Abstract
Mantidflies (Mantispidae) are an unusual and charismatic group of predatory lacewings (Neuroptera), whereby the adults represent a remarkable case of morphological and functional convergence with praying mantises (Mantodea). The evolutionary history of mantidflies remains largely unknown due to a scarcity of fossils. Here, we report the discovery of a highly diverse palaeofauna of mantidflies from the mid-Cretaceous (lowermost Cenomanian) of Myanmar. The raptorial forelegs of these mantidflies possess highly divergent morphological modifications, some of which are unknown among modern mantidflies, e.g. the presence of forked basal profemoral spines or even the complete loss of foreleg spine-like structures. A phylogenetic analysis of Mantispidae reveals a pattern of raptorial foreleg evolution across the family. The high species diversity and disparate foreleg characters might have been driven by diverse niches of predator–prey interplay in the complex tropical forest ecosystem of the mid-Cretaceous.
Keywords: Mantispidae, palaeodiversity, phylogeny, character evolution, Mesozoic
1. Introduction
Mantidflies comprise a lineage of charismatic lacewings (order Neuroptera) which have evolved a complex suite of biological and behavioural specializations associated with their predatory life history. Adults are characterized by an elongation of the pronotum posteriad, their characteristically raptorial forelegs which, along with their often highly mobile heads and large compound eyes, give them a distinct praying mantis-like appearance. Indeed, it is because of this striking morphological convergence with mantises (order Mantodea) that the group is commonly dubbed mantidflies. The larvae are hypermetamorphic and are known as specialist predators or parasites of the egg sacs of spiders or stinging wasps and bees [1]. Hitherto, 395 extant species have been documented and are collectively grouped into the family Mantispidae, with 44 genera occurring worldwide [2,3], and organized into four modern subfamilies, i.e. Symphrasinae, Drepanicinae, Calomantispinae and Mantispinae [4]. The Mantispinae are cosmopolitan, whereas the other three subfamilies have markedly narrower geographic ranges [2]. Symphrasinae are endemic to the New World; Drepanicinae are disjunctively distributed from Central and South America, Australia and southeastern Asia, while Calomantispinae occur in Australia and the New World.
Mantispidae extend back to at least the Early Jurassic as evidenced by the presumably oldest fossil mantidfly, Liassochrysa stigmatica [5] from Germany. This time of origin is also corroborated by divergence-time estimates using genomic data [6,7]. Currently, 25 named species of fossil mantidflies are recorded from the Early Jurassic to Miocene (see electronic supplementary material, table S2, revised from Jepson [8]).
Here, we report the discovery of a considerable diversity of fossil Mantispidae from the mid-Cretaceous, including six new genera and 10 new species, based on a variety of species representing several clades of the family in amber from northern Myanmar. These taxa reveal a hitherto unknown series of morphologies associated with the grasping structures of these distinctive predators, highlighting a greater anatomical disparity among mantidflies in the past. We also provide a phylogenetic analysis of the family integrating the fossil diversity based on a morphological dataset of Liu et al. [9] with newly added taxa and characters. The diversified morphological characters, particularly of the raptorial forelegs, in these Cretaceous amber mantidflies provide significant evidence for understanding the early disparity and evolution of characters related to the predatory feeding habits in Mantispidae.
2. Results
(a). Systematic palaeontology
Order Neuroptera Linnaeus, 1758
Family Mantispidae Leach, 1815
Note. See full description for all the following taxa in the electronic supplementary material, note S1.
Subfamily Symphrasinae Navás, 1909
Haplosymphrasites zouae gen. et sp. nov. (figure 1a; electronic supplementary material, figure S1)
Figure 1.
Habitus photographs of mantidflies from the Cretaceous amber of Myanmar. (a) Haplosymphrasites zouae gen. et sp. nov., holotype. (b) Habrosymphrasites xiai. (c) Parasymphrasites electrinus gen. et sp. nov., holotype. (d) Doratomantispa ares sp. nov., holotype. (e) D. pubescens sp. nov., holotype. (f) Paradoxomantispa jiaxiaoae gen. et sp. nov., holotype. (g) Acanthomantispa grandis gen. et sp. nov., holotype. (h) A. maculata gen. et sp. nov., paratype. (i) A. immaculata gen. et sp. nov., holotype. (j) Dicranomantispa zhouae gen. et sp. nov., holotype. (k) Psilomantispa abnormis gen. et sp. nov., holotype. Scale bar = 1.0 mm. (Online version in colour.)
Type species of the genus. Haplosymphrasites zouae sp. nov.
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:3E82DFDF-6533-4BEB-AF0C-B1A135E4B8EE; urn:lsid:zoobank.org:act:3F76BDA1-A945-447A-AE9F-2B316C48F0D0.
Diagnosis. The new genus is characterized by the following characters: (i) forewing 3sc-r perpendicular to ScP; (ii) one ra-rp crossvein present in both fore- and hindwing; (iii) most RP branches without marginal forks; (iv) a long crossvein present between forewing A2 and A3; (v) presence of 3–5 trichosors between neighbouring veins on distal margin. As the genus is monotypic, the diagnosis for the genus and species are identical.
Etymology. The genus-group name is a combination of haplos (single) and Symphrasites (a generic name among fossil Symphrasinae) and refers to the single ra-rp distinctive for this group. The gender is masculine. The new species is named after Mrs Jiaojie Zou, who kindly donated this specimen for our study.
Type material. Holotype: CAU-BA-WN-19001.
Habrosymphrasis Shi et al., 2020 (figures 1b and 2a,b; electronic supplementary material, figure S2)
Figure 2.
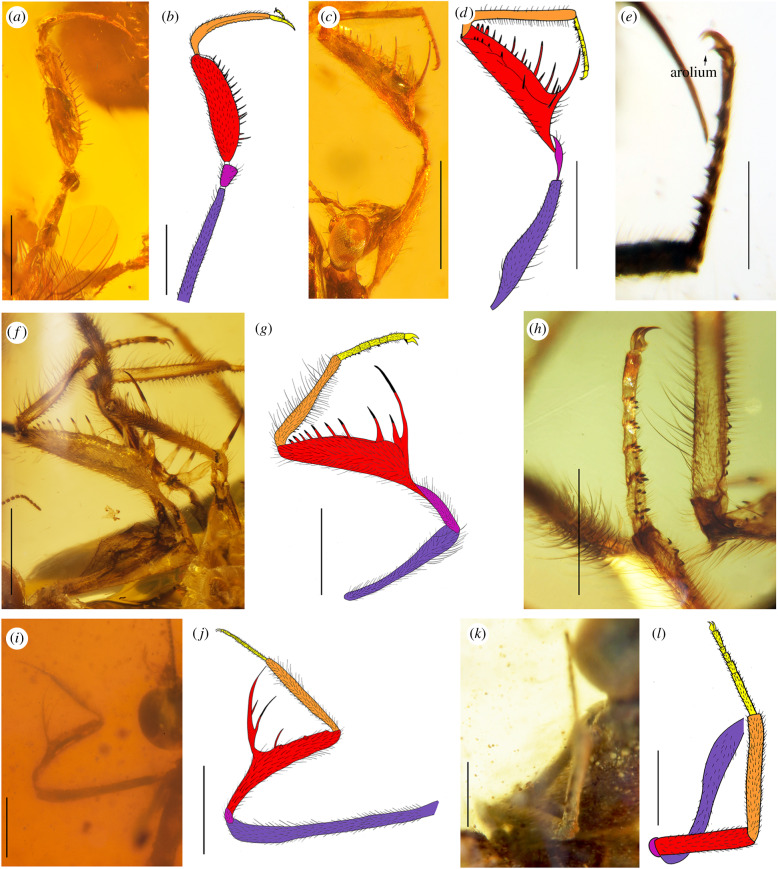
Different types of forelegs in mantidflies from the Cretaceous amber of Myanmar. (a) Habrosymphrasites xiai Shi et al., photograph, ectal view; (b) same, drawing. (c) Paradoxomantispa jiaxiaoae gen. et sp. nov., holotype, photograph, ental view; (d) same, drawing; (e) same, photograph of arolium; (f) Doratomantispa pubescens gen. et sp. nov., holotype, photograph, ectal view; (g) same, drawing; (h) same, photograph of tarsus; (i) Acanthomantispa maculata gen. et sp. nov., paratype, photograph, ectal view; (j) same, drawing; (k) Psilomantispa abnormis gen. et sp. nov., holotype, photograph, ectal view; (l) same, drawing. Procoxa (blue), protrochanter (purple), profemur (red), protibia (orange) and protarsus (yellow) coloured in drawings. Scale bar = 1.0 mm (a–d, f–g, i–l); 0.5 mm (e,h). (Online version in colour.)
Revised diagnosis. This genus is characterized by the following characters: (i) forewing 3sc-r inconspicuous associated with ScP abruptly bending toward RA; (ii) 1 m-cu approximating origin of MP; (iii) three forewing ra-rp and two hindwing ra-rp present; (iv) only one trichosor present between neighbouring veins on distal margin; (v) male gonocoxite IX short, distally bifurcated; (vi) male gonostylus X (pseudopenis) filamentous, not coilded.
Parasymphrasites electrinus gen. et sp. nov. (Figure 1c; eletronic supplementary material, figures S3, S12)
Type species of the genus. Parasymphrasites electrinus sp. nov.
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:E40CEA58-4274-4273-A384-54053BDE7F3A; urn:lsid:zoobank.org:act:6FC3296A-65EB-4011-9D31-0A5386152DE9.
Diagnosis. The new genus is characterized by the following characters: (i) forewing 3sc-r inconspicuous and associated with ScP abruptly bending toward RA; (ii) 1m-cu confluent with origin of MP; (iii) three ra-rp crossveins present in both fore- and hindwings; (iv) costal crossveins bifurcated marginally; (v) forewing CuP proximally strongly curved; (vi) only one trichosor present between neighbouring veins on distal margin. As the genus is monotypic, the diagnosis for the genus and species are identical.
Etymology. From the Greek ‘para-’ (meaning ‘similar’) and Symphrasites (a generic name of fossil Symphrasinae), in reference to the similar appearance of the new genus with Symphrasites. The gender is masculine. The specific epithet refers to the occurrence of the species in amber.
Type material. Holotype: EMTG BA-002162.
Subfamily Doratomantispinae subfam. nov.
Type genus. Doratomantispa Poinar in Poinar & Buckley, 2011.
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:F2104309-A635-4B85-BABC-39C26E87F81F.
Diagnosis. The new subfamily is characterized by the following characters: (i) prothorax strongly tubular, as long as or slightly longer than meso- plus metathorax, without transverse sulcus and maculae; (ii) profemur as long as protibia plus protarsus, with two rows of integumentary processes, and spine row ental to closed tibia with a long basal spine and 1–3 shorter spines separated at the same level, remaining spines distad basal spine comprising a series of long spines that are gradually shortened distad; (iii) protibia with a row of prostrate setae; (iv) protarsus pentamerous, with tarsomere I not prolonged and not acutely tapering; (v) two simple pretarsal claws and a small, acutely tapering arolium present on foreleg; (vi) trichosors well developed, only absent on proximal part of costal margin and posterior margin; (vii) forewing ScP abruptly bending toward RA and continuous with 3sc-r; (viii) pterostigma present between C and RA; (ix) a very short veinlet (MA stem or 1r-m) present between forewing R and MP near wing base; (x) hindwing costal space extending to pterostigma, with costal crossveins reduced on its distal part; (xi) male gonocoxites IX very short, distally with several claws; (xii) male gonostylus X (pseudopenis) filamentous, but not coiled; (xiii) female gonocoxites IX not specialized into a long ovipositor.
Remarks. The new subfamily appears to be a transitional lineage between Symphrasinae and Drepanicinae in light of the possession of some characters, respectively, shared by the latter two subfamilies. Doratomantispinae subfam. nov. and Symphrasinae share characters 3, 6–8 shown in the subfamilial diagnosis. The new subfamily and Drepanicinae share the characters 1, 3, 4, 9–13 shown in the subfamilial diagnosis, while some of these characters are also present in Calomantispinae and Mantispinae. The present phylogenetic analysis recovered characters 2 and 5 as autapomorphies of the new subfamily.
Doratomantispa Poinar in Poinar & Buckley, 2011
Diagnosis. Besides the subfamilial diagnosis, this genus is also characterized by the protibia with a row of prostrate setae that are sharply curved distad, distinctly forming angles and the absence of hindwing 1ra-rp.
Doratomantispa ares sp. nov. (Figure 1d; electronic supplementary material, figure S4)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:23672985-4CF2-466B-B053-C7C73F96336E.
Diagnosis. The new species differs from the type species, D. burmanica, and from D. pubescens sp. nov. by the many forked costal crossveins in the proximal half of the forewing. In D. burmanica and D. pubescens sp. nov., the forewing costal crossveins are mostly simple. The new species also differs from D. pubescens sp. nov. by the spotted forewing, which is immaculate in the latter species.
Type material. Holotype: NIGP173084, male.
Etymology. The specific epithet is from Ares, name of the Greek war god and son of Zeus and Hera, in reference to the aggressive appearance of the new species.
Doratomantispa pubescens sp. nov. (Figures 1e, 2f–h; electronic supplementary material, figure S5)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:4B01F062-6EEB-4B9A-A06D-74BD4ECA0878.
Diagnosis. Refer to diagnosis for D. ares (abovementioned).
Type material. Holotype: EMTG BU-001759.
Etymology. The specific epithet ‘pubescens’ refers to the presence of dense long setae on the pronotum in the new species.
Paradoxomantispa jiaxiaoae gen. et sp. nov. (Figures 1f, 2c–e; electronic supplementary material, figures S6, S12)
Type species of the genus. Paradoxomantispa jiaxiaoae sp. nov.
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:73F9BBE8-B4A3-4AD3-AA41-20CC113DDC38; urn:lsid:zoobank.org:act:BD10F941-4C53-4F55-8D72-6C76C213E3D7.
Diagnosis. This genus is characterized by the protibia with a row of prostrate setae that are smoothly curved distad and the presence of hindwing 1ra-rp. This new species is characterized by the presence of forewing markings and the simple forewing A3.
Etymology. From the Greek ‘paradoxos’ (paradoxical, strange) and Mantispa (a common genus-group name of Mantispidae), in reference to the foreleg that has profemoral spine-like structures that differ dramatically from those in other mantidfly subfamilies. The gender is feminine. The new species is named after and dedicated to Mrs Xiao Jia, who kindly provided the specimen for our study.
Type material. Holotype: CAM BA-0015, female.
Subfamily Drepanicinae Enderlein, 1910
Acanthomantispa gen. nov.
Type species of the genus. Acanthomantispa immaculata sp. nov.
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:C8ACEBD5-32D4-4571-A94E-C8B9F3245268.
Diagnosis. The new genus is characterized by the following characters: (i) head with vertex not domed; (ii) forewing ScP distally quite remote from RA, connected by a distinct crossvein; (iii) procoxa apparently longer than profemur; (iv) profemur with integumentary processes largely reduced, basal spine forked into two additional spines on proximal half; (v) forewing MP straight.
Etymology. From the Greek ‘acanthos’ (meaning ‘spine’) and Mantispa (a generic name of Mantispidae), in reference to the forked basal spine of profemur in the new genus. The gender is feminine.
Acanthomantispa grandis sp. nov. (Figure 1g; electronic supplementary material, figures S7, S12)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:523064C8-1466-4EC4-BAB0-C18B32AC6D43.
Diagnosis. The new species can be distinguished from A. immaculata sp. nov. and A. maculata sp. nov. by the broader wings, the presence of a dark stripe between forewing CuP and A1 and the presence of forewing ScA. In the latter two species, the wings are markedly narrower and the forewing ScA is absent. Furthermore, the wings are immaculate in A. immaculata sp. nov., while despite the presence of forewing dark markings, there is no marking between forewing CuP and A1 in A. maculata sp. nov. Considering the profemoral integumentary processes, A. grandis sp. nov. has an additional shorter spine separated at the same level with the large basal spine, while in the other species of Acanthomantispa, there is an additional shorter spine separated distinctly distad the basal spine.
Type material. Holotype: NIGP173085.
Etymology. The specific epithet ‘grandis’ refers to the presence of the relatively large body size of the new species in comparison to the other congeneric species.
Acanthomantispa immaculata sp. nov. (Figure 1i; electronic supplementary material, figures S8, S12)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:828D8C55-9C65-4D94-8AD7-B26777537B09.
Diagnosis. Refer to diagnosis for A. grandis (abovementioned).
Type material. Holotype: EMTG BU-001408, male.
Etymology. The specific epithet ‘immaculata’ refers to the immaculate wings in the new species.
Acanthomantispa maculata sp. nov. (Figures 1h, 2i,j; electronic supplementary material, figure S9)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:F2933A8A-C4E2-4289-A301-547A99982E7D.
Diagnosis. Refer to diagnosis for A. grandis (abovementioned).
Type material. Holotype: EMTG BU-002131, male. Paratype: EMTG BU-002136, male. Paratype: EMTG BU-002270.
Etymology. The specific epithet ‘maculata’ refers to the forewing with distinct dark markings in the new species.
Dicranomantispa zhouae gen. et sp. nov. (Figure 1j; electronic supplementary material, figures S10, S12G–H, L)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:9874641D-B41A-4C7F-985D-FFEDCF6A250C; urn:lsid:zoobank.org:act:6896C57B-2FFA-4055-B0CB-BCCF4ABE253D.
Type species of the genus. Dicranomantispa zhouae sp. nov.
Diagnosis. The new genus is characterized by the following characters: (i) forewing ScP distally quite remote from RA, connected by a distinct crossvein; (ii) pronotum shorter than mesothorax; (iii) profemur with integumentary processes largely reduced, only two closely spaced large spines remaining; (iv) only one sc-r crossvein (i.e. 3sc-r) present; (v) forewing MP straight. As the genus is monotypic, the diagnosis for the genus and species are identical.
Etymology. From the Greek ‘dicranos’ (meaning ‘bicuspid’) and Mantispa (a common generic stem for names of Mantispidae), in reference to the presence of two closely spaced, large spines on the profemur in the new genus. The gender is feminine. The new species is named after and dedicated to Mrs Meixu Zhou, who kindly donated the specimen of the new species for our research.
Type material. Holotype: CAU-BA-ZM-19003.
Psilomantispa abnormis gen. et sp. nov. (Figures 1k and 2k,l; electronic supplementary material, figure S11)
LSID (Life Science Identifier). urn:lsid:zoobank.org:act:974BB58E-A123-450C-BEFB-091BAFDAB897; urn:lsid:zoobank.org:act:A9B404CF-E2C5-4896-9563-50E594631213.
Type species of the genus. Psilomantispa abnormis sp. nov.
Diagnosis. The new genus is characterized by the following characters: (i) pterostigma quite remote from RA, connected by a distinct crossvein; (ii) profemoral spines absent; (iii) forewing MP straight; (iv) gradate series of crossveins absent. As the genus is monotypic, the diagnosis for the genus and species are identical.
Etymology. From ‘psilos’ (meaning ‘naked’) and Mantispa (a generic name of Mantispidae), in reference to the absence of profemoral spines in the new genus. The gender is feminine. The specific epithet ‘abnormis’ refers to the peculiar feature of the new species in Mantispidae with profemoral spines completely lost.
Type material. Holotype: CAU-BA-LX-19004, male.
(b). Phylogenetic analysis
The traditional search with TNT generated 18 most parsimonious trees (MPT) (Length = 170, consistency index = 53, retention index = 80). The strict consensus tree is shown in figure 3 and electronic supplementary material, figure S14. See detailed results in electronic supplementary material, note S2.
Figure 3.
Phylogeny of Mantispidae. Topology represents the strict consensus tree of the 18 most parsimonious trees yielded from TNT (see more detail information in electronic supplementary material, figure S14). Red stars indicate the mantidfly genera from the Cretaceous amber of Myanmar; black squares show the age of the remaining fossil mantidfly genera. Different types of raptorial forelegs and proportion of size between foreleg and pronotum are presented aside corresponding lineages. Profemoral basal spines (dark red), profemoral short spines (red) and protibial prostrate setae (green) are highlighted. (Online version in colour.)
3. Discussion
(a). Phylogeny of Mantispidae
The monophyly of Mantispidae was challenged by a recent phylogenomic study [7], in which Symphrasinae was recovered as the sister group of Rhachiberothidae (thorny lacewings), while the other mantidfly subfamilies formed a monophylum and as the sister group to Berothidae (beaded lacewings). So far, there has been no morphological evidence found to support the notion of paraphyly of Mantispidae, and our current result again recovered the monophyly of Mantispidae. The prothoracic elongation posteriad the procoxae and the presence of protibial prostrate setae as previously recovered in Liu et al. [9] are still convincing autapomorphies of Mantispidae, although the latter character state is not present in Mantispinae, undoubtedly as a secondary loss. The multiply branched recurrent humeral veinlet in the forewing is a newly recovered apomorphy of Mantispidae but probably not an autapomorphy of this family, because this character is only present in Mesomantispinae and Symphrasinae, but also broadly present in many other lacewing families [10]. Due to scarce data available on some key characters, the present analysis did not expand the sampling of the fossil mantispoid lineages beyond Mantispidae, such as Dipteromantispidae and Paraberothinae (Rhachiberothidae), both of which also have raptorial forelegs but are known only from the Cretaceous [11,12]. Thus, the complex evolutionary pattern of raptorial life styles must be further clarified in future studies when more finely preserved material of important fossil mantispoids can be assessed and new diversity considered.
The extinct subfamily Mesomantispinae is apparently sister to the remaining Mantispidae as recovered in a previous analysis [9]. Jepson et al. [13] questioned the monophyly of Mesomantispinae and believed that it represented a paraphyletic assemblage of stem-group mantidflies having many plesiomorphic characters. Nevertheless, all genera of Mesomantispinae herein form a natural group, with the profusely and pectinately branched forewing CuA as an autapomorphy uniting these early mantidflies. Although Jepson et al. [13] assumed a plesiomorphic condition for the configuration of forewing CuA in Mesomantispinae, this character state is actually not widely present in Mantispoidea and instead only developed in Mesomantispinae. In the studies of Wedmann & Makarkin [14] and Shi et al. [15], Mesomantispinae was considered to be close to Drepanicinae or the sister group of Symphrasinae, respectively. However, both hypotheses are not supported in our phylogenetic analysis. The single character supporting Mesomantispinae + Symphrasinae in Shi et al. [15] is the fusion of forewing ScP and RA, which is weak evidence because this character state is present in many heterogeneous lineages of Neuroptera and apparently rampantly homoplastic. Moreover, the fusion of forewing ScP and RA in Mesomantispinae and Symphrasinae was incorrectly interpretated by Shi et al. [15]. The distal part of forewing ScP is actually abruptly bent toward RA and continuous with 3sc-r in Mesomantispinae and some genera of Symphrasinae, and this character state is also found in Doratomantispinae subfam. nov.
Symphrasinae are herein recovered as monophyletic supported by the protarsus with tarsomere II markedly longer than the remaining tarsomeres and arising midway along tarsomere I. Liu et al. [9] recovered 13 apomorphic characters supporting the monophyly of extant Symphrasinae. Some of them are probably present in fossil symphrasines, especially the greatly elongate female gonocoxites 9 (i.e. long ovipositor), which needs to be carefully explored in forthcoming symphrasine fossils. The intergeneric relationships within Symphrasinae are largely congruent with that recovered previously [15]. Two Cretaceous amber genera, Habrosymphrasis and Parasymphrasites gen. nov., occupy relatively early positions, seemingly representing stem-group Symphrasinae as also considered in earlier studies [15]. However, it is notable that Haplosymphrasites gen. nov. is grouped with the three extant symphrasine genera based on the presence of two to four trichosors between longitudinal veins along the distal wing margin. Thus, the mid-Cretaceous fauna of Symphrasinae was composed of coexisting stem-group and crown-group species.
The subfamilial status of Doratomantispinae subfam. nov. is confirmed in the present analysis as Doratomantispa and Paradoxomantispa gen. nov. are not within any known mantidfly subfamily and share two unique autapomorphic characters. Thus, the original placement of Doratomantispa in Drepanicinae is rejected. Furthermore, this new subfamily represents a lineage intermingling plesiomorphic and apomorphic features between Symphrasinae and higher crown-group Mantispidae (i.e. Drepanicinae, Calomantispinae and Mantispinae), but not of stem-group Mantispidae as asserted by Shi et al. [16].
The remaining three Cretaceous amber genera, i.e. Acanthomantispa gen. nov., Dicranomantispa gen. nov. and Psilomantispa gen. nov., are a group of mantidflies with greatly specialized forelegs, which lack most or even all profemoral spines but have uniquely forked profemoral basal spines in some genera. Despite sharing similar wing and male genital characters with extant Drepanicinae, these three peculiar genera do not cluster with the other drepanicine genera. Nevertheless, among the other drepanicine groups, the extant genus Theristria is also not clustered with the clade including the remaining genera. In Liu et al. [9], a monophyletic Drepanicinae was recovered but based on only two apomorphic character states in the female gonocoxites and gonapophyses VIII. Unfortunately, the female genitalia of the three putative Cretaceous drepanicine genera remain unknown. The subfamilial identity of these new genera needs further clarification when additional material, especially females, are found.
(b). Evolution of raptorial forelegs in Mantispidae
Raptorial foreleg in arthropods as a prey-capture structure are usually formed by opposing spines or modified setae on the profemur and protibia which can be brought into rapid adjoinment. When coupled with an elongate procoxa and inflated profemur to allow for swift, powerful closure the result is a highly effective mechanism for subduing prey, made even more effective by refined visual acuity [17–19]. The spines or setae of the profemur allow the predator to sense prey movement and ensnare victims [20]. Many insects possess raptorial forelegs to enhance their predation efficiency, such as ambush bugs (Hemiptera: Reduviidae: Emesinae, Phymatinae), shore flies of the genus Ochthera (Diptera: Ephydridae), ancient predatory roach-like insects and praying mantises (Dictyoptera), and mantidflies and their relatives (Neuroptera: Mantispidae, Rhachiberothidae, Dipteromantispidae) [3,19,21–24]. However, the size and arrangement of profemoral spines or setae vary among these groups. Generally, the profemoral spines, if present, are irregularly arranged, or regularly arranged into a single row or two rows (mesal or ental to the closed protibia). Sometimes the spine row ental to the closed protibia includes a long basal spine that is usually present in many species of Mantodea and Mantispidae. By contrast, the profemoral spines are less developed in ambush bugs and Ochthera.
The newly reported amber mantidflies display diverse morphological modifications of the raptorial forelegs. By the mid-Cretaceous, most major types of mantidfly raptorial forelegs except those in Mantispinae and Calomantispinae, were already present. Moreover, certain morphological specializations of the forelegs were present that have subsequently been lost from the diversity of Mantispidae––e.g. the forked profemoral basal spine in Acanthomantispa gen. nov. and Dicranomantispa gen. nov., and the complete loss of profemoral spines in Psilomantispa gen. nov. The phylogeny estimated here provides a guide to understand the evolutionary history of raptorial forelegs in these charismatic predators.
First, the distinctly robust profemur, which is shared by Mesomantispinae and Symphrasinae, appears to be a plesiomorphic condition for the family. In the remaining, more derived mantidfly subfamilies, the profemur tends to be thinner or slightly flattened laterally. Second, the size of forelegs in these Cretaceous mantidflies significantly varies, approximately from 9.0 mm to 4.0 mm, although no clear pattern could be discerned regarding the evolution of foreleg size. Third, the profemoral spines or spinous macrosetae are arranged as two or more rows in Mesomantispinae, Symphrasinae and Doratomantispinae subfam. nov., while they are more closely spaced, generally arranged as a single row in most species of the more advanced mantidflies. Fourth, the development of a long basal profemoral spine is considered to have evolved once in the common ancestor of Doratomantispinae subfam. nov. and the more advanced mantidflies.
Considering the fine morphology of the foreleg spine-like structures in mantidflies, Pérez-de la Fuente & Peñalver [20] presented a detailed comparison among major lineages of Mantispoidea with raptorial forelegs. In Mantispidae, Drepanicinae have the most diverse morphological modifications of these foreleg spine-like structures. The presently described amber drepanicine mantidflies document another previously unknown trend, namely the large reduction of profemoral spine-like structures. Moreover, the paired or multiply forked profemoral basal spine in Acanthomantispa gen. nov. and Dicranomantispa gen. nov. represents a highly derived apomorphic condition. In Doratomantispinae subfam. nov., which is transitional between Symphrasinae and the more advanced mantidflies, the arrangement of the profemoral spines, as well as the presence of strongly curved prostrate setae on protibia, are unique in Mantispidae. The present finding of diverse types of raptorial forelegs in fossil Mantispidae provides significant evidence supporting a putative generality of biological evolution, which estimates the tendency for taxa to reach maximal morphological diversity (disparity) relatively early in the lifespan of their parent clade followed by subsequent canalization into narrower sets of variation [25–27].
The mid-Cretaceous of Myanmar harboured an extraordinarily diverse tropical forest biota [28], with rich and highly partitioned niches, reflected by a wide range of morphologically varied but closely related species. Furthermore, some species displayed morphological and behavioural specializations unknown in their modern counterparts. In the Cretaceous amber Neuroptera, larvae of stem-group Chrysopidae (green lacewings) have some spectacular morphological modifications, such as dramatically elongate legs, leaf-like thoracic and abdominal lobes, or extremely elongate, highly setigerous tubular processes on the thorax and abdomen, and these specialized characters are associated with predation, mimesis and camouflage in these species, respectively [29–32]. Some recently described Cretaceous myrmeleontiform larvae also had highly disparate morphological traits convergently evolved with chrysopoid larvae in relation to predatory habits [33–35]. Other, unrelated Cretaceous lacewing lineages had elongate mouthparts, in families which today lack such features, and were specializations for a variety of feeding strategies but including pollen and nectar [36–39].
Analogous to the above cases of greater niche variety, morphological specialization and convergence among Cretaceous lacewings, it is interesting to discover a diversity of predator morphologies among Mantispidae. Indeed, it appears that Neuroptera were considerably more varied in their life histories and morphologies during the Cretaceous than they were to become in the Cenozoic and today. It is likely that these Cretaceous amber mantidflies exhibited a diversity of feeding habits and behaviours, with different strategies present among the subfamilies and genera, and including habits that no longer persist. The most interesting feature among the mid-Cretaceous mantidflies is the complete loss of the foreleg spine-like structures in Psilomantispa gen. nov. Compared with their contemporaneous mantidflies (e.g. Doratomantispinae subfam. nov.) with well-developed foreleg spine-like strucutues, the foreleg of Psilomantispa gen. nov. likely could not function as in spiny raptorial forelegs. Modern resin bugs (Reduviidae: Harpactorinae) dip their forelegs in resin and smack the resulting ‘sticky’ foreleg against preys to achieve capture [40]. If Psilomantispa gen. nov. was predatory, perhaps it operated by a similar function. Alternatively, the adults of Psilomantispa gen. nov. might be polyphagous or even phytophagous, representing a unique reversal in feeding ecology. However, lacking raptorial forelegs is not necessarily an indication of non-predatory habits in Neuroptera as the adults of many green lacewings (Chrysopidae), brown lacewings (Hemerobiidae) and dustywings (Coniopterygidae) are predators [41]. Conversely, various groups of fossil and extant lacewings also fed on pollen and other food sources, although no Mantispidae are yet known to deviate from predatory behaviour [41,42]. Therefore, the behaviour and dietary specializations of Psilomantispa gen. nov. remain a mystery.
Hitherto, the species diversity of mantidflies from the mid-Cretaceous of Myanmar is known to be much richer than the other known palaeofauna of mantidflies as well as that of the Mesozoic praying mantises (Mantodea) or predatory cockroaches (Blattodea) with similarly configured raptorial forelegs. The raptorial foreleg might have acted as a ‘key innovation’ driving the high diversification of mantidfly species since their Jurassic origin. Thus, mantidflies might be among the earliest evolved insects with raptorial forelegs in adaption to previously underused niches of predator–prey interplay before the rise of praying mantises and other insects with raptorial forelegs [43].
(c). Origin of hypermetamorphosis in Mantispidae
Mantidfly larvae have an unusual developmental strategy, specifically hypermetamorphosis, whereby the first-instar larva is highly mobile and active and the remaining two larval stages are immobile and grub-like [1]. This remarkable life-history trait is also known among crown-group Berothidae (i.e. Berothinae), but specifically associated with termites [44,45]. To date, the larval development of mantidflies is mainly associated with either stinging wasps and bees (Hymenoptera) or spiders. Mantidfly larvae feeding on Hymenoptera are only known among Symphrasinae, and their larval development is completed in the nests of eusocial bees or several different hosts among wasps such as Vespidae and Sphecidae. [46–49]. The larvae of the other three extant mantidfly subfamilies are thought to be highly specialized predators of spiders and spider eggs [1,50], although most observations and empirical data are confined to Mantispinae. For Drepanicinae, there is only a single and brief report on a spider association between species of Theristria and Achaearanea (Theridiidae) in Australia [51]. The life history of Calomantispinae is also known only for Nolima pinal, which completed development to adulthood by feeding on spiders and immature insects under laboratory conditions [52].
The origin of this remarkable developmental ecology in mantidflies is poorly known. Ohl [53] first documented an Eocene Baltic amber mantidfly larva, which probably belongs to Mantispinae, attached to a clubionoid spider as a boarder searching for spider egg sacs. Haug et al. [50] recorded similar spider-boarding behaviour of a mantidfly larva from mid-Cretaceous amber, currently representing the earliest evidence of mantispid larvae specially associated with spiders. Notably, the mantidfly larva reported in Haug et al. [50] differs distinctly from mantispine larvae by the incurved mandibular–maxillary stylets, a feature however shared with Symphrasinae. As the incurved mandibular–maxillary stylets are plesiomorphic condition, their amber larva with spider-boarding behaviour could belong to stem-group Mantispidae, Symphrasinae, or even to Doratomantispinae subfam. nov., which shares many symplesiomorphies with Symphrasinae. As such, one must wonder whether an association with spiders is plesiomorphic for the entire family, perhaps even extending into the Jurassic. Given that spiders are truly ancient, with the lineage originating nearly 400 Mya [54], it is certainly possible that the establishment of mantifly spider associations could have occurred early in the appearance of Mantispidae, perhaps even fuelling their divergence from other stem mantispoid lineages. If this is the case, then predation on immature bees or wasps among Symphrasinae would assuredly be a secondary specialization that arose during the Cretaceous when these aculeate lineages were originating and diversifying [55]. The current discovery of crown-group Symphrasinae (i.e. Haplosymphrasite zouae gen. et sp. nov.) in the mid-Cretaceous is certainly consistent with the rise of aculeates at that time.
(d). Conclusions
The most diverse palaeofauna of Mantispidae hitherto known is uncovered from the mid-Cretaceous. Phylogenetic analysis of this unexpected diversity of mantidfly predators corroborates hypotheses of relationships among the major lineages of the family and highlights the greater morphological disparity once present in the family. The mid-Cretaceous fauna consisted of coexisting stem- and crown-group members of certain subfamilies (e.g. Symphrasinae), as well as transitional lineages between Symphrasinae and more advanced mantidflies. It is clear that Mantispidae reached a considerably diversified stage by the mid-Cretaceous and when the two major larval life styles of mantidflies associated with spiders and Aculeata could have originated. The high species diversity and disparate foreleg characters were likely driven by more diverse niches of predator–prey interplay in the complex tropical forest ecosystem of the mid-Cretaceous.
4. Material and methods
The amber specimens described here are from the Hukawng Valley, Tanai Township, Myitkyina District, Kachin State, northern Myanmar. Specimens are currently housed in the Entomological Museum of China Agricultural University (CAU), Beijing, before final deposition in the Three Gorges Entomological Museum (EMTG), Chongqing, the Century Amber Museum (CAM), Shenzhen, and the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing. We included most Mesozoic fossil mantidfly genera and representative genera of all extant subfamilies as ingroup taxa for the phylogenetic analysis and coded a total of 72 adult characters. See detailed methods in electronic supplementary material, note S3.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Mrs Xiao Jia, Mrs Meixu Zhou and Mr Ning Wang for kindly providing amber specimens herein studied. We also thank Mr Lukas Kirschy, Mr Bernhard Schurian and Mr Hongyu Li for kind help on photographing. Finally, sincere thanks go to the three referees who critically read the manuscript and offered valuable suggestions for improvement of this work.
Data accessibility
All data needed to evaluate the conclusions in the paper are present in the main text and/or electronic supplementary material.
Authors' contributions
X.Y.L. conceived and designed the experiments; X.M.L, X.Y.L., B.W., M.O. and M.S.E. performed the experiments and analyses; X.M.L, W.W.Z. and X.Y.L. prepared figures and collected data. X.M.L., X.Y.L., B.W., M.O. and M.S.E. wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no compteing interests.
Funding
This research was supported by the National Natural Science Foundation of China (grant nos. 31972871, 31900348, 31672322 and 41688103), the Changjiang Scholars Program (2018), the Shanghai Sailing Program (grant no. 19YF1443000) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB26000000).
References
- 1.Redborg KE. 1998. Biology of the Mantispidae. Annu. Rev. Entomol. 43, 175–194. ( 10.1146/annurev.ento.43.1.175) [DOI] [PubMed] [Google Scholar]
- 2.Ohl M. 2004. Annotated catalog of the Mantispidae of the World (Neuroptera). Contribut. Entomol. , Internat. 5, 131–262. [Google Scholar]
- 3.Engel MS, Winterton SL, Breitkreuz LCV. 2018. Phylogeny and evolution of Neuropterida: where have wings of lace taken us? Annu. Rev. Entomol. 63, 531–551. ( 10.1146/annurev-ento-020117-043127) [DOI] [PubMed] [Google Scholar]
- 4.Lambkin KJ. 1986. A revision of the Australian Mantispidae (Insecta: Neuroptera) with a contribution to the classification of the family. I. General and Drepanicinae. Aust. J. Zool. Suppl. Ser. 116, 1–142. ( 10.1071/AJZS116) [DOI] [Google Scholar]
- 5.Ansorge J, Schlüter T. 1990. The earliest chrysopid: Liassochrysa stigmatica n.g., n.sp. from the lower Jurassic of Dobbertin, Germany. Neuropt. Internat. 6, 87–93. [Google Scholar]
- 6.Wang YY, Liu XY, Garzón-Orduña IJ, Winterton SL, Yan Y, Aspöck U, Aspöck H, Ding Y. 2017. Mitochondrial phylogenomics illuminates the evolutionary history of Neuropterida. Cladistics 33, 617–636. ( 10.1111/cla.12186) [DOI] [PubMed] [Google Scholar]
- 7.Winterton SL, et al. 2018. Evolution of lacewings and allied orders using archored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 43, 330–354. ( 10.1111/syen.12278) [DOI] [Google Scholar]
- 8.Jepson JE. 2015. A review of the current state of knowledge of fossil Mantispidae (Insecta: Neuroptera). Zootaxa 3964, 419–432. ( 10.11646/zootaxa.3964.4.2) [DOI] [PubMed] [Google Scholar]
- 9.Liu XY, Winterton SL, Wu C, Piper R, Ohl M. 2015. A new genus of mantidflies discovered in the Oriental region, with a higher-level phylogeny of Mantispidae (Neuroptera) using DNA sequences and morphology. Syst. Entomol. 40, 183–206. ( 10.1111/syen.12096) [DOI] [Google Scholar]
- 10.Makarkin VN, Yang Q, Shi CF, Ren D. 2013. The presence of the recurrent veinlet in the Middle Jurassic Nymphidae (Neuroptera): a unique character condition in Myrmeleontoidea. Zookeys 325, 1–20. ( 10.3897/zookeys.325.5453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarkin VN. 2015. A new genus of the mantispid-like Paraberothinae (Neuroptera: Berothidae) from Burmese amber, with special consideration of its probasitarsus spine-like setation. Zootaxa 4007, 327–342. ( 10.11646/zootaxa.4007.3.2) [DOI] [PubMed] [Google Scholar]
- 12.Liu XY, Lu XM, Zhang WW. 2017. New genera and species of the family Dipteromantispidae (Insecta: Neuroptera) from the Cretaceous amber of Myanmar and New Jersey. Cretac. Res. 72, 18–25. ( 10.1016/j.cretres.2016.12.007) [DOI] [Google Scholar]
- 13.Jepson JE, Heads SW, Makarkin VN, Ren D. 2013. New fossil mantidflies (Insecta: Neuroptera: Mantispidae) from the Mesozoic of north-eastern China. Palaeontology 56, 603–613. ( 10.1111/pala.12005) [DOI] [Google Scholar]
- 14.Wedmann S, Makarkin VN. 2007. A new genus of Mantispidae (Insecta: Neuroptera) from the Eocene of Germany, with a review of the fossil record and palaeobiogeography of the family. Zool. J. Linn. Soc. 149, 701–716. ( 10.1111/j.1096-3642.2007.00273.x) [DOI] [Google Scholar]
- 15.Shi CF, Qiang Y, Winterton SL, Pang H, Ren D. 2020. Stem-group fossils of Symphrasinae shed light on early evolution of Mantispidae (Insects: Neuroptera). Pap. Palaeontol. 6, 143–154. ( 10.1002/spp2.1265) [DOI] [Google Scholar]
- 16.Shi CF, Qiang Y, Deng CS, Pang H, Ren D. 2019. New species of Doratomantispa from the mid-Cretaceous of northern Myanmar (Insecta, Neuroptera, Mantispidae). Palaeoentomology 002, 446–452. ( 10.11646/palaeoentomology.2.5.8) [DOI] [Google Scholar]
- 17.Loxton RG, Nicholls I. 1979. The functional morphology of the praying mantis forelimb (Dictyoptera: Mantodea). Zool. J. Linn. Soc. 66, 185–203. ( 10.1111/j.1096-3642.1979.tb01908.x) [DOI] [Google Scholar]
- 18.Willmann R. 1990. The phylogenetic position of the Rhachiberothinae and the basal sister-group relationships within the Mantispidae (Neuroptera). Syst. Entomol. 15, 253–265. ( 10.1111/j.1365-3113.1990.tb00316.x) [DOI] [Google Scholar]
- 19.Brannoch SK, Wieland F, Rivera J, Klass K, Béthoux O, Sevenson GJ. 2017. Manual of praying mantis morphology, nomenclature, and practices (Insecta, Mantodea). ZooKeys 696, 1–100. ( 10.3897/zookeys.696.12542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-de la Fuente R, Peñalver E. 2019. Mantidfly in Spanish Cretaceous amber illuminates the evolution of integumentary specialisations on the raptorial foreleg. Sci. Rep. 9, 13248 ( 10.1038/s41598-019-49398-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro-Huertas V, Forero D, Grazia J. 2019. Comparative morphology of the raptorial leg in thread-legged bugs of the tribe Metapterini Stål, 1859 (Hemiptera, Heteroptera, Reduviidae, Emesinae). Zoomorphology 138, 97–116. ( 10.1007/s00435-019-00431-x) [DOI] [Google Scholar]
- 22.Weirauch C, Forero D, Jacobs DH. 2011. On the evolution of raptorial legs—an insect example (Hemiptera: Reduviidae: Phymatinae). Cladistics 27, 138–149. ( 10.1111/j.1096-0031.2010.00325.x) [DOI] [PubMed] [Google Scholar]
- 23.Minakawa N, Futami K, Sonye G, Akweywa P, Kaneko S. 2007. Predatory capacity of a shorefly, Ochthera chalybescens, on malaria vectors. Malaria J. 6, 104 ( 10.1186/1475-2875-6-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dittmann IL, Hörnig MK, Haug JT, Haug C. 2015. Raptoblatta waddingtonae n. gen. nov. sp.—an Early Cretaceous roach-like insect with a mantodean-type raptorial foreleg. Palaeodiversity 8, 103–111. [Google Scholar]
- 25.Wills MA, Briggs DEG, Fortey RA. 1994. Disparity as an evolutionary index: a comparison of Cambrian and Recent arthropods. Paleobiology 20, 90–130. ( 10.1017/S009483730001263X) [DOI] [Google Scholar]
- 26.Erwin DH. 2007. Disparity: Morphological pattern and developmental context. Palaeontology 50, 57–73. ( 10.1111/j.1475-4983.2006.00614.x) [DOI] [Google Scholar]
- 27.Valentine JW, Erwin DH, Jablonski D. 1996. Developmental evolution of metazoan bodyplans: the fossil evidence. Dev. Biol. 173, 373–381. ( 10.1006/dbio.1996.0033) [DOI] [PubMed] [Google Scholar]
- 28.Grimaldi DA, Engel MS, Nascimbene PC. 2002. Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 3361, 1–72. () [DOI] [Google Scholar]
- 29.Liu XY, Zhang WW, Winterton SL, Breitkreuz LCV, Engel MS. 2016. Early morphological specialization for insect–spider associations in Mesozoic lacewings. Curr. Biol. 26, 1590–1594. ( 10.1016/j.cub.2016.04.039) [DOI] [PubMed] [Google Scholar]
- 30.Liu XY, Shi GL, Xia FY, Lu XM, Wang B, Engel MS. 2018. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 28, 1475–1481. ( 10.1016/j.cub.2018.03.060) [DOI] [PubMed] [Google Scholar]
- 31.Wang B, et al. 2016. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2, e1501918 ( 10.1126/sciadv.1501918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.la Fuente R Pérez-de, Peñalver E, Azar D, Engel MS.. 2018. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 8, 16663 ( 10.1038/s41598-018-34870-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badano D, Engel MS, Basso A, Bo W, Cerretti P. 2018. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Commun. 9, 3257 ( 10.1038/s41467-018-05484-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haug C, Herrera Flórez AF, Müller P, Haug JT. 2019. Cretaceous chimera—an unusual 100-million-year old neuropteran larva from the ‘experimental phase’ of insect evolution. Palaeodiversity 12, 1–11. ( 10.18476/pale.v12.a1) [DOI] [Google Scholar]
- 35.Haug C, Müller P, Haug JT. 2019. A 100-million-year old slim insectan predator with massive venom-injecting stylets—a new type of neuropteran larva from Burmese amber. Bull. Geosci. 94, 431–440. [Google Scholar]
- 36.Lu XM, Zhang WW, Liu XY. 2016. New long-proboscid lacewings of the mid-Cretaceous provide insights into the ancient plant–pollinator interactions. Sci. Rep. 6, 25382 ( 10.1038/srep25382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makarkin VN. 2016. Enormously long, siphonate mouthparts of a new, oldest known spongillafly (Neuroptera, Sisyridae) from Burmese amber imply nectarivory or hematophagy. Cretac. Res. 65, 126–137. ( 10.1016/j.cretres.2016.04.007) [DOI] [Google Scholar]
- 38.Liu Q, Lu XM, Zhang QQ, Chen J, Zheng XT, Zhang WW, Liu XY, Wang B. 2018. High niche diversity in Mesozoic pollinating lacewings. Nat. Commun. 9, 3793 ( 10.1038/s41467-018-06120-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khramov AV, Yan E, Kopylov DS. 2019. Nature's failed experiment: long-proboscid Neuroptera (Sisyridae: Paradoxosisyrinae) from upper Cretaceous amber of northern Myanmar. Cretac. Res. 104, 104180 ( 10.1016/j.cretres.2019.07.010) [DOI] [Google Scholar]
- 40.Silva AC, Gil-Sntana HR. 2004. Predation of Apiomerus pilipes (Fabricius) (Hemiptera, Reduviidae, Harpactorinae, Apiomerini) over Meliponinae bees (Hymenoptera, Apidae), in the State of Amnoazonas, Brazil. Rev.Bras. Zool. 21, 769–774. ( 10.1590/S0101-81752004000400007) [DOI] [Google Scholar]
- 41.New TR. 1989. Planipennia, lacewings. In Handbuch der Zoologie, vol. 4, chap. 30. Berlin, Germany: Walter de Gruyter. [Google Scholar]
- 42.Labandeira CC, et al. 2016. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B 283, 20152893 ( 10.1098/rspb.2015.2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornig MK, Haug JT, Haug C. 2017. An exceptionally preserved 110 million years old praying mantis provides new insights into the predatory behavior of early mantodeans. PeerJ 5, e3605 ( 10.7717/peerj.3605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tauber CA, Tauber MJ. 1968. Lomamyia latipennis (Neuroptera, Berothidae) life history and larval descriptions. Can. Entomol. 100, 623–629. ( 10.4039/Ent100623-6) [DOI] [Google Scholar]
- 45.Komatsu T. 2014. Larvae of the Japanese termitophilous predator Isoscelipteron okamotonis (Neuroptera, Berothidae) use their mandibles and silk web to prey on termites. Insectes Soc. 61, 203–205. ( 10.1007/s00040-014-0346-6) [DOI] [Google Scholar]
- 46.Maia-Silva C, Hrncir M, Koedam D, Machardo RJP, Imperatriz-Fonseca VL. 2013. Out with the garbage: the parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil. Naturwissenshaften 100, 101–105. ( 10.1007/s00114-012-0994-1) [DOI] [PubMed] [Google Scholar]
- 47.Dejean A, Canard M. 1990. Reproductive behaviour of Trichoscelia santareni (Navas) (Neuroptera: Mantispidae) and parasitization of the colonies of Polybia diguetana R. du Buysson (Hymenoptera: Vespidae). Neuropt. Internat. 6, 19–26. [Google Scholar]
- 48.Penny ND. 1982. Review of the generic level classification of the New World Mantispidae (Neuroptera). Acta Amazon. 12, 209–223. ( 10.1590/1809-43921982121209) [DOI] [Google Scholar]
- 49.Hook AW, Oswald JD, Neff JL. 2010. Plega hagenella (Neuroptera: Mantispidae) parasitism of Hylaeus (Hylaeopsis) sp. (Hymenoptera: Colletidae) reusing nests of Trypoxylon manni (Hymenoptera: Crabronidae) in Trinidad. J. Hymenopt. Res. 19, 77–83. [Google Scholar]
- 50.Haug JT, Müller P, Haug C. 2018. The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zool. Lett. 4, 31 ( 10.1186/s40851-018-0116-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Austin AD. 1985. The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna. J. Nat. Hist. 19, 359–376. ( 10.1080/00222938500770261) [DOI] [Google Scholar]
- 52.MacLeod EG, Redborg KE. 1982. Larval platymantispine mantispids (Neuroptera: Planipennia): possibly a subfamily of generalist predators. Neuropt. Internat. 2, 37–41. [Google Scholar]
- 53.Ohl M. 2011. Aboard a spider—a complex developmental strategy fossilized in amber. Naturwissenschaften 98, 453–456. ( 10.1007/s00114-011-0783-2) [DOI] [PubMed] [Google Scholar]
- 54.Fernández R, Kallal RJ, Dimitrov D, Ballesteros JA, Arnedo MA, Giribet G, Hormiga G. 2018. Phylogenomics, diversification dynamics, and comparative transcriptomics across the spider tree of life. Curr. Biol. 28, 1489–1497. ( 10.1016/j.cub.2018.03.064) [DOI] [PubMed] [Google Scholar]
- 55.Peters RS, et al. 2017. Evolutionary history of Hymenoptera. Curr. Biol. 27, 1013–1018. ( 10.1016/j.cub.2017.01.027) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the main text and/or electronic supplementary material.