Abstract
Biotic mechanisms associated with species diversity are expected to stabilize communities in theoretical and experimental studies but may be difficult to detect in natural communities exposed to large environmental variation. We investigated biotic stability mechanisms in a multi-site study across Inner Mongolian grassland characterized by large spatial variations in species richness and composition and temporal fluctuations in precipitation. We used a new additive-partitioning method to separate species synchrony and population dynamics within communities into different species-abundance groups. Community stability was independent of species richness but was regulated by species synchrony and population dynamics, especially of abundant species. Precipitation fluctuations synchronized population dynamics within communities, reducing their stability. Our results indicate generality of biotic stability mechanisms in natural ecosystems and suggest that for accurate predictions of community stability in changing environments uneven species composition should be considered by partitioning stabilizing mechanisms into different species-abundance groups.
Keywords: biodiversity, productivity, dominant species, precipitation variability, portfolio effect, species synchrony
1. Introduction
The ability of ecosystems to reliably provide biological products and services to humanity is being threatened by climatic changes and species loss [1–5], arousing interests in biodiversity–stability relationships. As an important ecosystem function, community biomass tracks environmental fluctuations [6–8]. This temporal variability is frequently estimated with the temporal coefficient of variation (CV = σ/μ, the inverse of community stability) [9–13], and, based on theoretical and experimental studies [14–16], is expected to be lower in species-rich than in species-poor communities owing to biotic mechanisms. First, asynchronous species dynamics can stabilize communities because the decreased biomass in one species can be compensated by increases in others [8,17–20]. Second, the mean–variance scaling relationship [21] suggests a stabilized community by distributing the total community biomass among more species when the scaling power ranges from 1 to 2 [10,13,22]. Third, the positive biodiversity–biomass relationship suggests that high species richness can stabilize communities by increasing total biomass, a phenomenon termed the overyielding effect [3,13].
Although these biotic mechanisms have been proposed to stabilize communities in theoretical analyses [10,12,20,23–25] and have been evidenced by single-site observational [8] and experimental [14,15,17,26] studies, as well as meta-analyses [16,21,27,28], they are still argued to be irrelevant in natural communities, especially at large spatial scales [2,29,30]. Community stability can vary spatially, potentially owing to spatial variations in abiotic and biotic factors. For example, a study in North America showed that community stability of grassland was lower than that of desert and forest because of its intermediate precipitation CV and potential productivity [7], suggesting that both abiotic and biotic factors affected community stability in natural ecosystems. Biotic stability mechanisms may be sensitive to climatic variation [27,28,31–34], masking their relevance in natural ecosystems. This environmental dependency suggests that stabilizing effects of biotic mechanisms on communities may vary across sites and ecosystems, and thus stabilizing effects found in a single-site study may not extrapolate to multiple sites across larger spatial scales. In addition, abiotic factors can affect community species composition [17,35] or even outweigh biotic factors in driving species richness [1,27,36] and species dynamics [28,31] at the regional scale, thus further modifying biotic stability mechanisms. Finally, abiotic factors may exert their influence on community stability via biotic factors or, in other words, biotic mechanisms may mediate the effects of abiotic factors on community stability. Therefore, investigating how biotic mechanisms affect community stability under varied environmental conditions and community species richness and composition in a multi-site study may give us deeper insights into biotic stability mechanisms in natural ecosystems than do single-site studies.
We conducted a multi-site observational study for five consecutive years across Inner Mongolian grassland in China (total area 78.8 million hectares [37]) (electronic supplementary material, figure S1). The 23 study sites were characterized by large between-site variation in climatic factors (e.g. precipitation and its interannual variation) and biotic factors (e.g. community biomass and species richness and composition) (electronic supplementary material, table S1 and figure S1). The Inner Mongolian grassland is a typical part of the Eurasian grassland biome and crucial in providing biological products and services to human populations living there [33,38]. In this region, precipitation is the dominant driver of biomass [8,33,36,39,40], species richness and species composition [36,41] in communities. Observational studies have shown that precipitation in Inner Mongolian grassland has dramatically changed during the past decades [42,43], while the ecological consequences of precipitation changes are still not fully understood. To investigate the community stability in this region, we employed a novel theoretical model relating its inverse (the community temporal CV) to species synchrony and weighted (by relative species biomass abundance) average species temporal CV, synthesizing the mean–variance scaling effect and the overyielding effect [13] (electronic supplementary material, appendices A.1 and A.2). We analysed whether the community stability and its biotic mechanisms depended on climatic factors (precipitation and its interannual variation) and how community stability was affected by those biotic mechanisms and species richness. We separated the biotic stability mechanisms into different species-abundance groups (electronic supplementary material, appendices A.3 and A.4) and hypothesized that community stability was more strongly affected by the most abundant, i.e. dominant, species than by total plant species richness. This is because abundant species contribute strongly to dynamic processes in communities of high unevenness [13,25,44] and the studied region is characterized by such high unevenness of species biomasses [8,33] (electronic supplementary material, figure S2).
2. Material and methods
(a). Study sites
The Inner Mongolian temperate grassland has a continental monsoon climate with a short and cool growing season (from May to October) and a long and cold non-growing season (from November to April) [36,45]. During the period of this study (from 2012 to 2016), the mean growing-season precipitation ranged from 186.2 to 398.0 mm, corresponding to about 90% of the annual precipitation (electronic supplementary material, table S1).
The Inner Mongolian grassland includes three main vegetation types: meadow steppe, typical steppe and desert steppe (electronic supplementary material, figure S1). The meadow steppe is dominated by perennial grasses such as Stipa baicalensis and Leymus chinensis and perennial forbs such as Convolvulus ammannii. The dominant species of the typical steppe are perennial grasses such as Stipa grandis, Leymus chinensis and Stipa krylovii. The desert steppe is dominated by perennial grasses such as Stipa caucasica and perennial forbs such as Allium polyrhizum (electronic supplementary material, table S1).
In this region, aboveground community biomass varied considerably from 20.9 to 180.9 g m–2 and species richness ranged from 6 to 25 (electronic supplementary material, table S1). The lowest community biomass (20.9–43.7 g m−2) and species richness (6–13) occurred in desert steppe and the highest values in meadow steppe (101.6–180.9 g m−2 for biomass and 11–25 for richness) (electronic supplementary material, table S1).
(b). Plant community survey and climate data collection
In 2012, we established a 23-site observational study along a transect across the Inner Mongolian grassland of China (latitudes ranged from 39.34 to 49.96°N and longitudes from 107.56 to 120.12°E) (electronic supplementary material, table S1 and figure S1). We recorded the geographical location (latitude and longitude) of these sites and resampled them in the following 4 years (2013–2016), resulting in a 5-year-long time series of field observations. For more than half of our sites (13 out of 23 sites), data for the whole 5 years were collected. For some sites (10 out of 23 sites), only data for 3 or 4 years were collected (two sites with data for 3 years because of land-use change and eight sites with data for 4 years because of mowing before the community survey could have been done) (electronic supplementary material, figure S3). A recent 39-site meta-analysis showed that investigating biodiversity–stability relationship with time series of less than or equal to 4 years can produce similar results to those using longer time series [46]. In addition, multiple short time series to some extent may compensate few long time series. We, therefore, expected that our dataset could provide reliable insights into the biodiversity–stability relationship of the studied region.
Plant communities were surveyed between late July and early August in each year with a method that has a well-documented efficiency to estimate aboveground biomass and plant species richness in grassland ecosystems [36,40,47]. To appropriately represent the natural community, the surveyed plant community at each site was randomly selected in each year, excluding areas with anthropogenic disturbances, e.g. heavy grazing or mowing. We positioned a plot of 10 × 10 m at each site and surveyed three 1 × 1 m quadrats along the diagonal. Subsequently, all living plant material in each quadrat was harvested and sorted into species. All material was oven-dried and weighed to obtain aboveground biomass and calculate effective species richness based on species biomass abundance (see below).
To obtain site-specific precipitation data, we collected the monthly climatic data from 119 climate stations across Inner Mongolia, and then calculated site-specific monthly precipitation using a kriging method with a 2 km resolution digital elevation model in ArcGIS software (Environmental Systems Research Institute, Redlands, CA, USA). A previous study has shown that the data interpolated using this method correlate well with in situ measured climatic data [47]. Using these interpolated site-specific precipitation data, we calculated the mean growing-season precipitation and its interannual variation (estimated as the temporal CV). In this study, we used these growing-season climatic variables because plants were most active during this period.
(c). Definitions of biotic stability mechanisms and species diversity indices
Based on a recent theoretical model, we related the community temporal CV to the species synchrony and the weighted average species temporal CV [13,25] (electronic supplementary material, appendices A.1 and A.2). In the current study, these two terms were estimated with either all species or only dominant species (relative species biomass greater than or equal to 5%), defined as the dominant species synchrony and the weighted average dominant species temporal CV (electronic supplementary material, appendices A.3 and A.4). According to a recent theoretical study, the weighted average species temporal CV can be affected by the mean–variance scaling relationship and the overyielding effect [13]. Here, the mean–variance scaling relationship is defined as the power function between temporal variance and mean biomass [13,21] and overyielding is defined as a positive effect of species richness on biomass [3,13]. The theoretical model shows that these two biotic stability mechanisms can interactively affect the weighted average species temporal CV, thus underpinning the effects of species richness [13]. In addition, the theoretical model suggests a weak effect of species richness on the weighted average species temporal CV when the mean–variance scaling relationship has a coefficient close to 2 [13].
In the current study, species richness was defined as the multi-year average number of species recorded in a 1 m2 quadrat. Considering the high unevenness of species biomasses in the studied grassland communities, we also used a measure of effective species richness, the antilog of the Shannon–Wiener diversity. This measure reflects how many species with an even abundance distribution would produce the same Shannon–Wiener diversity as observed for the actual uneven community [48]. These two methods were also used to estimate the species richness and effective species richness of the dominant species.
(d). Statistical analysis
The community temporal CV was estimated using aboveground biomass over the 5 years of the survey without detrending because biomass had no significant linear temporal trend (assessed using linear regression between biomass and year) except for one of the 23 study sites (electronic supplementary material, table S2). Taylor's power law [21] was used to estimate the mean–variance scaling coefficient (electronic supplementary material, figure S4). We did not explicitly estimate the strength of the overyielding effect [3] as we had no monoculture treatments, but could detect it via positive slopes of linear regressions between biomass as dependent and species richness as the independent variable (electronic supplementary material, figure S5).
We calculated the correlation coefficients between the climatic factors (precipitation and its interannual variation), biotic factors (species richness and effective species richness), biotic mechanisms (mean–variance scaling, weighted average species temporal CV and species synchrony) and the community temporal CV to develop causal hypotheses about relations between variables. Individual relationships were plotted and analysed with linear regression to assess how climatic and biotic factors directly influenced biotic stability mechanisms and the community temporal CV, and how biotic stability mechanisms directly influenced the community temporal CV.
To combine causal hypotheses about direct and indirect effects, we incorporated them into structural equation models (SEMs) and displayed their results with path-analysis graphs, using the lavaan package [49] of R 3.4.0 [50]. Specifically, we constructed SEMs that deliberately stayed as close as possible to a priori hypotheses proposed to be essential biotic stability mechanisms [13,19,20,27]. We did this even at the cost that the overall model fits might show significant deviations from a saturated model. Model-fit statistics such as χ2-tests or the goodness-of-fit index (GFI) comparing the deviation of a current SEM to a full SEM without residual degrees of freedom were only used as an additional guide, but we avoided searching for a best model post hoc (electronic supplementary material, appendix B).
All the above analyses were carried out with all species or only the dominant species included in biodiversity measures and biotic stability mechanisms, i.e. synchronous dynamics and weighted average species temporal CV of dominant species. All statistical analyses were conducted using R 3.4.0 [50] with the graphics package for plotting figures. For the correlation analyses, regression analyses and SEMs, relationships and pathways were considered significant if p < 0.05.
3. Results
(a). Effects of climatic factors on species diversity
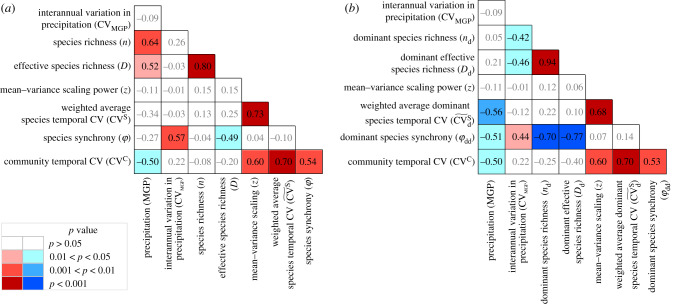
The mean growing-season precipitation and its interannual variation were strong drivers of species diversity of the studied Inner Mongolian grassland sites. Specifically, both species richness and effective species richness were positively associated with growing-season precipitation (figure 1a). In addition, the richness and effective richness of dominant species were negatively associated with the interannual variation in growing-season precipitation (figure 1b).
Figure 1.
Correlation matrix for climatic factors (mean growing-season precipitation and its interannual variation), biodiversity indices (species richness and effective species richness), biotic stability mechanisms (mean-variance scaling exponent, weighted average species temporal coefficient of variation (CV) and species synchrony) and community temporal CV. (a) Correlation matrix for variables calculated with all species and (b) correlation matrix for variables calculated only with dominant species (except climatic factors and community temporal CV). Black numbers with coloured background represent significant (p < 0.05) correlations and grey numbers with white background represent non-significant (p > 0.05) correlations. (Online version in colour.)
(b). Effects of climatic factors, species diversity and biotic stability mechanisms on the community temporal coefficient of variation
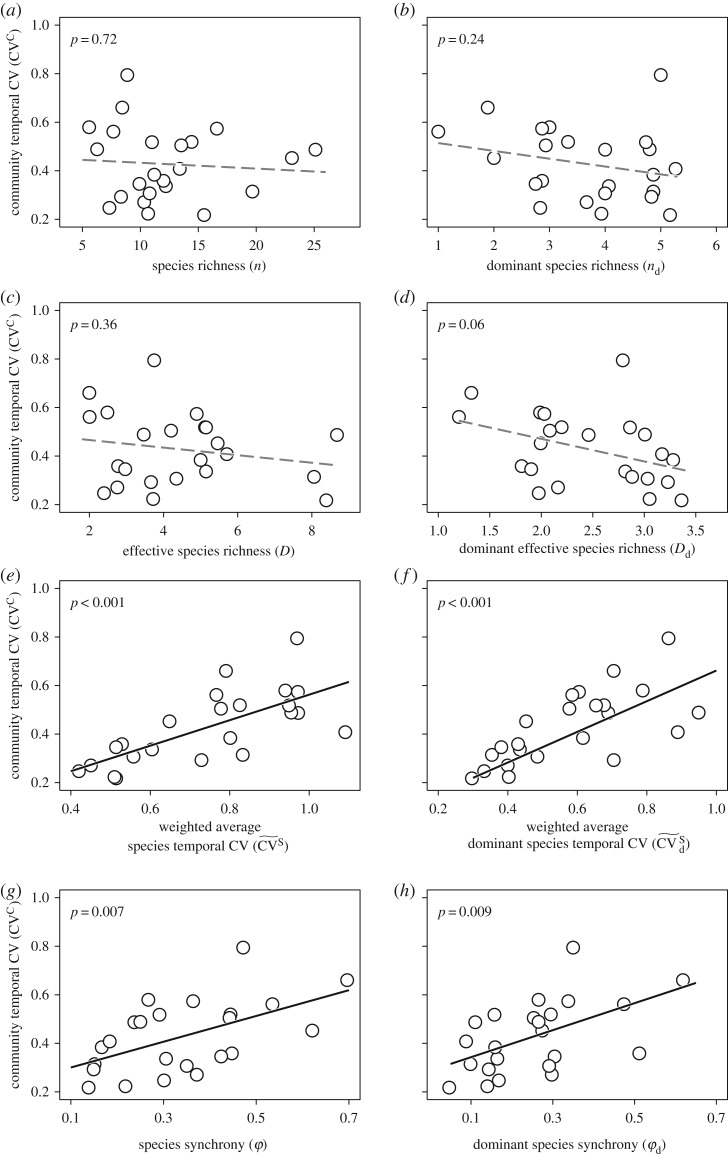
The community temporal CV was significantly affected by climatic factors but independent of species diversity. Specifically, growing-season precipitation had a negative effect on the community temporal CV, while its interannual variation had no significant effect (figure 1). In addition, species richness and effective species richness of all and of dominant species did not significantly affect the community temporal CV (figures 1 and 2a–d).
Figure 2.
Community temporal coefficient of variation (CV) in relation to species richness, effective species richness, weighted average species temporal CV, species synchrony (a,c,e,g, respectively) and the corresponding relations for dominant species only (b,d,f,h). Black solid lines represent significant linear relationships (p < 0.05) and grey dashed lines represent non-significant linear relationships (p > 0.05).
The community temporal CV was positively related to the mean–variance scaling exponent, the weighted average species temporal CV and species synchrony of all and of dominant species (figures 1 and 2e–h).
(c). Effects of climatic factors and species diversity on biotic stability mechanisms
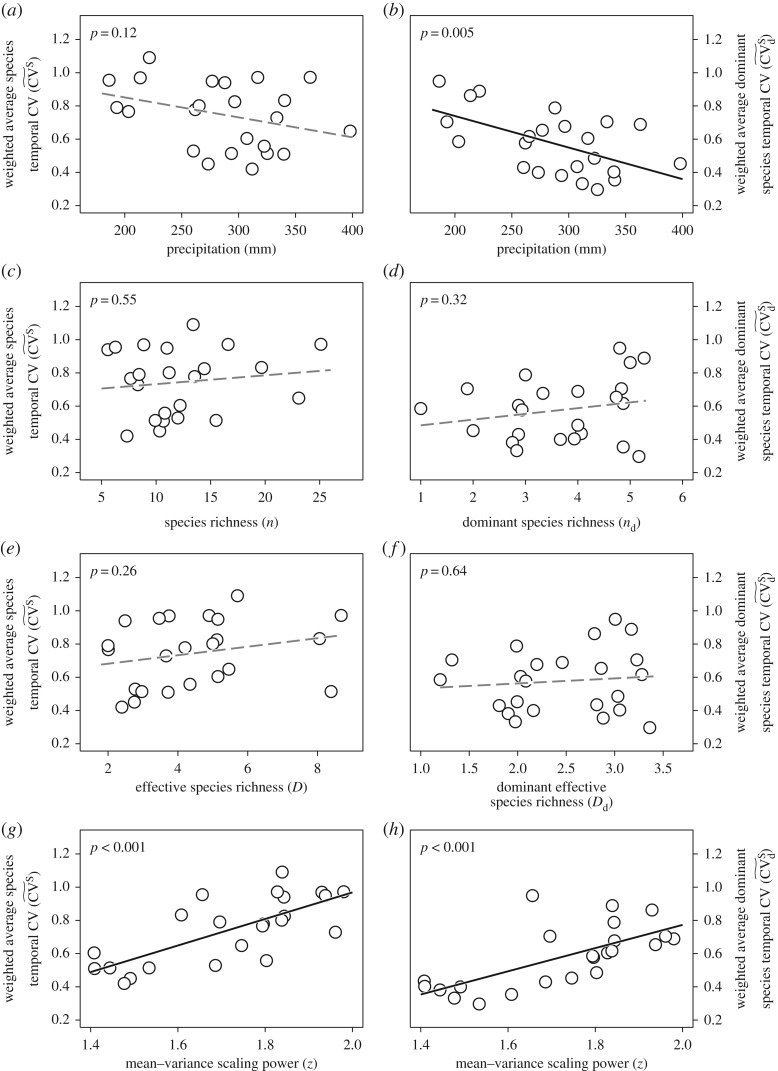
The weighted average species temporal CV of all species was not significantly influenced by growing-season precipitation but if it was calculated only for dominant species a negative relationship was found (figures 1 and 3a,b). Furthermore, the weighted average species temporal CV of all and of only the dominant species was not significantly related to the corresponding measures of species richness (figures 1 and 3c–f). However, the weighted average species temporal CV of all and of only the dominant species was positively associated with the mean–variance scaling exponent (figures 1 and 3g,h). The mean–variance scaling exponent was independent of climatic factors, i.e. the growing-season precipitation and its interannual variation, and species diversity indices measured with all species or only with dominant species (figure 1). We did not explicitly estimate the overyielding effect [3] but found significantly positive species richness–community biomass relationships at nine out of 23 sites (and only at one site was the relationship significantly negative; electronic supplementary material, figure S5), indicating that overyielding effects did occur.
Figure 3.
Weighted average species temporal coefficient of variation (CV) in relation to precipitation, species richness, effective species richness and mean–variance scaling exponent (a,c,e and g, respectively) and weighted average dominant species temporal CV in relation to precipitation, richness and effective richness of dominant species and mean–variance scaling exponent (b,d,f and h, respectively). Black solid lines represent significant linear relationships (p < 0.05) and grey dashed lines represent non-significant linear relationships (p > 0.05).
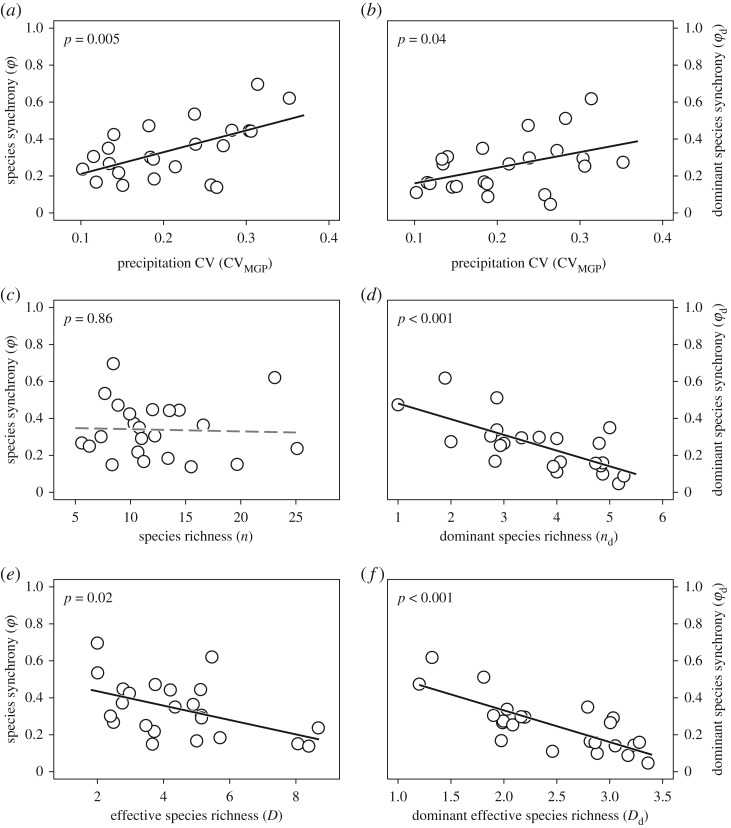
Both the community-wide species synchrony and the dominant species synchrony were positively affected by the interannual variation in growing-season precipitation (figures 1 and 4a,b). In addition, the community-wide species synchrony was negatively associated with effective species richness (figures 1a and 4e). The dominant species synchrony was negatively associated with both the dominant species richness and dominant effective species richness (figures 1b and 4d,f).
Figure 4.
Species synchrony in relation to the interannual variation in precipitation (precipitation CV), species richness and effective species richness (a,c and e, respectively) and dominant species synchrony in relation to precipitation CV and richness and effective richness of dominant species (b,d and f, respectively). Black solid lines represent significant linear relationships (p < 0.05) and grey dashed lines represent non-significant linear relationships (p > 0.05).
(d). Relating the community temporal coefficient of variation to climatic factors, biodiversity and biotic stability mechanisms
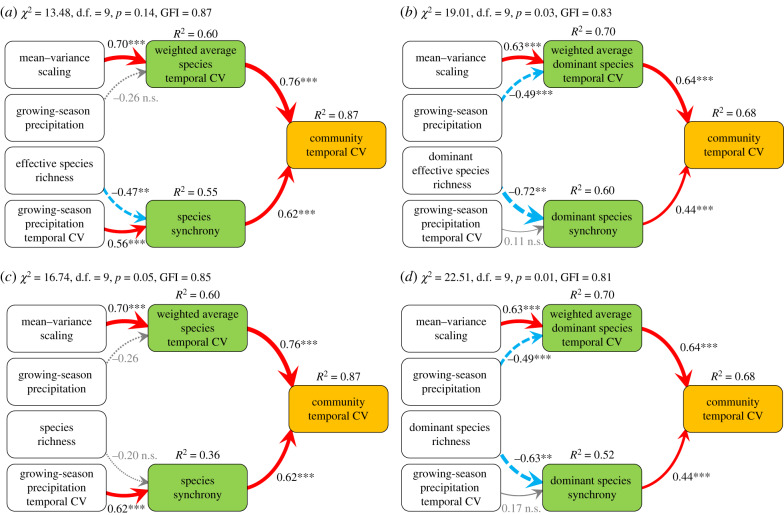
To test the hypotheses that the weighted average species temporal CV and species synchrony directly influenced the community temporal CV, we conducted path analysis to relate the community temporal CV to climatic factors, species diversity indices and biotic stability mechanisms using SEMs (electronic supplementary material, appendix B, table S3 and figure S6). This path analysis did confirm the hypotheses and had a total explanatory power of 0.87 (electronic supplementary material, appendix B; figure 5a). In addition, the mean–variance scaling exponent and the interannual variation in growing-season precipitation increased the community temporal CV indirectly via increasing the weighted average species temporal CV and the species synchrony, respectively (electronic supplementary material, appendix B; figure 5a). Effective species richness indirectly reduced the community temporal CV via decreasing species synchrony in this model (figure 5a). When we replaced effective species richness with species richness its effect on species synchrony was no longer significant (figure 5c).
Figure 5.
Path-analytic representations of structural equation models (SEMs) for relating the community temporal coefficient of variation (CV) to climatic factors (mean growing-season precipitation and its interannual variation), biodiversity indices (species richness and effective species richness) and biotic stability mechanisms (mean-variance scaling exponent, weighted average species temporal CV and species synchrony). (a) The best SEM based on our a priori hypotheses and statistical analysis (electronic supplementary material, appendix B, table S3 and figure S6), and (c) an SEM model in which effective species richness is replaced with uncorrected species richness. (b,d) SEMs corresponding to those in (a,c), respectively, but where biodiversity indices and biotic stability mechanisms were calculated only with dominant species (dominant species richness, the effective richness of dominant species, weighted average dominant species temporal CV and dominant species synchrony). Solid and dashed arrows represent positive and negative paths, respectively. Arrows with black numbers and asterisks represent significant paths (p < 0.05). Arrows with grey numbers represent non-significant paths (n.s., p > 0.05) that were nevertheless included in the SEMs because they corresponded to a priori hypotheses for causal relationships. All arrows are scaled in relation to the strength of the relationship, with numbers showing the standard path coefficients (i.e. indicating by how many standard deviations (s.d.) the variable at the end of an arrow would change if the variable at the beginning of the arrow would be changed by 1 s.d.). R2 values are proportions of variance explained by dependent variables in the model. Model-fit statistics such as χ2-test and GFI are shown in each panel. The significance level of each path is indicated by the number of asterisks (*P < 0.05, **p < 0.01, ***p < 0.001). (Online version in colour.)
(e). Effects of dominant species on the community temporal coefficient of variation
Based on the above SEMs, we furtherly analysed the effect of the dominant species alone on the community temporal CV by replacing community-wide biotic stability mechanisms and biodiversity indices with their counterparts calculated only with dominant species. These analyses showed that the weighted average dominant species temporal CV and the dominant species synchrony positively affected the community temporal CV with a total explanatory power of 0.68 (figure 5b,d). The mean–variance scaling exponent increased the community temporal CV via increasing the weighted average dominant species temporal CV; and growing-season precipitation decreased the community temporal CV via reducing the weighted average dominant species temporal CV (figure 5b,d). Furthermore, both the effective richness (figure 5b) and the uncorrected richness of dominant species (figure 5d) decreased the community temporal CV via reducing the synchrony of dominant species.
4. Discussion
In the present study, we investigated stabilizing effects of biotic mechanisms on natural community biomass at the regional scale of Inner Mongolia and analysed their dependencies on climatic factors, species diversity and dominant species dynamics. We found that the investigated biotic mechanisms strongly affected community stability in the 23 natural grasslands across a gradient of yearly precipitation and its variation. However, in contrast to expectations based on previous studies [27], the latter had negative rather than positive indirect effects on community stability because yearly precipitation fluctuations increased rather than decreased the synchrony of population dynamics of the different species within communities. This could compromise the reliability of the studied Inner Mongolian grassland in providing biological products and services to human populations under the ongoing increasing precipitation variability [42,43], as we discuss further below.
(a). Biotic stability mechanisms and the importance of dominant species in grasslands of Inner Mongolia
A recent theoretical analysis showed that both non-significant and negative biodiversity–stability relationships were possible when species synchrony, mean–variance scaling and overyielding interactively affected community stability [13]. In the present study, both species synchrony and the mean–variance scaling had significant effects on community stability. Furthermore, nine out of our 23 study sites showed significantly positive biodiversity–biomass relationships indicating overyielding (electronic supplementary material, figure S5). Community stability was independent of overall species richness but indirectly positively affected by a higher richness of dominant species, which decreased dominant species synchrony, which in turn decreased the community temporal CV. This indicates that in natural communities with highly uneven abundance distributions, rare species, which might be sink species not able to maintain independent populations in the community, can mask the influence of biodiversity variables on biotic stability mechanisms. Generally, theoretical and empirical studies predicted and found positive effects of species richness on community stability [14,15,27,28,32]. No effects have been reported from a recent single-site study in the same region as that of our study [33], potentially again for the above reason of highly uneven species abundance distributions in these grasslands.
Previous theoretical work found that unevenness can indeed weaken biodiversity–stability relationships [9,22], because the most diverse components of a community, namely rare species, may have limited effects on community stability owing to their low abundances [51]. This theoretical prediction is supported by a growing number of experimental investigations, as well as the current study, showing weak or non-significant biodiversity–stability relationships when dominant species regulate community stability [32–34,52–55]. In the present study, the dominant species as a group had low but variable richness across the 23 sites (1–5 species per square metre, accounting for 64.2–96.8% of community biomass; mean 82.8%) (electronic supplementary material, table S1). The weighted average dominant species temporal CV and the dominant species synchrony significantly impacted the community stability, with an explanatory power slightly lower than that of using community-wide counterparts and much higher than that of common- and rare-species groups (electronic supplementary material, figures S7 and S8). This suggests that theoretical studies assuming evenly distributed species abundances [10,12,18,23] may overestimate the regulatory effect of overall species richness on community stability, or, in other terms, that especially in natural ecosystems with highly uneven species abundance distributions it may be more appropriate to base predictions of community stability on the richness and population dynamics of the dominant species, as suggested above.
The weighted average species temporal CV provided the most important biotic stability mechanism across the 23 sites of the Inner Mongolian grassland, which was independent of overall or dominant species richness but was positively associated with the mean–variance scaling exponent. The mean–variance scaling has commonly been omitted in previous studies [26,32,33], while our results indicate that this biotic stability mechanism generally exists in natural grassland communities. Indeed, a recent theoretical analysis showed that the mean–variance scaling can determine the sign of the relationship between species richness and the weighted average species temporal CV [13]. The authors of that analysis found that species richness will negatively impact the weighted average species temporal CV when the mean–variance scaling has an exponent ranging from 1 to 2 [56]. However, a positive effect will occur when it is greater than 2 (some studies showed that such high values are not impossible, see e.g. [57,58]). In the present study, the estimated mean–variance scaling exponent had a mean value of 1.72 (ranging from 1.41 to 1.98). This value is consistent with the commonly considered range of 1–2 and the reported value (1.73) in a recent single-site study in this region [34]. However, this value is close to 2, and thus may in part explain the non-significant relationship between the weighted average species temporal CV and species richness.
The weighted average species temporal CV was strongly affected by the dominant species group and independent of the most diverse component of the community, the rare-species group (electronic supplementary material, figure S7), which was likely responsible for the lack of significant effects of total species richness. In addition, we found that higher growing-season precipitation can stabilize communities via decreasing the weighted average dominant species temporal CV. This is because of its stronger stimulation of the mean biomass than of its standard deviation (electronic supplementary material, figure S9), therefore, decreasing the standard deviation-to-mean ratio. More importantly, dominant species showed higher stability than other species (electronic supplementary material, figure S10), indicating that they are better able to maintain a stable biomass in a fluctuating environment than other species, potentially owing to their better abilities in acquiring nutrients, water and light via well-developed root systems and taller canopy [17,59,60]. Thus, these findings suggest that in the studied grassland communities dominant species are more important than other species for stabilizing community biomass.
(b). Yearly variation in precipitation synchronizes species and increases community temporal variation in grasslands of Inner Mongolia
To our knowledge, the current study is the first to find that precipitation variability can destabilize rather than stabilize natural grassland communities by increasing species synchrony, suggesting it may be responsible for the impaired stabilities of communities and vegetation activities under high precipitation variability in previous region-scale investigations [6,47]. Our results contrast with the results of a recent meta-analysis of nine sites across grasslands of North America, which found that there precipitation variability stabilized communities by promoting compensatory dynamics [27]. This discrepancy may be due to drier average conditions of the 23 sites here studied across the Inner Mongolian grassland (precipitation ranged from 186.2 to 398.0 mm), leading to a positive correlation between precipitation and biomass for all or at least most species [41], whereas under wetter average conditions in North American grassland (precipitation ranged from ca 250 to ca 900 mm at the sites analysed in [27]) some species may actually increase in biomass in drier than average years, thus maintaining more constant community biomass. Therefore, the pattern observed in the present study may also be characteristic for even drier regions with semi-arid and desert ecosystems.
In addition, the current study is also the first to quantify how different species-abundance groups affect species synchrony and found that here only dominant species impacted species synchrony significantly (electronic supplementary material, figure S8), potentially owing to differences in their responses to environmental fluctuations (electronic supplementary material, figure S3). Recent theoretical analyses have indicated that high unevenness of species abundance distributions can weaken the dependence of species synchrony on species richness and cause it to be strongly driven by a few abundant species [13,25]. Such a theoretical prediction has been supported by long-term (more than 20 years) single-site observational studies in grasslands of Inner Mongolia and the Qinghai-Tibet Plateau showing that compensatory dynamics between key functional groups maintain a stable community biomass [8,17]. Our results support such a theoretical prediction as well. Thus, the current study not only suggests that the strong dependence of species synchrony on few abundant species may be general in natural ecosystems characterized by high unevenness, but also provides a tool to examine and quantify this dependence.
In the current study, we investigated stabilizing effects of biotic mechanisms on temporal variation in plant community biomass. We did not quantify the effects of anthropogenic disturbances, e.g. grazing and mowing, although they can be large [16]. It is, of course, conceivable that anthropogenic disturbances can reduce the richness of dominant species within grassland communities and affect other variables in the systems depicted in our SEMs, thereby indirectly affecting grassland stability in Inner Mongolia. Indeed, Inner Mongolian grassland has been seriously disturbed by livestock overgrazing and coal mining during past decades [37,45,61]. Furthermore, predictions for the future climate include increased precipitation variation [42,43], which could decrease grassland stability, as shown in the present study.
Supplementary Material
Acknowledgements
The authors thank Zhuwen Xu, Shaopeng Wang and Jens-Christian Svenning for their constructive comments.
Data accessibility
The datasets and R code used for this study can be obtained from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ht76hdrc5 [62].
Authors' contributions
Y.W., X.N., L.Z., C.L. and W.M. designed the study. Y.W., X.N., L.Z., C.L., B.M., Q.Z., J.Z. and W.M. compiled the data. Y.W., B.S. and W.M. produced the results and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the National Nature Science Foundation of China (grant nos 31370454, 31960259, 31971434 and 31600385), the National Key Research and Development Program of China (grant no. 2016YFC0500602), the Ministry of Science and Technology of China (grant no. 2015BAC02B04) and the Natural Science Foundation of Inner Mongolia (grant nos. 2019MS03089, 2019MS03088 and 2015ZD05). B.S. was supported by the University Research Priority Program Global Change and Biodiversity of the University of Zurich.
References
- 1.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 3.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 4.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- 5.Harrison SP, Gornish ES, Copeland S. 2015. Climate-driven diversity loss in a grassland community. Proc. Natl Acad. Sci. USA 112, 8672–8677. ( 10.1073/pnas.1502074112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang J, Piao S, Tang Z, Peng C, Ji W. 2001. Interannual variability in net primary production and precipitation. Science 293, 1723 ( 10.1126/science.293.5536.1723a) [DOI] [PubMed] [Google Scholar]
- 7.Knapp AK, Smith MD. 2001. Variation among biomes in temporal dynamics of aboveground primary production. Science 291, 481–484. ( 10.1126/science.291.5503.481) [DOI] [PubMed] [Google Scholar]
- 8.Bai Y, Han X, Wu J, Chen Z, Li L. 2004. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431, 181–184. ( 10.1038/nature02850) [DOI] [PubMed] [Google Scholar]
- 9.Doak DF, Bigger D, Harding EK, Marvier MA, O'Malley RE, Thomson D. 1998. The statistical inevitability of stability-diversity relationships in community ecology. Am. Nat. 151, 264–276. ( 10.1086/286117) [DOI] [PubMed] [Google Scholar]
- 10.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474. ( 10.1890/0012-9658(1999)080[1455:TECOCI]2.0.CO;2) [DOI] [Google Scholar]
- 11.Lehman CL, Tilman D. 2000. Biodiversity, stability, and productivity in competitive communities. Am. Nat. 156, 534–552. ( 10.1086/303402) [DOI] [PubMed] [Google Scholar]
- 12.Loreau M. 2010. From populations to ecosystems: theoretical foundations for a new ecological synthesis. Princeton, NJ: Princeton University Press. [Google Scholar]
- 13.Thibaut LM, Connolly SR. 2013. Understanding diversity–stability relationships: towards a unified model of portfolio effects. Ecol. Lett. 16, 140–150. ( 10.1111/ele.12019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilman D, Reich PB, Knops JM. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632. ( 10.1038/nature04742) [DOI] [PubMed] [Google Scholar]
- 15.Hector A, et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220. ( 10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 16.Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. 2015. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348, 336–340. ( 10.1126/science.aaa1788) [DOI] [PubMed] [Google Scholar]
- 17.Liu H, et al. 2018. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl Acad. Sci. USA 115, 4051–4056. ( 10.1073/pnas.1700299114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loreau M, de Mazancourt C.. 2008. Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. Am. Nat. 172, E48–E66. ( 10.1086/589746) [DOI] [PubMed] [Google Scholar]
- 19.Wilcox KR, et al. 2017. Asynchrony among local communities stabilises ecosystem function of metacommunities. Ecol. Lett. 20, 1534–1545. ( 10.1111/ele.12861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Feng J, Loreau M, He N, Han X, Jiang L. 2019. Nitrogen addition does not reduce the role of spatial asynchrony in stabilising grassland communities. Ecol. Lett. 22, 563–571. ( 10.1111/ele.13212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor LR. 1961. Aggregation, variance and the mean. Nature 189, 732–735. ( 10.1038/189732a0) [DOI] [Google Scholar]
- 22.Tilman D, Lehman CL, Bristow CE. 1998. Diversity-stability relationships: statistical inevitability or ecological consequence? Am. Nat. 151, 277–282. ( 10.1086/286118) [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Loreau M. 2014. Ecosystem stability in space: α, β and γ variability. Ecol. Lett. 17, 891–901. ( 10.1111/ele.12292) [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Loreau M. 2016. Biodiversity and ecosystem stability across scales in metacommunities. Ecol. Lett. 19, 510–518. ( 10.1111/ele.12582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Lamy T, Hallett LM, Loreau M. 2019. Stability and synchrony across ecological hierarchies in heterogeneous metacommunities: linking theory to data. Ecography 42, 1200–1211. ( 10.1111/ecog.04290) [DOI] [Google Scholar]
- 26.Zhang Y, Loreau M, Lü X, He N, Zhang G, Han X. 2016. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Glob. Change Biol. 22, 1445–1455. ( 10.1111/gcb.13140) [DOI] [PubMed] [Google Scholar]
- 27.Hallett LM, et al. 2014. Biotic mechanisms of community stability shift along a precipitation gradient. Ecology 95, 1693–1700. ( 10.1890/13-0895.1) [DOI] [PubMed] [Google Scholar]
- 28.Hautier Y, et al. 2014. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 508, 521–525. ( 10.1038/nature13014) [DOI] [PubMed] [Google Scholar]
- 29.Srivastava DS, Vellend M. 2005. Biodiversity-ecosystem function research: is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 36, 267–294. ( 10.1146/annurev.ecolsys.36.102003.152636) [DOI] [Google Scholar]
- 30.Winfree R, Fox JW, Williams NM, Reilly JR, Cariveau DP. 2015. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 18, 626–635. ( 10.1111/ele.12424) [DOI] [PubMed] [Google Scholar]
- 31.Houlahan JE, et al. 2007. Compensatory dynamics are rare in natural ecological communities. Proc. Natl Acad. Sci. USA 104, 3273–3277. ( 10.1073/pnas.0603798104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Liu H, Mi Z, Zhang Z, Wang Y, Xu W, Jiang L, He J-S. 2017. Climate warming reduces the temporal stability of plant community biomass production. Nat. Commun. 8, 15378 ( 10.1038/ncomms15378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, et al. 2015. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J. Ecol. 103, 1308–1316. ( 10.1111/1365-2745.12441) [DOI] [Google Scholar]
- 34.Yang H, Jiang L, Li L, Li A, Wu M, Wan S. 2012. Diversity-dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecol. Lett. 15, 619–626. ( 10.1111/j.1461-0248.2012.01778.x) [DOI] [PubMed] [Google Scholar]
- 35.Gherardi LA, Sala OE. 2015. Enhanced precipitation variability decreases grass- and increases shrub-productivity. Proc. Natl Acad. Sci. USA 112, 12 735–12 740. ( 10.1073/pnas.1506433112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W, He J, Yang Y, Wang X, Liang C, Anwar M, Zeng H, Fang J, Schmid B. 2010. Environmental factors covary with plant diversity–productivity relationships among Chinese grassland sites. Glob. Ecol. Biogeogr. 19, 233–243. ( 10.1111/j.1466-8238.2009.00508.x) [DOI] [Google Scholar]
- 37.Wu J, Zhang Q, Li A, Liang C. 2015. Historical landscape dynamics of Inner Mongolia: patterns, drivers, and impacts. Landsc. Ecol. 30, 1579–1598. ( 10.1007/s10980-015-0209-1) [DOI] [Google Scholar]
- 38.Kang L, Han X, Zhang Z, Sun OJ. 2007. Grassland ecosystems in China: review of current knowledge and research advancement. Phil. Trans. R. Soc. B 362, 997–1008. ( 10.1098/rstb.2007.2029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai Y, Wu J, Xing Q, Pan Q, Huang J, Yang D, Han X. 2008. Primary production and rain use efficiency across a precipitation gradient on the Mongolia Plateau. Ecology 89, 2140–2153. ( 10.1890/07-0992.1) [DOI] [PubMed] [Google Scholar]
- 40.Hu Z, Guo Q, Li S, Piao S, Knapp AK, Ciais P, Li X, Yu G. 2018. Shifts in the dynamics of productivity signal ecosystem state transitions at the biome-scale. Ecol. Lett. 21, 1457–1466. ( 10.1111/ele.13126) [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Zhu J, Pan Q, Liu Y, Chen S, Chen D, Yan Y, Dou S, Han X. 2017. Grassland species respond differently to altered precipitation amount and pattern. Environ. Exp. Bot. 137, 166–176. ( 10.1016/j.envexpbot.2017.02.006) [DOI] [Google Scholar]
- 42.Piao S, et al. 2010. The impacts of climate change on water resources and agriculture in China. Nature 467, 43–51. ( 10.1038/nature09364) [DOI] [PubMed] [Google Scholar]
- 43.Huang J, Xue Y, Sun S, Zhang J. 2015. Spatial and temporal variability of drought during 1960–2012 in Inner Mongolia, north China. Quat. Int. 355, 134–144. ( 10.1016/j.quaint.2014.10.036) [DOI] [Google Scholar]
- 44.Arnoldi J, Loreau M, Haegeman B. 2019. The inherent multidimensionality of temporal variability: how common and rare species shape stability patterns. Ecol. Lett. 22, 1557–1567. ( 10.1111/ele.13345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Bai Y, Wu J. 2015. Towards a better understanding of landscape patterns and ecosystem processes of the Mongolian Plateau. Landsc. Ecol. 30, 1573–1578. ( 10.1007/s10980-015-0277-2) [DOI] [Google Scholar]
- 46.Craven D, et al. 2018. Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2, 1579–1587. ( 10.1038/s41559-018-0647-7) [DOI] [PubMed] [Google Scholar]
- 47.Shi Y, Wang Y, Ma Y, Ma W, Liang C, Flynn DFB, Schmid B, Fang J, He JS. 2014. Field-based observations of regional-scale, temporal variation in net primary production in Tibetan alpine grasslands. Biogeosciences 11, 2003–2016. ( 10.5194/bg-11-2003-2014) [DOI] [Google Scholar]
- 48.Klein JA, Harte J, Zhao XQ. 2004. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett. 7, 1170–1179. ( 10.1111/j.1461-0248.2004.00677.x) [DOI] [Google Scholar]
- 49.Rosseel Y. 2012. lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw. 48, 1–36. ( 10.18637/jss.v048.i02) [DOI] [Google Scholar]
- 50.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 51.Grime JP. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910. ( 10.1046/j.1365-2745.1998.00306.x) [DOI] [Google Scholar]
- 52.Lepš J. 2004. Variability in population and community biomass in a grassland community affected by environmental productivity and diversity. Oikos 107, 64–71. ( 10.1111/j.0030-1299.2004.13023.x) [DOI] [Google Scholar]
- 53.Polley HW, Wilsey BJ, Tischler CR. 2007. Species abundances influence the net biodiversity effect in mixtures of two plant species. Basic Appl. Ecol. 8, 209–218. ( 10.1016/j.baae.2006.02.006) [DOI] [Google Scholar]
- 54.Grman E, Lau JA, Schoolmaster DR, Gross KL. 2010. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 13, 1400–1410. ( 10.1111/j.1461-0248.2010.01533.x) [DOI] [PubMed] [Google Scholar]
- 55.Sasaki T, Lauenroth WK. 2011. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 166, 761–768. ( 10.1007/s00442-011-1916-1) [DOI] [PubMed] [Google Scholar]
- 56.Kilpatrick AM, Ives AR. 2003. Species interactions can explain Taylor's power law for ecological time series. Nature 422, 65–68. ( 10.1038/nature01471) [DOI] [PubMed] [Google Scholar]
- 57.Valone TJ, Hoffman CD. 2003. A mechanistic examination of diversity-stability relationships in annual plant communities. Oikos 103, 519–527. ( 10.1034/j.1600-0706.2003.12279.x) [DOI] [Google Scholar]
- 58.van Ruijven J, Berendse F.. 2007. Contrasting effects of diversity on the temporal stability of plant populations. Oikos 116, 1323–1330. ( 10.1111/j.2007.0030-1299.16005.x) [DOI] [Google Scholar]
- 59.Wang S, et al. 2012. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 93, 2365–2376. ( 10.1890/11-1408.1) [DOI] [PubMed] [Google Scholar]
- 60.Hautier Y, Niklaus PA, Hector A. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638. ( 10.1126/science.1169640) [DOI] [PubMed] [Google Scholar]
- 61.Tao S, Fang J, Zhao X, Zhao S, Shen H, Hu H, Tang Z, Wang Z, Guo Q. 2015. Rapid loss of lakes on the Mongolian Plateau. Proc. Natl Acad. Sci. USA 112, 2281–2286. ( 10.1073/pnas.1411748112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Niu X, Zhao L, Liang C, Miao B, Zhang Q, Zhang J, Schmid B, Ma W. 2020. Data from: Biotic stability mechanisms in Inner Mongolian grassland Dryad Digital Repository. ( 10.5061/dryad.ht76hdrc5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wang Y, Niu X, Zhao L, Liang C, Miao B, Zhang Q, Zhang J, Schmid B, Ma W. 2020. Data from: Biotic stability mechanisms in Inner Mongolian grassland Dryad Digital Repository. ( 10.5061/dryad.ht76hdrc5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets and R code used for this study can be obtained from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ht76hdrc5 [62].