Abstract
Prey naiveté—the failure of prey to recognize novel predators as threats—is thought to exacerbate the impact that exotic predators exert on prey populations. Prey naiveté varies under the influence of eco-evolutionary mediating factors, such as biogeographic isolation and prey adaptation, although an overall quantification of their influence is lacking. We conducted a global meta-analysis to test the effects of several hypothesized mediating factors on the expression of prey naiveté. Prey were overall naive towards exotic predators in marine and freshwater systems but not in terrestrial systems. Prey naiveté was most pronounced towards exotic predators that did not have native congeneric relatives in the recipient community. Time since introduction was relevant, as prey naiveté declined with the number of generations since introduction; on average, around 200 generations may be required to erode naiveté sufficiently for prey to display antipredator behaviour towards exotic predators. Given that exotic predators are a major cause of extinction, the global predictors and trends of prey naiveté presented here can inform efforts to meet conservation targets.
Keywords: invasive species, meta-analysis, naive prey, predator archetype, prey naiveté, prey behaviour
1. Introduction
The introduction of exotic species can cause substantial impacts on biodiversity and ecosystems [1–3], and such impacts are likely to exacerbate as rates of species introduction continue to increase [4,5]. Exotic predators are arguably the most disruptive group of introduced species [2,6], as they often exert impacts on native species far greater than those attributed to their native counterparts [7–9] and they are implicated in the extinction of hundreds of native species [1,2]. For instance, the accidental introduction of the brown tree snake (Boiga irregularis) onto the island of Guam, where there are no native arboreal vertebrate predators, caused the extinction of numerous species of birds, mammals, and reptiles [6]. The disproportionate impact of exotic predators on native communities is often attributed to prey naiveté—the failure of prey to recognize (or respond appropriately) to a novel predator species and/or the lack of an appropriate defence (sensu [10]). Such prey naiveté towards exotic predators likely derives from insufficient eco-evolutionary exposure [10–16]. For example, rats introduced to oceanic islands worldwide are implicated in numerous extinctions of mammals, birds, and reptiles that have no evolutionary experience with generalist mammalian nest predators [17]. However, rat impacts are reduced on islands that possess native rats or functionally similar land crabs, presumably because fauna on those islands are less naive to the effects of introduced omnivores [18].
Prey naiveté was originally conceived as a simplistic phenomenon where native animals become ‘easy prey’ to exotic predators owing to naive behaviour [11]. However, prey naiveté is now recognized as a more complex phenomenon and four levels of prey naiveté have been proposed [15,16,19]. Level-1 naive prey do not recognize the exotic predator as a threat, which precludes any antipredator behavioural responses [19]. Native animals experience level-2 naiveté if they recognize the exotic predator but show inappropriate antipredator behaviour [19]. Level-3 naive prey display an appropriate but ineffective behavioural response towards an exotic predator [19]. Lastly, level-4 naive prey over-respond to the exotic predator after experiencing excessive sublethal costs of predation [16]. In addition to exhibiting inadequate antipredator behaviour, prey species that lack evolutionary experience to exotic predation may also possess other morphological or physiological traits that make them susceptible to exotic predators such as insufficient armature, flightlessness, conspicuous scent, or inadequate camouflage [20]. Although prey naiveté is a well-accepted phenomenon [16], it varies under the influence of eco-evolutionary factors [14,15,21] whose relative importance and generality have yet to be quantified.
We hypothesize that the occurrence and strength of prey naiveté stems from several, non-exclusive factors that can be clustered into four themes (table 1). First, prey naiveté can be promoted by persistent biogeographic (hence evolutionary) isolation between predator and prey [13]. The pronounced isolation of freshwater biota has been hypothesized to render prey more sensitive to introduced predators compared with terrestrial or marine biota [10,22] (Hypothesis 1 in table 1). Prey naiveté is also presumed to be more prevalent on islands than on mainlands [23–25], owing to lack of eco-evolutionary experience with exotic predators—or even native ones on predator-free islands (Hypothesis 2 in table 1). Likewise, predators introduced to geographically isolated or species-poor biotas are more likely to represent a novel archetype—that is, prey will not display antipredator responses towards exotic predators that are unfamiliar, where a practical proxy for ‘archetype’ distinction has been proposed at the taxonomic level of genus or family [10,16,26,27] (Hypothesis 3 in table 1). The introduction of a predator from a different biogeographic realm enhances the probability that the predator will be distinct from those of the recipient biota and thus unfamiliar [10] (Hypothesis 4 in table 1). The second theme is related to the way animals acquire antipredator responses (and lose prey naiveté) over time through adaptation, which could be a function of the number of prey generations since the introduction of a predator [28–30] (Hypothesis 5 in table 1). The third theme is related to the mediating role of latitude on prey naiveté, as novel predator recognition could be higher in low latitude communities, which generally experience greater and more diverse predation pressure [31–33] and thus whose prey may display antipredator behaviours to a broader variety of predator archetypes (Hypothesis 6 in table 1). Finally, the fourth theme is related to taxonomic specificity, as the recognition of introduced predators might vary across taxa [34], such that certain predators are more recognizable than others and certain prey are better adapted to recognize certain predators or entire suites of predatory taxa (Hypotheses 7 and 8, respectively, in table 1).
Table 1.
Determinants of prey naiveté and the eight hypotheses tested in this study.
| eco-evolutionary theme | potential determinants of prey naiveté | hypotheses tested | predictions | references supporting predictions | did findings support predictions? |
|---|---|---|---|---|---|
| biogeographic isolation | system type | H1: Prey naiveté differs among system types (e.g. terrestrial, freshwater, or marine) | freshwater systems will experience higher levels of prey naiveté than terrestrial or marine systems, owing to higher biogeographic isolation | [13,22] | partially |
| insularity | H2: Prey naiveté differs between islands and continental mainlands | prey species on islands are more naive to novel terrestrial predators than on continents | [23–25] | yes | |
| archetype hypothesis | H3: Prey recognize introduced predators that are the same archetype as familiar local predators | predators introduced in locations that contain native congeners will encounter less naive prey | [10,16,26,27,59] | yes | |
| geographical scale | H4: The geographical scale of the predator introduction mediates prey naiveté | predators introduced in a foreign biogeographic realm will encounter prey species with higher levels of naiveté | [10] | yes | |
| adaptation | number of prey generations | H5: Prey naiveté varies with time of exposure to a novel predator | prey naiveté will decrease with the number of prey generations since the introduction of a predator | [28–30] | yes |
| latitude/biodiversity | latitude of the introduction | H6: Prey naiveté varies across latitudes | prey naiveté is less pronounced at low latitudes, which are more biodiverse and contain a broader range of predator types | [31–33] | no |
| taxonomic attribute | taxonomic group of the predator | H7: Prey naiveté differs among predator taxa | certain taxa of predators will be recognized by prey more than others | [38] | yes |
| taxonomic group of the prey | H8: Prey naiveté differs among prey taxa | some taxa of prey will recognize novel predators better than others | [34] | yes |
Many case studies suggest that these hypotheses are important predictors of prey naiveté [10,12,26,30,35,36], but no synthesis of global trends has been conducted. Here, we tested the generality of these eight hypothesized drivers of prey naiveté, with the goal of revealing which of these drivers can be used to effectively predict prey naiveté and conservation outcomes.
2. Materials and methods
We performed a search on 1 May 2019 following the guidelines of PRISMA (preferred reporting items for meta-analyses; [37]; electronic supplementary material, table S1). We entered the following terms in the Web of Science using the Advanced Search option: TS = (prey naiveté OR prey naivety OR naive prey OR lack of predator recognition OR antipredator behavio*) AND TS = (exotic OR invasive OR alien OR non-native), which produced 199 publications (electronic supplementary material, table S1). We also added 12 additional studies by examining the references of papers focused on prey naiveté (electronic supplementary material, table S1).
Studies were included if they met the following criteria. First, each study empirically compared—in field or laboratory experiments—the behavioural response of prey to an exotic and a native predator. In this study, we only evaluated evidence of predator recognition (Level-1 prey naiveté [19]), which has been proposed as the most fundamental form of prey naiveté [38]. Second, the studies quantified behavioural responses and reported the mean, some form of variance (standard deviation, standard error, or confidence interval) and the sample size. Third, experiments within published articles were included as individual observations (i.e. number of rows on the database) if (1) investigators used different species of prey, native predator, and/or exotic predator, (2) experiments were performed with individuals from different locations, and (3) studies provided measurements for distinct behavioural responses to the same set of species of predators and prey because antipredator responses can be contrasting (e.g. prey might reduce activity in the presence of an exotic predator but not alter refuge use). Finally, to avoid temporal pseudoreplication, if a study measured a behavioural response through time (e.g. longitudinal studies), then the mean response over time was calculated. This criterion was adopted to better represent the generality of the behavioural responses.
The effect size g was calculated as follows [39]:
where XE and XC are the mean of the experimental (exotic predator) and control groups (native predator), respectively. J corrects for bias because of different sample sizes by differentially weighing studies as follows [40]:
One can think of the effect size g as the difference in prey behaviour when in the presence of an exotic versus a native predator. Careful consideration was given when obtaining data from different metrics of predator avoidance, because the direction of the response variable depends on the specific behavioural response quantified. For instance, prey activity is a common metric of predator avoidance and decreases with increasing perception of risk, because prey are usually less active in the presence of a predator. On the other hand, refuge use—another common metric of antipredator behaviour—increases with increasing perception of risk. In order to standardize the direction of our metrics on antipredator behaviour, the data obtained from metrics that increased with increasing perception of risk were not changed and data obtained from metrics that decreased with increasing perception of risk were transformed to negative numbers (a negative symbol was added to the raw values for XE and XC). Therefore, g values near zero indicated predator recognition (e.g. prey respond similarly to an exotic and native predator), whereas values less than zero suggested prey naiveté (e.g. less perception of risk of the exotic predator than to the native predator), and positive values indicated prey perceiving an exotic predator to be more risky than a native predator. We obtained data from the text or tables of the studies or extracted measurements from figures in digital PDFs using ImageJ.
The pooled standard deviation (SDpooled) was calculated as [40,41]
where SD is the standard deviation of the experimental or control group and N is the sample size. When the standard error (SE) or the confidence interval (CI) was reported, the standard deviation was calculated. We weighted the effect sizes to account for inequality in study variance by using the inverse of the sampling variance, in which the variance for each effect size (Vg) was [40]
There were four studies (see electronic supplementary material, table S2) that compared the behavioural response of an exotic prey to native and exotic predators and the native predators were considered to be the novel consumers of the exotic prey, therefore qualifying as tests of prey naiveté. Three papers that investigated antipredator responses to the exotic green crab Carcinus maenas in the North-Western Atlantic [42–44] were not included in the meta-analysis because the native prey Nucella lapillus is sympatric with C. maenas in the North-Eastern Atlantic (the native range of the green crab), and, hence, did not meet our criteria.
In addition to the effect size, we recorded from each study the following factors: (1) ecosystem type (whether the exotic predator was introduced in a terrestrial, freshwater, or marine system), (2) insularity (whether the introduction was on an island or on a continental mainland including only data from terrestrial systems; Australia was considered a continental mainland, following [45]), (3) biogeographic realm difference (whether or not the location of introduction and the native range of the exotic species occupy the same biogeographic realm; terrestrial and freshwater systems were assigned to one of 11 biogeographic realms and marine ecosystems to 1 of 12—see electronic supplementary material, table S3), (4) taxonomic distinctiveness of the exotic predator (presence/absence of native predators in the introduced biogeographic region that belong to the same genus as the exotic predator), (5) the exotic predator taxonomic group (a posteriori categorized as six levels: fish, mammal, crustacean, herpetofauna, insect, and echinoderm), (6) the taxonomic group of the native prey (a posteriori categorized in six levels: fish, mammal, crustacean, herpetofauna, insect, and mollusc), (7) the number of prey generations since introduction (calculated by dividing the time passed since the exotic species was first recorded in the exotic region by the generation time of the prey species), and (8) the absolute latitude of the introduction of the novel predator measured in decimal degrees. We used the point of introduction instead of other potential spatial proxies—for instance, the midpoint of the full range of predator introduction—because the distributions of exotic predators and their native prey are usually patchy and often times unknown, and, more importantly, because many researchers used the patchiness of predator distributions to define the area of study (based on the presence or absence of the exotic predator).
(a). Statistical meta-analyses
Meta-analyses were performed using the metafor package for R [46]. Treatments with less than or equal to 10 observations were dropped from the analyses to improve statistical robustness, which only included the removal of exotic echinoderms and insects (3 and 4 observations, respectively) from the analysis (see electronic supplementary material, table S4). We ran six independent mixed-effect models with different fixed predictors and in which ‘study ID’ and ‘experiment ID’ were always included as nested random factors, to account for multiple observations attained from the same study and experiment. In addition, the number of generations since introduction was added in each of the six models as a covariate to account for the potential effects of adaptation on prey naiveté through time. These six independent models included the following fixed, categorical predictors: (1) the system type of the introduction (with three levels: terrestrial, freshwater, or marine), (2) insularity (with two levels: mainland or island), (3) taxonomic distinctiveness of the introduced predator (with two levels: yes or no), (4) difference in biogeographic realm (with two levels: same or different if the biogeographic realm of the exotic predator was the same or different than the biogeographic realm of the introduction), (5) the taxonomic group of the exotic predator (with four levels: fish, mammal, crustacean, and herpetofauna; insect and echinoderm were excluded because they had ≤10 replicates), and (6) the taxonomic group of the native prey (with six levels: fish, mammal, crustacean, herpetofauna, insect, and mollusc; some important taxa (e.g. birds) were not included as no publications were found that met our criteria for comparing the response of these groups towards native and exotic predators). Effect sizes were considered significant if the 95% CIs did not overlap with zero. We also ran two further independent mixed-effects models with the nested random factors described above and a continuous fixed factor: the number of prey generations since the introduction of the novel predator, and the absolute latitude of introduction. For these models with continuous predictors, their significance was determined by the p-value of the moderator [46].
Publication bias can distort the results in a meta-analysis [40] by, for instance, overestimating prey naiveté towards exotic species. The functions regtest and trimfill are not implemented in the metafor package for mixed-effects models. Therefore, potential publication bias was evaluated using Egger's regression test [47] by running models that included the standard error of the effect sizes (included as the square root of the variance) as a moderator [48]; bias was determined when the intercept of the model was different from zero at p-values ≤ 0.05. In addition, we examined the data for potential outliers by looking at the effect sizes with standardized residual values exceeding the absolute value of three [49] using the rstandard function in R. Adjusting for publication bias did not change the outcome of the analyses (by comparing fitted random-effects models with and without the influence of the potential outliers; electronic supplementary material, table S4), indicating minimal influence of potential outliers.
3. Results
We found 40 studies that met our criteria to be included in the final dataset (electronic supplementary material, table S2), which comprised a total of 214 observations. The studies were published between 1993 and 2018 (electronic supplementary material, table S2) and included 47 unique study locations of introduction (electronic supplementary material, figure S1). Overall, we included reports assessing prey naiveté in 61 species of prey, with 38 species of exotic predators and 57 species of native predators (electronic supplementary material, table S2). The majority of species of introduced predators in our study were from freshwater systems (54.6%; 117 observations out of 214) when compared with terrestrial (33.6%; 72 observations) and marine systems (11.7%; 25 observations). The models that included ‘number of prey generations’ had a lower Akaike's Information Criterion (AIC) than those that excluded this variable, so the variable was kept in the models, regardless of its significance.
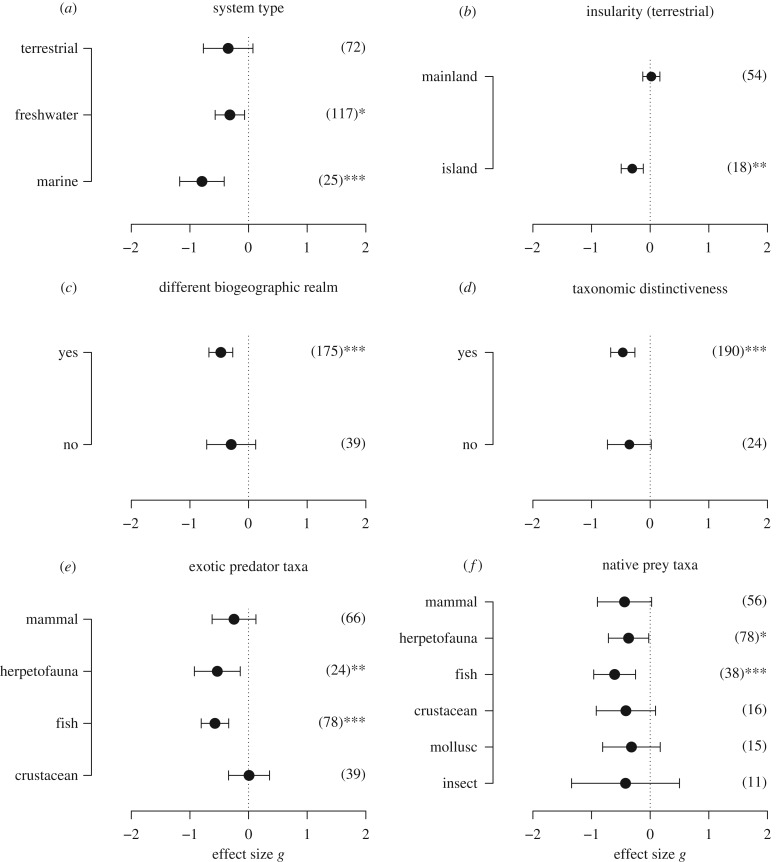
Naiveté was found to be significantly pronounced in animals from marine and freshwater systems (mean Hedge's g ± 95%CI = −0.79 ± 0.38 and −0.32 ± 0.25, p < 0.001, and p = 0.013, respectively; figure 1a) but not significant in terrestrial systems (g = −0.35 ± 0.42, p = 0.107; figure 1a). Likewise, significant levels of prey naiveté were exhibited by prey on islands (g = −0.31 ± 0.18, p = 0.001; figure 1b), but not by animals in terrestrial continents (g = −0.02 ± 0.15, p = 0.789; figure 1b). Prey naiveté was significant only when the original biogeographic realm of the exotic predator differed from the realm in which it was introduced (g = −0.47 ± 0.20, p < 0.001; figure 1c), but not if the introduction occurred within the same biogeographic realm (g = −0.30 ± 0.42, p = 0.165; figure 1c). Similarly, the taxonomic distinctiveness of the exotic predator in the introduced realm also predicted prey naiveté, as native prey were significantly naive to distinct exotic genera (g = −0.47 ± 0.21; p < 0.001; figure 1d), but not towards introduced species with a sympatric species in the same genus (g = −0.35 ± 0.37, p = 0.087; figure 1d).
Figure 1.
Determinants of prey naiveté. Influence of (a) system type, (b) insularity on terrestrial systems, (c) distinctiveness of the biogeographic realm, (d) taxonomic distinctiveness of the exotic predator (i.e. a congeneric species of the exotic predator does not exist within the recipient community), (e) exotic predator taxa, and (f) native prey taxa, which was assessed by comparing the behavioural response of native prey towards native and novel predators. Points indicate the mean effect sizes bracketed by 95% CIs estimated using mixed-effects models. Effect sizes less than zero indicate less antipredator response to a novel predator than to a native predator, and the opposite for effect sizes higher than zero. Effect sizes are considered significant if their 95% CIs do not overlap with zero. Number of observations used to calculate the effect sizes are indicated in parentheses.
We found significant evidence that two taxa of exotic predators (fish and herpetofauna—e.g. amphibians and reptiles) were not recognized by the native prey (g = −0.57 ± 0.23, and −0.53 ± 0.39, p = less than 0.001, and p = 0.007, respectively; figure 1e), whereas exotic mammals and crustaceans were recognized similar to native predators (g = −0.25 ± 0.37, and 0.008 ± 0.35, p = 0.193, and 0.962, respectively; figure 1e). We found significant evidence supporting that two taxa of native prey, herpetofauna and fish (g = −0.37 ± 0.34 and −0.60 ± 0.36, p = 0.036, and less than 0.001, respectively; figure 1f) were prone to be naive towards exotic predators, whereas species from four taxa (insects, molluscs, crustaceans, and mammals) did not exhibit overall prey naiveté (g = −0.42 ± 0.92, −0.32 ± 0.49, −0.41 ± 0.51, and −0.44 ± 0.46, p = 0.370, 0.202, 0.108, and 0.06, respectively; figure 1f).
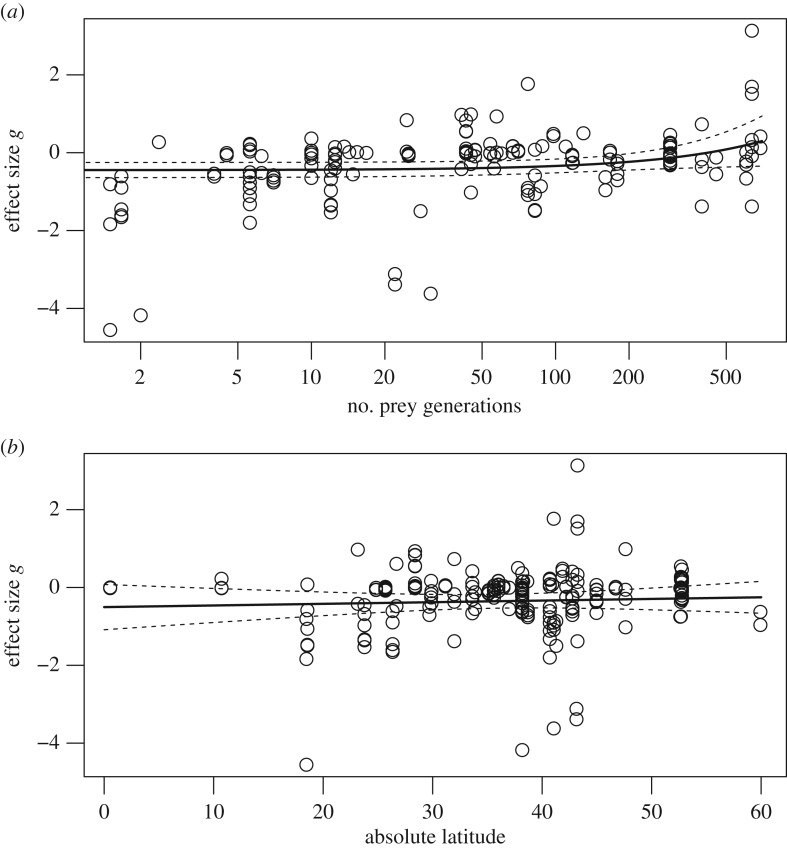
The probability of individuals expressing prey naiveté significantly decreased with the number of prey generations since introduction ((Q-test of the moderator (QM) = 4.332, p = 0.037; figure 2a). Prey species recognized novel predators as threatening as native predators after existing with the novel predators for an average of 215 prey generations, which coincided with the predicted 95% CI of the effect size overlapping with zero (figure 2a). The latitude of the introduction did not influence predator avoidance behaviour of prey to novel predators (QM = 0.287, p = 0.592; figure 2b).
Figure 2.
Determinants of prey naiveté. Influence of two continuous predictors: (a) number of prey generations and (b) absolute latitude of the introduction on prey naiveté. Solid line indicates the mean predicted effect sizes bracketed by 95% CIs (dashed lines) estimated using mixed-effects models.
4. Discussion
Our meta-analysis supports the generality of some, but not all of our hypotheses concerning invasions, and yields some novel insights (table 1). As postulated, we found that prey naiveté was pronounced in freshwater systems but not in terrestrial systems. In concordance with the aquatic-terrestrial dichotomy hypothesis, terrestrial animals were rarely naive towards exotic predators. This phenomenon in terrestrial systems has been attributed to the homogenizing effects of historical biotic interchanges across land masses, whereas the persistent isolation of freshwater systems might have rendered them less experienced to a broader suite of predatory archetypes [10]. Unexpectedly, marine environments appeared to be the most susceptible to introduced predators, contrary to the expectation that they are similar to terrestrial continents in terms of biotic connectivity [10]. Reports of prey naiveté in marine systems are rare and we gathered information from seven publications that compared antipredator responses with exotic and native marine predators. Four of these studies investigated fish naiveté to the exotic lionfish Pterois volitans in the Caribbean, reporting consistent naive fish behaviour. Two other studies [50,51] investigated the exotic marine green crab C. maenas, which found support for predator recognition by native crabs and gastropods. The other marine study [52] reported a pronounced degree of prey naiveté towards the exotic seastar Asterias amurensis by native scallops in Australia. Therefore, although exotic lionfish might have skewed our overall findings on marine prey naiveté, results from the exotic A. amurensis support this trend and suggest that exotic predation threats in marine systems might have been underestimated by conventional wisdom as opposed to actual data.
Evidence from this study supports the hypothesis that terrestrial animals on islands are generally naive towards exotic predators, representing the first global quantification of prey naiveté on islands. When isolated from predators, prey on islands can experience a rapid loss of antipredator behaviour through relaxed selection [53–56]. Indeed, some prey species lack predators in the isolated Galapagos Islands, which are often described as being naive to predatory risk [57]. Similar examples exist on less remote islands, such as snake-free Balearic Islands in the Mediterranean, where wall-lizards show a lack of antipredator behaviours such as tail-waving or slow-motion movement when exposed to introduced snakes [58]. Prey naiveté is a primary explanation for the more devastating impacts of introduced predators on oceanic islands compared with continental terrestrial systems [10]. However, only three studies in our database addressed prey naiveté on islands and two of those were coastal islands (the exception was New Zealand). We hypothesize that the degree of prey naiveté on remote oceanic islands likely exceeds that reported in this meta-analysis. Australia was included as a continental mainland in our study owing to its large size; we performed an additional test by including Australia in the island category, which did not change our findings (g = −0.16. ± 0.15.; p = 0.039 and g = −0.04 ± 0.15; p = 0.853 for island and mainland, respectively), suggesting that our results robustly support the hypothesis that terrestrial species on islands display pronounced levels of prey naiveté.
Prey species adapt to predators by accumulating eco-evolutionary experience [21] that familiarizes them with a particular species or archetype—a set of predatory species that have similar morphological and/or behavioural adaptations to obtain prey [10]. Recognition of a novel predation threat by a native prey species depends in part on the degree of similarity between the exotic predator and native predators present in the invaded community [14,16]. Hence, differences in predator archetypes between the area of origin and the area of introduction of an exotic species can profoundly influence the degree of prey naiveté. In the present study, we tested two proxies of distinctive predator archetype (allopatric origin and generic distinctiveness of the exotic predator [59]) and both were related to prey naiveté. The response towards exotic predators was limited when the introduced predator belonged to a novel genus in the invaded community or originated in a different biogeographic realm. Our results support the hypothesis that predator archetypes might be limited to congeneric species, as suggested previously [27,35], but phylogenetic analyses assessing evolutionary distance between predator species would be warranted to test this hypothesis. These results also substantiate a statistical synthesis [59] showing that high-impact invaders, including predators, are likely to belong to genera not present in the invaded community, which expectedly occurs more frequently if the predator is native to a foreign biogeographic realm.
Native prey appeared more likely to be naive towards reptile and fish predators. Indeed, many species from these groups have been implicated in extirpations and extinctions [60], although most attention has been given to iconic cases such as the Nile perch Lates niloticus [61] and the brown tree snake B. irregularis [6]. Native amphibians appeared to be sensitive to the introduction of predatory herpetofauna—mainly freshwater turtles and frogs (92% of the 24 observations)—where the majority of prey species were frogs (83% of 24 observations). On the other hand, exotic predatory fishes were represented broadly (17 freshwater and one marine exotic fish species with 78% and 22% of the 78 total observations, respectively) and their prey belong to four taxonomic groups (insects, fishes, herpetofauna, crustaceans), suggesting that the identification of exotic fish as a predation threat might be generally elusive. We performed an additional analysis to ascertain whether the high probability of herpetofauna and fish to encounter naive prey was due to taxonomic affiliation and not simply driven by ecosystem type (freshwater, terrestrial, marine). We found similar results for these two taxonomic groups, regardless of the ecosystem type (g = −0.39 ± 0.27; p = 0.005 and g = −1.07 ± 0.44; p < 0.001 for freshwater and marine fishes, respectively, and g = −0.39 ± 0.41; p = 0.060 and g = −1.29 ± 1.11; p = 0.022 for freshwater and terrestrial herpetofauna, respectively), supporting our findings of likelihood of prey naiveté towards fish and herpetofauna. A surprising result was that prey recognize exotic carnivorous mammals as a predation threat, despite that their exacerbated impacts have been commonly attributed to prey naiveté [23]. Similarly, a recent meta-analysis investigating prey naiveté towards exotic mammals in Australia found high-risk aversion towards canids: the European red fox Vulpes vulpes and the dingo/dog Canis lupus dingo/familiaris [38]. The majority (62%) of observations in our dataset involving exotic mammalian predators were for canids. When we re-ran our analysis excluding canids, prey were marginally naive to carnivorous mammals (g = −0.42 ± 0.49; p = 0.09). Thus, we speculate that the canid family, which has long been present in most continents (including Australia, where Canis lupus dingo was introduced 4000 years ago [62]), represents a predator archetype that could be more broadly recognized than many other archetypes, perhaps because of extensive evolutionary exposure associated with human domestication.
Our findings suggest that fish, amphibians, and reptiles are generally more naive to exotic predators than mammals and invertebrates (crustaceans, insects, and molluscs) and thus likely more sensitive to the introduction of predators. Exotic species have been identified as the number one threat associated with the extinctions of herpetofauna worldwide according to the International Union for Conservation of Nature (IUCN) Red List [63], but the extent to which prey naiveté drove these extinctions remains to be determined in many cases. We did not find significant levels of naiveté for mammalian prey in general, although exotic species are also the most frequent threat recorded for their extinctions [63]. Similar to our findings, a recent meta-analysis indicates that mammals in Australia identify exotic foxes and cats as a predation threat [38]. The authors argue that despite this lack of prey naiveté (level 1 sensu [19]) the rampant decline of prey by exotic mammals in Australia [64] might still be driven by inappropriate or ineffective prey responses (levels 2 and 3 naiveté sensu [19]), which are rarely quantified. Remarkably, although prey naiveté is invoked as responsible for the strong ecological impacts of exotic species on birds [2,6,65,66], we did not find any papers that met our criteria for quantifying prey naiveté in birds, mainly because the few studies addressing prey naiveté in birds lack a comparative treatment with a native predator. Finally, fish do not appear to respond to the risk of predation by novel fish. Collectively, these findings suggest global patterns that could strengthen predictions concerning evolutionary exposure.
The antipredator response of native prey to novel predators can evolve through time, if predation selects for predator recognition and avoidance behaviour [28]. Behaviours that determine the survival of individuals facing a novel predation threat can be subject to strong selection in the persistent presence of a predator [67]. If extinction is averted, evolutionary adaptation can be achieved in a small number of generations. For instance, the fence lizard Sceloporus undulatus acquired the capacity to avoid exotic predatory red fire ants Solenopsis invicta in North America within 40 generations [36]. Our meta-analysis shows that naiveté erodes with the number of prey generations following predator introduction, indicating a generalized pattern of adaptation [30,68]. Averaged across the various taxa in our study, approximately 200 generations are required for native prey to acquire an antipredator response towards exotic predators in the same manner as native predators. This phenomenon could explain, in part, the observed declines in negative ecological impacts of exotic predators over time. For example, the ecological impacts of the brown trout Salmo trutta—an exotic predator intentionally introduced globally—decreased linearly with time since introduction [69]. This reduction in the negative ecological impacts occurs circa one century after the introduction of the brown trout and it was hypothesized to result from either rapid evolutionary adaptation or prompt local extinction of native prey [69]. The capacity of prey to recognize exotic predators is conditional on the native prey averting extinction that often occurs before prey naiveté is assessed [1,2]. Our study might have underestimated the generation time required for predator recognition by omitting prey species that can never adapt or learn how to recognize exotic predators. We are aware of at least one extreme case in our dataset: several fishes exhibit limited antipredator behaviour in the presence of the exotic lionfish P. volitans in the Caribbean [35,70], where strong reductions [71,72] and even local extirpations [73] of fish populations have been reported.
We also predicted that prey at lower latitudes would be less naive towards novel predators owing to the large suite of predatory species and relative high intensity of predation in the tropics [32,33]. Although our results suggest that novel predator recognition is not influenced by latitude, data from the tropics were limited—perhaps reflecting actual low numbers of successful introduced predators [74] or historical low sampling effort of non-native species in the tropics [75].
There are several potential limitations to the data included in this meta-analysis. First, studies were excluded unless they met several criteria, with the disadvantage of not considering the totality of evidence generated globally on prey naiveté. We only considered experimental designs that included empirical comparisons between native and exotic predators, to ensure a direct and consistent way to quantify the perceived risk threat of an exotic predator. Consequently, we excluded studies with controls such as ‘absence of exotic predator’, as those comparisons often require cautious interpretation (e.g. does the behavioural response of prey towards the exotic predator as compared with an empty control indicate predator recognition or simply a response to the presence of an organism, regardless if it is perceived as a predatory threat?). Second, our study only included measurements of level-1 prey naiveté (sensu [16,19]), which interprets a lack of response to an exotic predator (when compared with a native predator) as a lack of recognition of the exotic predator as a threat. However, native animals experience additional levels of naiveté (level-2, -3, and -4), which relate to appropriate, effective, and/or commensurate responses to exotic predators, respectively. Therefore, wildlife might still experience heavy predation by exotic predators despite low level-1 naiveté. By focusing on level-1 naiveté, our study did not consider physiological responses to the presence of predators [76], which can be considered another important form of prey naiveté. Finally, our dataset did not include a random subset of all exotic predators, which might have biased our results towards the most notorious (and presumably detrimental) of exotic species.
Our meta-analysis identifies some global drivers of prey naiveté, paving the way for testing these drivers in different contexts. Assuming that prey naiveté results in increased mortality [14], our results point to several animal groups as being disproportionately sensitive to introduced predators. Some of these vulnerable cases were expected, such as insular terrestrial and freshwater fauna, whereas other cases were unpredicted, such as the high susceptibility of native prey to exotic predators in marine systems, or the vulnerability of specific prey taxa, including fishes and amphibians. The relationship between overall prey naiveté and the number of prey generations suggests that long-lived species could be particularly vulnerable to introduced predators. It remains to be determined how other eco-evolutionary factors influence the loss of prey naiveté through time—e.g. how does this rate differ across taxonomic groups and ecosystem types? Additionally, the most damaging groups of exotic predators were found to be animals that originate from a foreign biogeographic realm or that represent a new generic archetype. Particular attention should be given to the introduction of predatory fishes, reptiles, and mammals (perhaps with the exception of canids). This information could guide efforts to prioritize invasion threats to biodiversity and inform risk assessments of conservation schemes involving assisted colonization. Finally, we identified several areas in which the quantification of prey naiveté is notably scant (e.g. marine ecosystems, remote oceanic islands, and many common prey taxa) and these should be prioritized to clarify predictive patterns of prey naiveté.
Our results support the view that prey naiveté is shaped by multiple eco-evolutionary factors [16,19,21,38]. The phenomenon is of increasing relevance to conservation, given that species introductions to novel ecosystems are accelerating globally [4], along with other forms of global change that might promote ‘disturbed predator-prey interactions’ (sensu [16]). For example, the poleward migration of species driven by changing isotherms [77], including the imminent arrival of unique shell-breaking predators in Antarctica [78], will add novel predator-prey interactions even into historically isolated regions. Therefore, we recommend that factors influencing prey naiveté be given explicit consideration in biodiversity risk assessments.
Supplementary Material
Acknowledgements
We thank the School of Biological Sciences at Queen's University Belfast, the Queen's University Marine Laboratory, Julia Sigwart, Christine Maggs, and Bernie Curran for logistical support and Daniel Barrios-O'Neill for providing valuable feedback on an early draft of the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gqnk98sjh [79]. Code availability statement: the R script used in this manuscript is deposited here https://github.com/antongamazo/Determinants-of-prey-naivete.
Authors contributions
A.A. and N.R.G. conceived the study, A.A., N.R.G., A.R., and J.T.A.D. designed the study, A.A. collected data, A.A. and N.R.G. performed the analyses, A.A. wrote the first draft of the manuscript with substantial input from all authors, A.A., A.R., and N.R.G. contributed extensively to revisions, and all authors approved it for publication.
Competing interests
We declare we have no competing interests.
Funding
The Natural Environment Research Council (NERC) and the Natural Sciences and Engineering Research Council of Canada (NSERC) supported this study with grants to J.T.A.D. and A.R., respectively.
References
- 1.Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proc. Natl Acad. Sci. USA 113, 11 261–11 265. ( 10.1073/pnas.1602480113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina FM, et al. 2011. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Change Biol. 17, 3503–3510. ( 10.1111/j.1365-2486.2011.02464.x) [DOI] [Google Scholar]
- 3.Anton A, et al. 2019. Global ecological impacts of marine exotic species. Nat. Ecol. Evol. 3, 787–800. ( 10.1038/s41559-019-0851-0) [DOI] [PubMed] [Google Scholar]
- 4.Seebens H, et al. 2017. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435 ( 10.1038/ncomms14435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seebens H, et al. 2018. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl Acad. Sci. USA 115, E2264–E2273. ( 10.1073/pnas.1719429115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritts TH, Rodda GH. 1998. The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annu. Rev. Ecol. Syst. 29, 113–140. ( 10.1146/annurev.ecolsys.29.1.113) [DOI] [Google Scholar]
- 7.Barrios-O'Neill D, Dick JTA, Emmerson MC, Ricciardi A, MacIsaac HJ, Alexander ME, Bovy HC. 2014. Fortune favours the bold: a higher predator reduces the impact of a native but not an invasive intermediate predator. J. Anim. Ecol. 83, 693–701. ( 10.1111/1365-2656.12155) [DOI] [PubMed] [Google Scholar]
- 8.Paolucci EM, MacIsaac HJ, Ricciardi A. 2013. Origin matters: alien consumers inflict greater damage on prey populations than do native consumers. Divers. Distrib. 19, 988–995. ( 10.1111/ddi.12073) [DOI] [Google Scholar]
- 9.Salo P, Korpimaki E, Banks PB, Nordstrom M, Dickman CR. 2007. Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B 274, 1237–1243. ( 10.1098/rspb.2006.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox JG, Lima SL. 2006. Naivete and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21, 674–680. ( 10.1016/j.tree.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 11.Diamond J, Case TJ. 1986. Community ecology. New York: NY: Harpercollins College Div. [Google Scholar]
- 12.Banks PB. 1998. Responses of Australian bush rats, Rattus fuscipes, to the odor of introduced Vulpes vulpes. J. Mammal. 79, 1260–1264. ( 10.2307/1383017) [DOI] [Google Scholar]
- 13.Cox JG, Lima SL. 2007. Response to Banks and Dickman: prey naivete does indeed need more attention. Trends Ecol. Evol. 22, 230–231. ( 10.1016/j.tree.2007.02.007) [DOI] [Google Scholar]
- 14.Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR. 2010. Predator-prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. ( 10.1111/j.1600-0706.2009.18039.x) [DOI] [Google Scholar]
- 15.Carthey AJR, Banks PB. 2014. Naivete in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev. 89, 932–949. ( 10.1111/brv.12087) [DOI] [PubMed] [Google Scholar]
- 16.Carthey AJR, Blumstein DT. 2018. Predicting predator recognition in a changing world. Trends Ecol. Evol. 33, 106–115. ( 10.1016/j.tree.2017.10.009) [DOI] [PubMed] [Google Scholar]
- 17.Harper GA, Bunbury N. 2015. Invasive rats on tropical islands: their population biology and impacts on native species. Glob. Ecol. Conserv. 3, 607–627. ( 10.1016/j.gecco.2015.02.010) [DOI] [Google Scholar]
- 18.Jones TB, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR. 2008. Severity of the effects of invasive rats on seabirds: a global review. Conserv. Biol. 22, 16–26. ( 10.1111/j.1523-1739.2007.00859.x) [DOI] [PubMed] [Google Scholar]
- 19.Banks PB, Dickman CR. 2007. Alien predation and the effects of multiple levels of prey naivete. Trends Ecol. Evol. 22, 229–230. ( 10.1016/j.tree.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 20.Moseby KE, Blumstein DT, Letnic M. 2016. Harnessing natural selection to tackle the problem of prey naïveté. Evol. Appl. 9, 334–343. ( 10.1111/eva.12332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saul W-C, Jeschke JM. 2015. Eco-evolutionary experience in novel species interactions. Ecol. Lett. 18, 236–245. ( 10.1111/ele.12408) [DOI] [PubMed] [Google Scholar]
- 22.Ricciardi A, MacIsaac HJ. 2011. Impacts of biological invasions on freshwater ecosystems. In Fifty years of invasion ecology (ed. Richardson DM.), pp. 211–224. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 23.Ebenhard T. 1988. Introduced birds and mammals and their ecological effects. Swed. Wildl. Res. 13, 1–107. [Google Scholar]
- 24.Duncan RP, Blackburn TM. 2004. Extinction and endemism in the New Zealand avifauna. Glob. Ecol. Biogeogr. 13, 509–517. ( 10.1111/j.1466-822X.2004.00132.x) [DOI] [Google Scholar]
- 25.Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958. ( 10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 26.Fey K, Banks PB, Ylonen H, Korpimaki E. 2010. Behavioural responses of voles to simulated risk of predation by a native and an alien mustelid: an odour manipulation experiment. Wildl. Res. 37, 273–282. ( 10.1071/WR08031) [DOI] [Google Scholar]
- 27.Mitchell MD, McCormick MI, Chivers DP, Ferrari MCO. 2013. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Funct. Ecol. 27, 299–304. ( 10.1111/1365-2435.12043) [DOI] [Google Scholar]
- 28.Strauss SY, Lau JA, Carroll SP. 2006. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol. Lett. 9, 354–371. ( 10.1111/j.1461-0248.2005.00874.x) [DOI] [PubMed] [Google Scholar]
- 29.Carthey AJR, Banks PB. 2012. When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE 7, e31804 ( 10.1371/journal.pone.0031804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carthey AJR, Banks PB. 2016. Naiveté is not forever: responses of a vulnerable native rodent to its long term alien predators. Oikos 125, 918–926. ( 10.1111/oik.02723) [DOI] [Google Scholar]
- 31.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 32.Freestone AL, Ruiz GM, Torchin ME. 2013. Stronger biotic resistance in tropics relative to temperate zone: effects of predation on marine invasion dynamics. Ecology 94, 1370–1377. ( 10.1890/12-1382.1) [DOI] [PubMed] [Google Scholar]
- 33.Roslin T, et al. 2017. Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742–744. ( 10.1126/science.aaj1631) [DOI] [PubMed] [Google Scholar]
- 34.Dunlop-Hayden KL, Rehage JS. 2011. Antipredator behavior and cue recognition by multiple Everglades prey to a novel cichlid predator. Behaviour 148, 795–823. ( 10.1163/000579511X577256) [DOI] [Google Scholar]
- 35.Anton A, Cure K, Layman C, Puntila R, Simpson MS, Bruno JF. 2016. Prey naiveté to invasive lionfish (Pterois volitans) on Caribbean coral reefs. Mar. Ecol. Prog. Ser. 544, 257–269. ( 10.3354/meps11553) [DOI] [Google Scholar]
- 36.Langkilde T. 2009. Invasive fire ants alter behavior and morphology of native lizards. Ecology 90, 208–217. ( 10.1890/08-0355.1) [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks PB, Carthey AJR, Bytheway JP. 2018. Australian native mammals recognize and respond to alien predators: a meta-analysis. Proc. R. Soc. B 285, 20180857 ( 10.1098/rspb.2018.0857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. Malden, MA: Wiley-Blackwell. [Google Scholar]
- 40.Koricheva J, Gurevitch J, Mengersen K. 2013. Handbook of meta-analysis in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 41.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis, 1st edn Orlando, FL: Academic Press. [Google Scholar]
- 42.Large SI, Smee DL. 2010. Type and nature of cues used by Nucella lapillus to evaluate predation risk. J. Exp. Mar. Biol. Ecol. 396, 10–17. ( 10.1016/j.jembe.2010.10.005) [DOI] [Google Scholar]
- 43.Large SI, Smee DL. 2013. Biogeographic variation in behavioral and morphological responses to predation risk. Oecologia 171, 961–969. ( 10.1007/s00442-012-2450-5) [DOI] [PubMed] [Google Scholar]
- 44.Large SI, Torres P, Smee DL. 2012. Behavior and morphology of Nucella lapillus influenced by predator type and predator diet. Aquat. Biol. 16, 189–196. ( 10.3354/ab00452) [DOI] [Google Scholar]
- 45.Blackburn TM, Duncan RP. 2001. Determinants of establishment success in introduced birds. Nature 414, 195–197. ( 10.1038/35102557) [DOI] [PubMed] [Google Scholar]
- 46.Viechbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. [Google Scholar]
- 47.Egger M, Smith GD, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habeck CW, Schultz AK. 2015. Community-level impacts of white-tailed deer on understory plants in North American forests: a meta-analysis. Aob Plants 7, plv119 ( 10.1093/aobpla/plv119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viechtbauer W, Cheung MW-L. 2010. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1, 112–125. ( 10.1002/jrsm.11) [DOI] [PubMed] [Google Scholar]
- 50.Freeman AS, Wright JT, Hewitt CL, Campbell ML, Szeto K. 2013. A gastropod's induced behavioral and morphological responses to invasive Carcinus maenas in Australia indicate a lack of novelty advantage. Biol. Invasions 15, 1795–1805. ( 10.1007/s10530-013-0409-z) [DOI] [Google Scholar]
- 51.Coverdale TC, Axelman EE, Brisson CP, Young EW, Altieri AH, Bertness MD. 2013. New England salt marsh recovery: opportunistic colonization of an invasive species and its non-consumptive effects. PLoS ONE 8, e73823 ( 10.1371/journal.pone.0073823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutson KS, Ross DJ, Day RW, Ahern JJ. 2005. Australian scallops do not recognise the introduced predatory seastar Asterias amurensis. Mar. Ecol. Prog. Ser. 298, 305–309. ( 10.3354/meps298305) [DOI] [Google Scholar]
- 53.Beauchamp G. 2004. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. Lond. B 271, 1039–1042. ( 10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blumstein DT. 2002. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 29, 685–692. ( 10.1046/j.1365-2699.2002.00717.x) [DOI] [Google Scholar]
- 55.Blumstein DT. 2006. The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology 112, 209–217. ( 10.1111/j.1439-0310.2006.01209.x) [DOI] [Google Scholar]
- 56.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rödl T, Berger S, Michael Romero L, Wikelski M. 2007. Tameness and stress physiology in a predator-naive island species confronted with novel predation threat. Proc. R. Soc. B 274, 577–582. ( 10.1098/rspb.2006.3755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega Z, Mencia A, Perez-Mellado V. 2017. Rapid acquisition of antipredatory responses to new predators by an insular lizard. Behav. Ecol. Sociobiol. 71, 1 ( 10.1007/s00265-016-2246-4) [DOI] [Google Scholar]
- 59.Ricciardi A, Atkinson SK. 2004. Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecol. Lett. 7, 781–784. ( 10.1111/j.1461-0248.2004.00642.x) [DOI] [Google Scholar]
- 60.Alcaraz C, García-Berthou E. 2007. Life history variation of invasive mosquitofish (Gambusia holbrooki) along a salinity gradient. Biol. Conserv. 139, 83–92. ( 10.1016/j.biocon.2007.06.006) [DOI] [Google Scholar]
- 61.Aloo PA. 2003. Biological diversity of the Yala Swamp lakes, with special emphasis on fish species composition, in relation to changes in the Lake Victoria Basin (Kenya): threats and conservation measures. Biodivers. Conserv. 12, 905–920. ( 10.1023/A:1022869624524) [DOI] [Google Scholar]
- 62.Dickman CR. 1996. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 2, 185–195. ( 10.2981/wlb.1996.018) [DOI] [Google Scholar]
- 63.Bellard C, Cassey P, Blackburn TM. 2016. Alien species as a driver of recent extinctions. Biol. Lett. 12, 20150623 ( 10.1098/rsbl.2015.0623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woinarski JCZ, Burbidge AA, Harrison PL. 2015. Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc. Natl Acad. Sci. USA 112, 4531–4540. ( 10.1073/pnas.1417301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graham NAJ, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA. 2018. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559, 250–253. ( 10.1038/s41586-018-0202-3) [DOI] [PubMed] [Google Scholar]
- 66.Kurle CM, Croll DA, Tershy BR. 2008. Introduced rats indirectly change marine rocky intertidal communities from algae- to invertebrate-dominated. Proc. Natl Acad. Sci. USA 105, 3800–3804. ( 10.1073/pnas.0800570105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juliano SA, Gravel ME. 2002. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav. Ecol. 13, 301–311. ( 10.1093/beheco/13.3.301) [DOI] [Google Scholar]
- 68.Anderson RB, Lawler SP. 2016. Behavioral changes in tadpoles after multigenerational exposure to an invasive intraguild predator. Behav. Ecol. 27, 1790–1796. ( 10.1093/beheco/arw112) [DOI] [Google Scholar]
- 69.Závorka L, Buoro M, Cucherousset J. 2018. The negative ecological impacts of a globally introduced species decrease with time since introduction. Glob. Change Biol. 24, 4428–4437. ( 10.1111/gcb.14323) [DOI] [PubMed] [Google Scholar]
- 70.Benkwitt CE. 2017. Predator effects on reef fish settlement depend on predator origin and recruit density. Ecology 98, 896–902. ( 10.1002/ecy.1732) [DOI] [PubMed] [Google Scholar]
- 71.Green SJ, Akins JL, Maljković A, Côté IM. 2012. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 7, e32596 ( 10.1371/journal.pone.0032596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albins MA, Hixon MA. 2008. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar. Ecol. Prog. Ser. 367, 233–238. ( 10.3354/meps07620) [DOI] [Google Scholar]
- 73.Ingeman KE. 2016. Lionfish cause increased mortality rates and drive local extirpation of native prey. Mar. Ecol. Prog. Ser. 558, 235–245. ( 10.3354/meps11821) [DOI] [Google Scholar]
- 74.Sax DF. 2001. Latitudinal gradients and geographic ranges of exotic species: implications for biogeography. J. Biogeogr. 28, 139–150. ( 10.1046/j.1365-2699.2001.00536.x) [DOI] [Google Scholar]
- 75.Lockwood JL, Hoopes MF, Marchetti MP. 2013. Invasion ecology, 2nd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 76.Freeman AS, Byers JE. 2006. Divergent induced responses to an invasive predator in marine mussel populations. Science 313, 831–833. ( 10.1126/science.1125485) [DOI] [PubMed] [Google Scholar]
- 77.Fodrie FJ, Heck KL, Powers SP, Graham WM, Robinson KL. 2010. Climate-related, decadal-scale assemblage changes of seagrass-associated fishes in the northern Gulf of Mexico. Glob. Change Biol. 16, 48–59. ( 10.1111/j.1365-2486.2009.01889.x) [DOI] [Google Scholar]
- 78.Aronson RB, Frederich M, Price R, Thatje S. 2015. Prospects for the return of shell-crushing crabs to Antarctica. J. Biogeogr. 42, 1–7. ( 10.1111/jbi.12414) [DOI] [Google Scholar]
- 79.Anton A, Geraldi NR, Ricciardi A, Dick JTA.2020. Data from: Global determinants of prey naiveté to exotic predators. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Anton A, Geraldi NR, Ricciardi A, Dick JTA.2020. Data from: Global determinants of prey naiveté to exotic predators. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gqnk98sjh [79]. Code availability statement: the R script used in this manuscript is deposited here https://github.com/antongamazo/Determinants-of-prey-naivete.