Abstract
Migratory animals play vital ecological roles in ecosystems worldwide, yet many species are threatened by human activities. Understanding the detailed patterns of habitat use throughout the migration cycle is critical to developing effective conservation strategies for these species. Migratory shorebirds undertake some of the longest known migrations, but they are also declining precipitously worldwide. To better understand the dynamics of shorebird declines along the East Asian–Australasian Flyway, we quantified the spatiotemporal foraging distribution of 17 migratory shorebirds at two critical stopover sites. We found that shorebirds exhibit substantial interspecific and site-specific differences in their foraging distributions. Notwithstanding these differences, however, the upper tidal flats appear to be especially important to most shorebirds by providing more than 70% of the birds' cumulative foraging time, twofold greater than their proportional area. Because the upper tidal flats are also more prone to coastal development, our findings may help to explain why shorebird populations along the flyway have declined much faster than the overall rate of tidal flat loss. Our work highlights the importance of protecting upper tidal flats to conserve migratory shorebirds and demonstrates the value of a detailed ecological understanding of habitat usage by migratory animals for conservation planning.
Keywords: migration, shorebird, habitat loss, conservation, habitat use, tidal flat
1. Introduction
Animal migration is a ubiquitous global phenomenon, and migratory species play vital ecological roles that are crucial for the functioning of ecosystems [1]. Yet, many migratory animals are rapidly declining due to human activities [2]. Pinpointing the causes and magnitude of these declines is challenging, given the need to understand species’ habitat requirements at multiple sites, often over very long distances. Moreover, many migrants face multiple threats, which further complicate efforts to conserve them. While identifying the magnitude and causes of declines in migratory animals usually requires large-scale monitoring efforts [3–7], insights from site-level studies that consider explicitly the ecology of individual species and site-specific threats are crucial to develop and implement effective conservation strategies to conserve migratory species [8,9].
Migratory shorebirds undertake some of the longest migrations of any migratory animals [10], but they are also declining precipitously worldwide [4,5], especially in the East Asian–Australasian Flyway (EAAF) [11–13]. Shorebird declines along the EAAF have been largely attributed to the loss of tidal flats, which provide critical stopover habitats, in the Yellow Sea region due to coastal development [14–16]. During the 1950s–1980s, more than half of the tidal flats in the Yellow Sea disappeared (−3.0% yr−1); during the 1980s–2000s, the rate of loss slowed down but was still −1.2% yr−1 [17,18]. However, many species along the EAAF have experienced much steeper population declines than the rate of habitat loss. Spoon-billed sandpipers (Calidris pygmaea) declined at an estimated rate of 26% yr−1 during 2002–2009 [19]. Five of the fastest-declining shorebird populations that winter in Australia and New Zealand were all declining at rates ranging between 5.1 and 7.5% yr−1 during the 1990s–2010s [11]. A more in-depth understanding of the nature of shorebird declines along the EAAF may help to conserve not only these species but shorebirds along other flyways as well.
The discrepancy between the rates of habitat loss and population decline remains a puzzle. One part of the explanation possibly lies in the fact that not all stopover sites along the EAAF are of equal value to migrating shorebirds [11]; destroying the key stopover sites could have disproportionately detrimental impacts on migrant populations [16,20]. However, where exactly habitat loss occurs within the tidal flats could also matter greatly. Tidal flats are a spatially heterogeneous habitat due to the elevational gradient and the resulting changes in substrate characteristics and benthic invertebrate communities from the high-tide line to the low-tide line [21–24]. Moreover, because of the tidal cycle, only a portion of the tidal flats can be accessed for most of the day at a given site, making the tidal flats temporally heterogeneous. Most of the previous studies of shorebirds' habitat preferences and foraging distributions along other flyways have been conducted during the low-tide periods when all of the tidal flats are freely accessible (e.g. [23,25,26]), while shorebird population monitoring studies are focused on the high-tide periods when birds are packed into a small area and therefore easier to count [20].
Thus, we lack a complete picture as to how shorebirds use the tidal flats, one that takes into account both the spatial and temporal distributions of foraging shorebirds across the entire tidal cycle, especially during ebbing and flooding tides when only a portion of the tidal flats is available to the birds [26]. This gap in our knowledge may impede our ability to develop sound conservation practices for these birds in terms of identifying areas of higher conservation values within the tidal flats. If the portions of the tidal flats that are most important to shorebirds are also the portions that are disproportionately lost due to development, then treating the tidal flats as a homogeneous habitat and averaging the rate of tidal flat loss across the entire range would underestimate the intensity of the threat from coastal development and its potential impact on shorebird populations.
Here, we quantified the habitat use patterns and preferences exhibited by migratory shorebird communities at two major stopover sites in the Yellow Sea. We conducted field surveys to simultaneously map the spatial and temporal foraging habitat use of shorebirds throughout tidal cycles. We identify the foraging types based on the species’ use of tidal flats and compare the consistency of these patterns between sites. We also assess the importance of different portions of the tidal flats based on the cumulative time that different species spent foraging on them. In so doing, we identify the upper tidal flat as probably crucially important for the conservation of migratory shorebirds.
2. Material and methods
(a). Field sites and data collection
We conducted fieldwork at two well-known stopover sites for migratory shorebirds in the Yellow Sea region of China: Rudong (32.5 N, 121.2 E) from September to October, 2016, and Nanpu (39.1 N, 118.2 E) from April to May, 2017 (figure 1), coinciding with the peak migration at each site. Rudong is an important site for 18 coastal shorebirds, especially during the southward migration [20,27], and Nanpu supports large populations of at least 12 shorebirds during the northward migration [28,29].
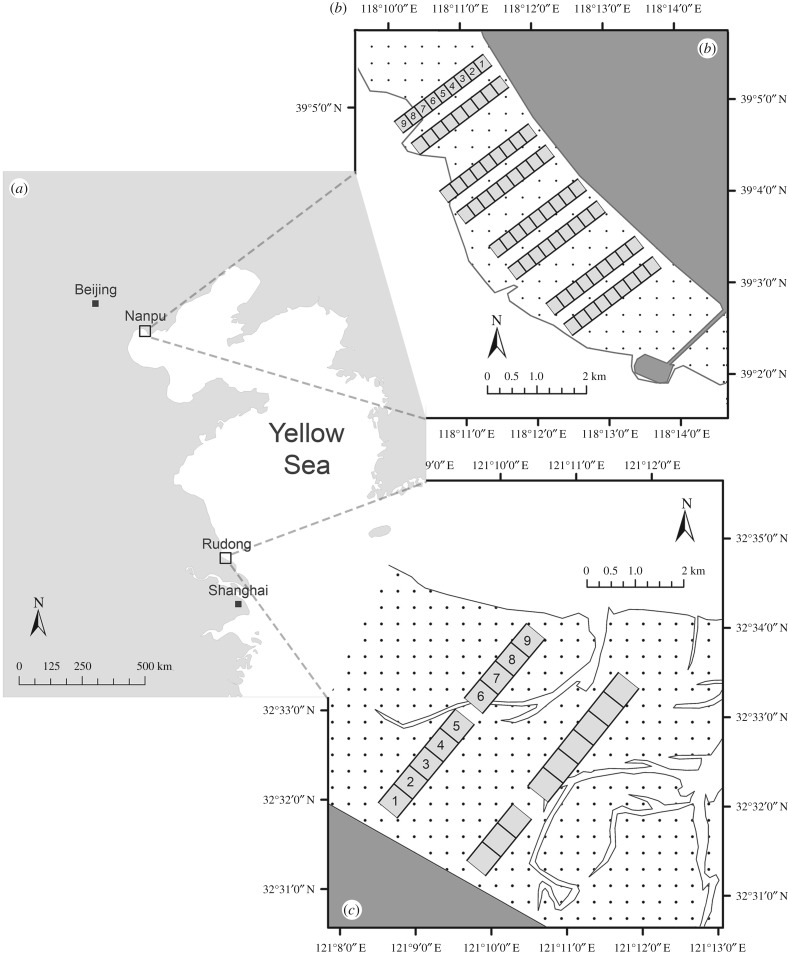
Figure 1.
Location of study sites in the Yellow Sea (a) and layout of transects at Nanpu (b) and Rudong (c). At Nanpu (b), each transect contained nine plots of 250 m × 250 m; adjacent transects constituted a transect pair whose data were combined and analysed together. At Rudong (c), each transect contained nine plots of 500 m × 500 m. Plots were numbered 1–9 based on their relative position to the seawall. In (b) and (c), dark grey areas represent land above the high-tide line; stippled areas represent tidal flats and light areas represent the sea beyond the low-tide line. Tidal flat data are from [35].
We set out survey transects on the tidal flats (figure 1) following the design of an earlier study [30]. The transects ran perpendicular to the tidal fronts and covered the entire elevational gradient from the seawall (approx. the average high-spring-tide line) to the low-tide line. The design and layout of transects differed slightly between the two sites because of local conditions: At Rudong, our two transects ran 4.5 km each and were set out so as to avoid areas of high human disturbance, or with numerous tidal channels, or covered by invasive Spartina grass; at Nanpu, four pairs of transects, running 2.25 km each, were set out to increase the coverage of the width of the tidal flat, and the data from each transect pair were analysed together to increase the temporal resolution of data. Along each of these transects, we delineated sets of nine adjacent survey plots, 500 m × 500 m at Rudong and 250 m × 250 m at Nanpu. Each plot was numbered 1 to 9, starting from the plots closest to the seawall, to indicate its relative position along the transects. At various steps of data analysis, we pooled the data from different transects at the same site in order to increase sample size by combining the value or data from plots of the same number. The average distance of each numbered plot to the seawall was calculated for the combined transects, using the distance to the seawall from the midpoint of each plot.
In daylight during the spring tides, two to four experienced observers conducted simultaneous surveys of foraging shorebirds in different plots along a given transect. We chose spring tides because they are the periods when the whole tidal flats are flooded and exposed over the course of a full tidal cycle. Each transect was surveyed for 2 to 4 days to ensure that every plot was counted at different times of the tidal cycle, with the cumulative data representing at least two entire tidal cycles pooled for the analysis. For each transect, observers walked parallel to the transects, staying at least 100 m outside the boundaries of each plot to avoid disturbing the birds, and recorded the species and numbers of foraging shorebirds within a plot at the time. The time spent surveying each plot varied depending on the number of shorebirds in it, and the surveyors started counting the next plot as soon as they finished the previous one. Time of exposure/immersion of each plot was also noted to estimate the speed of tidal front movements.
Shorebirds were identified to species in most cases. However, three pairs of similar-looking shorebirds were grouped together: great knot (Ca. tenuirostris) and red knot (Ca. canutus, ‘knots’; in Rudong only, as conditions permitted us to identify them to species at Nanpu); Eurasian curlew (Numenius arquata) and Far Eastern curlew (N. madagascariensis, ‘curlews’, both sites); lesser sand plover (Charadrius mongolus) and greater sand plover (Ch. leschenaultii, ‘sand plovers’, both sites). In total, 21 shorebird species (or species groups, referred to as ‘species’ hereafter) were recorded in 827 plot counts. Species recorded in 10 or fewer plot counts at a site were excluded from the analysis, leaving 13 species at Rudong and 13 species at Nanpu. With the data from different transects combined, the average interval (± s.d.) between two counts of the same plot was 15.7 (± 17.3) min, indicating a relatively high degree of temporal resolution in the counting data.
For easier interpretation of our analysis and results, we divided transects into three zones, each consisting of three plots, representing the upper (plot 1–3), middle (plot 4–6) and lower (7–9) tidal flats. We recognize that this does not follow the classical delineation of intertidal zones based on the tide levels of different tidal cycles [31]; however, due to the lack of detailed information on the topology of our study sites, we followed previous studies in delineating the zones by dividing each transect into three parts [30,32].
(b). Spatial and temporal distribution of shorebirds
To quantify shorebirds' spatial and temporal distributions, we calculated how the positions of shorebirds’ ‘abundance centroid’ changed with the proportion of tidal flats exposed. A species' ‘abundance centroid’ is the average position of all the individuals during a specific time, and the proportion of tidal flat exposed is a temporal index showing the relative time during a tidal cycle (figure 2). Because a given species may use tidal flats differently depending on the season and site [26], we analysed the counts of species that occur at both Rudong and Nanpu separately, and we will refer to them as two different populations hereafter because of the potential site-specific patterns.
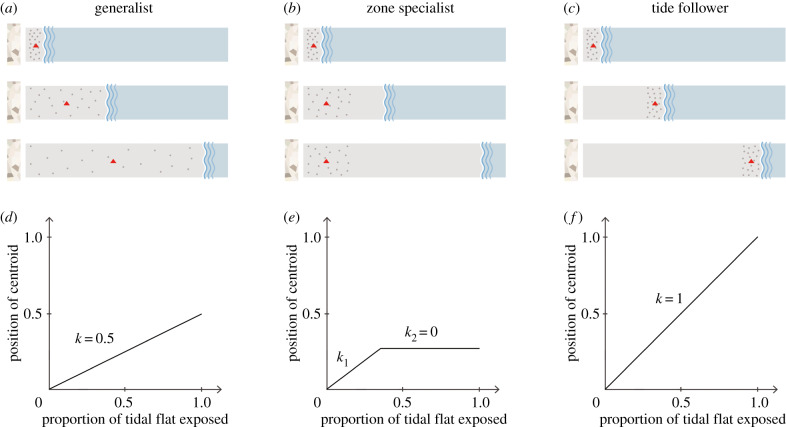
Figure 2.
Schematic representation of three foraging types exhibited by shorebirds. Boxes in (a, b, c) represent the same stretch of a tidal flat with the seawall (high-tide line) on the left and the low-tide line on the right, when varying proportions of the tidal flats are exposed, representing high, ebbing/flooding and low tides, from top to bottom. The leftmost blue wavy lines represent the location of the tidal front, with the areas to the right of the lines covered by water. Grey dots represent individual foraging shorebirds, and the red triangles represent the positions of the abundance centroid. (d, e, f) The centroid plots show the relationship between the position of the abundance centroid and the proportion of tidal flats exposed. (Online version in colour.)
Based on our field observations and consistent with previous studies [26,30], we proposed three foraging types for shorebirds using the tidal flats: generalists, zone specialists and tide followers (figure 2). Generalists feed whenever and wherever the tidal flats are exposed, thus, on a graph showing the positions of the abundance centroid against the proportion of tidal flat exposed (‘centroid plot’ in short), the slope k will be around 0.5, as the centroid will move towards the middle area when the entire tidal flat is exposed. Zone specialists exhibit a strong preference for a particular part of the tidal flats and remain there even when additional area becomes available. On the centroid plot, the line will be segmented: the slope will increase initially (k1) and then stay unchanged (k2, around 0). Tide followers, as the name suggests, follow the movement of the tidal front and feed in areas that are freshly exposed or about to be submerged. On the centroid plot, the slope k for tide followers will be around 1.
The abundance centroids were calculated in three steps. First, we transformed the time when each plot was counted into the position of the tidal front based on an average speed of tidal front movements which we measured during the survey separately for ebbing and flooding tides for each transect. When scaled to 0–1, with 0 representing the seawall and 1 representing the end of a transect (approx. the position of high- and low-tide lines, respectively), this relative position of the tidal front is also the proportion of tidal flats exposed. Next, we combined the counts from different transects at the same site and binned the plot counts into eight tidal periods based on the proportion of tidal flat exposed, in intervals of 0.125. The number, eight, was chosen subjectively as a trade-off between the number of time periods and the number of plot counts in each period. For each plot in a given tidal period, we calculated the average number of foraging individuals of each species. Finally, a species' abundance centroid for a given tidal period at a study site was calculated as the weighted average distance to the seawall, using the distance of each plot to the seawall and the average numbers of individuals of the species in the plot during the tidal period. We normalized the abundance centroids to the same scale as the proportion of tidal flat exposed.
To identify the foraging types, we tested the slope k using a linear model starting at (0, 0). We then used the segmented function in R Package segmented [33] to test how the relationship fits a broken line. We chose the following set of criteria to assign each population to a corresponding foraging type based on the results of statistical tests: generalists, 0.45 ≤ k ≤ 0.55 and r2 > 0.90; zone specialists, −0.05 ≤ k2 ≤ 0.05, r2 > 0.90; tide followers: 0.9 ≤ k ≤ 1.0 and r2 > 0.90. This set of criteria was chosen subjectively, and slightly altering them would not change our results and conclusion substantively. Testing whether the specific slope of the relationship falls in the given ranges was done by either testing whether the residuals of the specific linear model overlapped with 0 (for generalists and tide followers), or by overlapping the 95% CI (±1.96 SE) of k2 with the specified range (for zone specialists). Note that these criteria are not mutually exclusive or exhaustive: if a species was assigned to more than one type, we used Bayesian information criterion (BIC) to choose the better fit between the linear and segmented models [34]; if a species was not assigned to any type, we grouped that species as ‘not assigned’.
(c). The importance of different tidal flat zones
To quantify the importance of different tidal flat zones as foraging habitat to shorebirds, we calculated the cumulative foraging time in bird*minute(s) for each plot across entire tidal cycles. The cumulative foraging time takes into account both how many birds used the area and how long they spent feeding there, providing a comparable and quantitative measurement of habitat importance for within-site comparisons. Assuming the birds were constantly foraging between successive plot counts, for each plot in a transect, we plotted the changes in the number of foraging individuals for each species against the time during the tidal cycle and calculated the area under the curve, i.e. the cumulative foraging time of the species for the plot.
To determine the importance of different zones of the tidal flats to the overall foraging by shorebirds, we summed the cumulative foraging time for each species across all transects at the same site. This enables us to show the relative importance of each zone and to simulate the instantaneous impacts of coastal development of the tidal flats on shorebird foraging by calculating the loss of foraging time, assuming that the habitat loss progresses from the seawall to the low-tide line, with no rapid population response or reassortment by the benthic invertebrate fauna upon which the shorebirds feed in the remaining undeveloped portions of the tidal zone.
3. Results
(a). Generalists, zone specialists and tide followers
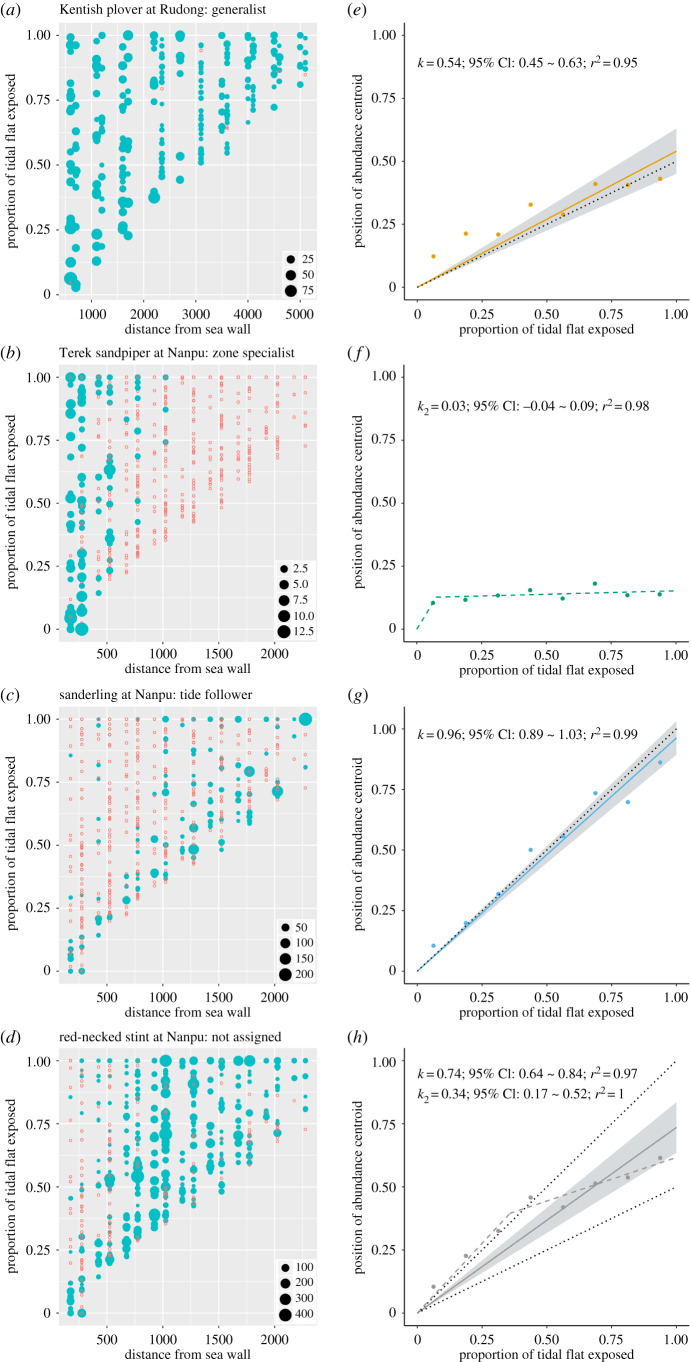
Among the 26 populations recorded, we identified eight generalists, 11 zone specialists, and three tide followers (table 1; figure 3; electronic supplementary material, figure S1). Four populations were not assigned to any type, mostly due to small sample sizes or intermediate patterns. Among the nine species that occur at both Rudong and Nanpu, seven species were assigned a foraging type at both sites, and among them, only two species, the Terek sandpiper (Xenus cinereus) and bar-tailed godwit (Limosa lapponica), exhibited the same foraging type at both sites.
Table 1.
Foraging types of shorebirds at Nanpu and Rudong. Each shorebird population was grouped into a foraging type by testing whether the slope of the relationship between the positions of the species' abundance centroid and the proportion of tidal flats exposed falls within the specified range of values. –: Not recorded or with too few observations for analysis.
| species | Nanpu | Rudong |
|---|---|---|
| grey plover Pluvialis squatarola | zone specialist | generalist |
| Kentish plover Charadrius alexandrinus | zone specialist | generalist |
| sand plovers Ch. mongolus and Ch. leschenaultii | – | generalist |
| curlews Numenius madagascariensis and N. arquata | not assigned | generalist |
| bar-tailed godwit Limosa lapponica | zone specialist | zone specialist |
| ruddy turnstone Arenaria interpres | generalist | zone specialist |
| great knot Calidris tenuirostris | tide follower | – |
| red knot Ca. canutus | generalist | – |
| knots Ca. tenuirostris and Ca. canutus | – | not assigned |
| curlew sandpiper Ca. ferruginea | zone specialist | – |
| red-necked stint Ca. ruficollis | not assigned | generalist |
| sanderling Ca. alba | tide follower | – |
| dunlin Ca. alpina | tide follower | zone specialist |
| Terek sandpiper Xenus cinereus | zone specialist | zone specialist |
| grey-tailed tattler Tringa brevipes | – | not assigned |
| common greenshank T. nebularia | zone specialist | generalist |
| marsh sandpiper T. stagnatilis | – | zone specialist |
Figure 3.
Distribution and centroid plots of four representative shorebird populations. We show here the representative populations of the three foraging types, and one population that was not assigned. In the spatiotemporal distribution plots (a, b, c, d), the number of foraging individuals recorded in a plot count is represented by the size of the solid blue circles, and the red open circles represent counts with 0 individuals. In the centroid plots (e, f, g, h), solid circles represent the positions of abundance centroid calculated for each tidal period along the tidal flats (0: seawall/high-tide line; 1: low-tide line). The solid line is the best-fitting line for the centroids passing through (0, 0), with 95% confidence intervals shown in grey. The dotted reference line has a slope of 0.5 (generalist) or 1 (tide follower). The dashed line represents the predicted broken-line relationship (zone specialists). All analysed populations are in electronic supplementary material, figure S1. (Online version in colour.)
(b). Upper tidal flats as critical foraging habitat
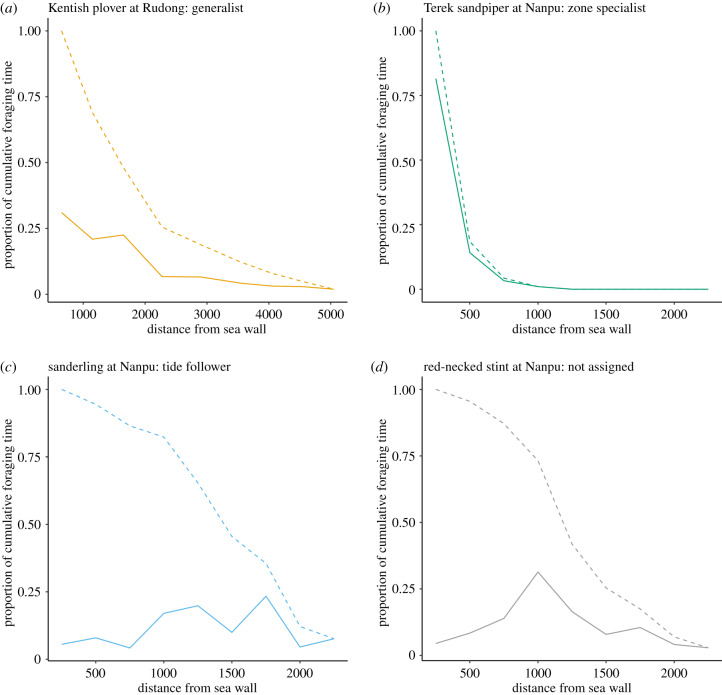
For most shorebirds among the 26 populations, the majority of their foraging time was spent in the plots closer to the seawall, shown by the cumulative foraging time along the tidal flats (figure 4; electronic supplementary material, figure S2). This is especially the case among generalists and zone specialists; tide followers predictably divided their foraging time roughly equally across the different plots or zones. For example, the proportions of cumulative foraging time spent by a generalist, the Kentish plover at Rudong, on the upper, middle and lower tidal flats, respectively, were 74.5%, 17.5% and 8.0%; for a zone specialist, the Terek sandpiper at Nanpu, these values were 99.0%, 1.0% and 0% and for a tide follower, the sanderling at Nanpu, they were 17.7%, 46.8% and 35.5%.
Figure 4.
(a–d) Cumulative foraging time along the tidal flat for four representative shorebird populations. The cumulative foraging time of the same species in figure 3 is shown as the proportion of total cumulative foraging time for a given population at a given site with transects summed. Solid lines show the distribution of cumulative foraging time of each plot along the tidal flat. Dashed lines show the reduction in total cumulative foraging time that would result from the hypothetical loss of tidal flats starting from the seawall and progressing seaward. (Online version in colour.)
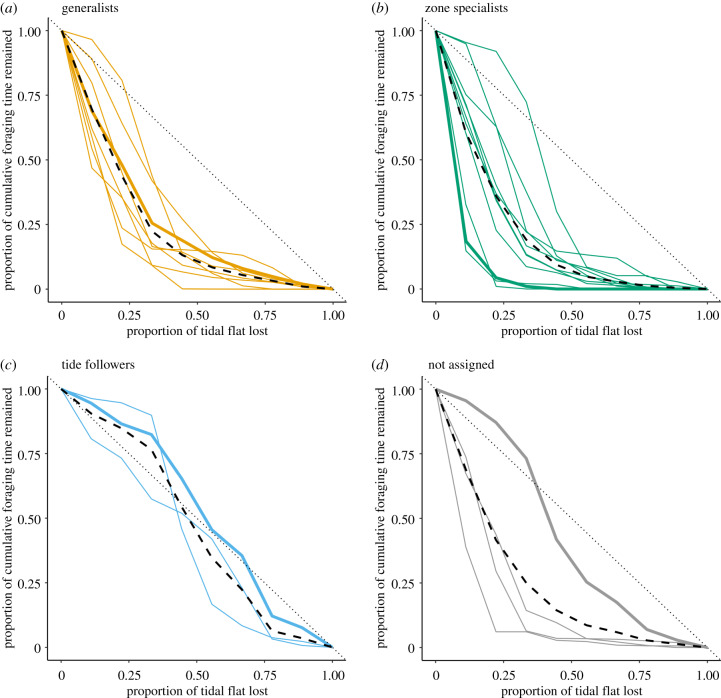
We aggregated the cumulative foraging time and plotted the loss of total foraging opportunity that would occur in response to a hypothetical loss of tidal flats that progresses from the seawall outward into the ocean to simulate the instantaneous effect of coastal development (dashed lines in figure 4; electronic supplementary material, figure S2 for individual species; solid lines in figure 5 for all species in a given foraging type). Losing the upper third of the current tidal flats would lead to a reduction in cumulative foraging time of, on average (± s.d.), 77.6% (± 13.6%) for generalists, 81.0% (± 20.9%) for zone specialists, 23.5% (± 17.0%) for tide followers, and 75.0% (± 32.3%) for species not assigned to any foraging types.
Figure 5.
(a–d) The importance of upper tidal flats to shorebirds of each foraging type. Solid lines in each plot show the reduction in total cumulative foraging time assuming habitat loss of the tidal flats starting from the seawall for all populations within the specified foraging type, in which the thicker line is the representative population in figure 4. The black dashed line shows the average proportion of decline of the type, and the dotted line is a reference line showing a proportional reduction when different portions of the tidal flat are of equal importance. (Online version in colour.)
4. Discussion
The conservation of migratory species requires an understanding of both their ecology at a fine spatial scale and the site-specific threats they face. By quantifying the spatial and temporal patterns in shorebird foraging distributions throughout the tidal cycle at two critical stopover sites in the Yellow Sea, we found that 17 migratory shorebirds exhibit substantial interspecific and even site-specific differences in their use of different portions of the tidal flats (table 1; figure 3; electronic supplementary material, figure S1). These populations can be mostly grouped into three foraging types: generalists, zone specialists and tide followers. Notwithstanding these differences in foraging behaviour, however, the upper tidal flat zone provides the majority of the cumulative foraging time of both the generalists and the zone specialists, far greater than the upper tidal zone's proportional area (figures 4 and 5; electronic supplementary material, figure S2). Because coastal development projects typically start near the high-tide line and proceed outward toward the sea [35,36], the upper tidal flats are also more prone to development than are the lower tidal flats, which may help to explain why shorebird populations along the EAAF have declined much faster than the overall rate of stopover habitat loss. Our work highlights the need to conserve as much of the upper tidal flats as possible within important stopover sites in order to protect today's diminished populations of migratory shorebirds. Our study also demonstrates the value of understanding the detailed patterns of habitat usage by migratory species throughout their journeys in order to properly conserve them.
(a). Differences in the foraging types
By taking into account the temporal changes in habitat availability, we show that most shorebird species at the two stopover sites fall into the three foraging types. Previous studies along other flyways about the foraging distribution of shorebird communities and their relationship with food availability have focused almost entirely on the spatial pattern during low tides [23,25], thereby overlooking the temporal changes in habitat (and thus food) availability and their effect on shorebirds' foraging behaviour during ebbing and flooding tides (see [26,30,37,38] for studies that considered tidal movements). Our analysis also shows that the same species may display different foraging behaviours at different stopover sites (table 1; figure 3). Of the seven species that occurred at and could be assigned to a foraging type at both sites, only two exhibited the same type, suggesting that these foraging behaviours may not be a species-specific behavioural property but, as suggested by earlier studies, may change in response to local environmental conditions [38–41], or differ between sexes or subspecies that constitute the local populations [42,43].
This result, while statistically robust for some species, needs to be validated by further studies. To simultaneously quantify the spatial and temporal aspects of shorebirds’ foraging distributions, our statistical tests rely on a single-derived and consolidated property (the position of shorebirds' abundance centroid in relation to the proportion of tidal flats exposed), which limits our ability to identify and account for finer patterns and changes in foraging distributions (e.g. red knot at Nanpu, in electronic supplementary material, figure S1). Our approach is expected to be especially poor at distinguishing between a generalist and a zone specialist, because a zone specialist will gradually turn into a generalist if its preferred foraging zone covers an increasingly large portion of the tidal flats. This was evident in our analysis when we had to rely on BIC to choose the better model describing the distributions of two populations that fell into both the generalist and zone specialist categories. There is clearly a need for more sophisticated statistical methods and more comprehensive field studies focused on the foraging behaviours of shorebirds using tidal flat habitat.
(b). The importance of upper tidal flats
We show that most shorebird populations at the two stopover sites spend disproportionately large amounts of time foraging on the upper tidal flats, particularly the generalists and zone specialists (figure 5), a combined result of the upper zone's longer exposure time and the shorebirds' preference for it. It may seem counterintuitive that the upper tidal flats are almost as important for the generalists (77.6% ± 13.6%) as they are for the specialists (81.0% ± 20.9%), but this reflects the relatively minor contributions of the middle and lower tidal flats to the cumulative foraging time of the generalists, a consequence of the limited exposure time relative to the upper tidal zone.
Unfortunately, the upper tidal flats are also the part of the habitat that is most prone to coastal development [30,35,36]. Decreases in the amount of available foraging habitat and foraging time could lower shorebirds’ survival rates and/or breeding success, directly or via carryover effects [8,44,45]. By simulating progressive seaward development of the tidal flats, we predicted that the loss of upper tidal flats may cause substantial and disproportionately severe reductions in overall foraging opportunities (figures 4 and 5), assuming the benthic invertebrate species upon which the shorebirds feed do not reassort in a way that partially compensates for the loss of foraging opportunities. Note that our results were generated from surveys conducted only during the spring tides, when the entire tidal flats are covered and then exposed during a tidal cycle, leading to more uniform distributions of foraging time among different zones. During a neap tide, the upper tidal zone would be exposed even longer relative to the lower tidal zone, suggesting that the overall importance of upper tidal flats to the foraging shorebirds could be much higher than we showed in the current study.
We suggest that the disproportionate importance of the upper tidal flats to EAAF shorebirds, combined with their heightened historical and current vulnerability to coastal development, may help to explain the discrepancy between the overall rates of coastal habitat loss (−1.2% yr−1 [17]) and the rate of population decline in EAAF shorebirds (up to −26% yr−1 in a similar period [11,19]). Thus, analyses based on the average rate of habitat loss may have significantly underestimated the severity of the threat that coastal development poses to EAAF shorebird populations. However, we could not perform any correlational analysis to confirm this hypothesis, as we lack detailed information on the changes in the total area of the different tidal flat zones in the Yellow Sea region, especially with respect to exactly how much more of the upper tidal flats was lost relative to the lower tidal flats, apart from the fact that most of the development has proceeded from the high-tide line outward to the sea, impacting the upper tidal flats most severely [17,35,36,46].
We recognize that our measure of tidal flat importance looked only at the number of foraging individuals and their foraging time, but not their intake rates. However, other studies have indicated that the higher intake rates often achieved at lower tidal flats are insufficient to compensate for the shorter exposure times [30,47] and that shorebirds select areas where the tidal flats are exposed for longer periods [48].
Another limitation of our study is that our simulation of upper tidal development takes into account only the instantaneous impact of habitat loss and assumes no reassortment of the benthic invertebrate species upon which the birds feed or other changes in local conditions. Coastal development in the long term, however, not only leads to direct habitat loss, but also alters the remaining habitat by changing its hydrodynamic and deposition patterns in ways that can lead to erosion or siltation [35], which, in turn, affect the substrate structure, size, physiochemical conditions and benthic invertebrate faunas of stopover sites [21,49]. A comprehensive and long-term monitoring programme focusing on the changes in both benthic fauna and shorebird distributions is needed to provide a mechanistic understanding on how coastal development affects local shorebirds both instantaneously and in the long term, as well as whether and how fast the tidal flats may recover their sizes and benthic invertebrate fauna.
(c). Conservation recommendations
As the most valuable yet most vulnerable parts of the tidal flat for shorebirds, the upper tidal zones should be the focus of conservation actions within the Yellow Sea region, through a combination of protecting them from further development and improving the quality of existing habitat. The planning of future development projects in this region should entail careful consideration of any activities that disproportionately affect the upper tidal flats and/or areas providing major foraging opportunities identified by local studies following our approach. For threatened species with very small population sizes (e.g. spoon-billed sandpipers), tracking the movement of individuals throughout the tidal cycle using a combination of direct observations and telemetry may be needed to understand their habitat preferences [9], in addition to our population-level approach.
We encourage researchers to conduct similar studies on the spatiotemporal distribution of foraging shorebirds elsewhere along the EAAF and along other flyways to better understand how shorebird species are using the tidal flats throughout their annual migration and to determine if the upper tidal flats are of disproportionate importance to these birds elsewhere. Development of tidal flats is not limited to the Yellow Sea. A recent study showed extensive losses of tidal flats around the world: more than 15%, or 20 000 km2 worldwide between 1984 and 2016 [35]. Such development, combined with sea-level rises triggered by global climate change [50], simultaneously threatens the tidal flats and many of the world's shorebirds. Absent a concerted, international effort to protect the intertidal stopover sites these birds depend upon, the world stands to lose some of its most remarkable long-distance migrants.
Supplementary Material
Acknowledgements
We thank U. Srinivasan, F. Hua, H. Peng and the Drongos for useful discussions throughout the study. We thank Y. Wang, X. Shi, J. Zhang, C. Yu, Y. Zhong, X. Chen, W. Liu, J. Li, J. Chen, F. Yan, C. Liu, J. Loghry, T. Zhao, L. Zhang, J. Li, W. Lei, Y.C. Chan, A. Boyle, C. Hassell, Z. Zhang and Z. Ma for their assistance and logistical support in the field. We thank X. Ren and X. Shang for preparing figures 1 and 2. We also thank the editors and reviewers who greatly improved the manuscript.
Ethics
The field survey protocol was approved by the Institutional Animal Care and Use Committee of Princeton University.
Data accessibility
Data and R code are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gmsbcc2jb [51].
Authors' contributions
T.M. perceived and designed the study, collected and analysed the data, and drafted the manuscript. D.S.W. codesigned the study, provided critical input for analysis and extensively revised the manuscript. All authors gave final approval for publication.
Funding
This work was supported by the High Meadows Foundation.
References
- 1.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 2.Wilcove DS, Wikelski M. 2008. Going, going, gone: is animal migration disappearing. PLoS Biol. 6, e188 ( 10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison CA, Robinson RA, Clark JA, Risely K, Gill JA, Duncan R. 2013. Recent population declines in Afro-Palaearctic migratory birds: the influence of breeding and non-breeding seasons. Divers. Distrib. 19, 1051–1058. ( 10.1111/ddi.12084) [DOI] [Google Scholar]
- 4.Rosenberg KV, et al. 2019. Decline of the North American avifauna. Science 366, 120 ( 10.1126/science.aaw1313) [DOI] [PubMed] [Google Scholar]
- 5.Delany S, Scott D, Dodman T, Stroud D. 2009. An atlas of wader populations in Africa and western Eurasia. Wageningen, The Netherlands: Wetlands International. [Google Scholar]
- 6.Gilroy JJ, Gill JA, Butchart SH, Jones VR, Franco AM. 2016. Migratory diversity predicts population declines in birds. Ecol. Lett. 19, 308–317. ( 10.1111/ele.12569) [DOI] [PubMed] [Google Scholar]
- 7.Moller AP, Rubolini D, Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl. Acad. Sci. USA 105, 16 195–16 200. ( 10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker AJ, et al. 2004. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proc. Biol. Sci. 271, 875–882. ( 10.1098/rspb.2003.2663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan Y-C, Peng H-B, Han Y-X, Chung SS-W, Li J, Zhang L, Piersma T. 2019. Conserving unprotected important coastal habitats in the Yellow Sea: shorebird occurrence, distribution and food resources at Lianyungang. Glob. Ecol. Conserv. 20, e00724 ( 10.1016/j.gecco.2019.e00724) [DOI] [Google Scholar]
- 10.Battley PF, et al. 2012. Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica. J. Avian Biol. 43, 21–32. ( 10.1111/j.1600-048X.2011.05473.x) [DOI] [Google Scholar]
- 11.Studds CE, et al. 2017. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nat. Commun. 8, 14895 ( 10.1038/ncomms14895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piersma T, et al. 2016. Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. J. App. Ecol. 53, 479–490. ( 10.1111/1365-2664.12582) [DOI] [Google Scholar]
- 13.Amano T, Székely T, Koyama K, Amano H, Sutherland WJ. 2010. A framework for monitoring the status of populations: an example from wader populations in the East Asian–Australasian flyway. Biol. Conserv. 143, 2238–2247. ( 10.1016/j.biocon.2010.06.010) [DOI] [Google Scholar]
- 14.MacKinnon J, Verkuil YI, Murray N. 2012. IUCN situation analysis on East and Southeast Asian intertidal habitats, with particular reference to the Yellow Sea (including the Bohai Sea). Gland, Switzerland and Cambridge, UK: IUCN. [Google Scholar]
- 15.Ma Z, Melville DS, Liu J, Chen Y, Yang H, Ren W, Zhang Z, Piersma T, Li B. 2014. Rethinking China's new great wall. Science 346, 912 ( 10.1126/science.1257258) [DOI] [PubMed] [Google Scholar]
- 16.Moores N, Rogers DI, Rogers K, Hansbro PM. 2016. Reclamation of tidal flats and shorebird declines in Saemangeum and elsewhere in the Republic of Korea. Emu - Austral Ornithology 116, 136–146. ( 10.1071/mu16006) [DOI] [Google Scholar]
- 17.Murray NJ, Clemens RS, Phinn SR, Possingham HP, Fuller RA. 2014. Tracking the rapid loss of tidal wetlands in the Yellow Sea. Front. Ecol. Environ. 12, 267–272. ( 10.1890/130260) [DOI] [Google Scholar]
- 18.Murray N, Phinn S, Clemens R, Roelfsema C, Fuller R. 2012. Continental scale mapping of tidal flats across East Asia using the Landsat Archive. Remote Sens. 4, 3417–3426. ( 10.3390/rs4113417) [DOI] [Google Scholar]
- 19.Zöckler C, Syroechkovskiy EE, Atkinson PW. 2010. Rapid and continued population decline in the spoon-billed sandpiper Eurynorhynchus pygmeus indicates imminent extinction unless conservation action is taken. Bird Conserv. Int. 20, 95–111. ( 10.1017/S0959270910000316) [DOI] [Google Scholar]
- 20.Bai Q, et al. 2015. Identification of coastal wetlands of international importance for waterbirds: a review of China Coastal Waterbird Surveys 2005–2013. Avian Res. 6, 1 ( 10.1186/s40657-015-0021-2) [DOI] [Google Scholar]
- 21.Wang Y, et al. 2002. Definition, properties, and classification of muddy coasts. In Muddy coasts of the world: processes, deposits and function (eds Healy T, Wang Y, Healy J-A), pp. 9–18/ Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 22.Choi C-Y, Battley PF, Potter MA, Ma Z, Liu W. 2014. Factors affecting the distribution patterns of benthic invertebrates at a major shorebird staging site in the Yellow Sea, China. Wetlands 34, 1085–1096. ( 10.1007/s13157-014-0568-4) [DOI] [Google Scholar]
- 23.Yates MG, et al. 1993. Sediment characteristics, invertebrate densities and shorebird densities on the inner banks of the wash. J. App. Ecol. 30, 599 ( 10.2307/2404240) [DOI] [Google Scholar]
- 24.Dyer KR, Christie MC, Wright EW. 2000. The classification of intertidal mudflats. Cont. Shelf Res. 20, 1039–1060. ( 10.1016/S0278-4343(00)00011-X) [DOI] [Google Scholar]
- 25.Goss-Custard J, Jones R, Newbery P. 1977. The ecology of the Wash. I. Distribution and diet of wading birds (Charadrii). J. App. Ecol. 14, 681–700. ( 10.2307/2402803) [DOI] [Google Scholar]
- 26.Nehls G, Tiedemann R. 1993. What determines the densities of feeding birds on tidal flats? A case study on dunlin, Calidris alpina, in the Wadden Sea. Neth. J. Sea Res. 31, 375–384. ( 10.1016/0077-7579(93)90054-v) [DOI] [Google Scholar]
- 27.Peng H-B, et al. 2017. The intertidal wetlands of southern Jiangsu Province, China—globally important for spoon-billed sandpipers and other threatened waterbirds, but facing multiple serious threats. Bird Conserv. Int. 27, 305–322. ( 10.1017/s0959270917000223) [DOI] [Google Scholar]
- 28.Lei W, Masero JA, Piersma T, Zhu B, Yang H-Y, Zhang Z. 2018. Alternative habitat: the importance of the Nanpu Saltpans for migratory waterbirds in the Chinese Yellow Sea. Bird Conserv. Int. 28, 549–566. ( 10.1017/s0959270917000508) [DOI] [Google Scholar]
- 29.Yang H-Y, Chen B, Barter M, Piersma T, Zhou C-F, Li F-S, Zhang Z-W. 2011. Impacts of tidal land reclamation in Bohai Bay, China: ongoing losses of critical Yellow Sea waterbird staging and wintering sites. Bird Conserv. Int. 21, 241–259. ( 10.1017/s0959270911000086) [DOI] [Google Scholar]
- 30.Granadeiro JP, Dias MP, Martins RC, Palmeirim JM. 2006. Variation in numbers and behaviour of waders during the tidal cycle: implications for the use of estuarine sediment flats. Acta Oecologica 29, 293–300. ( 10.1016/j.actao.2005.11.008) [DOI] [Google Scholar]
- 31.Klein G.d. 1985. Intertidal flats and intertidal sand bodies. In Coastal sedimentary environments, pp. 187–224, Berlin, Germany: Springer. [Google Scholar]
- 32.Li C, Wang P, Daidu F, Bing D, Tiesong L. 2000. Open-coast intertidal deposits and the preservation potential of individual laminae: a case study from east-central China. Sedimentology 47, 1039–1051. ( 10.1046/j.1365-3091.2000.00338.x) [DOI] [Google Scholar]
- 33.Muggeo VM. 2008. Segmented: an R package to fit regression models with broken-line relationships. R news 8, 20–25. [Google Scholar]
- 34.Kass RE, Raftery AE. 1995. Bayes factors. J. am. stat. assoc. 90, 773–795. ( 10.1080/01621459.1995.10476572) [DOI] [Google Scholar]
- 35.Murray NJ, Phinn SR, DeWitt M, Ferrari R, Johnston R, Lyons MB, Clinton N, Thau D, Fuller RA. 2019. The global distribution and trajectory of tidal flats. Nature 565, 222–225. ( 10.1038/s41586-018-0805-8) [DOI] [PubMed] [Google Scholar]
- 36.Choi C-Y, Jackson MV, Gallo-Cajiao E, Murray NJ, Clemens RS, Gan X, Fuller RA. 2018. Biodiversity and China's new Great Wall. Divers. Distrib. 24, 137–143. ( 10.1111/ddi.12675) [DOI] [Google Scholar]
- 37.Gils J, Spaans B, Dekinga A, Piersma T. 2006. Foraging in a tidally structured environment by red knots (Calidris Canutus): ideal, but not free. Ecology 87, 1189–1202. ( 10.1890/0012-9658(2006)87[1189:fiatse]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 38.Oudman T, Piersma T, Ahmedou Salem MV, Feis ME, Dekinga A, Holthuijsen S, Ten Horn J, van Gils JA, Bijleveld AI. 2018. Resource landscapes explain contrasting patterns of aggregation and site fidelity by red knots at two wintering sites. Mov. Ecol. 6, 24 ( 10.1186/s40462-018-0142-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zwarts L, Wanink JH. 1993. How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body weight, biomass, burying depth and behaviour of tidal-flat invertebrates. Neth. J. Sea Res. 31, 441–476. ( 10.1016/0077-7579(93)90059-2) [DOI] [Google Scholar]
- 40.Goss-Custard JD, Jenyon RA, Jones RE, Newbery PE, Williams RLB. 1977. The ecology of the wash. II. Seasonal variation in the feeding conditions of wading birds (Charadrii). J. App. Ecol. 14, 701 ( 10.2307/2402804) [DOI] [Google Scholar]
- 41.Yang H-Y, Chen B, Piersma T, Zhang Z, Ding C. 2016. Molluscs of an intertidal soft-sediment area in China: does overfishing explain a high density but low diversity community that benefits staging shorebirds? J. Sea Res. 109, 20–28. ( 10.1016/j.seares.2016.01.006) [DOI] [Google Scholar]
- 42.Duijns S, Piersma T. 2014. Interference competition in a sexually dimorphic shorebird: prey behaviour explains intraspecific competition. Anim. Behav. 92, 195–201. ( 10.1016/j.anbehav.2014.04.007) [DOI] [Google Scholar]
- 43.Duijns S, van Gils JA, Spaans B, Ten Horn J, Brugge M, Piersma T. 2014. Sex-specific winter distribution in a sexually dimorphic shorebird is explained by resource partitioning. Ecol. Evol. 4, 4009–4018. ( 10.1002/ece3.1213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buehler DM, Piersma T. 2008. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Philos. Trans. R Soc. Lond. B Biol. Sci. 363, 247–266. ( 10.1098/rstb.2007.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Dong J, Xiao X, Zhang M, Tian B, Zhou Y, Li B, Ma Z. 2016. Land claim and loss of tidal flats in the Yangtze Estuary. Sci. Rep. 6, 24018 ( 10.1038/srep24018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwarts L, Blomert A-M, Ens BJ, Hupkes R, Van Spanje T. 1990. Why do waders reach high feeding densities on the intertidal flats of the Banc d'Arguin, Mauritania. Ardea 78, 39–52. [Google Scholar]
- 48.Jackson MV, et al. 2019. Multiple habitat use by declining migratory birds necessitates joined-up conservation. Ecol. Evol. 9, 2505–2515. ( 10.1002/ece3.4895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolet C, Spilmont N, Davoult D, Goberville E, Luczak C. 2015. Anthropogenic impact on macrobenthic communities and consequences for shorebirds in Northern France: a complex response. Biol. Conserv. 184, 396–404. ( 10.1016/j.biocon.2015.02.016) [DOI] [Google Scholar]
- 50.Spencer T, Schuerch M, Nicholls RJ, Hinkel J, Lincke D, Vafeidis AT, Reef R, McFadden L, Brown S. 2016. Global coastal wetland change under sea-level rise and related stresses: the DIVA wetland change model. Glob. Planet. Change 139, 15–30. ( 10.1016/j.gloplacha.2015.12.018) [DOI] [Google Scholar]
- 51.Mu T, Wilcove DS. 2020. Data from: Upper tidal flats are disproportionately important for the conservation of migratory shorebirds. Dryad Digital Repository ( 10.5061/dryad.gmsbcc2jb) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mu T, Wilcove DS. 2020. Data from: Upper tidal flats are disproportionately important for the conservation of migratory shorebirds. Dryad Digital Repository ( 10.5061/dryad.gmsbcc2jb) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R code are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gmsbcc2jb [51].