Abstract
Counterillumination, the masking of an animal's silhouette with ventral photophores, is found in a number of mesopelagic taxa but is difficult to employ because it requires that the animal match the intensity of downwelling light without seeing its own ventral photophores. It has been proposed that the myctophid, Tarletonbeania crenularis, uses a photophore directed towards the eye, termed an eye-facing photophore, as a reference standard that it adjusts to match downwelling light. The potential use of this mechanism, however, has not been evaluated in other fishes. Here, we use micro-computed tomography, photography and dissection to evaluate the presence/absence of eye-facing photophores in three families of stomiiform fishes. We found that all sampled species with ventral photophores capable of counterillumination possess an eye-facing photophore that is pigmented on the anterior and lateral sides, thus preventing its use as a laterally directed signal, lure or searchlight. The two species that are incapable of counterillumination, Cyclothone obscura and Sigmops bathyphilus, lack an eye-facing photophore. After determining the phylogenetic distribution of eye-facing photophores, we used histology to examine the morphology of the cranial tissue in Argyropelecus aculeatus and determined that light from the eye-facing photophore passes through a transparent layer of tissue, then the lens, and finally strikes the accessory retina. Additionally, eight of the 14 species for which fresh specimens were available had an aphakic gap that aligned with the path of emitted light from the eye-facing photophore, while the remaining six had no aphakic gap. These findings, combined with records of eye-facing photophores from distantly related taxa, strongly suggest that eye-facing photophores serve as a reference for counterillumination in these fishes.
Keywords: camouflage, deep-sea, bioluminescence, stomiiformes
1. Introduction
The open ocean is the largest habitable volume on earth and poses particular difficulties for the organisms living there. At mesopelagic depths (200 m–1000 m), the intensity of downward radiance is approximately 200 times that of upward radiance [1], so opaque organisms––even those with white ventral surfaces––create a silhouette that can be detected from below. To counteract this, a number of mesopelagic taxa (e.g. certain squid, crustaceans and fishes, including sharks) have arrays of ventral photophores that replace the downwelling light blocked by the body of the animal [2–6]. This form of camouflage, called counterillumination, is particularly common in stomiiform and myctophiform fishes, two of the most speciose and abundant orders of mesopelagic fishes [7].
The primary challenges for effective counterillumination are matching the intensity, spectrum and angular distribution of downwelling light. The ventral photophores of mesopelagic fishes are known to emit light with a spectrum that is a close match to the spectrum of downwelling light [6,8]. Additionally, at least two stomiiform species, Chauliodus sloani and Argyropelecus affinis, have photophores that use guanine reflectors to match the angular distribution of downwelling light [9]. The photophores of Stomiiformes and Myctophiformes are under neural control [10,11], and certain mesopelagic fishes (Dasyscopelus obtusirostris and Dasyscopelus spinosus sensu Martin et al. [12], formerly Myctophum obtusirostre and Myctophum spinosum, respectively) adjust their ventral light emission in response to changes in downwelling light intensity over a range of up to 15 000-fold [6,13].
Despite evidence that counterillumination matches the intensity of downwelling light, little is known about the feedback system that mediates this process, given that these fishes are unable to see their own ventral photophores. It has been hypothesized that fishes may use a reference photophore, with an intensity correlated with the intensity of the ventral photophores, to emit light towards the eye (termed eye-facing photophore hereafter), allowing modulation of ventral bioluminescence until it matches the downwelling light viewed by the eye [4,14]. The potential use of such a reference photophore mechanism, however, has only been examined in one species, the myctophid Tarletonbeania crenularis [14]. To evaluate the role of an eye-facing photophore as part of a more general mechanism of regulating counterillumination, we characterized the presence/absence and orientation (eye-facing or not) of the orbital photophores of 36 species of stem group Stomiiformes in a phylogenetic context (we sampled 15 of 18 genera in the tree from Rabosky et al. [15]). In addition to those traits, we assessed the presence of an aphakic gap, a lensless section of pupil that allows for increased light capture, for the 14 species for which we were able to acquire photographs of fresh specimens. We then examined the morphology of the eye-facing photophores and surrounding tissues in one species in which the morphology of the retina is well characterized, the hatchetfish Argyropelecus aculeatus, to determine whether light from its eye-facing photophore reaches the eye, and to explore other potential functions of the emitted light.
2. Material and methods
(a). Specimen acquisition
We focused on stem stomiiform fishes, members of the families Gonostomatidae (bristlemouths), Sternoptychidae (hatchetfishes) and Phosichthyidae (lightfishes). We chose these families because they are known to possess either a pre-, ant-, or suborbital photophore, but do not have the proliferation of cranial photophores seen in the crown group Stomiidae. The additional cranial photophores in stomiids suggests functional diversification, with many of these photophores hypothesized to function as searchlights, a process that does not necessarily require the same feedback as counterillumination [16,17].
Twenty-one specimens from 21 species were collected via high-speed rope trawl or 10 m2 MOCNESS in the Gulf of Mexico between 2009 and 2017. Immediately following collection, animals were placed in 10% formalin in buffered seawater and stored until use, at which point they were transferred to 70% ethanol. One specimen of A. aculeatus was collected via midwater Tucker trawl from Baltimore Canyon near 38.05° N 73.7° W and immediately placed in 2% glutaraldehyde in buffered seawater for use in histology (the A. aculeatus specimen collected previously in the Gulf of Mexico was stained for micro-CT). The remaining 15 specimens were acquired via loan from the Smithsonian Institution Museum of Natural History, Washington, DC, USA. One specimen per species was used because, while there are intraspecific differences in photophore patterning in some deep-sea fishes associated with changes in body size and population divergence [18–20], to our knowledge there are no shifts in the overall direction of photophores within a species (i.e. a photophore directed into the eye or away from the eye). Additional details on specimen collection, including museum acquisition numbers and trawl depth, can be found in electronic supplementary material, table S1.
(b). Fitting models of discrete trait evolution
Of the 36 species considered here, 25 from 13 genera were included in a comprehensive molecular phylogeny of ray-finned fishes [15]. Those that were not found on the phylogeny were not included in the models of discrete trait evolution because they could not be unambiguously placed on the tree. The full tree was pruned to include only the species surveyed in this analysis using the packages ‘ape v5.3’ [21] and ‘phytools v0.6–60’ [22] in R [23]. To determine if there is a phylogenetic signal in the distribution of eye-facing photophores or ventral photophores that are capable of effective counterillumination, we calculated Fritz and Purvis's D statistic using 1000 permutations with the package ‘caper’ [24,25]. To consider the coevolution of ventral photophores capable of counterillumination and the eye-facing photophore, we fitted independent and dependent models of discrete trait evolution in BayesTraits v. 3.0.1 [26]. All parameters were set to their default values and the results of each model were compared using maximum likelihood.
(c). Eye-facing photophore identification
Given their proximity to the skin and the transparent tissue surrounding them, many eye-facing photophores could be identified under a stereo microscope (M5, Wild, Heerbrugg, Switzerland). For specimens (5/36) where the presence and orientation of an orbital photophore could not be inferred from standard imaging, it was characterized using micro-computed tomography (micro-CT). Prior to micro-CT scanning, whole fish were stained for two to five days in a 50 : 50 mixture of 2% aqueous solution of Lugol's iodine (J. Crows LLC, Ipswich, NH, USA) and the initial preservation medium (either 70% ethanol or 10% buffered formalin solution). Micro-CT was performed using a Nikon XTH 225 ST scanner at the Duke University Shared Materials Instrumentation Facility, which produced voxels with edge lengths between 1.2 µm and 4.1 µm with beam settings of 110 kV–190 kV and 43 µA–114 µA. We confirmed via dissection that micro-CT can be used to accurately identify both the presence of a photophore and its orientation because of differential staining of the lens and photocytes and the asymmetrical morphology of the photophore (figure 1).
Figure 1.
Micro-CT images of (a) Sternoptyx pseudobscura, (b) Argyropelecus hemigymnus and (c) Sternoptyx diaphana showing the presence and orientation of the photophores from iodine-stained specimens. The eye-facing photophore and the lens are shown in the inset panels. Eye-facing photophores and ventral photophores denoted by the arrows and brackets, respectively. Top: lateral view. Bottom: ventral view. All scale bars are 5 mm. (Online version in colour.)
(d). Histology of eye-facing photophore
A block of tissue from Argyropelecus aculeatus containing the photophore, part of the upper jaw, and the tissue between the cornea and the photophore was excised with a razor blade and partially dehydrated in 95% ethanol for 24 h. The tissue was embedded in glycol methacrylate plastic (GMA) to minimize tissue distortion (Technovit 7100, Kulzer GmbH, Hanau, Germany), and 2 µm thick sections were cut using a glass knife (Reichert-Jung, Leica, Wetzlar, Germany). Sections were stained with Picrosirius/Fast Green stain (0.3 g Sirius Red, 0.3 g Fast Green FCF, 300 ml saturated picric acid) for 3 h at 60°C to differentiate between tissue types. Following staining, samples were rinsed with deionized water, dried and mounted under a coverslip. Composite images were taken with a Zeiss Axiocam HRc digital camera on a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany).
(e). Characterization of aphakic gaps
To further assess potential morphological specialization that may permit light from the eye-facing photophore to enter the eye, we assessed the presence and location of the aphakic gap for species in which we were able to acquire photographs of fresh specimens (14/36). We used fresh specimens only because many long-preserved specimens have distortions of the iris and tapetum that make assessment of the aphakic gap difficult. In total, we acquired aphakic gap data for 14 of the 36 species included in the study.
3. Results
(a). Phylogenetic distribution of eye-facing orbital photophores
Eye-facing photophores were present in all species with ventral photophores that are capable of counterillumination (34/36 species; for examples of light paths, see figure 2). Additionally, in at least A. aculeatus, S. diaphana and Maurolicus spp., the path of the light emitted from the eye-facing photophore intersects regions of high retinal cell density [27–29]. All eye-facing photophores were surrounded by a melanin layer on the side opposite the eye (i.e. anterior side for a photophore located in front of the eye) and the lateral face (figure 3; full trait data in electronic supplementary material, table S2). This pigment layer ostensibly absorbs light that is not propagating towards the eye and thus prevents the eye-facing photophore from being used as a searchlight, lure, or laterally directed signal. Conversely, we did not find eye-facing photophores in Cyclothone obscura and Sigmops bathyphilus, two primarily bathypelagic species [30]. While C. obscura has lost all photophores, S. bathyphilus maintains small masses of bioluminescent tissue known as secondary photophores and one short series of organized photophores on the ventral surface. These photophore arrays are unlikely to be sufficient for effective counterillumination, although it is possible they are used to disrupt the silhouette [31,32].
Figure 2.
Light path from eye-facing photophore of three representative species. Micro-CT images from (a) A. hemigymnus, (b) S. diaphana and (c) M. weitzmani, showing zoomed-in side and top views of the eye-facing photophore and the eye. The dashed lines for all views are parallel to face of the photophore closest to the eye. Arrows are perpendicular to the dashed lines and denote the predicted light path. The top view for S. diaphana is in the plane of the dorsal surface of the photophore, not in the plane of the most-dorsal surface of the body, because the photophore is obscured dorsally by the musculature (note––the musculature does not extend into the light path between the photophore and the eye). (Online version in colour.)
Figure 3.
(a) Phylogeny of sampled species of Gonostomatidae, Sternoptychidae and Phosichthyidae pruned from a comprehensive molecular phylogeny of fishes [15]. Colours indicate the presence or absence of the ventral photophores, the eye-facing photophore and an aphakic gap (where available). Only 25/36 species are included in the tree, but the remaining 11 species all exhibit ventral photophores capable of counterillumination and eye-facing photophores (Ariaophos eastropas, Agyripnus atlanticus, A. brocki, A. ephippiatus, Argyropelecus pacificus, Cyclothone braueri, Maurolicus muelleri, Polyipnus aquavitus, P. nuttingi, P. spinifer, Vinciguerria poweriae). Dissecting scope images show examples of the eye-facing photophore surrounded by melanin in (b) Cyclothone pallida, (c) Argyropelecus lychnus and (d) Maurolicus japonicus. The eye-facing photophores are denoted by the white arrows. (Online version in colour.)
The phylogenetic distributions of ventral photophores capable of counterillumination and the eye-facing photophore, when considered independently, were not significantly different than expected under random phylogenetic structure or Brownian motion (BM) (D = −0.431; Random: p = 0.164; BM: p = 0.635); however, this may be an underestimate of the phylogenetic signal because it does not include the 11 species that could not be unambiguously placed on the tree. When considering the coevolution of the two traits, a model of dependent evolution of eye-facing photophores and ventral photophores capable of counterillumination provided a better fit for the distribution of both traits than a model treating both traits independently (chi-square: p = 0.007). Our findings indicate that the ventral photophores and eye-facing photophores evolved in a correlated fashion, where the rate of transitioning to each presence–absence state for each trait depends on the presence–absence state of the other. This result is in line with the expected outcome given the one-to-one relationship between the presence of ventral photophores capable of counterillumination and eye-facing photophores (figure 3; electronic supplementary material, table S2). It should be noted, however, that there are only two transitions between character states, and no species that have one trait but not the other.
(b). Eye-facing photophore morphology in Argyropelecus aculeatus
We found that the eye-facing photophore in A. aculeatus is enclosed on the dorsal and ventral sides by two layers of connective tissue that flare away from the light-emitting end of the photophore to allow light propagation towards the eye (figure 4). Additionally, the lateral and anterior sides of the photophore are shielded by a layer of melanin that is several granules (1 µm–21 µm) thick. This pigment layer, like the layer found in other species with an eye-facing photophore, prevents the use of the orbital photophore as a searchlight for finding prey, a proposed function of the orbital photophores of many stomiid dragonfishes (the crown group of the order) [33]. Between the eye-facing photophore and the cornea of the eye is a region of tissue that is transparent in fresh specimens, translucent in preserved specimens and does not stain with Fast Green, suggesting little protein content (and thus presumably minimal scattering). Light exiting the eye-facing photophore and travelling through this transparent tissue would be absorbed by the iris in the tubular eyes of other deep-sea fishes (e.g. Opisthoproctus soleatus) but is able to pass through a dip in the nasal edge of the iris that is reported by Collin et al. [27]. It then presumably passes through the lens and falls on the accessory retina, likely as an unfocused spot because the distance between the accessory retina and the lens is far less than 2.55 times the lens radius (the predicted focal length for fishes from Matthiessen's ratio [34]; figure 4).
Figure 4.
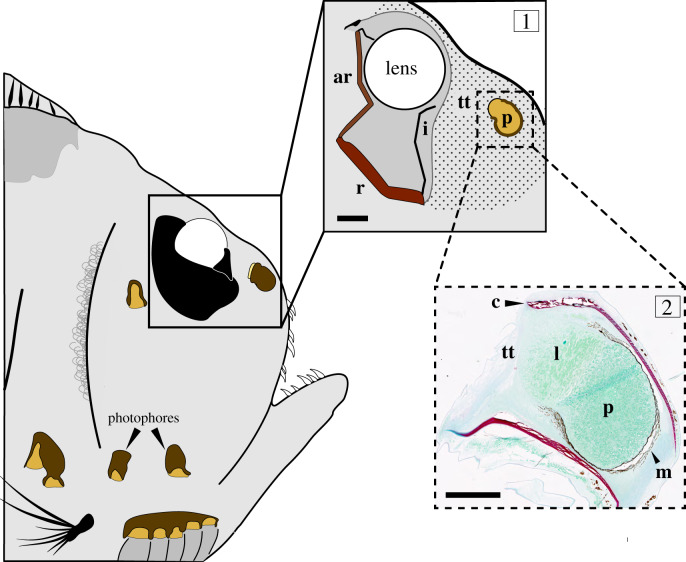
Digital illustration of Argyropelecus aculeatus showing the morphology of the eye and eye-facing photophore constructed from micro-CT, histological measurements, and previously described retinal morphology [27]. Box 1: the eye-facing photophore (p) emits light that passes through a volume of transparent tissue (tt) and through a dip in the iris (i) reported by Collin et al. [27]. After travelling through the lens, light strikes the accessory retina (ar) while downwelling light illuminates the main retina (r). Box 2: histological section through the middle of the eye-facing photophore shows photocytes (p), the photophore lens (l) and a layer of melanin (m) surrounding three sides of the photophore, preventing light from escaping anteriorly or laterally. Note, there is no reflector or filter in the eye-facing photophore. Additionally, collagen layers (c) flare out from the end of the photophore that the light exits. Once leaving the photophore, light travels through tissue (tt) that is largely transparent and does not stain with Fast Green. Scale bars: box 1, 500 µm; box 2, 100 µm. (Online version in colour.)
(c). Presence and location of aphakic gaps
We found aphakic gaps in eight of the 14 species for which we could get photographs of fresh specimens (trait data: figure 3). In A. aculeatus, A. affinis, A. sladeni, I. ovatus and V. tripunctulatus the aphakic gap consisted of a dip in the nasal edge of the iris that exposed the lens to the eye-facing photophore. Typically, without this dip, the pigmented iris found in other fishes with tubular eyes would block light coming from the photophore. In S. diaphana and S. pseudobscura, the aphakic gap is located ventral to the lens. The eye-facing photophore in these species is located on the orbit posterior and dorsal to the lens and directs light ventrally and anteriorly (figures 1 and 2). The ventral aphakic gap in Sternoptyx spp. aligns with the light path from the photophore, allowing the emitted light to enter the eye. Additionally, at least in S. diaphana, the ventral part of the retina where the photophore light would fall has a high density of cells in the ganglion cell layer [28]. In P. clarus, the aphakic gap is located along the ventral margin of the lens and the eye-facing photophore is directed posteriorly and ventrally, roughly aligning with the location of the aphakic gap. The remaining six species (C. braueri, C. obscura, C. pallida, P. mauli, S. elongatus and V. poweriae) do not possess an aphakic gap. There were no species for which we recorded an aphakic gap that did not align with the direction of the eye-facing photophore.
4. Discussion
Here, we show that 34 of 36 sampled species of stem Stomiiformes (of the 53 found on a phylogeny) [15] are counterilluminators based on the presence of ventral photophores, and that these species have a photophore directed into the eye that is pigmented in a way that rules out a searchlight function or use as a laterally directed signal. While behavioural experiments directly linking the eye-facing photophore to counterillumination regulation were not possible because mesopelagic fishes caught in trawl nets are typically deceased or moribund, we found that the distribution of ventral photophores and the eye-facing photophore are best explained by a dependent model of discrete trait evolution. That is, considering complete rows of ventral photophores (a proxy for counterillumination) and the eye-facing photophore as correlated traits provides a higher maximum likelihood than treating the two traits independently. Additionally, a number of species possess morphological specializations such as aphakic gaps that permit light from the eye-facing photophore to enter the eye and strike the retina.
Further supporting the role of the eye-facing photophore in regulating counterillumination are reports of similar photophores in distantly related counterilluminating species. Lawry [14] found that the myctophid Tarletonbeania crenularis has a small photophore dorsal to the eye that casts light on the ventral part of the retina. Also, barracudinas (Paralepididae) in the genus Lestrolepis have a small organ anterior to the eye that is pigmented on the anterior and lateral sides that is presumed to be bioluminescent [35]. Finally, etmopterid sharks have small photophores dorsal to the eye that may cast light on the retina in a similar manner to T. crenularis [36]. While future work is necessary to systematically describe the distribution and morphology of eye-facing photophores in these groups of counterilluminating fishes, the widespread distribution of eye-facing photophores suggests that this mechanism of counterillumination regulation could extend beyond Stomiiformes.
The only suggested alternative hypothesis for the function of the eye-facing photophore is that, by shining light in the eye, it changes the adaptation state to ‘prime’ the eye (i.e. lower its sensitivity) and allow the fish to see any potential prey that are illuminated by a searchlight photophore without blinding itself. Priming the eye is unlikely to be the function of the eye-facing photophore in these families because they do not possess a searchlight photophore. Given this, there is no likely alternative function, to our knowledge, for a photophore that reduces sensitivity and increases glare, especially in a light-limited environment characterized by ocular adaptations that increase sensitivity (e.g. large pupil, tubular shape, long photoreceptors, spatial summation, aphakic gaps and accessory retinae [28]). Further investigation may reveal associations between these other ocular adaptations and eye-facing photophores. In sum, our results suggest that stem stomiiformes use a photophore directed into the eye to calibrate and thereby aid in the regulation of the intensity of their ventral bioluminescence for counterillumination camouflage.
Supplementary Material
Acknowledgements
We thank Justin Gladman and Dr. Carlos Taboada for assistance with micro-CT, and Jesse Granger, Sarah Solie, Dr Eleanor Caves and Dr Robert Fitak for helpful comments on an earlier version of the manuscript. We also thank the crew of the R/V Hugh R. Sharp, R/V Point Sur and M/V Meg Skansi for assistance at sea.
Data accessibility
All data are available in the text and the electronic supplementary material.
Authors' contributions
A.L.D and S.J. conceived the work; T.S. provided specimens; S.J. acquired funding; A.L.D. and W.M.K. did the histology; A.L.D performed the dissection, micro-CT, data analysis and wrote the manuscript. All authors were involved in the discussion of the results and revision of the manuscript.
Competing interests
Authors declare no competing interests.
Funding
Funding for this work was provided by the Duke University Department of Biology, the National Science Foundation (IOS 1557754 to W.M.K.) and was made possible in part by a grant from The Gulf of Mexico Research Initiative. Data are publically available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (doi:10.7266/N7VX0DK2; doi:10.7266/N7R49NTN). A.L.D. conducted this research with Government support under and awarded by DoD, Army Research Office, National Defense Science and Engineering Graduate Fellowship (NDSEG).
References
- 1.Johnsen S. 2014. Hide and seek in the open sea: pelagic camouflage and visual countermeasures. Ann. Rev. Mar. Sci. 6, 369–392. ( 10.1146/annurev-marine-010213-135018) [DOI] [PubMed] [Google Scholar]
- 2.Claes JM, Mallefet J. 2010. The lantern shark's light switch: turning shallow water crypsis into midwater camouflage. Biol. Lett. 6, 685–687. ( 10.1098/rsbl.2010.0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke WD. 1963. Function of bioluminescence in mesopelagic organisms. Nature 198, 1244–1246. ( 10.1038/1981244a0) [DOI] [Google Scholar]
- 4.Herring PJ. 1977. Bioluminescence of marine organisms. Nature 267, 788–793. ( 10.1038/267788a0) [DOI] [Google Scholar]
- 5.Widder EA. 1999. Bioluminescence. In Adaptive mechanisms in the ecology of vision (eds Archer SN, Djamgoz MBA, Loew ER, Partridge JC, Vallerga S), pp. 555–581. Boston, MA: Kluwer Academic. [Google Scholar]
- 6.Young RE, Kampa EM, Maynard SD, Mencher FM, Roper CF. 1980. Counterillumination and the upper depth limits of midwater animals. Deep Sea Res. A. 27, 671–691. ( 10.1016/0198-0149(80)90022-9) [DOI] [Google Scholar]
- 7.Gjøsæter J, Kawaguchi K. 1980. A review of the world resources of mesopelagic fish Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 8.Widder EA, Latz MI, Case JF. 1983. Marine bioluminescence spectra measured with an optical multichannel detection system. Biol. Bull. 165, 791–810. ( 10.2307/1541479) [DOI] [PubMed] [Google Scholar]
- 9.Denton EJ, Gilpin-Brown JB, Wright PG. 1972. The angular distribution of the light produced by some mesopelagic fish in relation to their camouflage. Proc. R. Soc. B 182, 145–158. ( 10.1098/rspb.1972.0071) [DOI] [Google Scholar]
- 10.Krönström J, Holmgren S, Baguet F, Salpietro L, Mallefet J. 2005. Nitric oxide in control of luminescence from hatchetfish (Argyropelecus hemigymnus) photophores. J. Exp. Biol. 208, 2951–2961. ( 10.1242/jeb.01712) [DOI] [PubMed] [Google Scholar]
- 11.Krönström J, Mallefet J. 2010. Evidence for a widespread involvement of NO in control of photogenesis in bioluminescent fish. Acta Zool. 91, 474–483. ( 10.1111/j.1463-6395.2009.00438.x) [DOI] [Google Scholar]
- 12.Martin RP, Olson EE, Girard MG, Smith WL, Davis MP. 2018. Light in the darkness: new perspective on lanternfish relationships and classification using genomic and morphological data. Mol. Phylo. Evo. 121, 71–85. ( 10.1016/j.ympev.2017.12.029) [DOI] [PubMed] [Google Scholar]
- 13.Case JF, Warner J, Barnes AT, Lowenstine M. 1977. Bioluminescence of lantern fish (Myctophidae) in response to changes in light intensity. Nature 265, 179–181. ( 10.1038/265179a0) [DOI] [PubMed] [Google Scholar]
- 14.Lawry JV. 1974. Lantern fish compare downwelling light and bioluminescence. Nature 247, 155–157. ( 10.1038/247155a0) [DOI] [Google Scholar]
- 15.Rabosky DL, et al. 2018. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395. ( 10.1038/s41586-018-0273-1) [DOI] [PubMed] [Google Scholar]
- 16.Fink WL. 1985. Phylogenetic interrelationships of the stomiid fishes (Teleostei: Stomiiformes) Ann Arbor, MI: Museum of Zoology, University of Michigan. [Google Scholar]
- 17.Kenaley CP. 2010. Comparative innervation of cephalic photophores of the loosejaw dragonfishes (Teleostei: Stomiiformes: Stomiidae): evidence for parallel evolution of long-wave bioluminescence. J. Morph. 271, 418–437. ( 10.1002/jmor.10807) [DOI] [PubMed] [Google Scholar]
- 18.Gordeeva NV, Nanova OG. 2017. Application of geometric morphometrics for intraspecific variability analysis in mesopelagic fishes of Sternoptychidae and Myctophidae families. J. Ichthyol. 57, 29–36. ( 10.1134/S0032945217010052) [DOI] [Google Scholar]
- 19.Herring PJ. 2007. Sex with the lights on? A review of bioluminescent sexual dimorphism in the sea. J. Mar. Biol. Assoc. U. K. 87, 829–842. ( 10.1017/S0025315407056433) [DOI] [Google Scholar]
- 20.Lima AT, Costa PA, Braga AC, Nunan GW, Mincarone MM. 2011. Fishes of the family Sternoptychidae (Stomiiformes) collected on the Brazilian continental slope between 11 and 23 S. Zootaxa 2742, 34–48. ( 10.11646/zootaxa.2742.1.2) [DOI] [Google Scholar]
- 21.Paradis E, Schliep K. 2018. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 35, 526–528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 22.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 23.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.Fritz SA, Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051. ( 10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 25.Orme D, Freckleton R, Thomas G, Petzoldt T. 2013. The caper package: comparative analysis of phylogenetics and evolution in R. See http://caper.r-forge.r-project.org.
- 26.Pagel M, Meade A. 2007. BayesTraits. See http://www.evolution.rdg.ac.uk/BayesTraits.html.
- 27.Collin SP, Hoskins RV, Partridge JC. 1997. Tubular eyes of deep-sea fishes: a comparative study of retinal topography (part 1 of 2). Brain Behav. Ev. 50, 335–346. ( 10.1159/000113345) [DOI] [PubMed] [Google Scholar]
- 28.Wagner HJ, Fröhlich E, Negishi K, Collin SP. 1998. The eyes of deep-sea fish II. Functional morphology of the retina. Prog. Ret. Eye Res. 17, 637–685. ( 10.1016/S1350-9462(98)00003-2) [DOI] [PubMed] [Google Scholar]
- 29.de Busserolles F, et al. 2017. Pushing the limits of photoreception in twilight conditions: the rod-like cone retina of the deep-sea pearlsides. Sci. Adv. 3, aao4709 ( 10.1126/sciadv.aao4709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quéro JC, Njock JC, de la Hoz MM. 1990. Gonostomatidae. In Check-list of the fishes of the eastern tropical atlantic (CLOFETA) (eds Quero JC, Hureau JC, Karrer C, Post A, Saldanha L), pp. 283–292. Lisbon, Portugal: JNICT. [Google Scholar]
- 31.McFall-Ngai M, Morin JG. 1991. Camouflage by disruptive illumination in leiognathids, a family of shallow-water, bioluminescent fishes. J. Exp. Biol. 156, 119–137. [Google Scholar]
- 32.Harper RD, Case JF. 1999. Disruptive counterillumination and its anti-predatory value in the plainfish midshipman, Porichthys notatus. Mar. Biol. 134, 529–540. ( 10.1007/s002270050568) [DOI] [Google Scholar]
- 33.Young RE. 1983. Oceanic bioluminescence: an overview of general functions. Bull. Mar. Sci. 33, 829–845. [Google Scholar]
- 34.Matthiessen L. 1882. Ueber die Beziehungen, welche zwischen dem Brechungsindex des Kerncentrums der Krystalllinse und den Dimensionen des Auges bestehen. Pflügers Archiv Eur. J. Physiol. 27, 510–523. ( 10.1007/BF01802978) [DOI] [Google Scholar]
- 35.Ghedotti MJ, Barton RW, Simons AM, Davis MP. 2015. The first report of luminescent liver tissue in fishes: evolution and structure of bioluminescent organs in the deep-sea naked barracudinas (Aulopiformes: Lestidiidae). J. Morph. 276, 310–318. ( 10.1002/jmor.20341) [DOI] [PubMed] [Google Scholar]
- 36.Claes JM, Partridge JC, Hart NS, Garza-Gisholt E, Ho HC, Mallefet J, Collin SP. 2014. Photon hunting in the twilight zone: visual features of mesopelagic bioluminescent sharks. PLoS ONE 9, 104213 ( 10.1371/journal.pone.0104213) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the text and the electronic supplementary material.