Abstract
This article summarizes the likely attenuation properties of RRx-001 in COVID-19 based on its mechanism of action and the putative pathogenesis of the disease, which appears to activate inflammatory, oxidative, and immune cascades with the potential to culminate in acute respiratory distress syndrome, cytokine storm and death. An ongoing pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), COVID-19 appears to present with 3 major patterns of clinical symptomatology: (1) mild upper respiratory tract infection, (2) non–life-threatening pneumonia, and (3) severe pneumonia and acute respiratory distress syndrome that initially manifest as a mild prodrome lasting for 7–8 days before rapid clinical and radiological deterioration requiring ICU transfer.
RRx-001 is a targeted nitric oxide donor. This small molecule, which has been evaluated in multiple Phase 1–2 clinical trials for cancer as well as a Phase 3 clinical trial for the treatment of small cell lung cancer called REPLATINUM (NCT03699956), is minimally toxic and demonstrates clear evidence of antitumor activity. During the course of these clinical trials it was noted that the rate of chronic obstructive pulmonary disease exacerbation and pneumonia in actively smoking small cell lung cancer patients treated with RRx-001 is less than 1%. Due to extensive history of tobacco use, 40%–70% of patients with lung cancer have chronic obstructive pulmonary disease and the expected rate of pulmonary infection in this population is 50%–70%, which was not observed in RRx-001 clinical trials. Moreover, in preclinical studies of pulmonary hypertension, RRx-001 was found to be comparable with or more effective than the FDA approved agent, Bosentan. The potential pulmonary protective effects of RRx-001 in patients with recurrent lung infections coupled with preclinical models demonstrating RRx-001-mediated reversal of pulmonary hypertension suggests RRx-001 may have therapeutic activity in patients with acute respiratory symptoms due to COVID 19. Clinical trials have been initiated to confirm the hypothesis that RRx-001 may be repurposed to treat SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, RRx-001, Nitric oxide
Introduction
In 2019, an enveloped positive-sense, single-stranded bat-borne [1], [2], [3] highly pathogenic RNA virus, SARS-CoV-2 jumped to humans (Fig. 1 ). The pandemicity of the virus [4] and the ensuing economic, social, and political disruption from widespread morbidity and mortality [5] has galvanized the clinical community and regulatory authorities to accelerate the pace of vaccine and treatment trials.
Fig. 1.
Zoonotic origin of SARS-CoV-2. Severe acute respiratory syndrome (SARS-CoV-2) was originally a bat virus that spread to humans.
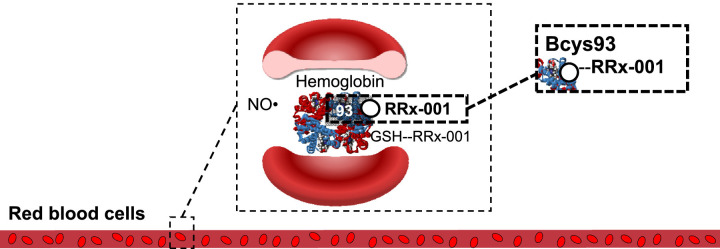
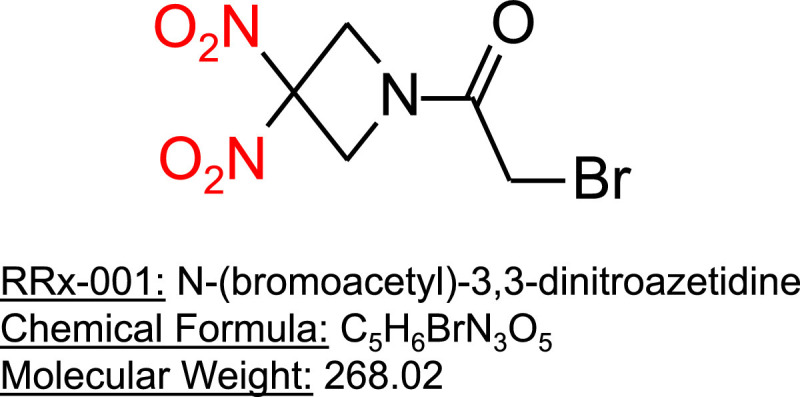
RRx-001 (N-(bromoacetyl)-3,3-dinitroazetidine) (Fig. 2 ) is a targeted, small-molecule nitric oxide (NO) donor with anticancer activity. This agent is being studied in a Phase 3 clinical trial to treat advanced small cell lung cancer (SCLC) [6]. Like chloroquine [7] and hydroxychloroquine, RRx-001 is coupled to red blood cells [8] (RBCs), which mostly mediate the normal tissue protective, antitumor and antipathogenic properties of RRx-001 (Fig. 3 ). In preclinical testing, RRx-001 has demonstrated activity against malaria [9], Ebola, tuberculosis, and leishmaniasis. Importantly, under hypoxic conditions, RRx-001 is a NO donor, resulting in targeted NO delivery to hypoxic tissues such as tumors. NO improves oxygenation through pulmonary vasodilation [10]. Consequently, targeted NO delivery to hypoxic lung tissue may lead to reversal of hypoxic pulmonary vasoconstriction caused by infection, inflammation, and edema and might treat or help prevent acute respiratory distress syndrome (ARDS) [11]. In preclinical models, RRx-001 has been demonstrated to reduce pulmonary artery hypertension (PAH) (manuscript in preparation). We review the evidence that RRx-001 potentially alleviates pulmonary pathology including pneumonia, acute lung injury (ALI) and ARDS and the potential for use of this agent to manage pulmonary symptoms caused by COVID-19.
Fig. 2.
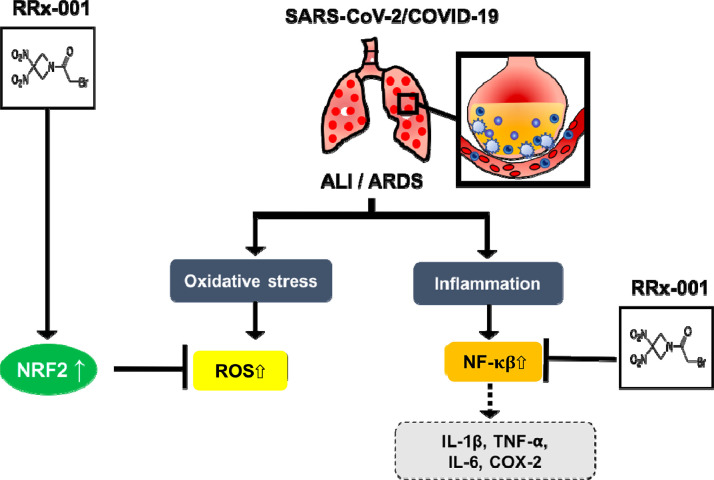
Potential protective antioxidant and anti-inflammatory effects of RRx-001 on SARS-CoV-2-induced lung injury. RRx-001 may provide support for patients with ALI/ARDS and related complications. It is associated with Nrf2/antioxidant response element signaling, which effectively reduces ROS levels, which are elevated in acute lung injury (ALI), and inhibition of the proinflammatory NF-κB pathway. ARDS = acute respiratory distress syndrome.
Fig. 3.
RRx-001 binding to hemoglobin. RRx-001 selectively, rapidly, and irreversibly binds glutathione (GSH) and a specific thiol on hemoglobin on Cys 93 With permission from: Scicinski et al. Discovery and Development of RRx-001, a Novel Nitric Oxide and ROS Mediated Epigenetic Modulator in Nitric Oxide and Cancer: Pathogenesis and Therapy pp 259-277.
COVID-19 pathogenesis and the potential role for RRx-001 as an acute treatment
Patients with COVID-19 due to SARS-CoV-2 infection present with a spectrum of symptomatology ranging from mild (upper respiratory infection and non–life-threatening pneumonia) to critical (hypoxemic pneumonia and ARDS); severity is related to advanced age and/or medical comorbidities such as cardiovascular disease, diabetes mellitus, morbid obesity, chronic lung disease, hypertension, and cancer [12]. Major complications of COVID-19 [13] include ALI/ARDS, sepsis, respiratory failure, heart failure, cardiomyopathies, sudden cardiac arrest, and cytokine storm, which is associated with an overproduction of IL-1β, IL-2, IL-6, IL-8, both IFN-α/β, tumor necrosis factor (TNF), C-C motif chemokine 3 (CCL3), CCL5, CCL2, and IP-10 [14].
A preexisting proinflammatory state, which is common to old age, obesity, tobacco abuse, infections, malignancies, autoimmune, and cardiopulmonary disease, may be the common denominator that underlies susceptibility to cytokine storm and adverse outcomes in COVID-19 [15] hence, the rationale to treat with immune/cytokine modulators such as chloroquine/hydroxychloroquine +/− azithromycin [16], tocilizumab, an IL-6 inhibitor [17], anakinra, an IL-1β antagonist, and certolizumab pegol, an anti-TNF blocker. The preexisting proinflammatory response is intensified at sites of SARS-CoV-2-tissue injury due to the release of chemokines and growth factors, which initiate an influx of neutrophils and monocytes that lead to more tissue injury with endothelial cell damage, vascular permeability, edema generation, and increased inflammatory cell accumulation, constituting a vicious circle [18].
The mechanisms by which RRx-001, which has been evaluated extensively in both SCLC and non–small cell lung cancer may attenuate the severity of SARS-Co-V-2 infection include NO release under hypoxia, antioxidation through Nrf2 generation, and NFκB inhibition.
RRx-001-induced release of NO under hypoxia
NO, a short-lived free radical gas, binds to soluble guanylyl cyclase, and increases the formation of guanosine 3′,5′-monophosphate from guanosine triphosphate, an increase which is responsible for a range of effects including blood vessel relaxation and increased blood flow, bronchodilation, and antiviral activity including against SARS coronavirus [19,20]. Three distinct isoforms of the enzyme nitric oxide synthase (NOS) generate nitric oxide. These are neuronal nNOS (or NOS I), inducible iNOS (or NOS II), and endothelial eNOS (or NOS III) [21]. All NOS isoforms depend on the presence of molecular oxygen to produce NO from L-arginine [22]. In ischemic/hypoxic conditions, when the endogenous NOS pathway is inactivated, the main source of NO is from deoxyhemoglobin-mediated nitrite reduction. Therefore, red blood cells improve perfusion and oxygen supply to hypoxic tissues through NO generation [23].
RRx-001 binds to a ubiquitous residue on hemoglobin called beta cysteine 93, which enhances the enzymatic conversion of nitrite to NO and leads to its overproduction and release only under hypoxic conditions only [24,25]. (Fig. 3) Unlike approved NO donors such as nitroglycerin, isosorbide dinitrate and nitroprusside, where systemic-wide NO release occurs, RRx-001-mediated NO production is selectively targeted to ischemic tissues. Consequently, side effects such as methemoglobinemia, hypotension, dizziness, or headache are not observed [26]. Clinical [27] and preclinical data has shown that RRx-001 administration increases blood flow to profoundly hypoxic tumors [28] in an NO dependent manner [29]. Studies, have also demonstrated that RRx-001 improves tissue blood flow in other ischemic disorders such as sickle cell disease, hypoxic pulmonary hypertension (manuscript in preparation), doxorubicin-induced heart failure [30], cerebral malaria-induced vasospasm [31], ischemia-reperfusion injury [32] and hemorrhagic shock [33] while additional experiments are planned or ongoing in peripheral artery disease, critical limb ischemia, and vasculitis.
Potentially beneficial characteristics of nitric oxide for the management of COVID-19 include:
-
•
Treatment of pulmonary arterial hypertension in ARDS.
-
•
Direct antiviral potential of NO since S-nitroso-N-acetylpenicillamine, an NO donor, prevented SARS coronavirus (SARS-CoV-1) viral replication in vitro [34].
PAH is a ubiquitous complication of ARDS [35]. The main consequence of PAH is right heart failure with reduced systemic oxygen delivery. RRx-001 was tested in an animal model of PAH against the FDA approved endothelial receptor antagonist, Bosentan. The results demonstrated that, similar to Bosentan, RRx-001 attenuated pulmonary artery and right ventricular remodeling.
Inhaled NO has been shown to decrease V/Q mismatch and shunt [36] and to improve arterial oxygenation in patients with ARDS [37] since the vasodilatory effects are limited to well-ventilated lung regions. However, with RRx-001, oxygenation is expected to improve due to improved cardiac output and reduced pulmonary venous remodeling.
Nrf2 activation and NFκB inhibition
In the pathogenesis of ALI/ARDS, with occurs in COVID-19, oxidative stress and inflammation [38] are 2 key interrelated factors [39]. RRx-001 has previously been demonstrated to activate Nrf2 [40] and inhibit NF-κB. Inflammation induces oxidative stress, which, in turn, feeds forward and amplifies inflammation through the activation of the proinflammatory mediator, nuclear factor kappa B (NF-κB). In ALI/ARDS, excessive reactive oxygen species mediates epithelial and endothelial injury, lung edema, and protein leakage. Nrf2, a master regulator of antioxidative responses, induces genes that reduce overall reactive oxygen species levels. Consequently, RRx-001 is expected to help protect pulmonary parenchyma by induction of Nrf2.
At the other end of the spectrum, NF-κB, a p50/p65 heterodimer is one of the main transcription factors, which produce proinflammatory cytokines and enzymes such as IL-1β, TNF-α, cyclooxygenase-2 (COX-2), and IL-6 and induce lung injury. Thus, as a dual Nrf2 inducer and NF-κB inhibitor, RRx-001 may provide antioxidant protection against COVID-19-induced lung injury, as illustrated in Figs. 2, 3, 4 .
Fig. 4.
Potential protective antioxidant and anti-inflammatory effects of RRx-001 on SARS-CoV-2-induced lung injury. RRx-001 may provide support for patients with ALI/ARDS and related complications. It is associated with Nrf2/antioxidant response element signaling, which effectively reduces ROS levels, which are elevated in acute lung injury (ALI), and inhibition of the proinflammatory NF-κB pathway. ARDS = acute respiratory distress syndrome.
Safety
RRx-001 has been evaluated and well tolerated in over 300 patients treated in clinical trials that have enrolled patients with cancer (Table 1). The main RRx-001-related adverse event is localized pain or discomfort at the site of infusion, which is mitigated with corticosteroid premedication. No maximally tolerated dose was ever reached with RRx-001 and no dose-limiting toxicities have been reported to date either as monotherapy or in combination with chemotherapy.
Conclusion
In the face of the dire global threat presented by the SARS-CoV-2 virus, more than 20 medicines [41], including human immunoglobulin from convalescent sera, interferons, inhaled nitric oxide, chloroquine, hydroxychloroquine, arbidol, remdesivir, favipiravir, lopinavir, ritonavir, oseltamivir, methylprednisolone, bevacizumab, and traditional Chinese medicines have been therapeutically repurposed or repositioned and used to treat patients with COVID-19. Hopefully, like with the Ebola virus, a fully protective vaccine candidate will emerge. [42] However, in the meantime, new treatment strategies are urgently needed given the high mortality rate and the economic and social upheaval, which has ensued.
Evidence in both animal models and cancer clinical trials has demonstrated that RRx-001 is protective for the lungs against the toxicities of chemotherapy and radiation potentially through Nrf2 activation and NF-κB inhibition. Furthermore, in animal models of pulmonary hypertension, RRx-001 has been shown to blunt adverse pulmonary vascular remodeling and reduce right ventricular hypertrophy. In clinical studies involving RRx-001, the incidence of chronic obstructive pulmonary disease exacerbations and pneumonia in a heavily smoking SCLC population has been significantly less than expected, suggesting protection of lung parenchyma.
On the basis of its targeted production of NO in hypoxic tissue, RRx-001 has the potential to reduce the pulmonary toxicity associated with the COVID-19 infection. RRx-001 has demonstrated a favorable safety profile in clinical studies of over 300 cancer patients and will be evaluated for safety and activity in clinical trials for patients experiencing infection with COVID-19. As a red blood cell based therapeutic, RRx-001 may serve as a less toxic alternative to chloroquine for the treatment of COVID-19. Finally, because RRx-001 experimentally ameliorated ischemia-reperfusion injury, it may also have the potential to reduce the morbidity of oxygen toxicity and ventilator-induced lung injury.
In summary, while protease inhibitors may decrease viral burden, inhaled nitric oxide may reduce V/Q mismatch, anticytokine therapy may treat cytokine storm or prevent its progression, and immunomodulators like chloroquine/hydroxychloroquine may inhibit viral replication, RRx-001 may serve as an all-in-one therapy, which improves cardiopulmonary function, decreases viral burden and reduces the systemic inflammatory response. Results from pending clinical trials of RRx-001 in COVID-19 disease are eagerly awaited.
Declaration of Competing Interest
The authors declare that EpicentRx, Inc. funds research of RRx-001.
References
- 1.Wang W., Dou S., Dong W. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis. 2018;13:3767–3776. doi: 10.2147/COPD.S168048. Published 2018 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valvani A., Martin A., Devarajan A., Chandy D. Postobstructive pneumonia in lung cancer. Ann Transl Med. 2019;7(15):357. doi: 10.21037/atm.2019.05.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allocati N., Petrucci A., Di Giovanni P. Bat–man disease transmission: zoonotic pathogens from wildlife reservoirs to human populations. Cell Death Discov. 2016;2:16048. doi: 10.1038/cddiscovery.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2019Novel coronavirus, Wuhan, China. Information for Healthcare Professionals. Available at: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html(Accessed on February 14, 2020).
- 5.Cascella M., Rajnik M., Cuomo A., et al. Features, Evaluation and treatment coronavirus (COVID-19) [Updated 2020 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed]
- 6.Oronsky B., Reid T.R., Larson C. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 2019;15(30):3427–3433. doi: 10.2217/fon-2019-0317. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari V., Cutler D.J. Uptake of chloroquine by human erythrocytes. Biochem Pharmacol. 1990;39(4):753–762. doi: 10.1016/0006-2952(90)90155-e. [DOI] [PubMed] [Google Scholar]
- 8.Cabrales P., Scicinski J., Reid T. A look inside the mechanistic black box: are red blood cells the critical effectors of RRx-001 cytotoxicity? Med Oncol (Northwood, London, England. 2016;33(7):63. doi: 10.1007/s12032-016-0775-3. [DOI] [PubMed] [Google Scholar]
- 9.Yalcin O., Oronsky B., Carvalho L.J., Kuypers F.A., Scicinski J., Cabrales P. From METS to malaria: RRx-001, a multi-faceted anticancer agent with activity in cerebral malaria. Malar J. 2015;14:218. doi: 10.1186/s12936-015-0720-5. Published 2015 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akmal A.H., Hasan M. Role of nitric oxide in management of acute respiratory distress syndrome. Ann Thorac Med. 2008;3(3):100–103. doi: 10.4103/1817-1737.41914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolin A., Bjertnaes L. Hypoxic pulmonary vasoconstriction in the adult respiratory distress syndrome. Acta Anaesthesiol Scand Suppl. 1991;95:40–52. doi: 10.1111/j.1399-6576.1991.tb03399.x. discussion 53-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2019;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautret P., Lagier J., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. medRxiv 2020.03.16.20037135; In Press. [DOI] [PMC free article] [PubMed] [Retracted]
- 17.Reuters. China approves use of Roche drug in battle against coronavirus complications. Available at: https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF
- 18.Bratton D.L., Henson P.M. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32:350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oronsky B.T., Knox S.J., Scicinski J.J. Is nitric oxide (NO) the last word in radiosensitization? A Review. Transl Oncol. 2012;5(2):66–71. doi: 10.1593/tlo.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marletta M.A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- 23.Fens M.H., Larkin S.K., Oronsky B., Scicinski J., Morris C.R., Kuypers F.A. The capacity of red blood cells to reduce nitrite determines nitric oxide generation under hypoxic conditions. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scicinski J., Oronsky B., Ning S. NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015;6:1–8. doi: 10.1016/j.redox.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scicinski J. Presentation at 3rd International Workshop on Nitric Oxide in Cancer Therapy, May 31 – Jun3 1. 2013. RRx-001, a hypoxia activated, nitric oxide generating cytotoxic agent: Phase 1 study results’. Kingston Ontario. [Google Scholar]
- 26.Reid T., Oronsky B., Scicinski J. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1133–1142. doi: 10.1016/S1470-2045(15)00089-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim M.M. et al. Initial Clinical and Advanced Imaging Outcomes from a Multi-Institutional Phase I Dose-Escalation Trial of RRx-001 Plus Whole Brain Radiation for Patients with Brain Metastases. Int J Radiat Oncol Biol Phys, Volume 102, Issue 3, e266
- 28.Ning S., Bednarski M., Oronsky B., Scicinski J., Saul G., Knox S.J. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials. Cancer Res. 2012;72(10):2600–2608. doi: 10.1158/0008-5472.CAN-11-2303. [DOI] [PubMed] [Google Scholar]
- 29.Fens M.H., Cabrales P., Scicinski J. Targeting tumor hypoxia with the epigenetic anticancer agent, RRx-001: a superagonist of nitric oxide generation. Med Oncol. 2016;33(8):85. doi: 10.1007/s12032-016-0798-9. [DOI] [PubMed] [Google Scholar]
- 30.Oronsky B., Ao-Ieong E.S.Y., Yalcin O., Carter C.A., Cabrales P. Cardioprotective effect of phase 3 clinical anticancer agent, RRx-001, in doxorubicin-induced acute cardiotoxicity in mice. Mol Pharm. 2019;16(7):2929–2934. doi: 10.1021/acs.molpharmaceut.9b00150. [DOI] [PubMed] [Google Scholar]
- 31.Yalcin O., Oronsky B., Carvalho L.J., Kuypers F.A., Scicinski J., Cabrales P. From METS to malaria: RRx-001, a multi-faceted anticancer agent with activity in cerebral malaria. Malar J. 2015;14:218. doi: 10.1186/s12936-015-0720-5. Published 2015 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrales P., Caroen S., Oronsky A. The macrophage stimulating anti-cancer agent, RRx-001, protects against ischemia-reperfusion injury. Expert Rev Hematol. 2017;10(6):575–582. doi: 10.1080/17474086.2017.1324779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouse C., Ortiz D., Su Y., Oronsky B., Scicinski J., Cabrales P. Impact of hemoglobin nitrite to nitric oxide reductase on blood transfusion for resuscitation from hemorrhagic shock. Asian J Transfus Sci. 2015;9(1):55–60. doi: 10.4103/0973-6247.150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8(4):223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ñamendys-Silva S.A., Santos-Martínez L.E., Pulido T. Pulmonary hypertension due to acute respiratory distress syndrome. Braz J Med Biol Res. 2014;47(10):904–910. doi: 10.1590/1414-431X20143316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossaint R., Falke K.J., López F. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths M.J., Evans T.W. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 38.Sarma J.V., Ward P.A. Oxidants and redox signaling in acute lung injury. Compr Physiol. 2011;3:1365–1381. doi: 10.1002/cphy.c100068. [DOI] [PubMed] [Google Scholar]
- 39.Baetz D., Shaw J., Kirshenbaum L.A. Nuclear factor-kappaB decoys suppress endotoxin-induced lung injury. Mol Pharmacol. 2005;4:977–979. doi: 10.1124/mol.105.011296. [DOI] [PubMed] [Google Scholar]
- 40.Oronsky B., Scribner C., Aggarwal R., Cabrales P. RRx-001 protects normal tissues but not tumors via Nrf2 induction and Bcl-2 inhibition. J Cancer Res Clin Oncol. 2019 Aug;145(8):2045–2050. doi: 10.1007/s00432-019-02958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.https://www.paho.org/journal/sites/default/files/2020-03/40-20-249-Rosa-prelim.pdf?ua=1
- 42.Henao-Restrepo A.M., Camacho A., Longini I.M. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]