Abstract
Introduction
As has happened in other emerging respiratory pandemics, demand for N95 filtering facemask respirators (FFRs) has far exceeded their manufacturing production and availability in the context of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. One of the proposed strategies for mitigating the massive demand for N95 FFRs is their reuse after a process of decontamination that allows the inactivation of any potentially infectious material on their surfaces. This article aims to summarize all of the available evidence on the different decontamination methods that might allow disposable N95 FFRs to be reused, with emphasis on decontamination from SARS-CoV-2.
Methods
We performed a systematic review of the literature in order to identify studies reporting outcomes of at least 1 decontamination method for inactivating or removing any potentially infectious material from the surface of N95 FFRs, specifically addressing issues related to reduction of the microbial threat (including SARS-CoV-2 when available), maintaining the function of N95 FFRs and a lack of residual toxicity.
Results
We identified a total of 15 studies reporting on the different decontamination methods that might allow disposable N95 FFRs to be reused, including small-scale energetic methods and disinfecting solutions/spray/wipes. Among these decontamination methods, ultraviolet germicidal irradiation and vaporized hydrogen peroxide seem to be the most promising decontamination methods for N95 FFRs, based on their biocidal efficacy, filtration performance, fitting characteristics, and residual chemical toxicity, as well as other practical aspects such as the equipment required for their implementation and the maximum number of decontamination cycles.
Conclusions
Although all the methods for the decontamination and reuse of N95 FFRs have advantages and disadvantages, ultraviolet germicidal irradiation and vaporized hydrogen peroxide seem to be the most promising methods.
Key Words: Equipment reuse, Microbial viability, Virus inactivation, Disinfection, Respiratory protective devices
Introduction
An outbreak starting in December 2019 caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), declared a global pandemic by the World Health Organization on March 11, 2020, has posed a severe threat to public health and local economies around the globe.1 As the pandemic accelerates, the increased risk of infection of health care workers due to a rise in their demand makes their safety, including an adequate provision of personal protective equipment (PPE), a cause of great concern.2 Recent guidelines proposed by the Infectious Diseases Society of America recommend the use of a reprocessed N95 respirator for reuse during respirator shortage based on laboratory evidence, due to lack of clinical experience with the decontamination process.3 Among the various PPEs, disposable N95 filtering facemask respirators (FFRs) are of critical importance for confronting the SARS-CoV-2 pandemic because of their tight fit and their filtration capability of at least 95% of airborne particles, including large and small particles.4 However, as has happened in other emerging respiratory pandemics, demand for N95 FFRs has far exceeded their manufacturing production and availability. This is, among other factors, due to the minimum number of N95 FFRs required for each health care worker involved in direct patient contact, assuming adequate care is taken. For a recent respiratory pandemic outbreak of influenza, there were estimations of requirements of as many as 360 million FFRs, in scenarios assuming a pandemic duration of 24 weeks.5
Ideally, disposable N95 FFRs should be discarded after each patient encounter and after aerosol-generating procedures (considering that they are potentially fomites because they remove pathogenic microorganisms from aerosols generated by infected individuals), when they become damaged or deformed, when they no longer form an effective seal to the face, when they become wet or visibly dirty, when breathing becomes difficult, as well as when they become contaminated with blood, respiratory or nasal secretions, or other bodily fluids.6 Some of these recommendations are supported by evidence showing the probability of viral contamination and the viability of respiratory viruses on N95 FFRs and other inanimate surfaces for variable periods, the ability of the influenza virus to persist for 6 days on the outer side of the N95 FFRs having been demonstrated.7 , 8 Specifically for SARS-CoV-2, although it is more stable on plastic and stainless steel than on copper and cardboard, viable virus can be detected for up to 72 hours.9
One of the proposed strategies for mitigating the massive demand for N95 FFRs not met by manufacturing supply that typically occurs during a respiratory pandemic that helps to ensure their continued availability in health care environments is their reuse after a process of decontamination that allows the inactivation of any potentially infectious material on their surfaces.10 Although various decontamination methods have been used, there are concerns over certain characteristics of the N95 FFRs with respect to their utilization, such as alterations in their physical appearance/odor, structural integrity, filtration efficiency, fit and seal and filter airflow resistance, degradation of their material, and chemical residues that are potentially toxic or irritate the skin (due to the chemical disinfectants required for rinsing and drying). The requirement for specialized equipment for using the decontamination methods must also be considered, as well as their speed and ease of use, cost, and the maximum allowed number of decontamination cycles.6 However, to the best of our knowledge, no systematic review has summarized the findings of studies that have assessed the above-mentioned advantages and disadvantages of all available decontamination methods for N95 FFRs.
The present article aims to summarize all of the available evidence on the different decontamination methods that might allow disposable N95 FFRs to be reused, with emphasis on decontamination from SARS-CoV-2.
Methods
Search strategy and selection of included studies
Potentially relevant studies were identified thorough a search of the literature in the electronic databases MEDLINE, EMBASE, CINAHL, and SCOPUS up to July, 2020, using the terms ("Decontamination"(Mesh) OR "Equipment Reuse"(Mesh) OR "Microbial Viability"(Mesh) OR "Virus Inactivation"(Mesh) OR "Disinfection"(Mesh) OR "Respiratory Tract Infections/prevention and control"(Mesh)) AND ("Respiratory Protective Devices"(Mesh) OR "N95 respirator" OR "filtering facepiece respirator"). Two review authors (C.R.M. and M.P.S.) scanned the abstracts and titles of articles retrieved by the electronic databases according to the eligibility criteria, retrieving full copies of all those deemed potentially eligible for closer examination. Disagreement was resolved by consensus. The electronic database searches were supplemented by information obtained from the references of the identified studies. We included citations in any language.
Inclusion and exclusion criteria
To be included, the studies had to meet the following criteria: studies reporting outcomes of at least 1 decontamination method for inactivating or removing any potentially infectious material from the surface of N95 FFRs, including filtration performance, structural integrity, and potentially toxic or chemical residues postdecontamination. Studies that reported on the efficacy of decontamination methods on elastomeric respirators were excluded. Likewise, studies in which it was not possible to extract outcomes of interest separately for the different decontamination methods, or studies published solely in abstract form, were not eligible for inclusion in the review.
Data extraction
Two reviewers (C.E.R.M. and J.A.C.L.) independently used a data extraction sheet designed a priori to obtain the specific data required for this review. From the included studies, we extracted descriptive data (first author, year), type of decontamination method(s) used, and details of their implementation, such as the equipment required, speed and ease of use, cost, and the maximum allowed number of decontamination cycles. Likewise, we extracted data on outcomes of relevance, including alterations in the physical appearance/odor, structural integrity, filtration efficiency, fit and seal, and filter airflow resistance of the N95 FFRs, as well as chemical residues that are potentially toxic or that irritate the skin upon use.
Results
Results of the search
Figure 1 shows the selection process of the studies. The systematic search of databases retrieved 244 studies. Among those, we excluded 237 studies. The most common reason for excluding studies was that they did not meet the eligibility criteria. Eight additional studies that met the inclusion criteria were identified from the references of eligible articles. In the end, a total of 15 studies reporting on the different decontamination methods that might allow disposable N95 FFRs to be reused were included in the review and presented by type of decontamination method.
Fig 1.
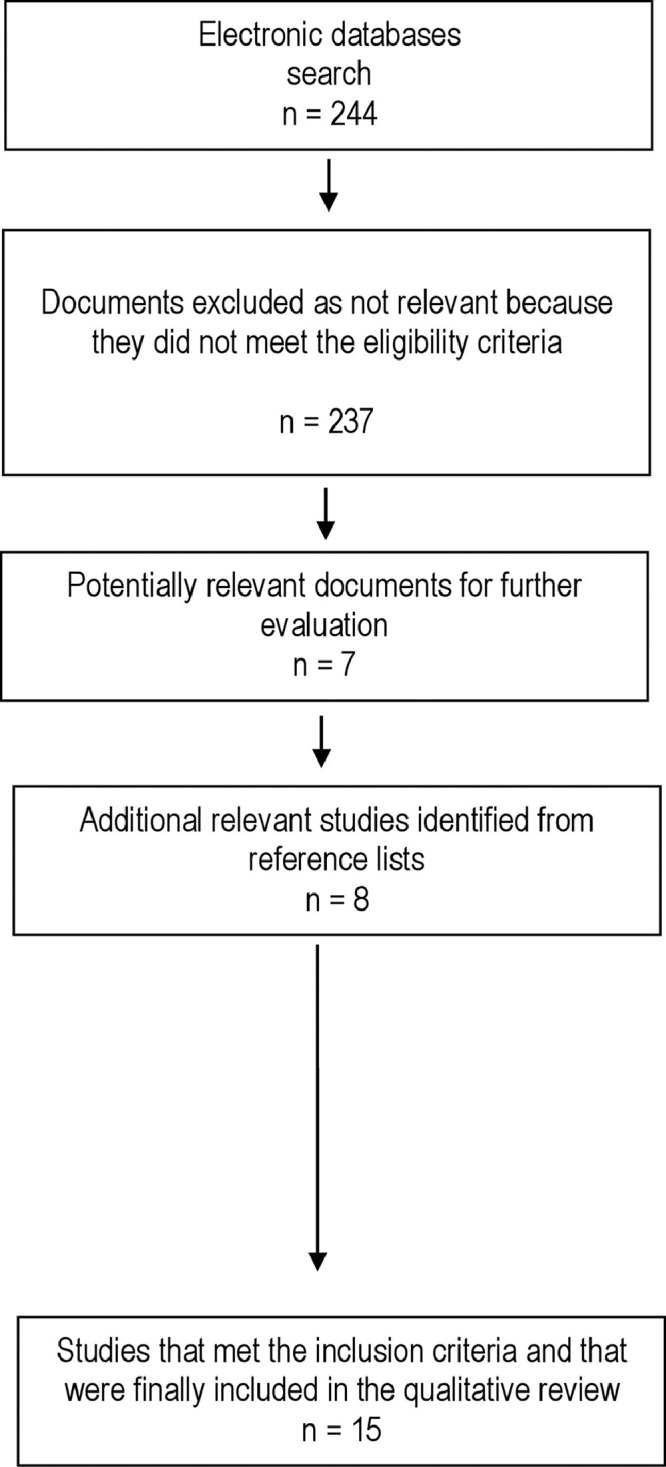
Flowchart of selection of studies for inclusion in the systematic review.
Ultraviolet germicidal irradiation
Viscusi et al evaluated the filtration performance of an N95 FFR after ultraviolet germicidal irradiation (UVGI) decontamination (0.24 mW/cm2) under 2 different conditions, using a poly-dispersed sodium chloride aerosol test method. The N95 FFR was placed on the working surface of a laminar flow hood, Sterilgard III, (The Baker Company, Sanford, ME), fitted with a 40 W ultraviolet light for general decontamination. The intensity was reported as the average obtained at 9 positions over the area used, with a UVX Digital Radiometer with MODEL UVX-25 sensor (254 nm filter; VWR Lab Shop, Batavia, IL). For both treatments, samples were turned over after 50% exposure to allow treatment of the inside as well as the outside of the respirator. No significant visible changes were observed for any samples after either treatment. The average penetration results for N95 respirators were not significantly affected by either treatment (mean and standard deviation % penetration: 0.7 ± 0.267 vs 0.57 ± 0.152 vs 0.79 ± 0.082, for as-received and postdecontamination under 2 different conditions).11
Lore et al examined the effectiveness of UVGI (using viral cultures and a quantitative molecular amplification assay), as well as the filtration performance of 2 models of N95 FFRs. An aerosol settling chamber was used to apply virus-laden droplets (influenza A/H5N1) to FFRs in a method designed to simulate respiratory deposition of droplets onto surfaces. The decontamination procedure was as follows: a 126- (L) %15.2- (W) % 10.8-cm (H), dual-bulb, 15-W UV-C (254-nm wavelength) lamp (Ultraviolet Products, Upland, CA) was placed in a Labgard class II, type A2, laminar flow cabinet (NuAire, Inc., Plymouth, MN) set to a height of 25 cm above the cabinet's working surface. As measured by a UVX digital radiometer (UVP Inc., Upland, CA), the lamp's UV-C wavelength irradiance ranged between 1.6 mW cm2 and 2.2 mW cm2. Virus-laden respirators were placed inside the cabinet directly under the ultraviolet lamp, with the convex panel facing the treatment, and were exposed for a total of 15 minutes at a UV-C wavelength dose of 18 kJ m2. After the decontamination procedure, the reduction in viral recovery (expressed as log10 TCID50/mL reduction) was ≥4.54 and ≥4.65 for the FFRs models, respectively, and no viable virus was detectable, with the number of amplification cycles to detect vRNA of 2.97 and 5.60 for the FFRs models, respectively. Additionally, the postdecontamination filter performance analyses showed that the mean penetration of 1% NaCl aerosol at 300-nm particle size was 0.99 and 0.37 for the FFR models, respectively.12
Viscusi et al placed N95 FFRs on the working surface of a Sterilgard III laminar flow cabinet (The Baker Company, Sanford, ME) fitted with a 40-WUV-C light (average UV intensity experimentally measured to range from 0.18 to 0.20 mW cm2), 15-minute exposure to each side (outer and inner), 176-181 mJ cm-2 exposure to each side of the FFR, for a total time of 30 minutes. All laboratory experiments were conducted under standard laboratory conditions (21°C ± 2°C and relative humidity of 50%-10%) on triplicate sets of FFRs. Ultraviolet germicidal irradiation treatment did not affect the filter aerosol penetration (pre- and postdecontamination average percentage of sodium chloride penetration of the 3 surgical N95 FFRs evaluated: 1.57 ± 0.83 vs 1.86 ± 0.97, 0.335 ± 0.19 vs 0.371 ± 0.21, and 0.716 ± 0.37 vs 0.720 ± 0.37) or physical appearance of the FFRs. There were statistically significant, although not clinically significant, differences between pre- and postdecontamination measures of filter airflow resistance in 2 of the 3 surgical N95 FFRs evaluated (pre- and postaverage decontamination resistance in mmH2O: 8.4 ± 0.50 vs 9.2 ± 0.44, 6.1 ± 0.15 vs 7.1 ± 0.61, and 6.7 ± 0.17 vs 6.6 ± 0.26). No known health risks to the user were identified.13 In addition to these results, Viscusi et al also showed that N95 FFR users would be unlikely to experience a clinically meaningful reduction in fit, increase in odor, increase in discomfort, or increase in difficulty in donning after UVGI decontamination.14 Lindsley et al exposed both sides of material coupons and respirator straps from 4 models of N95 FFRs to UVGI doses from 120 to 950 J/cm2 and evaluated the particle penetration, flow resistance, bursting strengths of the individual respirator coupon layers, and breaking strength of the respirator straps. N95 FFRs were exposed to ultraviolet light with a primary wavelength of 254 nm (UV-C) in a custom-made 91 cm × 31 cm × 64 cm high chamber. The chamber was fitted with 2 15-watt T-150 254 nm UV-C lamps in a reflective housing and lined with black felt to minimize reflections. UV-C irradiance was measured using a radiometer (ILT-1700, International Light Technologies, Peabody, MA). UVGI exposure led to only a small increase in particle penetration (up to 1.25%) and had little effect on the flow resistance. UVGI exposure had a more pronounced effect on the strengths of the respirator materials. At the higher UVGI doses, the strength of the layers of respirator material was substantially reduced (in some cases, by >90%). The changes in the strengths of the respirator materials varied considerably among the different models of respirators. UVGI had less of an effect on the respirator straps; a dose of 2,360 J/cm2 reduced the breaking strength of the straps by 20%-51%.15
Bergman et al evaluated changes in physical appearance, odor, and laboratory performance (filter aerosol penetration and filter airflow resistance) of 6 N95 FFRs models after 3 cycles of decontamination with UVGI. The experimental conditions and parameters were as follows: UV bench lamp (UV-C, 254 nm, 40 W), Model XX-40S (UVP, LLC, Upland, CA). Forty-five-minute exposure at intensity 1.8 mW/cm2 (note: one 45-minute continuous exposure constitutes the 3× cycle). Test tube racks were placed beneath both ends of the lamp to lift the lamp ∼25 cm from the working surface of the laboratory hood. The UV intensity was reported as the mean of 27 measurements over the rectangular area used at the surface of the hood, using a UVX digital radiometer with a model UVX-25 sensor (254 nm filter; UVP, LLC, Upland, CA). Only the exteriors of the FFRs were exposed. The duckbill and flat-fold style FFRs were placed over beakers to facilitate exposure to the FFR surface. A Model 8130 automated filter tester (AFT; TSI, Inc., St Paul, MN) was used to measure initial percent filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.34% to 1.59%) and filter airflow resistance (mean values ranging from 7.9 to 17.6 mmH2O). UVGI decontamination did not cause any observable physical change in the FFRs.16
Mills et al evaluated the UVGI decontamination efficiency of 15 samples of influenza-contaminated N95 FFR models (facepiece and strap) and then covered them with a soiling agent-artificial saliva or artificial skin oil. Contaminated FFRs were treated for 60-70 seconds at an irradiance of approximately 17 mW/cm2, resulting in a dose of 1 J/cm2 UVGI. The custom UVGI device was made of polished aluminum (Alloy 6061-T6 and Alloy 2024-T3). Eight 32-in 254-nm UV-C bulbs with an irradiance of 0.39 W/cm2 at 1 m (Fresh-Aire UV; Jupiter, FL) were incorporated into the device to deliver a UV dose of 1 J/cm2 in approximately 1 minute. The titers of viable influenza viruses were determined by a 50% tissue culture infectious dose (TCID50/mL) assay. Significant reductions (≥3 log) in influenza viability for both soiling conditions were observed on facepieces from 12 of 15 FFR models (with a mean log reduction ranging from 1.42 to 4.84 log TCID50/mL for mucin-soiled facepieces, and from 1.25 to 4.64 log TCID50/mL for sebum-soiled facepieces) and straps from 7 of the 15 FFR models (with a mean log reduction ranging from 0.00 to 4.31 log TCID50/mL for mucin-soiled straps, and from 0.08 to 4.40 log TCID50/mL for sebum-soiled straps).17
Heimbuch et al evaluated the decontaminant efficacy and the durability and functionality of fifteen N95 FFR models after multiple cycles of UVGI. Using a Mineralight XX-20S 20-W UV bench lamp, 4 UV doses were evaluated: 1 × 103, 5 × 105, 1 × 106, and 2 × 106 µJ/cm2. For each test, FFRs were inoculated in a Class II biological safety cabinet (BSC) with ten 1-µL droplets of ∼109 TCID50/mL H1N1 influenza onto each of the 4 surfaces selected for inoculation. After the droplets had dried, a soiling agent (synthetic skin oil or artificial saliva buffer) was applied over each inoculated surface to act as a protective factor. The test results were reported as the reduction of the virus titer due to treatment with UV, expressed as log10. The TCID50/mL was determined using the Spearman-Karber method. UVGI performance varied considerably for all 15 FFR models tested, with log reductions ranging from 0.00 to 4.85 log10 TCID50/mL, based on inoculation location, soiling agent, and control recovery. For all 3 soiling conditions, a direct relationship was demonstrated between UV dosage and influenza decontamination, with no viable virus detected after UV treatment ≥1 J/cm2. Additionally, it was demonstrated that up to 20 cycles of UVGI treatment (approximately 1 J/cm2 per cycle) do not exert a meaningfully significant effect on fit, airflow resistance, or particle penetration for the 15 FFR models tested. Strap tension data indicated that 10 UVGI cycles do not have a significant effect on FFR straps, but 20 UVGI cycles may have a significant effect on straps from some N95 FFR models (3M 1860, 3M 1870, and Kimberly-Clark PFR models).10
Lin et al determined the relative survival of Bacillus subtilis spores loaded onto N95 FFRs after UVGI (UVA 365 nm, UVC 254 nm) decontamination under a worst-case temperature (37°C, similar to body temperature) and humidity (95% relative humidity, the maximum feasible relative humidity value) that prevails when an FFR is placed in a zipper bag in a health care worker's pocket. The treatment proceeded as follows: an N95 FFR was placed 10 cm below a 6 W handheld UV lamp (model UVGL-58, VUP LLC, Upland, CA) that emitted a wavelength of 254 nm (UVC, 18.9 mW/cm2) or 365 nm (UVA, 31. 2 mW/cm2). Both sides of each N95 FFR were exposed for different lengths of time: 1, 2, 5, 10, and 20 minutes, in a BSC. The UV intensity was measured using a handheld laser power and energy meter (OPHIR NOVA II, model Nova II PD300-UV) and was reported as a mean of 5 measurements over a 10 × 10 mm aperture with a swivel mount and a removable filter. Colony-forming units (CFUs) were counted, and their relative survival was calculated. Without decontamination, 59%± 8% of the loaded spores survived for 24 hours. No colony was recovered after exposure to UV-C for as little as 5 minutes. However, RS remained above 20% after 20 minutes of irradiation by UV-A, exponentially decaying with increased exposure time.18
Fischer et al analyzed the ability of UV radiation to inactivate SARS-CoV-2 on N95 FFRs (50 µL of 105 TCID50/mL of SARS-CoV was applied on N95 and stainless steel) and used quantitative fit testings to measure their filtration performance after each decontamination run and 2 hours of wear, for 3 consecutive decontamination and wear sessions. Plates with fabric and steel discs were placed under an LED high-power UV germicidal lamp (effective UV wavelength 260-285 nm) without the titanium mesh plate (LEDi2, Houston, Tx) 50 cm from the UV source. At 50 cm, the UVAB power was measured at 5 µW/cm2 using a General UVAB digital light meter (General Tools and Instruments New York, NY). The plates were removed at 10, 30, and 60 minutes, and 1 mL of cell culture medium added. N95 FFRs integrity was quantitatively determined using the fit factor, a measure of filtration performance: the ratio of the concentration of particles outside the mask to the concentration inside, requiring a minimum fit factor of 100 for a mask to pass a fit test. UV inactivated SARS-CoV-2 rapidly from steel (decay rates of viable virus titers over time and half-lives; median, interquartile range: 0.733, 0.649-0.802 minutes), but more slowly on N95 fabric (decay rates of viable virus titers over time and half-lives; median, interquartile range: 6.26, 5.31-7.15 minutes). The UV-treated masks retained filtration performance comparable to the control group after 2 cycles of decontamination and maintained acceptable performance after 3 cycles.19
Liao et al placed 3 models of N95 FFRs into a UV sterilizer cabinet (CHS-208A), with a 254 nm, 8 W lamp, and 475 cm2 internal area. Samples were irradiated for 30 minutes and left to stand under ambient conditions for 10 minutes per cycle. Samples were either returned to the chamber for the next cycle or tested. UV-treated FFRs were able to withstand 10 cycles of treatment (with filtration efficiency of 80.65% ± 2.97% and a pressure drop of 8.3Pa ± 1.2), but efficiency eventually decayed to 93% at 20 cycles, making it unsuitable for N95-grade FFRs by itself.20
Ethylene oxide
Viscusi et al evaluated the filtration performance of an N95 FFR after ethylene oxide (EtO) decontamination under 2 different conditions, using a poly-dispersed sodium chloride aerosol test method. The 2 treatment conditions (EtO 3M Steri-Vac 4XL [3M, St. Paul, MN] sterilizer processed in the warm cycle of 55°C and 883 mg/L ethylene oxide gas and EtO 3M Steri-Vac 5XL [3M, St. Paul, MN] sterilizer processed in the warm cycle of 55°C and 725 mg/L ethylene oxide gas) are different model instruments using the same process but differing in some aspects (eg, gas concentration, chamber volume, and duration). Respirators tested using these treatments were shipped to and from a commercial facility specializing in low-temperature sterilization methods. Four FFR samples were placed in standard poly/paper pouches and treated with EtO. All respirator samples were exposed to EtO for 1 hour, followed by a 4-hour aeration interval. The respirators were shipped back to the investigators and subsequently tested in house for filtration efficiency within 72 hours of receipt. After EtO decontamination, the average penetration slightly increased for N95 FFRs, though not beyond their respective certification criteria (mean and standard deviation % penetration: 0.7 ± 0.267 vs 0.729 ± 0.136 vs 0.35 ± 0.019, for as-received, EtO 3M 5XL, and EtO 3M 4XL postdecontamination, respectively). EtO 3M 5XL was found to be slightly less degraded than EtO 3M 4XL.11
Viscusi et al used a Steri-Vac 5XL sterilizer (3M, St Paul, MN) in a single warm cycle (55°C and 725 mg l-1 100%EtO gas). FFRs and a chemical indicator were placed in an individual standard poly/paper pouch. EtO exposure for 1 hour followed by 4 hours of aeration, for a total time of 5 hours. FFRs were shipped to and from a commercial facility specializing in low-temperature sterilization methods and were tested within 72 hours of receipt. EtO decontamination did not affect the filter aerosol penetration (pre- and postdecontamination average percentage of sodium chloride penetration of the 3 surgical N95 FFRs evaluated: 1.57± 0.83 vs 0.90 ± 0.49, 0.335± 0.19 vs 0.498 ± 0.32, and 0.716± 0.37 vs 0.687 ± 0.35), filter airflow resistance (pre- and postdecontamination average resistance in mmH2O of the 3 surgical N95 FFRs evaluated: 8.4± 0.50 vs 8.1 ± 0.32, 6.1 ± 0.15 vs 6.7 ± 0.40, and 6.7± 0.17 vs 6.3 ± 0.25), or physical appearance of the FFRs in this study.13
Bergman et al evaluated changes in the physical appearance, odor, and laboratory performance of six N95 FFR models after 3 cycles of decontamination with EtO. The experimental conditions and parameters were as follows: Amsco Eagle 3017 100% EtO Sterilizer/Aerator (STERIS Corp., Mentor, OH) on HI-TEMP setting (55°C); 1-hour EtO exposure (736.4 mg/L) followed by 12-hours aeration. Samples were packaged in Steris Vis-U-All Low-Temperature Tyvek/polypropylene-polyethylene heat-seal sterilization pouches (6 samples per pouch with a chemical indicator strip). All samples were physically accommodated by a single EtO cycle. Samples were processed at a university medical center (1 treatment per day for 3 consecutive days). The same pouch was used for all 3 treatments. A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure the initial percent filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following the decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.25% to 2.55%) and filter airflow resistance (mean values ranging from 8.0 to 16.9 mmH2O). EtO decontamination did not cause any observable physical change in the FFRs.16
Vaporized hydrogen peroxide
Viscusi et al evaluated the filtration performance of an N95 FFR after vaporized hydrogen peroxide (VHP) decontamination under 2 different conditions using a poly-dispersed sodium chloride aerosol test method. The 2 treatment conditions (STERRAD NX Standard cycle and STERRAD 100S [Advanced Sterilization Products, Irvine, CA] Standard cycle) were different model instruments using the same process (hydrogen peroxide gas plasma [HPGP]), differing only in duration and capacity. Respirators tested using these treatments were shipped to and from a commercial facility specializing in low-temperature sterilization methods and were tested in-house for filtration efficiency within 72 hours of receipt from the commercial facility. The STERRAD sterilization process is less effective when used on cellulose-based products; hence the use of Tyvek/Mylar pouches was required. Since there were no inherent hazardous residues as a result of the STERRAD process, no aeration interval was necessary. Aluminum nosebands were slightly tarnished and visibly not as shiny when compared with their as-received counterparts after both STERRAD treatments. For both treatments, the average penetration of the 95 FFR model did not significantly increase and remained below certification limits. STERRAD NX was found to be slightly less degraded than STERRAD 100S, but the difference was not statistically significant (mean and standard deviation % penetration: 0.7± 0.267 vs 0.213 ± 0.094 vs 0.166 ± 0.339, for as-received, STERRAD NX, and STERRAD 100S postdecontamination, respectively).11
Viscusi et al used a STERRAD 100S H2O2 gas plasma sterilizer (Advanced Sterilization Products, Irvine, CA), single 55-minute standard cycle. FFRs and a chemical indicator were placed in an individual Mylar/Tyvek self-seal pouch. FFRs were shipped to and from a commercial facility specializing in low-temperature sterilization methods and were tested within 72 hours of receipt. VHP decontamination for a single warm cycle did not significantly affect FFR filter aerosol penetration (pre- and postdecontamination average percentage of sodium chloride penetration of the 3 surgical N95 FFRs evaluated: 1.57± 0.83 vs 0.71 ± 0.50, 0.335± 0.19 vs 0.542 ± 0.32, and 0.716± 0.37 vs 0.727 ± 0.37) or filter airflow resistance (pre- and postdecontamination average resistance in mmH2O of the 3 surgical N95 FFRs evaluated: 8.4± 0.50 vs 8.6 ± 1.04, 6.1± 15 vs 7.1 ± 1.28, and 6.7± 0.17 vs 6.5 ± 0.29). The only visible physical effect on the FFRs was a slight tarnishing of the metallic nosebands.13
A more recent report prepared by Battelle Memorial Institute evaluated the efficacy of VHP decontamination of N95 FFRs and characterized the impact of VHP exposure on the mechanical integrity and performance of the FFRs. Using the Bioquell Clarus C VHP generator, the parameters of the VHP decontamination cycle to ensure a 6-log reduction in organism viability (ie, complete inactivation of Geobacillus stearothermophilus spores inoculated to the surfaces of N95 FFRs) were: a 10-minute conditioning phase, a 20-minute gassing phase at 2 g/min, a 150-minute dwell phase at 0.5 g/min, and a 300-minute aeration phase such that no “off-gassing” of hydrogen peroxide from the FFR was detected, that is a total cycle duration of 480 minutes (8 hours). Performance tests included inert aerosol collection efficiency, biological aerosol collection efficiency, inhalation resistance, and respirator fit on a manikin head form. The aerosol collection efficiency (with a filtration efficiency above 99% for both the inert and biological aerosol tests) and the airflow resistance (with a mean inhalation resistance 9 ± 0.4 mmH2O) were not affected over 50 cycles of VHP exposure. Complete inactivation was demonstrated following 50 repeat aerosol inoculation/decontamination cycles. No visible degradation was observed after exposure to 10 or 20 HPV cycles. However, after 30 HPV cycles, it was observed that that elastic material in the straps fragmented when stretched.21
Kenney et al evaluated the virucidal activity of VHP using a BQ-50 system (Bioquell, Horsham, PA) after inoculating 3M 1870 N95 FFRs with 3 aerosolized bacteriophages (T1, T7, and Pseudomonas phage phi-6) that are a reasonable proxy for SARS-CoV-2. Concentrations were selected to approximate viral titers necessary for TCID50/mL of SARS-CoV-2. N95 FFRs were suspended by their elastic on racks in a 33 m3 room and sterilized with BQ-50 using a 10-minute conditioning phase, 30-40 minute gassing phase (varies with humidity and room size) at 16 g/min, 25-minute dwell phase, and 150-minute aeration phase (varies with the number of respirators and room size), with this long duration intended to reduce HP vapors. The virucidal activity was measured by a standard plaquing assay on a lawn of host bacteria before and after sterilization. A single VHP cycle resulted in complete eradication of phage from masks (limit of detection 10 PFU, lower than the infectious dose of the majority of respiratory viral pathogens). After 5 VHP cycles, the respirators appeared to be similar to new ones, with no deformity.22
Bergman et al evaluated changes in the physical appearance, odor, and laboratory performance of six N95 FFR models after 3 cycles of decontamination with HPGP. The experimental conditions and parameters were as follows: STERRAD 100S H2O2 Gas Plasma Sterilizer (Advanced Sterilization Products, Irvine, CA), 59% H2O2, cycle time ∼55 minutes (short cycle); 45°C-50°C. Samples were packaged in Steris Vis-U-All Low Temperature Tyvek/polypropylene–polyethylene heat seal sterilization pouches (6 samples per pouch with a chemical indicator strip). Samples were processed at a university medical center (1 treatment per day for 3 consecutive days). The same pouch was used for all 3 treatments. A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure the initial percent of filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, HPGP treatment resulted in mean penetration levels >5% for 4 of the 6 FFR models (mean values ranging from 1.71% to 8.76%). N95 FFRs treated by means of HPGP had the expected levels of filter airflow resistance (mean values ranging from 7.7 to 14.4 mmH2O). HPGP decontamination did not cause any observable physical change in the FFRs.16
Fischer et al analyzed the ability of VHP to inactivate SARS-CoV-2 on N95 FFRs (50 µL of 105 TCID50/mL of SARS CoV was applied on N95 and stainless steel) and used quantitative fit testings to measure their filtration performance after each decontamination run and 2 hours of wear, for 3 consecutive decontamination and wear sessions. Plates with fabric and steel discs were placed into a Panasonic MCO-19AIC-PT (PHC Corp. of North America Wood Dale, IL) incubator with VHP generation capabilities and exposed to hydrogen peroxide (approximately 1,000 ppm). The exposure to VHP was 10 minutes; after the inactivation of the hydrogen peroxide, the plate was removed and 1 mL of cell culture medium was added. N95 FFRs integrity was quantitatively determined using the fit factor, a measure of filtration performance: the ratio of the concentration of particles outside the mask to the concentration inside, requiring a minimum fit factor of 100 for a mask to pass the fit test. VHP yielded extremely rapid inactivation, both on N95 (decay rates of viable virus titers over time and half-lives; median, interquartile range: 0.78, 0.685-0.858 minutes) and on stainless steel (decay rates of viable virus titers over time and half-lives; median, interquartile range: 0.765, 0.669-0.843 minutes). The VHP-treated masks retained filtration performance comparable to the control group after 2 rounds of decontamination and maintained acceptable performance after 3 rounds.19
Microwave oven use
Lore et al examined the effectiveness of microwave-generated steam (MGS), as well as the filtration performance of 2 models of N95 FFRs. An aerosol settling chamber was used to apply virus-laden droplets (influenza A/H5N1) to FFRs in a method designed to simulate respiratory deposition of droplets onto surfaces. The decontamination procedure was as follows: a 1,250-W (2,450 MHz) commercially available microwave oven (Panasonic Corp., Secaucus, NJ) with a rotating glass plate was used to irradiate a single respirator per treatment. Samples were placed above a plastic box filled with 50 mL of room-temperature tap water. The top of the box was perforated with 96 holes (7-mm diameter) evenly distributed over the entire surface in order to allow MGS to vent through the respirator. The virus-contaminated respirator was placed with the convex surface pointed toward the steam source, and the FFR was then irradiated for 2 minutes at full power. After the decontamination procedure, the reduction in viral recovery (expressed as log10 TCID50/mL reduction) was ≥4.81 and ≥4.79 for the FFRs models, respectively, and no viable virus was detectable, with a number of amplification cycles to detect vRNA of 5.87 and 4.01 for the FFR models, respectively. Additionally, the postdecontamination filter performance analyses showed that the mean penetration of 1% NaCl aerosol at 300-nm particle size was 1.51 and 0.99 for the FFR models, respectively.12
Viscusi et al evaluated the filtration performance of an N95 FFR after microwave decontamination under 2 different conditions, using a poly-dispersed sodium chloride aerosol test method. Exposures were carried out in a standard, commercially available 2,450-MHz microwave oven, Sharp Model R-305KS (Sharp Electronics, Mahwah, NJ) with a revolving glass carousel. Although rated at 1,100 W on 100% full-power setting, an average power measurement of 750 W/ft3 from 4 evaluations at various evenly spaced, representative locations in the oven, using the power determination method recommended by the manufacturer, was obtained. In both treatments (2 and 4 minutes), the samples were irradiated for half the time, promptly turned over, and irradiation was repeated for the remainder of the allotted time. For treatments of a duration of 2 minutes, no visible changes were observed for any sample. The average penetration increased slightly for N95 FFRs. After 4 minutes of microwave exposure, the N95 filter media melted at the ends of the aluminum nosebands and formed visible holes. N95 filter penetration significantly increased (mean and standard deviation % penetration: 0.7± 0. 267 vs 0.662 ± 0.148 vs 0.617 ± 0.022, for as-received, 2 minutes, and 4 minutes postdecontamination, respectively).11
Viscusi et al used a commercially available 2450 MHz, Sharp Model R-305KS (Sharp Electronics, Mahwah, NJ) microwave oven with revolving glass carousel, 1,100 W (manufacturer rated); 750 Wft-3 experimentally measured; 2-minute total exposure (1 minute each side of FFR). A paper towel was placed on the revolving glass plate for insulation to protect the FFRs from melting onto the glass plate. Filter aerosol penetration (pre- and postdecontamination average percentage of sodium chloride penetration: 1.57 ± 0.83 vs 0.711 ± 0.44, and 0.716± 0.37 vs 0.652 ± 0.33) and filter airflow resistance (pre- and postdecontamination average resistance in mmH2O: 8.4± 0.50 vs 8.7 ± 0.64, and 6.7± 0.17 vs 5.4 ± 0.72) were not affected for 2 of the 3 surgical N95 FFRs evaluated. The material component melted on the remaining FFR. No known health risks to the user were identified.13 Viscusi et al also showed that N95 FFR users would be unlikely to experience a clinically meaningful reduction in fit, increase in odor, increase in discomfort, or increased difficulty in donning after MGS decontamination.14
Bergman et al evaluated changes in physical appearance, odor, and laboratory performance of six N95 FFRs models after 3 cycles of decontamination with MGS. The experimental conditions and parameters were as follows: commercially available 2,450-MHz, Sharp Model R-305KS (Sharp Electronics, Mahwah, NJ) microwave oven with revolving glass carousel, 1,100 W (manufacturer rated); 750 W/ft3 experimentally measured; 2-minute total exposure duration at a power setting of 10 (maximum power). Two pipette tip boxes placed side-by-side (each 11.7 cm x 8.0 cm x 5.0 cm) filled with 50 mL room-temperature tap water (∼20°C). An FFR was placed outer-side down on top of pipette-tip boxes. FFR samples were dried for 1 hour between each exposure. A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure initial percent filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.08% to 2.14%) and filter airflow resistance (mean values ranging from 8.8 to 14.4 mmH2O). MGS decontamination caused a partial separation of the inner foam nose cushion and a slight melting of the head straps from some FFR samples.16
Fisher et al evaluated the use of 2 commercially-available microwave steam bags marketed to the public for disinfecting infant feeding equipment (Medela Quick Clean MICRO-STEAM BAGS (Medela, McHenry, IL) and the Munchkin Steam Guard Bags (Munchkin Inc., North Hills, CA) for N95 FFR decontamination. Following contamination with experimentally inoculated bacteriophage MS2 droplets as a surrogate for a pathogenic virus and up to 3 cycles of steam bag sterilization, which included a 30-minute drying period between treatments, FFRs were sequentially evaluated for filtration performance, water absorbency/retention, and decontamination efficacy. Following the manufacturer's instructions, the bags were sealed using the bag's integrated zipper-lock seal and placed in a commercially-available Sharp Model R-305KS (2,450 MHz, 1,100 W) microwave oven (Sharp Electronics, Mahwah, NJ). The FFRs in the sealed steam bags were irradiated at high power for 90 seconds, the prescribed time for a microwave with a rating of 1,100 W. Water absorption of the FFRs was found to be model-specific, since FFRs constructed with hydrophilic materials absorbed more water. The steam had little effect on FFR performance, since the filtration efficiency of the treated FFRs remained above 95% (values ranging from 95.5% to 99.0%). The tested steam bags were found to be 99.9% (values ranging from 99.86% to 99.99%) effective for inactivating MS2 on FFRs.23
Steam
Liao et al stacked 3 models of N95 FFRs on top of a beaker with boiling water inside (at around 15 cm above the water). The samples were left on top of the beaker and steamed for 10 minutes, and afterward they were left to air-dry completely (to touch). Samples were either tested or placed back on top of the beaker to continue the next treatment cycle. Filtration efficiency and pressure drop after 10 cycles of treatment were 80.65% ± 2.97% and 8.3Pa ± 1.2, respectively.20
Bleach
Viscusi et al evaluated the filtration performance of an N95 FFR after bleach decontamination at 2 different concentrations (0.525% and 5.25%), using a poly-dispersed sodium chloride aerosol test method. The bleach solution was made by mixing Fisher 5.25% sodium hypochlorite (NaOCl) with 0.20% sodium hydroxide (NaOH). The aluminum nosebands were tarnished by both treatments (0.525% and 5.25% bleach) after a 30-minute submersion. For the 0.525% treatment, the average penetration did not significantly change for N95 samples. Treatment with 5.25% bleach resulted in the stiffening of the filter media and elastic straps for N95 FFRs, though to a lesser degree for the more diluted treatment. The N95 average penetration increased from baseline but was still less than the 5% maximum specified by National Institute for Occupational Safety and Health certification (mean and standard deviation % penetration: 0.7± 0.267 vs 0.68 ± 0.124 vs 3.79 ± 3.446, for as-received, 0.525%, and 5.25% postdecontamination, respectively).11
Viscusi et al evaluated 3 surgical N95 FFRs after thirty minutes submersion in 0.6% (1 part bleach to 9 parts of deionized water) aqueous solution of sodium hypochlorite (initial concentration 5.6% available as Cl2). Manufacturing specification: 6.00%-0.06% (w/w) available chlorine; Cat no. 7495.7-1, CAS no. 7732-18-5 (Ricca Chemical Company, Pequannock, NJ). After treatment, the FFRs were hung on a laboratory pegboard and allowed to air-dry overnight with assistance from a freestanding fan. Bleach decontamination did not affect the FFRs’ filter aerosol penetration (pre- and postdecontamination average percentage of sodium chloride penetration of the 3 surgical N95 FFRs evaluated: 1.57± 0.83 vs 0.561 ± 0.38, 0.335± 0.19 vs 0.233 ± 0.12, and 0.716 ± 0.37 vs 0.692 ± 0.35) and filter airflow resistance (pre- and postdecontamination average resistance in mmH2O of the 3 surgical N95 FFRs evaluated: 8.4± 0.50 vs 9.6 ± 0.29, 6.1 ± 0.15 vs 6.6 ± 0.56, and 6.7± 0.17 vs 5.9 ± 0.46). The metallic nosebands were slightly tarnished and visibly not as shiny when compared with their as-received counterparts, and the inner nose cushion of 1 of the 3 surgical N95 FFRs evaluated was discolored. Following air-drying overnight (16 hours), all the FFRs were dry to the touch, and all still had a characteristic smell of bleach.13
Lin et al determined the relative survival of Bacillus subtilis spores loaded onto N95 FFRs after bleach decontamination. The treatment proceeded as follows: a 0.4 mL volume of bleach with various concentrations (5.4% (w/w) as Cl2: original; 2.7%: 1 part bleach to 1 part of deionized water; 0.54%: 1 part bleach to 9 parts of deionized water) was added to the center of the surface of the N95 FFR using a pipette, the FFR was then dried in a petri dish in a BSC for 10 minutes, the CFUs were counted, and their (RS) was calculated. Without decontamination, 59± % 8% of the loaded spores survived for 24 hours. In the bleach decontamination test, no colony was recovered after 5.4%, 2.7%, or 0.54% sodium hypochlorite was used, constituting no dilution, 2-fold, and 10-fold dilution, respectively.18
Bergman et al evaluated changes in the physical appearance, odor, and laboratory performance of six N95 FFR models after 3 cycles of decontamination with bleach. The experimental conditions and parameters were as follows: 30-minute submersion in 0.6% (1 part bleach to 9 parts of deionized water) solution of sodium hypochlorite (original concentration = 6% available as Cl2). Manufacturing specification: 6.00± % 0.06% (w/w) available chlorine; Cat no. 7495.7-1, CAS No. 7732-18-5 (Ricca Chemical Company, Pequannock, NJ). A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure initial percent filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.24% to 4.01%) and filter airflow resistance (mean values ranging from 6.9 to 12.1 mmH2O). Bleach decontamination caused various effects: for all FFR models, metallic nosebands were slightly tarnished and visibly not as shiny when compared with their as-received counterparts. For those models with staples, the staples were oxidized to varying degrees. Some models had dissolution of nose pads, and discoloration (yellowing) of inner nose pads, material adjacent to nose pad, and other areas of the FFRs (bleeding of printed ink lettering). Additionally, following air-drying between exposure cycles (at least 16 hours), all FFRs that were exposed to bleach were dry to the touch, and all still had a characteristic bleach odor.16
Liao et al sprayed 3 models of N95 FFRs with approximately 0.3-0.5 mL of household chlorine-based disinfectant (∼2% NaClO). Samples were left to air-dry and off-gas completely, while hanging. From the first disinfection, ethanol drastically degraded the filtration efficiency (73.11% ± 7.32%), while the pressure drop remained comparable (9.0Pa ± 1.0).20
Heat treatment
Viscusi et al evaluated the filtration performance of an N95 FFR after dry heat (oven) decontamination under 2 different conditions, using a poly-dispersed sodium chloride aerosol test method. Respirators were placed in a metal pan on racks of a Fisher Isotemp 500 Series (Fisher Scientific, Pittsburgh, PA) laboratory oven at the specified temperature and turned over midway through the exposure period. At a temperature of 80°C, no visible changes were observed after 60 minutes; however a small increase in average penetration was observed for N95 FFRs (mean and standard deviation % penetration: 0.7± 0.267 and 0.84 ± 0.258, for as-received and 80°C postdecontamination, respectively). For treatment at 160°C, N95 FFRs were largely melted and unusable after only 22 minutes. No further penetration tests were attempted using dry heat.11
Lore et al examined the effectiveness of moist heat, as well as the filtration performance of 2 models of N95 FFRs. An aerosol settling chamber was used to apply virus-laden droplets (Influenza A/H5N1) to FFRs in a method designed to simulate respiratory deposition of droplets onto surfaces. The decontamination procedure was as follows: a 6-l sealable container (19 × 19 × 17cm) was filled with 1l of tap water, placed in an oven (Thermo Fisher Scientific Inc., Marietta, OH), and heated to 65°C ± 5°C for 3 hours. This allowed the liquid to reach the desired temperature before any decontamination tests. For testing, the container was removed from the oven, and a single virus-contaminated respirator was placed on the rack. For each decontamination procedure, the container was opened and the FFR placed onto the rack with the convex surface pointed toward the water layer. The container was then sealed and returned to the oven for the 20-minute treatment. After the decontamination procedure, the reduction in viral recovery (expressed as log10 TCID50/mL reduction) was ≥4.62 and ≥4.65 for the FFR models, respectively, and no viable virus was detectable, with several amplification cycles to detect vRNA of 5.62 and 42.45 for the FFR models, respectively. Additionally, the postdecontamination filter performance analyses showed that the mean penetration of 1% NaCl aerosol at 300-nm particle size was 1.04 and 0.99 for the FFR models, respectively.12
Fischer et al analyzed the ability of heat to inactivate SARS-CoV-2 on N95 FFRs (50 µL of 105 TCID50/mL of SARS-CoV was applied on N95 and stainless steel) and used quantitative fit testings to measure their filtration performance after each decontamination run and 2 hours of wear, for 3 consecutive decontamination and wear sessions. Plates with fabric and steel discs were placed in a 70°C oven. Plates were removed at 10, 20, 30, and 60 minutes, and 1 mL of cell culture medium added. The N95 FFRs’ integrity was quantitatively determined using the fit factor, a measure of filtration performance: the ratio of the concentration of particles outside the mask to the concentration inside, requiring a minimum fit factor of 100 for a mask to pass a fit test. The heat caused more rapid inactivation on N95 (decay rates of viable virus titers over time and half-lives; median, interquartile range: 4.64, 3.87-5.41 minutes) than on steel (decay rates of viable virus titers over time and half-lives; median, interquartile range: 8.83, 7.49-10.1 minutes), with inactivation rates on N95 comparable to UV. Quantitative fit tests showed that the filtration performance of N95 FFRs was not markedly reduced after a single decontamination with heat, but subsequent rounds of decontamination caused sharp drops in their filtration performance.19
Bergman et al evaluated changes in the physical appearance, odor, and laboratory performance of six N95 FFR models after 3 cycles of decontamination with moist heat incubation/pasteurization (MHI). The experimental conditions and parameters were as follows: 30-minute incubation at 60°C, 80% RH in a Caron model 6010 laboratory incubator (Marietta, OH). Following the first incubation, the samples were removed from the incubator and air-dried overnight. Following the second and third incubations, the samples were removed from the incubator and air-dried for 30 minutes with the aid of a fan. A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure initial percent filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.43 to 2.16%), and filter airflow resistance (mean values ranging from 7.5 to 15.0 mmH2O). MHI decontamination did not cause any observable physical change in the FFRs.16 Viscusi et al also showed that N95 FFR users would be unlikely to experience a clinically meaningful reduction in fit, increase in odor, increase in discomfort, or increase in difficulty in donning after MHI decontamination.14
Liao et al loaded 3 models of N95 FFRs into a preheated 5-sided heating chamber (Across International, LLC or SH-642, ESPEC). Dry heat (≤85°C) under various humidities (≤100% relative humidity, RH) was applied using the Across International vacuum heating oven under ambient conditions. The resting time between cycles was 10 minutes for the 75°C and 85°C treatments and 5 minutes for the 100°C and 125°C treatments. After resting, the samples were returned to the chamber to begin the next cycle. The highest temperature that the FFR could be subjected to while allowing repeated use with ≥95% efficiency was <100°C. At temperatures of ≤85°C, humidity did not seem to play a crucial role in the filtration properties, since FFRs tested at near 100% RH at 85°C were unaffected. The drop in filtration efficiency and pressure after 10 cycles of treatment was 97.25% ± 0.34% and 8.0Pa ± 1.0, respectively.20
Ethanol
Lin et al determined the relative survival rate of Bacillus subtilis spores loaded onto N95 FFRs after ethanol decontamination. Ethanol in various concentrations and volumes was added to the center of the surface of the N95 FFR using a pipette; the FFR was then dried in a petri dish that was placed in a biosafety cabinet (BSC) for 10 minutes. CFUs were counted, and their RS was calculated. Without decontamination, 59%± 8% of the loaded spores survived for 24 hours. Just after spiking with ethanol, the RS was found to have declined from 100% to 68%-75%. When 0.4 mL of 70% ethanol was applied, the RS fell to 22% in 24 hours. The RS fell to 20% when 80% ethanol was used.18
Fischer et al analyzed the ability of 70% ethanol to inactivate SARS-CoV-2 on N95 FFRs (50 µL of 105 TCID50/mL of SARS CoV-2 was applied on N95 and stainless steel) and used quantitative fit testings to measure their filtration performance after each decontamination run and 2 hours of wear, for 3 consecutive decontamination and wear sessions. Fabric and steel discs were placed into the wells of one 24-well plate per time-point and sprayed with 70% ethanol to saturation. The plate was tipped to near vertical, and 5 passes of ethanol were sprayed onto the discs from approximately 10 cm. After 10 minutes, 1 mL of cell culture medium was added. The N95 FFRs’ integrity was quantitatively determined using the fit factor, a measure of filtration performance: the ratio of the concentration of particles outside the mask to the concentration inside, requiring a minimal fit factor of 100 for a mask to pass a fit test. Ethanol yielded extremely rapid inactivation both on N95 (decay rates of viable virus titers over time and half-lives; median, interquartile range: 0.639, 0.55- 0.721 min) and on stainless steel (decay rates of viable virus titers over time and half-lives; median, interquartile range: 1.06, 0.888-1.23 minutes). Quantitative fit tests showed that the filtration performance of the N95 FFRs was not markedly reduced after a single decontamination with 70% ethanol. However, subsequent rounds of decontamination caused sharp drops in the filtration performance of the ethanol-treated masks.19
Liao et al immersed 3 models of N95 FFRs into a solution of 75% ethanol and left them to air-dry (hanging), and they were subsequently tested. At the first disinfection, ethanol drastically degraded the filtration efficiency (56.33% ± 3.03%), while the pressure drop remained comparable (7.7Pa ± 0.6).20
Liquid hydrogen peroxide
Viscusi et al evaluated the filtration performance of an N95 FFR liquid hydrogen peroxide (Fisher 30% stabilized H2O2) decontamination at 2 different conditions, using a poly-dispersed sodium chloride aerosol test method. Submersion in 3% hydrogen peroxide for 30 minutes produced no unaided observable changes for any of the samples. The average penetration did not significantly increase for N95 FFRs. The same treatment with 6% hydrogen peroxide for 30 minutes slightly faded the label ink on the fabric of the respirator. The average penetration did not significantly change for the N95 respirator when compared to baseline and remained well below the National Institute for Occupational Safety and Health 5% criteria (means and standard deviation % penetration: 0.7 ± 0.267 vs 0.75 ± 0.242 vs 0.71 ± 0.148, for as-received and postdecontamination under 2 different conditions).11
Bergman et al evaluated the changes in physical appearance, odor, and laboratory performance of six N95 FFR models after 3 cycles of decontamination with liquid hydrogen peroxide. The experimental conditions and parameters were as follows: 30-minute submersion in 6% (1 part hydrogen peroxide to 4 parts of deionized water) solution of hydrogen peroxide. Manufacturing specification: 30% hydrogen peroxide; Cat no. H325-500, CAS Nos.7722-84-1, 7732-18-5, 12058-66-1 (Fisher Scientific, Fair Lawn, NJ). A Model 8130 AFT (TSI, Inc., St Paul, MN) was used to measure the initial percent of filter aerosol penetration (%P) and filter airflow resistance (pressure drop in mmH2O column height pressure) for all FFR samples. Following decontamination treatment, all the N95 FFR samples had the expected levels of both filter aerosol penetration (mean values ranging from 0.12% to 3.35%) and filter airflow resistance (mean values ranging from 6.2 to 11.7 mmH2O). For those models that had staples, liquid hydrogen peroxide treatment caused the staples to oxidize to varying degrees.16
Autoclave
Viscusi et al evaluated the filtration performance of a N95 FFR after autoclave decontamination at 121°C (15 psi) under 2 different conditions (30- and 15 minutes), using a poly-dispersed sodium chloride aerosol test method. All samples were sealed in a standard poly/paper autoclave bag and treated in a Market Forge Automatic Sterilmatic steam pressure sterilizer (Everett, MA) for the specified period. The respirators were then air-dried for 72 hours prior to filter testing. For both treatment conditions (30- and 15 minutes), the N95 FFRs were deformed, shrunken, stiff, and mottled. Both treatment conditions markedly increased the average penetration for N95 FFRs (means and standard deviation % penetration: 0.7± 0.267 vs 1.13 ± 0.662 vs 1.77 ± 0.617, for as-received, 15- and 30 minutes postdecontamination, respectively).11
Lin et al determined the relative survival of Bacillus subtilis spores loaded onto N95 FFRs after autoclave decontamination. The N95 FFR was heated for 15 minutes at 121°C and 103 kPa. CFUs were counted and their RS was calculated. Without decontamination, 59± % 8% of the loaded spores survived for 24 hours. Autoclave effectively sterilized almost 100% of the bacteria.18
Isopropyl alcohol
Viscusi et al evaluated the filtration performance of an N95 FFR after isopropyl alcohol (IPA) at 2 different times of submersion (1 second and 1 minute), using a poly-dispersed sodium chloride aerosol test method. Fading of strap ink was the only visible change observed. As expected, both treatment conditions (1-second and 1-minute submersion) resulted in markedly increased average penetration for N95 FFRs (mean and standard deviation % penetration: 0.7± 0.267 vs 17.8 ± 5.508 vs 21.6 ± 1.337, for as-received, 1-second and 1-minute postdecontamination, respectively).11
Wipe products
Heimbuch et al evaluated the ability of commercially available wipe products to clean 3 models of surgical N95 FFRs contaminated with aerosols of mucin or viable Staphylococcus aureus. After cleaning, FFRs were separated into components (nose pad, fabrics, and perforated strip), and contaminants were extracted and quantified. Filtration performance was assessed for cleaned FFRs. Wipe products selected for this study were 504/07065 respirator cleaning wipes (3M Company, St. Paul, MN),15, which contain benzalkonium chloride (BAC); Hype-Wipes (Current Technologies, Inc, Crawfordsville, IN),16, which contain 0.9% hypochlorite (OCL); and Pampers wipes (Proctor &Gamble, Cincinnati, OH),17, which contain no active antimicrobial ingredients (ie, inert). S. aureus was applied to both interior and exterior FFR surfaces (in separate experiments) to provide sufficient sensitivity for reliable analysis. Mucin was applied as a heavy loading (1 mg/cm2) only to exterior surfaces. After cleaning, the FFRs were incubated for 15 minutes at room temperature before quantification of contaminants. The filters’ performance was evaluated after 3 cleaning cycles using a model 8130 automated filter tester (TSI Inc, Shore-view, MN). While no mucin was detected in replicates using the OCL wipes, the mean removal efficiency of mucin by BAC and inert wipes ranged from 21.47% to 76.41%. Otherwise, the mean removal efficiency of Staphylococcus aureus by OCL wipes ranged from 98.98% to >99.99%, the mean removal efficiency of S. aureus by BAC wipes ranged from 68.92% to >99.99%, and the mean removal efficiency of S. aureus by inert wipes ranged from 59.37% to >96.53%. Removal was less effective from nose pads and perforated edges. Although particle penetration following cleaning yielded mean values <5%, BAC wipes caused more penetration than the other wipes, this difference being significant for 2 models of FFRs.24
Tap water
Viscusi et al evaluated the filtration performance of an N95 FFR after tap water, using a poly-dispersed sodium chloride aerosol test method. This treatment involved submerging the test respirators in tap water for 30 minutes and was included as a control to reveal any effect due to immersion in tap water and air drying. No significant visible changes were detected. As expected, the average filter penetration was unchanged for N95 FFRs (mean and standard deviation % penetration: 0.7± 0.267 and 0.72 ± 0.202, for as-received and tap water postdecontamination, respectively).11
Soap and water
Viscusi et al evaluated the filtration performance of an N95 FFR after soap and water at 2 different time intervals, using a poly-dispersed sodium chloride aerosol test method. For treatments of submersion in the soap and water solution for 2 and 20 minutes, no visible changes were detected for any samples. The average penetration markedly increased for N95 respirators at both time intervals (mean and standard deviation % penetration: 0.7± 0.267 vs 38.3 ± 1.269 vs 34.9 ± 4.599, for as-received, 2- and 20 minutes postdecontamination, respectively).11
Traditional electric rice cooker
Lin et al determined the relative survival rate of Bacillus subtilis spores loaded onto N95 FFRs after traditional electric rice cooker decontamination. The N95 FFR was placed in an electric rice cooker for dry heating for 3 minutes (149°C-164°C, without added water). CFUs were counted, and their RS was calculated. Without decontamination, 59± % 8% of the loaded spores survived for 24 hours. The traditional electric rice cooker effectively sterilized almost 100% of the bacteria.18
Discussion
This systematic review summarized all the existing evidence on the different decontamination methods that might allow disposable N95 FFRs to be reused, specifically addressing issues related to biocidal efficacy, filtration performance, fitting characteristics, and residual chemical toxicity, as well as other practical aspects such as the equipment required for their implementation and the maximum number of decontamination cycles. Our findings show that the issues mentioned above largely depend not only on the decontamination method itself, but also on other factors, such as the N95 FFR model, the presence of soiling agents, the surface type, the directness of exposure to the target surface for decontamination, the type of nucleic acid of the virus, the water-vapor content on the surface, and specific characteristics of each decontamination method, such as dose, intensity, concentration, and time of exposure. Our review shows that although not all methods have been evaluated for their efficacy in virus inactivation, UVGI, VHP, heat, and ethanol are specifically efficacious against SARS-CoV-2. Our review supports the recent recommendations made by the Infectious Diseases Society of America related to the use of reprocessed N95 for reuse during a contingency3 and provides wider support for alternative methods. It must also be recognized that clinical evidence for the reuse of decontaminated N95 is lacking, and such a study would be of interest in crisis-capacity settings but also relevant for less-developed countries.
Although all the methods have advantages and disadvantages (Table 1 ), UVGI and VHP seem to be the most promising decontamination methods for N95 FFRs. UVGI decontamination has many benefits: it is the most frequently studied and reported decontamination method for N95 FFRs that has demonstrated significant reductions in influenza virus recovery and viability even with soiling conditions (mucin and sebum-soiled facepieces),17 and it has shown substantial reductions in the recovery of pathogens such as B. subtilis after exposure to UVGI for as little as 5 minutes,18 inactivation of 99.9%-99.999% of respiratory viruses such as Influenza A (H1N1), Avian Influenza A virus (H5N1), Influenza A (H7N9) A/Anhui/1/2013, Influenza A (H7N9) A/Shanghai/1/2013, MERS-CoV, and SARS-CoV,10 and inactivation of SARS-CoV-2.19 All these biocidal effects have been demonstrated without known health risks to the users nor a meaningfully significant effect on filter aerosol penetration, filter airflow resistance,11, 12, 13 , 15 fit and seal, odor (although a singed smell has been reported on FFRs following UVGI treatment, this does not necessarily indicate toxicity, and it generally dissipates naturally within 4 hours),25 discomfort, difficulty in donning, or physical appearance14 after up to 20 of cycles of decontamination.10 Additionally, UVGI is the most viable treatment for large-scale applications, due to its simplicity of use and its ability to rapidly scale up the process by adding inexpensive FFR UVGI exposure units. UVGI technology has also been developed for whole-room decontamination for hospitals, which provides opportunities for dual-use technologies and reduction of implementation costs.13 However, several aspects need to be taken into account before being truly optimistic: UVGI decontamination is based on supplying an adequate dose to the contaminated area.26 The UVGI dose required for decontamination, which is microbe specific (eg, a direct relationship between UV dosage and influenza decontamination has been demonstrated, with no viable virus detected after UV treatment ≥1 J/cm2 10), is a function of irradiance and time.26 However, at the higher UVGI doses, there are greater reductions in the strength of the layers of FFR material.15 Furthermore, UVGI efficiency is hampered by shadowing produced by the multiple layers of the N95 FFRs.17 An additional factor to consider is that UVGI performance can vary among different models of N95 FFRs, different parts of the respirators, distinct types of UVGI, and number of cycles of decontamination. Although 10 UVGI cycles do not have a significant effect on FFR straps, 20 cycles may have a considerable impact on the strap tension of some N95 FFR models (3M 1860, 3M 1870, and Kimberly-Clark PFR models).10 While no colony of B. subtilis was recovered after 5 minutes of exposure to UV-C, RS remained above 20% after 20 minutes of irradiation by UV-A.18 Moreover, UVGI has been demonstrated to inactivate SARS-CoV-2 rapidly on steel but more slowly on N95 fabric, likely due to its porous nature.19 Finally, UVGI decontamination is limited by the available working surface area of a biosafety cabinet equipped with a UV-C source or other area being irradiated by a UVGI source.13
Table 1.
Summary of advantages and disadvantages of decontamination methods that might allow N95 filtering facemask respirators (FFRs) to be reused
| Method of decontamination | N95 FFR models tested | Advantages | Disadvantages/ N95 models with their disadvantages |
|---|---|---|---|
| Ultraviolet germicidal irradiation (UVGI) | 3M 1860 3M 1870 3M 8000 3M 8210 3M 9210 3M 9211 3M VFlex 1805 Alpha Protech 695 Gerson 1730 Kimberly-Clark 46727 Kimberly-Clark PFR95-270 Moldex 1512 Moldex 1712 Moldex EZ-22 Moldex 2200 Precept 65-3395 Prestige Ameritech RP88020 Sperian HC-NB095 Sperian HC-NB295F U.S. Safety AD2N95A U.S. Safety AD4N95 N95-A N95-B N95-C SN95-D SN95-E SN95-F GE 1730 KC 46727 4C Air, Inc. (GB2626 KN95) ESound (GB2626 KN95) Onnuriplan (KFDA KF94) |
Significant reductions in Influenza virus recovery and viability even under soiled conditions17 Substantial reduction in the recovery of pathogens such as Bacillus subtilis spores after exposure to UVGI for as little as 5 minutes18 Inactivation of influenza A (H1N1), avian influenza A (H5N1), influenza A (H7N9) A/Anhui/1/2013, influenza A (H7N9) A/Shanghai/1/2013, MERS-CoV, SARS-CoV, and SARS-CoV-210,19 No significant effect on filter aerosol penetration, filter airflow resistance, fit and seal, odor, discomfort, difficulty in donning, or physical appearance after up to 20 of cycles of decontamination10, 11, 12, 13, 14, 15,25 No significant effect on filtration efficiency after 10 cycles of decontamination.20 |
Reduction in the strength of the layers of FFR material (at higher UVGI doses)/ (3M 1860, 3M 9210, Gerson 1730, Kimberly-Clark 46727)15 UVGI efficiency hampered by shadowing produced by the multiple layers of the N95 FFRs/(all FFR models)17 UVGI performance can vary among different models of N95 FFRs, different parts of the respirators, distinct types of UVGI, and number of cycles of decontamination Considerable impact on the strap tension of some N95 FFR models (with 20 cycles of decontamination)/ (3M 1860, 3M 1870, Kimberly-Clark PFR)10 Better reduction in B. subtilis recovery with UV-C than with UV-A/ (3M 8210)18 UV-treated FFRs were able to withstand 10 cycles of treatment, but efficiency eventually decayed to 93% at 20 cycles, making it unsuitable for N95-grade FFRs by itself/ (3M 8210, 4C Air, Inc. (GB2626 KN95), ESound (GB2626 KN95), Onnuriplan (KFDA KF94)).20 |
| Vaporized hydrogen peroxide (VHP) | 3M 1860 3M 1870 3M 9211 N95-A N95-B N95-C SN95-D SN95-E SN95-F |
Complete inactivation of G. stearothermophilus spores following 50 repeated aerosol inoculation/decontamination cycles21 Complete eradication after a single VHP cycle of 3 aerosolized bacteriophages (T1, T7, and Pseudomonas phage phi-6)22 Extremely rapid inactivation of SARS-CoV-2 both on N95 and on stainless steel.19 No significant effects on filtration performance, filter aerosol penetration, fit, and filter airflow resistance after up to 50 cycles of decontamination21 No vapors potentially toxic to humans nor environmentally hazardous residues as a result of the VHP decontamination process11 |
Fragmentation of the elastic material in the straps when stretched (after 30HPV, but not after 10 or 20 VHP cycles)/ (3M 1860)21 Slight tarnishing of the metallic nosebands/ (all FFR models)13 |
| Ethylene oxide (EtO) | N95-A N95-B N95-C SN95-D SN95-E SN95-F |
No significant affection of filter aerosol penetration, filter airflow resistance, or physical appearance of the FFRs11,13,16 | None |
| Microwave oven use | 3M 1860 3M 1870 N95-A N95-B N95-C SN95-D SN95-E SN95-F 3M 1860 3M 1870 3M 8000 3M 8210 Moldex 2200 Kimberly-Clark PFR95-270 Cardinal Health N95 |
Satisfactory decontamination against influenza virus on N95 FFRs as measured by a virus culture method12 High efficacy for inactivating bacteriophage MS2 droplets as a surrogate for a pathogenic virus on FFRs23 No significant affection of filter aerosol penetration or filter airflow resistance of FFRs (after 2 minutes of microwave exposure)11, 12, 13,16 No significant reduction in fit, increase in odor, increase in discomfort, or increased difficulty in donning14 No known health risks to the users13 |
Melting of the filter media at the ends of the aluminum nosebands, forming visible holes. Significant increase in filter penetration (after 4 minutes of microwave exposure)/ (all FFR models)11 Partial separation of the inner foam nose cushion and slight melting of the head straps from some FFR samples/ (SN95-E and SN95-D, respectively)16 Microwave oven irradiation melted samples from 1 FFR model/ (SN95-E)13 Filtration material melted in areas adjacent to the metallic nosebands/ (SN95-E)13 |
| Bleach | N95-A N95-B N95-C SN95-D SN95-E SN95-F 3M 8210 4C Air, Inc. (GB2626 KN95) ESound (GB2626 KN95) Onnuriplan (KFDA KF94) |
99-100% biocidal efficacy, indicating effective sterilization of Bacillus subtilis spores18 No significant affection of filter aerosol penetration or filter airflow resistance |
Tarnishing of the aluminum nosebands/ (all FFR models)11,13,16 Stiffening of the filter media and elastic straps (treatment with 5.25% bleach)11 Discoloration of the inner nose cushion of 1 of the 3 N95 FFRs evaluated/(SN95-E)13 Characteristic smell of bleach/(all FFR models)13 Oxidation of staples/ (N95-B, N95-C, SN95-E, SN95-F)16 Dissolution of nose pads/ (SN95-E)16 Discoloration (yellowing) of inner nose pads/ (N95-A, SN95-E, SN95-F)16 Discoloration of other areas of the FFR (bleeding of printed ink lettering)/ (SN95-F)16 Yellowing of the material adjacent to nose pad/ (SN95-E)16 From the first disinfection, bleach drastically degraded the filtration efficiency/ (3M 8210, 4C Air, Inc. (GB2626 KN95), ESound (GB2626 KN95), Onnuriplan (KFDA KF94)).20 |
| Heath treatment | 3M 1860 3M 1870 3M 8000 3M 8210 3M 9211 N95-A N95-B N95-C SN95-D SN95-E SN95-F Moldex 2200 Kimberly-Clark PFR95-270 4C Air, Inc. (GB2626 KN95) ESound (GB2626 KN95) Onnuriplan (KFDA KF94) |
Satisfactory decontamination of influenza virus on N95 FFRs as measured by a virus culture method12 No significant affection of filter aerosol penetration of FFRs12,16,19 No significant affection of filter airflow resistance of FFRs16 No observable physical change in FFRs16 No clinically significant reduction in fit, increase in odor, increase in discomfort, or increase in difficulty in donning14 No significant affection of filtration efficiency or pressure after 10 cycles of decontamination.20 |
Increase in filtration penetration (at a temperature of 80°C)11 More than 1 cycle of decontamination caused sharp drops in the filtration performance of N95 FFRs/ (3M 9211)19 N95 FFRs were largely melted and unusable after only 22 minutes of treatment at 160°C11 Partial separation of the inner foam nose cushion and slight melting of the head straps from some FFR samples/ (SN95-E and SN95-D, respectively)16 |
| Ethanol | 3M 8210 3M 9211 4C Air, Inc. (GB2626 KN95) ESound (GB2626 KN95) Onnuriplan (KFDA KF94) |
Extremely rapid inactivation of SARS-CoV-2 both on N95 and on stainless steel19 Filtration performance of N95 FFRs was not markedly reduced after a single decontamination with 70% ethanol19 |
Higher survival of Bacillus subtilis spores than with other decontamination methods/ (3M 8210)18 More than 1 cycle of decontamination caused sharp drops in the filtration performance of the ethanol-treated masks/(3M 9211)19 From the first disinfection, ethanol drastically degraded the filtration efficiency/(3M 8210, 4C Air, Inc. (GB2626 KN95), ESound (GB2626 KN95), Onnuriplan (KFDA KF94)).20 |
| Liquid hydrogen peroxide (LHP) | N95-A N95-B N95-C SN95-D SN95-E SN95-F |
No significant affection of filter aerosol penetration of FFRs11,16 No significant affection of filter airflow resistance of FFRs16 |
Slight fading of the label ink on the fabric of FFRs (treatment with 6% LHP for 30 minutes)11 Oxidation of the staples to varying degrees (for those models that use staples)/ (N95-B, N95-C, SN95-E, SN95-F)16 |
| Autoclave | 3M 8210 | Effective sterilization of almost 100% of Bacillus subtilis spores18 | N95 FFRs were deformed, shrunken, stiff, and mottled (for both 15- and 30 minutes of treatment)11 Marked increase of filter aerosol penetration of FFRs (for both 15- and 30 minutes of treatment)11 |
| Isopropyl alcohol | N95 (model not specified) | None | Fading of strap ink of FFRs11 Marked increase of filter aerosol penetration of FFRs (for both 1 second and 1 minute of treatment)11 |
| Wipe products | 3M 1860 3M 1870 Kimberly-Clark PFR |
No mucin detected in replicates using the 0.9% hypochlorite wipes24 Mean removal efficiency of S. aureus by 0.9% hypochlorite wipes ranged from 98.98 to >99.99%24 |
Removal of S. aureus less effective from nose pads and perforated edges/ (Kimberly-Clark PFR)24 Mean removal efficiency of mucin by benzalkonium chloride and inert wipes ranged from 21.47% to 76.41%24 Benzalkonium chloride wipes caused more penetration than the other wipes/ (all FFR models)24 |
| Tap water | N95 (model not specified) | Average filter penetration was unchanged for N95 FFRs11 No significant visible changes in FFRs detected11 |
None |
| Soap and water | N95 (model not specified) | No visible changes detected in FFR samples11 | Average penetration markedly increased for N95 FFRs at both time intervals (2- and 20 minutes)11 |
| Traditional electric rice cooker | 3M 8210 | Effective sterilization of almost 100% of Bacillus subtilis spores18 | None |
VHP is another widely-studied decontamination method for N95 FFRs that has been shown to have several advantages: complete inactivation of G. stearothermophilus spores following 50 repeat aerosol inoculation/decontamination cycles,21 complete eradication after a single VHP cycle of 3 aerosolized bacteriophages (T1, T7, and Pseudomonas phage phi-6), which are a reasonable proxy for SARS-CoV-2,22 and extremely rapid inactivation of SARS-CoV-2 both on N95 and on stainless steel.19 All these biocidal effects have been demonstrated without meaningfully significant effects on filtration performance, filter aerosol penetration, fit, and filter airflow resistance after up to 50 cycles of decontamination.21 Additionally, there are no vapors potentially toxic to humans nor environmentally hazardous residues as a result of the VHP decontamination process.11 However, after VHP decontamination, some studies have reported a fragmentation of the elastic material in the straps when stretched (after 30 HPV, but not after 10 or 20 HPV cycles)21 and a slight tarnishing of the metallic nosebands.13 A potential disadvantage is that although the total VHP cycle time is short compared to other decontamination methods, the throughput capability of VHP processing is limited by the fact that cellulose-based products (eg, cotton, which may be present in some head straps or some FFR layers) absorb hydrogen peroxide and can cause the STERRAD cycle to abort due to low hydrogen peroxide vapor concentration.13 As in the case of UV treatment, some information has cast doubt on the number of cycles allowed while maintaining the integrity of the fit testing after more than 2 wear sessions.19
The results of our review support and build on previous recommendations for the most viable decontamination methods for N95 FFRs. Concerning decontamination and reuse of FFRs, the Centers for Disease Control and Prevention recognizes UVGI, VHP, and moist heat as the most promising potential methods for decontaminating FFRs. Additionally, the Centers for Disease Control and Prevention also accept steam treatment and liquid hydrogen peroxide as promising methods, but with some limitations, and EtO as a promising method but with serious limitations.6 The Asia Pacific Center for Evidence-Based Healthcare concluded, based on laboratory-based studies, that UVGI, MGS, warm moist heath, and VHP were shown to be effective in reducing either viral or bacterial load while still maintaining the integrity of N95 FFRs.27 Hamzavi et al consider that given that many of our health care providers are using substitutes for N95 FFRs that offer very limited degree of protection, using UVGI and repurposing phototherapy devices could be the best practical solution at this time.28 Also, supporting the findings of our review, VHP and UVGI for decontaminating and reusing N95 respirators are being used at the Duke University Health Systemy29 and at the University of Nebraska Medical Center,30 respectively. As an additional contribution, our review provides information about the N95 FFR models tested with each decontamination method, as well as the specific models that had some problem with each decontamination method (Table 1).
In conclusion, although all the methods for decontaminating and reusing N95 FFRs have advantages and disadvantages, UVGI and VHP seem to be the most promising methods, based on their reduction of the microbial threat (including SARS-CoV-2) while maintaining the function of N95 FFRs as well as the lack of residual toxicity. Future studies are required in order to establish the efficacy and security of these decontamination methods on different N95 FFR models and the maximum allowed number of cycles of decontamination under different conditions.
Acknowledgments
The authors thank Mr. Charlie Barret for his editorial assistance.
Footnotes
Conflicts of interest: None of the authors have conflicts of interest to declare.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet (London, England) 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The L COVID-19: protecting health-care workers. Lancet (London, England) 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch JB, Davitkov P, Anderson DJ, et al. Infectious Diseases Society of America Guidelines on Infection Prevention for Health Care Personnel Caring for Patients with Suspected or Known COVID-19. Available at: https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/infection-prevention/idsa-covid-19-guideline_ip_v1.0.1.pdf. Accessed June 3, 2020. [DOI] [PMC free article] [PubMed]
- 4.Hospital Respiratory Protection Program Toolkit. Resources for Respirator Program Administrators. Available at:https://www.cdc.gov/niosh/docs/2015-117/pdfs/2015-117.pdf. Accessed June 3, 2020.
- 5.OSHA . Occupational Safety and Health Administration; Washington, DC: 2009. Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers. Report No. OSHA3328-05R 2009. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Decontamination and reuse of filtering facepiece respirators. Available at:https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html. Accessed June 3, 2020
- 7.Coulliette AD, Perry KA, Edwards JR, Noble-Wang JA. Persistence of the 2009 pandemic influenza A (H1N1) virus on N95 respirators. Appl Environ Microbiol. 2013;79:2148–2155. doi: 10.1128/AEM.03850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady TM, Strauch AL, Almaguer CM, et al. Transfer of bacteriophage MS2 and fluorescein from N95 filtering facepiece respirators to hands: measuring fomite potential. J Occup Environ Hyg. 2017;14:898–906. doi: 10.1080/15459624.2017.1346799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbuch B, Harnish D. Applied Research Associates, Inc. (ARA). Research to mitigate a shortage of respiratory protection devices during public health emergencies. Available at: https://www.ara.com/sites/default/files/MitigateShortageofRespiratoryProtectionDevices_3.pdf. Accessed June 3, 2020.
- 11.Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007;24:93–107. [Google Scholar]
- 12.Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 13.Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscusi DJ, Bergman MS, Novak DA, et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 15.Lindsley WG, Martin SB, Jr., Thewlis RE, et al. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR. Evaluation of multiple (3-Cycle) decontamination processing for filtering facepiece respirators. J Eng Fiber Fabr. 2010;5:33–41. [Google Scholar]
- 17.Mills D, Harnish DA, Lawrence C, Sandoval-Powers M, Heimbuch BK. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TH, Tang FC, Hung PC, Hua ZC, Lai CY. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer RJ, Morris DH, Van Doremalen N, et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2 [e-pub ahead of print] MedRxiv. 2020 doi: 10.1101/2020.04.11.20062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao L, Xiao W, Zhao M, et al. Can N95 respirators be reused after disinfection? how many times? ACS Nano. 2020;14:6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- 21.Richter W, Hofacre K, Willenberg Z. Final report for the bioquell hydrogen peroxide vapor (HPV) decontamination for reuse of n95 respirators. Available at: https://www.fda.gov/media/136386/download. Accessed June 3, 2020.
- 22.Kenney PA, Chan BK, Kortright K, et al. Hydrogen peroxide vapor sterilization of N95 respirators for reuse [e-pub ahead of print] MedRxiv. 2020 doi: 10.1001/jamaoto.2020.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher EM, Williams JL, Shaffer RE. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS One. 2011;6:e18585. doi: 10.1371/journal.pone.0018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimbuch BK, Kinney K, Lumley AE, Harnish DA, Bergman M, Wander JD. Cleaning of filtering facepiece respirators contaminated with mucin and staphylococcus aureus. Am J Infect Control. 2014;42:265–270. doi: 10.1016/j.ajic.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimbuch B, Harnish D. Applied Research Associates, Inc. (ARA). Amendment 1—limited study evaluating UVGI-treated FFR odor. Available at:https://www.ara.com/sites/default/files/Amendment1_LimitedStudyofUVGITreatedFFROdor.pdf. Accessed June 3, 2020.
- 26.Fisher EM, Shaffer RE. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J Appl Microbiol. 2011;110:287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabaluna ITG, Melicor A. What are the effective methods of decontaminating N95 mask for reuse?Asia Pacific Center for Evidence Based Healthcare. Avaiable at:https://www.psmid.org/wp-content/uploads/2020/04/Decontamination-of-N95-mask-Abdriged-IGC-AM-02Apr-2020.pdf. Accessed June 3, 2020.
- 28.Hamzavi IH, Lyons AB, Kohli I, et al. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82:1511–1512. doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz A, Stiegel M, Greeson N, et al. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS‐CoV‐2 (COVID‐19) pandemic. Available at:https://www.safety.duke.edu/sites/default/files/N-95_VHP-Decon-Re-Use.pdf. Accessed June 3, 2020 [DOI] [PMC free article] [PubMed]
- 30.Lowe JJ, Paladino KD, Farke JD, et al. N95 filtering facemask respirator ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse. Available at: https://cleanhospital.com/wp-content/uploads/2020/03/N-95-decon-process.pdf. Accessed June 3, 2020.


