to EGFR effects), and dry skin (150). These side effects are usually mild and self-limited (146, 150). Dry skin is successfully treated with moisturizers. EGFR-mediated acne can be treated with oral doxycycline or minocycline (151).
Palmar-plantar erythrodysesthesia syndrome, or hand-foot skin reaction, is the most frequent and challenging cutaneous side effect associated with kinase inhibitors. The severity of this condition is proportional to the dose (146, 152). Patients with hand-foot skin reaction may have hyperkeratosis, painful vesicles, skin desquamation, and even ulcers, especially in areas of friction or pressure (hands, soles, toes, elbows, knees, interdigital areas) (55, 146, 152). Ulcers may occasionally become infected (153). Manifestations of cutaneous erythrodysesthesia may appear in unusual places, such as the groin and genitalia (154). Symptoms usually develop 2 to 4 weeks after treatment starts. Patients with hand-foot skin reaction may complain of pain, burning sensation, paresthesia, and tingling (150). Hand-foot skin reaction can substantially alter the patient’s QOL, and in severe cases, it may lead to disability (155). Patients may discontinue therapy with kinase inhibitors because of hand-foot skin reaction (155–157). Preventable measures include the use of soft, wide shoes; thick socks made of cotton; avoidance of exposure to hot water; use of gentle bar soaps and refreshing towels with minimal friction; avoidance of chemicals and sanitizers (cleaning products, detergents); and use of moisturizers. Limiting physical activities that lead to friction or pressure on the hands and feet is important. Hyperkeratosis is usually the initial manifestation of hand-foot skin reaction (146). Hyperkeratotic lesions may be treated with 20% to 40% urea-based creams, and referral to a dermatologist and/or podiatrist is recommended (158). Topical steroids may improve inflammation. Clobetasol, triamcinolone, desonide, and other topical steroids may help reduce redness and pain (150, 159). Retinoids such as tazarotene help improve hyperkeratotic lesions and decrease inflammation (150). Topical treatments should be used two or three times daily. An important fact to remember is that excessive use of these medications may cause skin atrophy and/or irritation (150). Pain may be treated with nonsteroidal anti-inflammatory medications such as ibuprofen, naproxen, and celecoxib (150, 160). Patients with severe manifestations may have to discontinue treatment with the kinase inhibitor for a few days and reinitiate treatment upon recovery at a lower dose.
Patients given kinase inhibitors may also experience stomatitis, mucositis, and sore throat. Vandetanib is one of the few kinase inhibitors not associated with these complications (139). Measures to prevent mucositis include the use of lip balm, free alcohol mouthwash, and nonabrasive toothpaste (i.e., children’s toothpaste). For patients with severe mucositis, treatment with compounded mouthwashes (e.g., tetracycline/dexamethasone/nystatin or sucralfate/magnesium hydroxide and aluminum hydroxide/diphenhydramine) is indicated. Over-the-counter mouthwashes with mint flavor or alcohol should be avoided. Temporary holding of treatment with the kinase inhibitor and/or dose reduction may be needed (139). Avoiding salty, acidic, pungent, or spicy foods is recommended, as they irritate the mucosa (139). Occasionally, patients experience alopecia (150), which is reversed by discontinuing use of the kinase inhibitor.
Some skin side effects are mainly associated with specific kinase inhibitors. Sorafenib may predispose patients to hyperpigmentation, whereas sunitinib and pazopanib may cause hypopigmentation of the skin and hair (150). Also, patients given vandetanib are prone to sunburns and should avoid prolonged sun exposure (139, 142, 161). Photosensitivity is also common in patients given vemurafenib (162). Sunburn may develop a few minutes after sunlight exposure and can be severe (161). These patients should wear large-brimmed hats, cover their upper and lower extremities, and wear broad-spectrum sunscreen (161). In general, sunscreen use is recommended to everyone given kinase inhibitors, as photosensitivity reactions have been described with other TKIs (163).
Selective BRAF inhibitors such as dabrafenib and vemurafenib, as well as sorafenib, which has weak BRAF activity, may cause papules, pruritus, dry skin, paronychia, and alopecia (164–167). Dabrafenib and especially vemurafenib may predispose patients to hand-foot skin reaction, papilloma, keratoacanthoma, and squamous cell carcinoma (168). Keratoacanthomas and squamous cell carcinomas are easily treated with cryoablation. Patients given BRAF inhibitors may also experience new common nevi, dysplastic nevi, or even melanomas (169, 170). Patients should undergo periodic dermatologic assessment with a particular focus on skin cancer screening.
MEK inhibitors (trametinib, cobimetinib, selumetinib, and binimetinib) may predispose patients to dry skin, acne, and pruritus (164). Of note, patients given combinations of BRAF and MEK inhibitors exhibit lower rates of cutaneous toxicity (squamous cell carcinomas, keratoacanthomas, and hand-foot skin reaction) than do patients given single-agent BRAF inhibitors (171). MEK inhibitors inhibit RAS signaling, preventing abnormal skin cell proliferation (171).
Gastrointestinal side effects
Diarrhea is the most common gastrointestinal adverse event associated with treatment with kinase inhibitors, occurring in 50% to 60% of patients (172). The severity of the diarrhea is usually mild to moderate. About 10% of patients given TKIs may have severe diarrhea. Diarrhea is related to targeting of the VEGFRs and EGFR in the intestinal mucosa (172). Up to 30% of patients given vemurafenib have diarrhea, but it is less common with the use of dabrafenib (164). To prevent diarrhea, patients given TKIs may have to avoid intake of fat, dairy products, food with a high fiber content, and coffee. Patients should be well hydrated, as well. Treatment of diarrhea in these patients varies. For patients with grade 1 or 2 diarrhea, symptomatic treatment is recommended. Patients can be given 2 to 4 mg of loperamide followed by 2 mg every 4 hours after loose stool, 5 mg/0.05 mg of diphenoxylate/atropine every 6 hours, or alternating doses of these drugs (164). Treatment with cholestyramine may help some patients (139). For patients with severe diarrhea, treatment must be interrupted. These patients may restart treatment at a lower dose once they are properly hydrated and asymptomatic.
“A routine of mild to moderate cardiovascular and weight-bearing exercises may help some patients to preserve muscle mass and strength.”
Tincture of opium has been used in patients with MTC and calcitonin-mediated diarrhea (173). It may also palliate severe diarrhea in patients given kinase inhibitors (139). Many patients with MTC successfully treated with kinase inhibitors may exhibit disappearance of calcitonin-mediated diarrhea.
Constitutional symptoms
Fatigue is perhaps the most common adverse event noted in clinical practice in patients given molecularly targeted therapies. Most patients experience grade 1 or 2 fatigue. About 10% of patients exhibit grade 3 fatigue (55, 64, 76, 174). Fatigue may be self-limited or persistent and progressive. QOL may be substantially altered because of fatigue, and some patients may discontinue treatment permanently. Patients with fatigue must be evaluated for other underlying causes of this symptom, such as primary hypothyroidism, diarrhea, depression, tumor progression, and anemia. Treatment of fatigue is challenging. Patients may have to take frequent naps, hold treatment of a few days, and/or have a dose reduction. A routine of mild to moderate cardiovascular and weight-bearing exercises may help some patients to preserve muscle mass and strength. However, exercise may exacerbate hand-foot skin reaction.
Some patients given kinase inhibitors, particularly lenvatinib and cabozantinib, may experience weight loss (55, 64). Weight loss is usually mild to moderate and indolent. Nevertheless, careful monitoring of the patient’s nutritional status is recommended (139). Of note, overweight patients may benefit from this side effect. Clinical practice has demonstrated that some patients experience improvement of blood pressure, insulin resistance, diabetes mellitus, and other manifestations of metabolic syndromes.
Fever has been described in patients given BRAF inhibitors, particularly dabrafenib (164, 175). In patients who have fever, ruling out an infection before attributing the fever to the BRAF inhibitor is important. Guidelines for management of fever are outlined in Table 5 (164).
Table 5.
Suggested Management of Fever, Which Is Commonly Seen in Patients Receiving Dabrafenib
| Recommended Evaluations | Recommended Treatment | |
|---|---|---|
| Uncomplicated first occurrence | Evaluate patient for signs and symptoms of infection and consider imaging/testing as appropriate | Hold drug until fever resolves |
| Routine laboratory testing should include: | Encourage oral hydration | |
| • CBC with differential | Give antipyretic until fever resolves: | |
| • Blood cultures | • Acetaminophen every 6 h | |
| • Renal function testing | • Consider alternating acetaminophen and ibuprofen (ibuprofen should be used cautiously owing to possible renal insufficiency) | |
| • Consider a short trial of oral corticosteroids | ||
| Resume drug at same dose when fever is resolved | ||
| Consider fever prophylaxis: | ||
| • Acetaminophen and/or ibuprofen are reasonable for prophylaxis, but they may not be as effective as prednisone | ||
| • Prednisone (e.g., 10–20 mg daily); titrate down each week over 2–4 weeks | ||
| Uncomplicated second or subsequent occurrence | Evaluate patient for signs and symptoms of infection and consider imaging/testing as appropriate | Hold drug until fever resolves |
| Routine laboratory testing should include: | Encourage oral hydration | |
| • CBC with differential | Give antipyretic until fever resolves: | |
| • Blood cultures | • Acetaminophen every 6 h | |
| • Renal function testing | • Consider alternating acetaminophen and ibuprofen (ibuprofen should be used cautiously owing to possible renal insufficiency) | |
| • Consider oral corticosteroids | ||
| If uncomplicated fever recurs in spite of fever prophylaxis: | ||
| • If acetaminophen or ibuprofen was used for prophylaxis, switch to prednisone | ||
| • If prednisone was used as prophylaxis, use a higher dose of prednisone; once fever resolves, restart at the same dose | ||
| • If uncomplicated fever recurs in spite of a higher dose of steroid-based prophylaxis (e.g., 40 mg daily), reduce dabrafenib dose to the next lowest dose (e.g., from 150 mg to 100 mg) | ||
| Complicated first occurrence | Patient should be evaluated immediately by a physician to determine the severity of symptoms | Hold drug until fever resolves |
| Evaluate patient for signs and symptoms of infection and consider imaging/testing as appropriate | Encourage oral hydration or provide IV hydration | |
| Acute/severe issues (e.g., hypotension, arrhythmia) should be managed aggressively and require hospitalization | Give antipyretic until fever resolves (see guidelines for uncomplicated fever): | |
| Routine laboratory testing should include: | • Oral steroids are recommended; a higher dose may be necessary (e.g., prednisone at 20–40 mg depending on the severity of the event); titrate steroids down over a period of 2 wk | |
| • CBC with differential | Consider pyrexia prophylaxis; titrate steroids down slowly (e.g., over 2–4 wk) | |
| • Blood cultures | Once fever resolves, reduce dabrafenib dose to the next lowest dose (e.g., from 150 mg to 100 mg) | |
| • Renal function testing | In patients who experience severe symptoms (e.g., hypotension, arrhythmia), consider discontinuing dabrafenib permanently | |
| Complicated second occurrence | Repeat evaluations described above for complicated first occurrence | If severe symptoms recur in spite of dose reduction and pyrexia prophylaxis, consider discontinuing dabrafenib permanently |
Note: Adverse events are defined using the Common Terminology Criteria for Adverse Events (CTCAE), v5.0, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 27 November 2017. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Definitions: uncomplicated: temperature <104°F with no hypotension, dehydration, dizziness, arrhythmia, rigor, or grade 3–related symptoms; complicated: temperature ≥104°F with hypotension, dehydration, dizziness, arrhythmia, rigor, and/or grade 3–related symptoms.
Abbreviation: CBC, complete blood count.
[Reprinted with permission from Copyright Clearance Center: Springer, Hormones & Cancer, Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G. BRAF inhibitors: experience in thyroid cancer and general review of toxicity. Horm Cancer 2015;6:21–36.]
Arthralgias are more common in patients taking vemurafenib (176). Treatment with nonsteroidal anti-inflammatory medications (e.g., celecoxib) is recommended (164).
Hemorrhage, gastrointestinal perforation, and fistula formation
Patients given antiangiogenic kinase inhibitors may be at risk for bleeding, perforation, and fistula formation. These side effects are likely secondary to the antiangiogenic properties of many of these drugs (55, 112). Patients at risk for fistula formation include those with tumors that infiltrate the respiratory (larynx, trachea, bronchi) or gastrointestinal (esophagus) tract and patients who previously underwent radiation therapy to the neck and/or mediastinum. Patients at risk for major/fatal bleeding include those with brain metastases, tumors that infiltrate or encase major vessels, or chronic inflammatory conditions, such as ulcerative colitis, Crohn disease, peptic ulcers, and diverticulitis. These complications have been reported in patients taking cabozantinib, lenvatinib, sunitinib, and vandetanib (55, 112). Cabozantinib has a black box warning for fistula formation and bleeding (55). Patients at risk for these complications should explore other non-antiangiogenic treatments (e.g., BRAF inhibitors in patients with BRAF-mutated thyroid cancer, NTRK inhibitors for NTRK-fusion thyroid cancer, selective RET inhibitors for RET-driven thyroid cancers). Patients with hemoptysis or gastrointestinal bleeding should not receive antiangiogenic kinase inhibitors. If any of these complications develop, treatment with kinase inhibitors should be discontinued.
Wound healing
The antiangiogenic properties of several kinase inhibitors may predispose patients to delayed wound healing (172). Therefore, discontinuing these drugs at least 2 weeks before any elective surgery is prudent. Treatment may be resumed after surgery based on clinical judgment of good wound healing.
Neurologic and ocular side effects
Rare cases of reversible posterior leukoencephalopathy syndrome have been described in patients given different antiangiogenic kinase inhibitors (e.g., axitinib, lenvatinib, pazopanib, sorafenib, sunitinib) (177, 178). These patients often have poorly controlled hypertension and present with seizures, and several types of neurologic deficits that cannot be attributed to brain metastases. MRI of the brain may identify band-like hyperintense lesions in the gray and white matter of the central nervous system, especially in the parietal and occipital regions in association with vasogenic edema. Patients should discontinue taking TKIs and receive symptom therapy (e.g., blood pressure control, antiepileptic medications) (179). Reversible posterior leukoencephalopathy syndrome is usually reversible after discontinuation of the offending agent and blood pressure control (179). Nevertheless, use of the kinase inhibitors should be discontinued, and other non‐antiangiogenic therapies should be considered.
Uveitis has been described in patients given BRAF inhibitors such as dabrafenib and vemurafenib (180–183). This complication is rare, occurring in 1% to 2% of patients given these drugs (183). The manifestations of uveitis are several and overwhelming, including blurred vision, photophobia, pain, and temporal or permanent blindness (183). Patients must be referred for ophthalmologic evaluation, and use of BRAF inhibitors should be discontinued.
Primary hypothyroidism
All antiangiogenic TKIs may cause primary hypothyroidism due to thyroidal capillary regression. This complication occurs in a substantial number of patients given these drugs (184). Primary hypothyroidism is usually easily treatable with replacement thyroid hormone or an increase in the existing thyroid hormone dose (185). A previous study of sunitinib demonstrated that it induces activation of the type 3 deiodinase in peripheral organs such as the liver and that this likely explains why patients receiving other antiangiogenic kinase inhibitors may need larger than expected doses of thyroid hormone replacement therapy (186). Patients should undergo periodic follow-up measurement of thyroid hormone levels (every 2 to 3 months). Thyroid hormone replacement or suppressive therapies should be adjusted based on the TSH level.
QOL Implications
To date, no clinical trial for MTC or DTC has demonstrated that patients on systemic therapy receive a QOL benefit. In the DECISION trial, QOL was measured but scores were worse in patients on sorafenib compared with those treated with placebo (187). Unfortunately, in the SELECT and ZETA trials, no QOL questionnaires were collected. These were collected in the EXAM trial but the data were never reported. Patient-reported outcomes were collected in the clinical trial in patients with BRAF-mutated ATC (96). Patients who achieved a PR or CR to therapy had improvement in patient-reported outcome scores.
The financial toxicity should also be addressed with QOL, as financial toxicity has been associated with worse QOL. Financial toxicity has been evaluated in a large group of patients with thyroid cancer but included only 11 patients who were on kinase inhibitors. A significant number of patients experienced financial toxicity (188). It has been reported that patients with thyroid cancer have a higher risk of bankruptcy when compared with other types of cancers (189). These patients tended to be younger, which may not reflect the usual population of patients with thyroid cancer who will need kinase inhibitors. However, note that Bible et al. (190) have reported that kinase inhibitor therapy ranges from approximately US$95,000 to US$142,000 annually per person treated based on average retail prices in 2014.
Concluding Remarks
A better understanding of the molecular basis of thyroid cancers as well as development of more effective cancer therapies has revolutionized the treatment approach in patients with advanced thyroid cancer. During the last decade, we have seen five new indications for targeted therapies for thyroid cancer of all subtypes. Despite this, whether OS is improved with the use of these agents is unclear. Also, a fact that should be made clear is that although these drugs shrink tumors and delay progression, they are not curative. Thus, finding new approaches to treatment of thyroid cancer that lead to a cure will be the challenge during the next decade and beyond.
Glossary
Abbreviations:
- 90Y
yttrium-90
- 131I
iodine-131
- 177Lu
lutetium-177
- ATC
anaplastic thyroid cancer
- cfDNA
cell-free DNA
- CR
complete response
- DSS
disease-specific survival
- DTC
differentiated thyroid cancer
- DTP
dabrafenib/trametinib/pembrolizumab
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FDA
US Food and Drug Administration
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- FNA
fine-needle aspiration
- FTC
follicular thyroid cancer
- HR
hazard ratio
- HTC
Hürthle cell cancer
- IHC
immunohistochemistry
- MEK
MAPK kinase
- MEN
multiple endocrine neoplasia
- MIBG
meta-iodobenzylguanidine
- MTC
medullary thyroid cancer
- mTOR
mammalian target of rapamycin
- NGS
next-generation sequencing
- NIS
sodium iodide symporter
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PD-1
programmed cell death protein 1
- PDGF
platelet-derived growth factor
- PD-L1
programmed death-ligand 1
- PDTC
poorly differentiated thyroid cancer
- PFS
progression-free survival
- PR
partial response
- PTC
papillary thyroid cancer
- QOL
quality of life
- RAI
radioactive iodine
- SD
stable disease
- SSTR2
somatostatin receptor 2
- TCGA
The Cancer Genome Atlas
- TKI
tyrosine kinase inhibitor
- TRK
tropomyosin receptor kinase
- VEGFR
vascular endothelial growth factor receptor
Additional Information
Disclosure Summary: M.E.C. has received research funding from Genentech and Eisai and has participated in advisory boards for LOXO, Blueprint, and Ignyta. C.J. has received research funding from Molecular Insight Pharmaceuticals and Exelixis. M.R. has nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 2. Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 3. Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med. 1987;83(3):479–488. [DOI] [PubMed] [Google Scholar]
- 4. Gopal RK, Kubler K, Calvo SE, Polak P, Livitz D, Rosebrock D, Sadow PM, Campbell B, Donovan SE, Amin S, Gigliotti BJ, Grabarek Z, Hess JM, Stewart C, Braunstein LZ, Arndt PF, Mordecai S, Shih AR, Chaves F, Zhan T, Lubitz CC, Kim J, Iafrate AJ, Wirth L, Parangi S, Leshchiner I, Daniels GH, Mootha VK, Dias-Santagata D, Getz G, McFadden DG. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in hurthle cell carcinoma. Cancer Cell. 2018;34(2):242–255.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA. Integrated genomic analysis of hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34(2):256–270.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106(6):1286–1295. [DOI] [PubMed] [Google Scholar]
- 7. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, Bussolati G, Rosai J. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31(8):1256–1264. [DOI] [PubMed] [Google Scholar]
- 8. Xu B, Ibrahimpasic T, Wang L, Sabra MM, Migliacci JC, Tuttle RM, Ganly I, Ghossein R. Clinicopathologic features of fatal non-anaplastic follicular cell-derived thyroid carcinomas. Thyroid. 2016;26(11):1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, Lee NY, Shaha AR, Patel SG, Tuttle RM, Balm AJ, Shah JP, Ganly I. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986–2009 Memorial Sloan-Kettering Cancer Center experience. Thyroid. 2013;23(8):997–1002. [DOI] [PubMed] [Google Scholar]
- 10. Haugen BR, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–1057, discussion 1057–1058. [PubMed] [Google Scholar]
- 12. Nam SH, Bae MR, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, Kim SY. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018;87: 158–164. [DOI] [PubMed] [Google Scholar]
- 13. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maciel RM, Camacho CP, Assumpção LV, Bufalo NE, Carvalho AL, de Carvalho GA, Castroneves LA, de Castro FM Jr, Ceolin L, Cerutti JM, Corbo R, Ferraz TM, Ferreira CV, França MI, Galvão HCR, Germano-Neto F, Graf H, Jorge AA, Kunii IS, Lauria MW, Leal VL, Lindsey SC, Lourenço DM Jr, Maciel LM, Magalhães PK, Martins JR, Martins-Costa MC, Mazeto GM, Impellizzeri AI, Nogueira CR, Palmero EI, Pessoa CH, Prada B, Siqueira DR, Sousa MS, Toledo RA, Valente FO, Vaisman F, Ward LS, Weber SS, Weiss RV, Yang JH, Dias-da-Silva MR, Hoff AO, Toledo SPA, Maia AL. Genotype and phenotype landscape of MEN2 in 554 medullary thyroid cancer patients: the BrasMEN study. Endocr Connect. 2019;8(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, van Amstel HK, Lips CJ, Nishisho I, Takai SI, Marsh DJ, Robinson BG, Frank-Raue K, Raue F, Xue F, Noll WW, Romei C, Pacini F, Fink M, Niederle B, Zedenius J, Nordenskjöld M, Komminoth P, Hendy GN, Gharib H, Thibodeau SN, Lacroix A, Frilling A, Ponder BA, Mulligan LM. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276(19):1575–1579. [PubMed] [Google Scholar]
- 16. Saltiki K, Simeakis G, Anagnostou E, Zapanti E, Anastasiou E, Alevizaki M. Different outcomes in sporadic versus familial medullary thyroid cancer. Head Neck. 2019;41(1):154–161. [DOI] [PubMed] [Google Scholar]
- 17. Meijer JA, le Cessie S, van den Hout WB, Kievit J, Schoones JW, Romijn JA, Smit JW. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf). 2010;72(4):534–542. [DOI] [PubMed] [Google Scholar]
- 18. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13(11):644–660. [DOI] [PubMed] [Google Scholar]
- 19. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15(4):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasongsook N, Kumar A, Chintakuntlawar AV, Foote RL, Kasperbauer J, Molina J, Garces Y, Ma D, Wittich MA, Rubin J, Richardson R, Morris J, Hay I, Fatourechi V, McIver B, Ryder M, Thompson G, Grant C, Richards M, Sebo TJ, Rivera M, Suman V, Jenkins SM, Smallridge RC, Bible KC. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2017;102(12):4506–4514. [DOI] [PubMed] [Google Scholar]
- 21. Foote RL, Molina JR, Kasperbauer JL, Lloyd RV, McIver B, Morris JC, Grant CS, Thompson GB, Richards ML, Hay ID, Smallridge RC, Bible KC. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid. 2011;21(1):25–30. [DOI] [PubMed] [Google Scholar]
- 22. Edge SB, Compton CC. The American Joint Committee on Cancer: 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 23. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasad ML, Vyas M, Horne MJ, Virk RK, Morotti R, Liu Z, Tallini G, Nikiforova MN, Christison-Lagay ER, Udelsman R, Dinauer CA, Nikiforov YE. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122(7):1097–1107. [DOI] [PubMed] [Google Scholar]
- 26. Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, Gandhi M, Carty SE, Hodak SP, Luo J, Dacic S, Yu YP, Nikiforova MN, Ferris RL, Altschuler DL, Nikiforov YE. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci USA. 2014;111(11):4233–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritterhouse LL, Wirth LJ, Randolph GW, Sadow PM, Ross DS, Liddy W, Lennerz JK. ROS1 rearrangement in thyroid cancer. Thyroid. 2016;26(6):794–797. [DOI] [PubMed] [Google Scholar]
- 28. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, Ghossein RA, Fagin JA. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–E1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20(4):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, Murugan AK, Guan H, Yu H, Wang Y, Sun H, Shan Z, Teng W, Xing M. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99(6):E1130–E1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, Castro P, Prazeres H, Lima J, Amaro T, Lobo C, Martins MJ, Moura M, Cavaco B, Leite V, Cameselle-Teijeiro JM, Carrilho F, Carvalheiro M, Máximo V, Sobrinho-Simões M, Soares P. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99(5):E754–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krishnamoorthy GP, Davidson NR, Leach SD, Zhao Z, Lowe SW, Lee G, Landa I, Nagarajah J, Saqcena M, Singh K, Wendel HG, Dogan S, Tamarapu PP, Blenis J, Ghossein R, Knauf JA, Ratsch G, Fagin JA. EIF1AX and RAS mutations cooperate to drive thyroid tumorigenesis through ATF4 and c-MYC. Cancer Discov. 2019;9(2): 264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sherman EJ, Dunn LA, Ho AL, Baxi SS, Ghossein RA, Fury MG, Haque S, Sima CS, Cullen G, Fagin JA, Pfister DG. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer. 2017;123(21):4114–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS One. 2013;8(1):e54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, Morris JC, Ryder M, Alsidawi S, Hilger C, Bible KC. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017;102(6):1943–1950. [DOI] [PubMed] [Google Scholar]
- 39. Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. [DOI] [PubMed] [Google Scholar]
- 41. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. [DOI] [PubMed] [Google Scholar]
- 42. Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. 2018;28(9):1083–1093. [DOI] [PubMed] [Google Scholar]
- 43. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, McIver B, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sippel R, Smallridge RC, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Johnson-Chilla A, Hoffmann KG, Gurski LA. NCCN guidelines insights: thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. 2018;16(12):1429–1440. [DOI] [PubMed] [Google Scholar]
- 44. Gianoukakis AG, Dutcus CE, Batty N, Guo M, Baig M. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer. 2018;25(6):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cabanillas M, Zafereo M, Williams MD, Ferrarotto R, Dadu R, Gross N, Gunn GB, Skinner H, Cote G, Grosu HB, Iyer P, Busaidy NL. Recent advances and emerging therapies in anaplastic thyroid carcinoma. F1000 Res. 2018;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME. Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid. 2017;27(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith AL, Williams MD, Stewart J, Wang WL, Krishnamurthy S, Cabanillas ME, Roy-Chowdhuri S. Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma. Cancer Cytopathol. 2018;126(6):406–413. [DOI] [PubMed] [Google Scholar]
- 48. Bisschop C, Ter Elst A, Bosman LJ, Platteel I, Jalving M, van den Berg A, Diepstra A, van Hemel B, Diercks GFH, Hospers GAP, Schuuring E. Rapid BRAF mutation tests in patients with advanced melanoma: comparison of immunohistochemistry, Droplet Digital PCR, and the Idylla mutation platform. Melanoma Res. 2018;28(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sandulache VC, Williams MD, Lai SY, Lu C, William WN, Busaidy NL, Cote GJ, Singh RR, Luthra R, Cabanillas ME. Real-time genomic characterization utilizing circulating cell-free DNA in patients with anaplastic thyroid carcinoma. Thyroid. 2017;27(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iyer PC, Cote GJ, Hai T, Gule-Monroe M, Bui-Griffith J, Williams MD, Hess K, Hofmann MC, Dadu R, Zafereo M, Busaidy NL, Ferrarotto R, Subbiah V, Gross N, Gunn BG, Skinner HD, Garden AS, Cabanillas ME. Circulating BRAF V600E cell-free DNA as a biomarker in the management of anaplastic thyroid carcinoma. JCO Precis Oncol. 2018;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fussey JM, Bryant JL, Batis N, Spruce RJ, Hartley A, Good JS, McCabe CJ, Boelaert K, Sharma N, Mehanna H. The clinical utility of cell-free DNA measurement in differentiated thyroid cancer: a systematic review. Front Oncol. 2018;8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pupilli C, Pinzani P, Salvianti F, Fibbi B, Rossi M, Petrone L, Perigli G, De Feo ML, Vezzosi V, Pazzagli M, Orlando C, Forti G. Circulating BRAFV600E in the diagnosis and follow-up of differentiated papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98(8):3359–3365. [DOI] [PubMed] [Google Scholar]
- 53. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Capdevila J, Trigo JM, Aller J, Manzano JL, Adrián SG, Llopis CZ, Reig Ò, Bohn U, Cajal TR, Duran-Poveda M, Astorga BG, López-Alfonso A, Martínez JM, Porras I, Reina JJ, Palacios N, Grande E, Cillán E, Matos I, Grau JJ. Axitinib treatment in advanced RAI-resistant differentiated thyroid cancer (DTC) and refractory medullary thyroid cancer (MTC). Eur J Endocrinol. 2017;177(4):309–317. [DOI] [PubMed] [Google Scholar]
- 55. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, Shah K, Wright J, Shah MH. Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: results of a multicenter phase II International Thyroid Oncology Group trial. J Clin Oncol. 2017;35(29):3315–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, Cabanillas ME, Sherman SI, Ma B, Curtis M, Goodman V, Kurzrock R. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. 2015;25(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JH, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, Park CS, Nam KH, Kang SW, Kim MK, Kim SB, Lee SH, Kim HG, Na II, Kim YS, Choi MY, Kim JG, Park KU, Yun HJ, Kim JH, Cho BC. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24(12):3089–3094. [DOI] [PubMed] [Google Scholar]
- 60. Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, Roozen IC, Bos M, Corver WE, van Wezel T, Smit JW, Morreau H, Guchelaar HJ, Kapiteijn E. Everolimus in patients with advanced follicular-derived thyroid cancer: results of a phase II clinical trial. J Clin Endocrinol Metab. 2017;102(2):698–707. [DOI] [PubMed] [Google Scholar]
- 61. Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, van Wezel T, van Eijk R, Morreau H, Guchelaar HJ, Kapiteijn E. Beneficial effects of the mTOR inhibitor everolimus in patients with advanced medullary thyroid carcinoma: subgroup results of a phase II trial. Int J Endocrinol. 2015;2015:348124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, Haddad RI, Kraft S, Cabanillas ME, Rabinowits G, O’Neill A, Limaye SA, Alexander EK, Moore FD Jr, Misiwkeiwicz K, Thomas T, Nehs M, Marqusee E, Lee SL, Jänne PA, Lorch JH. Genomic correlates of response to everolimus in aggressive radioiodine-refractory thyroid cancer: a phase II study. Clin Cancer Res. 2018;24(7):1546–1553. [DOI] [PubMed] [Google Scholar]
- 63. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. [DOI] [PubMed] [Google Scholar]
- 64. Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R, Martins RG, Licitra LF, Shah MH, Bodenner D, Elisei R, Burmeister L, Funahashi Y, Ren M, O’Brien JP, Sherman SI. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22(1):44–53. [DOI] [PubMed] [Google Scholar]
- 65. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Sasaki T, Suzuki T, Fujino K, Dutcus CE, Takahashi S. Lenvatinib for anaplastic thyroid cancer. Front Oncol. 2017;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC III, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C; Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11(10):962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC, Lim WT, Bossou AR, Isham CR, Webster KP, Kukla AK, Bieber C, Burton JK, Harris P, Erlichman C; Mayo Phase 2 Consortium; Mayo Clinic Endocrine Malignances Disease Oriented Group. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97(9):3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Karlin N, Sideras K, Morris JC III, McIver B, Hay I, Fatourechi V, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Cher Goh B, Isham CR, Harris P, Erlichman C; Endocrine Malignancies Disease Oriented Group, Mayo Clinic Cancer Center, and the Mayo Phase 2 Consortium. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab. 2014;99(5):1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, Sammet S, Hall NC, Wakely PE Jr, Vasko VV, Saji M, Snyder PJ, Wei L, Arbogast D, Collamore M, Wright JJ, Moley JF, Villalona-Calero MA, Shah MH. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28(14):2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Capdevila J, Iglesias L, Halperin I, Segura A, Martínez-Trufero J, Vaz MA, Corral J, Obiols G, Grande E, Grau JJ, Tabernero J. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19(2):209–216. [DOI] [PubMed] [Google Scholar]
- 73. Bikas A, Kundra P, Desale S, Mete M, O’Keefe K, Clark BG, Wray L, Gandhi R, Barett C, Jelinek JS, Wexler JA, Wartofsky L, Burman KD. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur J Endocrinol. 2016;174(3):373–380. [DOI] [PubMed] [Google Scholar]
- 74. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. Phase II study of daily sunitinib in FDG-PET–positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16(21):5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ravaud A, de la Fouchardière C, Caron P, Doussau A, Do Cao C, Asselineau J, Rodien P, Pouessel D, Nicolli-Sire P, Klein M, Bournaud-Salinas C, Wemeau JL, Gimbert A, Picat MQ, Pedenon D, Digue L, Daste A, Catargi B, Delord JP. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer. 2017;76:110–117. [DOI] [PubMed] [Google Scholar]
- 76. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13(9):897–905. [DOI] [PubMed] [Google Scholar]
- 78. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner DL, Topliss D, Andresen C, O’Brien JP, Ren M, Funahashi Y, Allison R, Elisei R, Newbold K, Licitra LF, Sherman SI, Ball DW. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer. 2015;121(16):2749–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schlumberger M, Elisei R, Müller S, Schöffski P, Brose M, Shah M, Licitra L, Krajewska J, Kreissl MC, Niederle B, Cohen EE, Wirth L, Ali H, Clary DO, Yaron Y, Mangeshkar M, Ball D, Nelkin B, Sherman S. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28(5):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM, Wells SA, Balis FM. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19(15):4239–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nella AA, Lodish MB, Fox E, Balis FM, Quezado MM, Whitcomb PO, Derdak J, Kebebew E, Widemann BC, Stratakis CA. Vandetanib successfully controls medullary thyroid cancer-related Cushing syndrome in an adolescent patient. J Clin Endocrinol Metab. 2014;99(9):3055–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baudry C, Paepegaey AC, Groussin L. Reversal of Cushing’s syndrome by vandetanib in medullary thyroid carcinoma. N Engl J Med. 2013;369(6):584–586. [DOI] [PubMed] [Google Scholar]
- 86. Drilon A, Hu ZI, Lai GG, Tan DS. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes [published correction appears in Nat Rev Clin Oncol. 2018;15(3):150]. Nat Rev Clin Oncol. 2018;15(3):150. [DOI] [PubMed] [Google Scholar]
- 87. Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Müller T, Ratain MJ, Salgia R. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cabanillas ME, Hu MI, Jimenez C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat—and with which drug—those are the questions. J Clin Endocrinol Metab. 2014;99:4390–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24(10):1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O’Dwyer PJ, Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26(29):4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27(10):1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. 2017;35(23):2692–2699. [DOI] [PubMed] [Google Scholar]
- 93. Lassen U, Albert CM, Kummar S, van Tilburg C, DuBois SG, Geoerger B, Mascarenhas L, Federman N, Schilder R, Doz F, Berlin J, Oh DO, Bielack S, McDermott R, Tan D, Cruickshank S, Ku NC, Cox MC, Drilon A, Hong DS. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach [abstract]. Ann Oncol. 2018;29(Suppl 8):mdy279.397. [Google Scholar]
- 94. Demetri GD, Paz-Ares L, Farago AF, Liu SV, Chawla SP, Tosi D, Kim ES, Blakley C, Krauss JC, Sigal D, Bazhenova L, John T, Besse B, Wolf J, Seto T, Chow-Maneval E, Multani PS, Johnson AD, Simmons B, Doebele RC. Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372-001 [abstract]. Ann Oncol. 2018;29(Suppl 8): mdy424.017. [Google Scholar]
- 95. Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, Doebele R, Giannetta L, Cerea G, Marrapese G, Schirru M, Amatu A, Bencardino K, Palmeri L, Sartore-Bianchi A, Vanzulli A, Cresta S, Damian S, Duca M, Ardini E, Li G, Christiansen J, Kowalski K, Johnson AD, Patel R, Luo D, Chow-Maneval E, Hornby Z, Multani PS, Shaw AT, De Braud FG. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7(4):400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Keam B, Kreitman RJ, Wainberg ZA, Cabanillas ME, Cho DC, Italiano A, Stein A, Cho JY, Schellens JHM, Wen PY, Zielinski CC, Boran AD, Mookerjee B, Burgess P, Rangwala F, Subbiah V. Updated efficacy and safety data of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated anaplastic thyroid cancer (ATC) [abstract]. Ann Oncol. 2018;29(Suppl 8):mdy302.002. [Google Scholar]
- 97. Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li L, Wang F, Hao Y, Li C, Chi Y. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sun Y, Du F, Gao M, Ji Q, Li Z, Zhang Y, Guo Z, Wang J, Chen X, Wang J, Chi Y, Tang P. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid. 2018;28(11):1455–1461. [DOI] [PubMed] [Google Scholar]
- 99. Lim SM, Chung WY, Nam KH, Kang SW, Lim JY, Kim HG, Shin SH, Sun JM, Kim SG, Kim JH, Kang CW, Kim HR, Cho BC. An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer. Eur J Cancer. 2015;51(12):1588–1595. [DOI] [PubMed] [Google Scholar]
- 100. Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, Nolop K, Lee RJ, Sherman SI. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23(10):1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dadu R, Shah K, Busaidy NL, Waguespack SG, Habra MA, Ying AK, Hu MI, Bassett R, Jimenez C, Sherman SI, Cabanillas ME. Efficacy and tolerability of vemurafenib in patients with BRAFV600E-positive papillary thyroid cancer: M.D. Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100(1):E77–E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shah MH, Wei L, Wirth LJ, Daniels GH, De Souza JA, Timmers CD, Sexton JL, Beshara M, Nichols D, Snyder N, Devine CE, Konda B, Busaidy NL. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma [abstract]. J Clin Oncol. 2017;35(15 Suppl):6022 https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.6022. [Google Scholar]
- 104. Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, Gray N, Barletta JA, Guo Y, Swanson SJ, Ruan DT, Hanna GJ, Haddad RI, Getz G, Kwiatkowski DJ, Carter SL, Sabatini DM, Jänne PA, Garraway LA, Lorch JH. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sherman E, Ho AL, Fury M, Baxi SS, Dunn L, Lee JS, Lipson BL, Pfister D. Combination of everolimus and sorafenib in the treatment of thyroid cancer: update on phase II study [abstract]. J Clin Oncol. 2015;33(15 Suppl):6069 https://ascopubs.org/action/showCitFormats?doi=10.1200/jco.2015.33.15_suppl.6069. [Google Scholar]
- 106. Drilon A, Subbiah V, Oxnard GR, Bauer TM, Velcheti V, Lakhani NJ, Besse B, Park K, Petel JD, Cabanillas ME, Johnson ML, Reckamp KL, Boni V, Loong HHF, Wirth LJ. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers [abstract]. J Clin Oncol. 2018;36(15 Suppl):102 https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.102. [Google Scholar]
- 107. Subbiah V, Taylor M, Lin J, Hu M, Ou SI, Brose M, Garralda E, Clifford C, Palmer M, Tugnait M, Evans E, Shi H, Wolf B, Gainor J. Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in ARROW, a phase 1 study of advanced, RET-altered solid tumors. In: Proceedings of the American Association for Cancer Research Annual Meeting; 14–18 April 2018; Chicago, IL. Abstract CT043.
- 108. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ho A, Dedecjus M, Wirth LJ, Tuttle R, Tennaval J, So K, Carroll D, Hovey T, Thakre B, Fagin J. ASTRA: a phase III, randomized, placebo-controlled study evaluating complete remission rate (CRR) with short-course selumetinib plus adjuvant radioactive iodine (RAI) in patients (pts) with differentiated thyroid cancer (DTC). In: Proceedings of the 88th Annual Meeting of the American Thyroid Association; 3–7 October 2018; Washington, DC. Abstract short call oral 8.
- 110. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028–1035. [DOI] [PubMed] [Google Scholar]
- 111. Jaber T, Waguespack SG, Cabanillas ME, Elbanan M, Vu T, Dadu R, Sherman SI, Amit M, Santos EB, Zafereo M, Busaidy NL. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018;103(10):3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Blevins DP, Dadu R, Hu M, Baik C, Balachandran D, Ross W, Gunn B, Cabanillas ME. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014;24(5):918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. DuBois SG, Laetsch TW, Federman N, Turpin BK, Albert CM, Nagasubramanian R, Anderson ME, Davis JL, Qamoos HE, Reynolds ME, Cruickshank S, Cox MC, Hawkins DS, Mascarenhas L, Pappo AS. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer. 2018;124(21):4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cabanillas ME, Busaidy NL, Zafereo M, Waguespack SG, Hu MI, Hofmann MC, Sturgis EM, Sherman SI. Neoadjuvant vemurafenib in patients with locally advanced papillary thyroid cancer (PTC). Eur Thyroid J. 2017;6(Suppl 1):38. [Google Scholar]
- 115. Cabanillas ME, Ferrarotto R, Garden AS, Ahmed S, Busaidy NL, Dadu R, Williams MD, Skinner H, Gunn GB, Grosu H, Iyer P, Hofmann MC, Zafereo M. Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid. 2018;28(7):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Treglia G, Tamburello A, Giovanella L. Detection rate of somatostatin receptor PET in patients with recurrent medullary thyroid carcinoma: a systematic review and a meta-analysis. Hormones (Athens). 2017;16(4):362–372. [DOI] [PubMed] [Google Scholar]
- 117. Ilias I, Divgi C, Pacak K. Current role of metaiodobenzylguanidine in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin Nucl Med. 2011;41(5):364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L, Kostakoglu L, Serafini AN, Pampaloni MH, Jensen J, Armor T, Lin T, White T, Stambler N, Apfel S, DiPippo V, Mahmood S, Wong V, Jimenez C. Efficacy and safety of high-specific-activity 131I MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019;60:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Papotti M, Kumar U, Volante M, Pecchioni C, Patel YC. Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol (Oxf). 2001;54(5):641–649. [DOI] [PubMed] [Google Scholar]
- 121. Frank-Raue K, Ziegler R, Raue F. The use of octreotide in the treatment of medullary thyroid carcinoma. Horm Metab Res Suppl. 1993;27:44–47. [PubMed] [Google Scholar]
- 122. Iten F, Müller B, Schindler C, Rochlitz C, Oertli D, Mäcke HR, Müller-Brand J, Walter MA. Response to [90yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res. 2007;13(22 Pt 1):6696–6702. [DOI] [PubMed] [Google Scholar]
- 123. Waldherr C, Schumacher T, Pless M, Crazzolara A, Maecke HR, Nitzsche EU, Haldemann A, Mueller-Brand J. Radiopeptide transmitted internal irradiation of non-iodophil thyroid cancer and conventionally untreatable medullary thyroid cancer using. Nucl Med Commun. 2001;22(6):673–678. [DOI] [PubMed] [Google Scholar]
- 124. Bodei L, Handkiewicz-Junak D, Grana C, Mazzetta C, Rocca P, Bartolomei M, Lopera Sierra M, Cremonesi M, Chinol M, Mäcke HR, Paganelli G. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother Radiopharm. 2004;19(1):65–71. [DOI] [PubMed] [Google Scholar]
- 125. Vaisman F, Rosado de Castro PH, Lopes FP, Kendler DB, Pessoa CH, Bulzico DA, de Carvalho Leal D, Vilhena B, Vaisman M, Carneiro M, Corbo R. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clin Nucl Med. 2015;40(2):123–127. [DOI] [PubMed] [Google Scholar]
- 126. Castellani MR, Seregni E, Maccauro M, Chiesa C, Aliberti G, Orunesu E, Bombardieri E. MIBG for diagnosis and therapy of medullary thyroid carcinoma: is there still a role? Q J Nucl Med Mol Imaging. 2008;52(4):430–440. [PubMed] [Google Scholar]
- 127. Mehnert JM, Varga A, Brose MS, Agarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wirth LJ, Eigendorff E, Capdevila J, Pas-Ares LG, Lin C, Taylor MH, Ramlau R, Butler M, Delord JP, Horvath Z, Gelderblom H, Ascierto PA, Fasolo A, Fuhrer D, Wu H, Bostel G, Cameron S, Faris JE, Varga AI. Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with anaplastic thyroid cancer [abstract]. J Clin Oncol. 2018;36(15 Suppl):6024. https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.6024. [Google Scholar]
- 130. Gunda V, Gigliotti B, Ndishabandi D, Ashry T, McCarthy M, Zhou Z, Amin S, Freeman GJ, Alessandrini A, Parangi S. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer. 2018;119(10):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bongiovanni M, Rebecchini C, Saglietti C, Bulliard JL, Marino L, de Leval L, Sykiotis GP. Very low expression of PD-L1 in medullary thyroid carcinoma. Endocr Relat Cancer. 2017;24(6):L35–L38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dadu R, Canales JR, Wistuba I, Tian W, Lui H, Grubbs E, Cote G, Ray G, Williams MD, Cabanillas M. Immune markers in medullary thyroid cancer (MTC) and their clinical significance [abstract]. Thyroid. 2015;25(Suppl 1). Abstract 491. [Google Scholar]
- 133. Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, Wirth L, Robinson B, Sherman S, Suzuki T, Fujino K, Gupta A, Hayato S, Tahara M. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015;106(12):1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9(3):182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56(4):675–681. [DOI] [PubMed] [Google Scholar]
- 136. Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59(1):151–157. [DOI] [PubMed] [Google Scholar]
- 137. Herrmann J. Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Curr Oncol Rep. 2016;18(6):33. [DOI] [PubMed] [Google Scholar]
- 138. Bilbao-Meseguer I, Jose BS, Lopez-Gimenez LR, Gil MA, Serrano L, Castaño M, Sautua S, Basagoiti AD, Belaustegui A, Baza B, Baskaran Z, Bustinza A. Drug interactions with sunitinib. J Oncol Pharm Pract. 2015;21(1):52–66. [DOI] [PubMed] [Google Scholar]
- 139. Brose MS, Bible KC, Chow LQM, Gilbert J, Grande C, Worden F, Haddad R. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat Rev. 2018;66:64–73. [DOI] [PubMed] [Google Scholar]
- 140. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. [DOI] [PubMed] [Google Scholar]
- 141. Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, Dunner K Jr, Pati S, Bankson JA, Pasqualini R, Arap W, Bryan NS, Taegtmeyer H, Langley RR, Yao H, Kupferman ME, Entman ML, Dickinson ME, Khakoo AY. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5(187):187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cabanillas ME, Hu MI, Jimenez C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat—and with which drug—those are the questions. J Clin Endocrinol Metab. 2014;99(12):4390–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10(5):470–481. [DOI] [PubMed] [Google Scholar]
- 144. Lupu I, Voiculescu N, Bacalbasa N, Cojocaru I, Vrancian V, Giurcaneanu C. Cutaneous complications of molecular targeted therapy used in oncology. J Med Life. 2016;9(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 145. Shi VJ, Levy LL, Choi JN. Cutaneous manifestations of nontargeted and targeted chemotherapies. Semin Oncol. 2016;43(3):419–425. [DOI] [PubMed] [Google Scholar]
- 146. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72(2):221–236. [DOI] [PubMed] [Google Scholar]
- 147. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72(2):203–218. [DOI] [PubMed] [Google Scholar]
- 148. Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: part II. Targeted therapy. J Am Acad Dermatol. 2014;71(2):217.e1–217.e11. [DOI] [PubMed] [Google Scholar]
- 149. Zimmerman EI, Gibson AA, Hu S, Vasilyeva A, Orwick SJ, Du G, Mascara GP, Ong SS, Chen T, Vogel P, Inaba H, Maitland ML, Sparreboom A, Baker SD. Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP3K7-driven cell death. Cancer Res. 2016;76(1):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stanculeanu DL, Zob D, Toma OC, Georgescu B, Papagheorghe L, Mihaila RI. Cutaneous toxicities of molecular targeted therapies. Maedica (Buchar). 2017;12(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- 151. Lynch TJ Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12(5):610–621. [DOI] [PubMed] [Google Scholar]
- 152. Stănculeanu DL, Ardeleanu CM, Zob DL, Mihăilă RI, Toma OC, Simion L, Stovicek PO, Schenker M. Adenocarcinoma versus pancreatic neuroendocrine tumor—case report. Rom J Morphol Embryol. 2017;58(3):1091–1097. [PubMed] [Google Scholar]
- 153. Hoesly FJ, Baker SG, Gunawardane ND, Cotliar JA. Capecitabine-induced hand-foot syndrome complicated by pseudomonal superinfection resulting in bacterial sepsis and death: case report and review of the literature. Arch Dermatol. 2011;147(12):1418–1423. [DOI] [PubMed] [Google Scholar]
- 154. Guerra JR, Suelves AM, Bella A, Lolo D. Hand, foot and scrotal blisters in a patient with cancer receiving oral chemotherapy. BMJ Case Rep. 2014;2014:bcr2013202822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sibaud V, Dalenc F, Chevreau C, Roché H, Delord JP, Mourey L, Lacaze JL, Rahhali N, Taïeb C. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011;16(10):1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, Scotte F, Lorusso D, Dummer R, Lacouture ME, Lademann J, Hauschild A. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44(6):781–790. [DOI] [PubMed] [Google Scholar]
- 157. Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22(6):1585–1593. [DOI] [PubMed] [Google Scholar]
- 158. Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, Song T, Zhou W, Wang H, Yang W, Wang X, Yang Y, Shi L, Bai Y, Guo X, Ye SL. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894–900. [DOI] [PubMed] [Google Scholar]
- 159. Latif S, Fraga G, Gadzia J. Increased mast cell density in capecitabine-induced hand-foot syndrome: a new pathologic finding. J Drugs Dermatol. 2010;9(3):268–270. [PubMed] [Google Scholar]
- 160. Lin E, Morris JS, Ayers GD. Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology (Williston Park). 2002;16(12Suppl No. 14):31–37. [PubMed] [Google Scholar]
- 161. Giacchero D, Ramacciotti C, Arnault JP, Brassard M, Baudin E, Maksimovic L, Mateus C, Tomasic G, Wechsler J, Schlumberger M, Robert C. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch Dermatol. 2012;148(12):1418–1420. [DOI] [PubMed] [Google Scholar]
- 162. Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med. 2012;366(5):480–481. [DOI] [PubMed] [Google Scholar]
- 163. Udompanich S, Chanprapaph K, Rajatanavin N. Phototoxic reaction induced by pazopanib. Case Rep Dermatol. 2018;10(3):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G. BRAF inhibitors: experience in thyroid cancer and general review of toxicity. Horm Cancer. 2015;6(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600–mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Breaker K, Naam M, La Rosa FG, Flaig IP, Flaig TW. Skin cancer associated with the use of sorafenib and sunitinib for renal cell carcinoma. Dermatol Surg. 2013;39(7):981–987. [DOI] [PubMed] [Google Scholar]
- 168. Cebollero A, Puértolas T, Pajares I, Calera L, Antón A. Comparative safety of BRAF and MEK inhibitors (vemurafenib, dabrafenib and trametinib) in first-line therapy for BRAF-mutated metastatic melanoma. Mol Clin Oncol. 2016;5(4):458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zimmer L, Hillen U, Livingstone E, Lacouture ME, Busam K, Carvajal RD, Egberts F, Hauschild A, Kashani-Sabet M, Goldinger SM, Dummer R, Long GV, McArthur G, Scherag A, Sucker A, Schadendorf D. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30(19):2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Dalle S, Poulalhon N, Debarbieux S, Zaharia D, Mihm MC, Lacouture ME, Rosen A, Marghoob AA, Busam KJ, Depaepe L, Bringuier PP, Richez P, Baurain JF, Bressac-de Paillerets B, Balme B, Thomas L. Tracking of second primary melanomas in vemurafenib-treated patients. JAMA Dermatol. 2013;149(4):488–490. [DOI] [PubMed] [Google Scholar]
- 171. Chen FW, Tseng D, Reddy S, Daud AI, Swetter SM. Involution of eruptive melanocytic nevi on combination BRAF and MEK inhibitor therapy. JAMA Dermatol. 2014;150(11):1209–1212. [DOI] [PubMed] [Google Scholar]
- 172. Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63(4):249–279. [DOI] [PubMed] [Google Scholar]
- 173. Jiménez C, Hu MI, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am. 2008;37(2):481–496, x–xi. [DOI] [PubMed] [Google Scholar]
- 174. Kim G, McKee AE, Ning YM, Hazarika M, Theoret M, Johnson JR, Xu QC, Tang S, Sridhara R, Jiang X, He K, Roscoe D, McGuinn WD, Helms WS, Russell AM, Miksinski SP, Zirkelbach JF, Earp J, Liu Q, Ibrahim A, Justice R, Pazdur R. FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation. Clin Cancer Res. 2014;20(19):4994–5000. [DOI] [PubMed] [Google Scholar]
- 175. Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, Martín-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 176. Oneal PA, Kwitkowski V, Luo L, Shen YL, Subramaniam S, Shord S, Goldberg KB, McKee AE, Kaminskas E, Farrell A, Pazdur R. FDA approval summary: vemurafenib for the treatment of patients with Erdheim-Chester disease with the BRAFV600 mutation. Oncologist. 2018;23(12):1520–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shah RR. Anti-angiogenic tyrosine kinase inhibitors and reversible posterior leukoencephalopathy syndrome: could hypomagnesaemia be the trigger? Drug Saf. 2017;40(5):373–386. [DOI] [PubMed] [Google Scholar]
- 178. Osawa Y, Gozawa R, Koyama K, Nakayama T, Sagoh T, Sunaga H. Posterior reversible encephalopathy syndrome after lenvatinib therapy in a patient with anaplastic thyroid carcinoma. Intern Med. 2018;57(7):1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017;264(8):1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Joshi L, Karydis A, Gemenetzi M, Shao EH, Taylor SR. Uveitis as a result of MAP kinase pathway inhibition. Case Rep Ophthalmol. 2013;4(3):279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Draganova D, Kerger J, Caspers L, Willermain F. Severe bilateral panuveitis during melanoma treatment by dabrafenib and trametinib. J Ophthalmic Inflamm Infect. 2015;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Guedj M, Quéant A, Funck-Brentano E, Kramkimel N, Lellouch J, Monnet D, Longvert C, Gantzer A, Brézin AP. Uveitis in patients with late-stage cutaneous melanoma treated with vemurafenib. JAMA Ophthalmol. 2014;132(12):1421–1425. [DOI] [PubMed] [Google Scholar]
- 183. Choe CH, McArthur GA, Caro I, Kempen JH, Amaravadi RK. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol. 2014;158(4):831–837.e2. [DOI] [PubMed] [Google Scholar]
- 184. Bianchi L, Rossi L, Tomao F, Papa A, Zoratto F, Tomao S. Thyroid dysfunction and tyrosine kinase inhibitors in renal cell carcinoma. Endocr Relat Cancer. 2013;20(5):R233–R245. [DOI] [PubMed] [Google Scholar]
- 185. Del Fabbro E, Dev R, Cabanillas ME, Busaidy NL, Rodriguez EC, Bruera E. Extreme hypothyroidism associated with sunitinib treatment for metastatic renal cancer. J Chemother. 2012;24(4):221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kappers MH, van Esch JH, Smedts FM, de Krijger RR, Eechoute K, Mathijssen RH, Sleijfer S, Leijten F, Danser AH, van den Meiracker AH, Visser TJ. Sunitinib-induced hypothyroidism is due to induction of type 3 deiodinase activity and thyroidal capillary regression. J Clin Endocrinol Metab. 2011;96(10):3087–3094. [DOI] [PubMed] [Google Scholar]
- 187. Schlumberger M, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Worden F, Bockisch A, Nutting C, Shong Y, Sherman SI, Smit J, Chung J, Kappeler C, Molnar I, Keating K, Cella D, Brose MS. Phase III randomized, double-blinded, placebo-controlled trial of sorafenib in locally advanced or metastatic patients with radioactive iodine (Rai)-refractory differentiated thyroid cancer (Dtc)—exploratory analyses of patient-reported outcomes. In: Proceedings of the 83rd Annual American Thyroid Association meeting; 16–20 October 2013; San Juan, Puerto Rico. Oral abstract 100.
- 188. Souza JA, Grogan R, Aschebrook-Kilfoy B. Financial toxicity in thyroid cancer: an analysis from the North American Thyroid Cancer Survivorship study. J Clin Oncol. 2016;34(3 Suppl):17. [Google Scholar]
- 189. Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, Newcomb P, Hollingworth W, Overstreet K. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bible KC, Ain KB, Rosenthal MS. Protein kinase inhibitor therapy in advanced thyroid cancer: ethical challenges and potential solutions. Int J Endocr Oncol. 2014;2(2):145–151. [Google Scholar]