Abstract
Background
Severe acute respiratory syndrome coronavirus 2 is a novel coronavirus first diagnosed in U.S. hospitals in January 2020. Typical presenting symptoms include fever, dry cough, dyspnea, and hypoxia. However, several other symptoms have been reported, including fatigue, weakness, diarrhea, and abdominal pain. We have identified a series of patients with diabetic ketoacidosis (DKA) likely precipitated by coronavirus disease 2019 (COVID-19).
Case Series
We describe 5 patients with previously known type 2 diabetes and no history of DKA, who presented to the emergency department with new-onset DKA and COVID-19.
Why Should an Emergency Physician Be Aware of This?
Diabetes mellitus is a known risk factor for poor outcomes in viral respiratory illnesses, including COVID-19. Infection may precipitate DKA in patients with type 2 diabetes. Aggressive management of these patients is recommended; however, management guidelines have not yet been put forth for this unique subset of patients.
Keywords: COVID-19, SARS-CoV-2, coronavirus, diabetic ketoacidosis, diabetes
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus first discovered in Wuhan, China, in late December 2019 and first identified in the United States in mid-January 2020 (1). Presenting symptoms vary, but include fever, dry cough, fatigue, myalgias, abdominal pain, and diarrhea (1,2). Patients with advanced age and medical comorbidities are at higher risk for mortality, morbidity, and need for intensive care unit (ICU) admission (2,3). Cardiovascular disease and diabetes are associated with particularly high risk for death among patients with coronavirus disease 2019 (COVID-19) (3).
Diabetic ketoacidosis (DKA) is a life-threatening condition seen most commonly in patients with type 1 diabetes mellitus; however, physiologically stressful conditions, such as surgery, trauma, or infection, can precipitate DKA in type 2 diabetes mellitus (DM2), with approximately 30% to 50% of DKA cases triggered by infection (4,5). DKA confers a mortality rate of approximately 5%, but notably higher mortality rates occur in the elderly and patients with concurrent acute illnesses (5). The overall mortality in patients with COVID-19 is likely to be approximately 1% to 3%. However, among patients with diabetes, mortality may be > 7%, and likely higher in the elderly diabetic population (3,6). We are unaware of any reports of DKA among COVID-19 patients or the associated mortality risk. It is unknown whether the mortality of these two conditions is additive or even exponential. Given the high-risk nature of both DKA and COVID-19, it is paramount that DKA be recognized quickly in patients with concern for COVID-19 and, conversely, that COVID-19 is considered as a precipitant for DKA.
To our knowledge, there are no reported cases of new-onset DKA in patients with DM2 and COVID-19 infection. In this novel case series, we report 5 patients who presented to the emergency department (ED) with a spectrum of respiratory symptoms and were found to be in DKA likely precipitated by COVID-19.
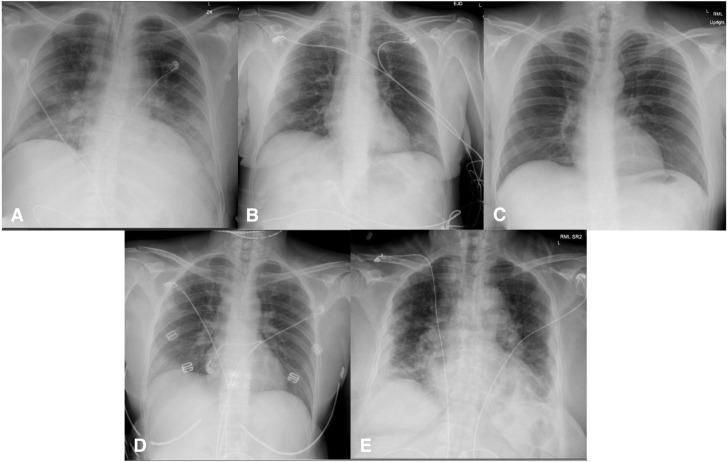
This case series was granted exempt status by the local Institutional Review Board. Table 1 provides demographic and laboratory details for each patient. Figure 1 shows the chest x-ray study findings of the presented cases.
Table 1.
Characteristics of Patients Presenting with Diabetic Ketoacidosis and COVID-19 Infection
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Average |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (years) | 55 | 57 | 38 | 45 | 63 | 49 |
| Sex | Male | Female | Male | Female | Female | — |
| Body mass index | 31.1 | 19.8 | 29.1 | 21.4 | 36.2 | 27.5 |
| Patient disease characteristics | ||||||
| Diabetes medications | Glipizide | Metformin, glipizide | Insulin detemir and aspart | None | Insulin | — |
| Hemoglobin A1C (%) | 9.5 | 11.3 | 11.9 | 15 | 9.8 | 11.5 |
| Presentation characteristics | ||||||
| Heart rate (beats/min) | 91 | 122 | 129 | 116 | 97 | 114 |
| Blood pressure (mm Hg) | 161/77 | 128/75 | 99/68 | 115/75 | 150/90 | 126/74 |
| Respiratory rate (breaths/min) | 55 | 32 | 20 | 18 | 53 | 31 |
| SpO2∗ (%) | 66 on NRB | 97 | 99 | 100 | 95 on 6 L | 91 |
| Glasgow Coma Scale score | 11 | 15 | 15 | 15 | 15 | 14 |
| Initial glucose (mg/dL) | 948 | 227 | 399 | 342 | 749 | 533 |
| pH | 7.13 | 7.11 | 7.02 | 6.99 | 7.21 | 7.06 |
| Anion gap | 21 | 24 | 22 | 18 | 18 | 21 |
| β-hydroxy butyrate (mmol/L) | 0.75 | 9.36 | n/o | 7.83 | n/o | 5.98 |
| COVID-19 laboratory values | ||||||
| Creatine kinase (units/L) | 545 | n/o | n/o | n/o | 329 | 437 |
| C-reactive protein (mg/dL) | >19.0 | 18.2 | n/o | n/o | >19.0 | 18.7 |
| d-dimer (mcg feu/mL) | 5.44 | 1.06 | n/o | n/o | >35.2 | 13.9 |
| Ferritin (ng/mL) | 1214 | 337 | n/o | n/o | >16,500 | 6017 |
| Fibrinogen | 600 | n/o | n/o | n/o | n/o | — |
| Lactate dehydrogenase (units/L) | 931 | n/o | n/o | n/o | 3934 | 2,433 |
| Procalcitonin (ng/mL) | 1.38 | 0.16 | n/o | n/o | 3.65 | 1.73 |
| Troponin (ng/mL) | n/o | <0.02 | n/o | n/o | 2.23 | 1.13 |
| Hospitalization characteristics | ||||||
| Fluid to gap closure (mL) | 2125 | 4625 | 3100 | 5550 | 6160 | 3850 |
| Time to gap closure (min) | 38 | 900 | 194 | 720 | 650 | 463 |
| Length of stay (days) | 8 | 4 | 3 | 4 | 4.75 | |
| Outcome | Hospitalized at time of printing | Discharge home | Discharge home | Discharge home | Death | — |
COVID-19 = coronavirus disease 2019; n/o = not obtained; NRB = non-rebreather mask.
Room air oxygen saturations (SpO2) were not available for all patients.
Figure 1.
Features of chest radiograph of selected cases. (A) Case 1, bilateral interstitial opacifications with endotracheal tube in place. (B) Case 2, mild interstitial prominence of left lower lobe. (C) Case 3, clear chest. (D) Case 4, developing right upper and lower interstitial prominences. (E) Case 5, extensive severe bilateral disease with interstitial and basilar predominance.
Case 1
A 55-year old African American man with DM2 was brought in from home for altered mental status and hypoxia. He reported a cough for several days prior and on the day of presentation had become confused. His initial prehospital oxygen saturation was 35%. He was given oxygen via non-rebreather mask and brought to the ED, where he was persistently hypoxic to 66% and was subsequently intubated. Initial laboratory results were consistent with DKA and a chest x-ray study found bilateral pneumonia (Figure 1A). He was started on a DKA protocol with an i.v. insulin drip and crystalloid fluid. He was started on antibiotics and hydroxychloroquine for presumed COVID-19, which was confirmed by polymerase chain reaction testing on hospital day 2.
On arrival to the ICU, the patient's anion gap had closed and he was transitioned to subcutaneous insulin. As of the time of article completion, he was on hospital day 15, still intubated in the ICU, with slowly improving ventilator parameters.
Case 2
A 57-year-old Hispanic woman with a history of DM2 on glipizide and metformin presented to the ED with 3 days of increasing dyspnea associated with fevers and cough. She had been evaluated in the ED 12 h earlier with a chest radiograph, electrocardiogram, an unremarkable metabolic panel, and COVID-19 testing. She was discharged with a diagnosis of pneumonia. After discharge, her vomiting and dyspnea worsened and she returned to the ED.
Laboratory workup at the second visit revealed laboratory abnormalities consistent with DKA (Table 1). She was tachycardic but normotensive, with tachypnea and moderate respiratory distress. She was started on an i.v. insulin drip and i.v. crystalloid fluids in addition to antibiotics and hydroxychloroquine for multifocal pneumonia. She was admitted to a medical ward, her anion gap closed, and she was transitioned to subcutaneous insulin before being discharged from the hospital. She tested positive for COVID-19 on hospital day 2. On follow-up, she was doing well at home.
Case 3
A 38-year-old Hispanic man with a medical history of DM2 on insulin detemir and aspart presented to the ED for 1 day of vomiting associated with subjective fevers, cough, and shortness of breath. He noted his blood sugars had recently been elevated to > 300 mg/dL. Laboratory findings were consistent with DKA. His chest x-ray study showed with bilateral infiltrates.
He was started on an i.v. insulin drip and given crystalloid fluids. He was admitted to a medical ward where his anion gap resolved, and he was transitioned to a subcutaneous insulin regimen. He tested positive for COVID-19 on hospital day 2 and was discharged on hospital day 3. He has remained stable and recently was seen for follow-up with improved glycemic control.
Case 4
A 45-year-old Hispanic woman with a history of DM2 and nonadherent with medication presented to the ED with concerns for COVID-19 after developing headache, myalgias, anorexia, and fever. She had previously been managed with oral antihyperglycemics but had stopped taking her medications 2 months prior to presentation.
The patient was ill-appearing, with tachypnea and clinical dehydration. Initial laboratory findings showed DKA. She was admitted to a medical ward and treated with i.v. insulin and crystalloid fluids. Her anion gap resolved after 12 h, and she was transitioned to a subcutaneous insulin regimen. Her COVID-19 testing returned positive on hospital day 2. She was discharged after 2 days and has not returned to the ED.
Case 5
A 63-year-old African American woman with a history of coronary artery disease, asthma, hypertension, and insulin-dependent DM2 presented with dyspnea and cough for 1 week. She was severely tachypneic on arrival with a respiratory rate of 53 breaths/min and an oxygen saturation of 95% on 6 L of oxygen via nasal cannula. A chest x-ray study showed bilateral pulmonary infiltrates (Figure 1E). Given the respiratory distress and bilateral pneumonia, the patient was started on antimicrobial coverage as well as hydroxychloroquine, and swabbed for COVID-19, which returned positive on hospital day 2.
Initial laboratory studies (Table 1) were consistent with DKA. She was intubated for refractory hypoxia. Despite attempts to match her intrinsically high minute ventilation, the patient's acidosis continued to worsen, resulting in near cardiac arrest several hours later with severe hemodynamic instability. She received bicarbonate with improvement of circulation and perfusion. Although the patient's anion gap closed and DKA resolved, she developed worsening multi-organ system failure despite maximal ventilatory and vasopressor support. Ultimately, the patient was made Do Not Resuscitate and was palliatively extubated on hospital day 4.
Discussion
These 5 cases of new-onset DKA in DM2 highlight a previously undescribed presentation of patients with COVID-19. Although COVID-19 has not previously been reported as a precipitant of DKA, cases of fulminant DKA have been observed with other viral infections, such as influenza. For example, Moghadami et al. described 2 patients who presented to emergency care in DKA presumed secondary to H1N1 influenza; both ultimately died (7). It is well known that DKA has a strong association with activated innate immune cells secondary to infection (2,8). What is unusual about our cases is the severity of DKA in previously controlled diabetics. Notably, glipizide, a drug inherently dependent on endogenous pancreatic function, was used for hyperglycemic control in cases 1 and 2. Although these patients may have been advancing in their disease, their rapid evolution from DM2 to DKA suggests a more acute precipitant. This rapid progression, in conjunction with the known cytokine release of COVID-19, raises the possibility that the intense cytokine release associated with COVID-19 may play a role in the insulin dysregulation seen in these patients (9,10). Another possibility is that these patients were sustaining pancreatic injury to β-islet cells, as a recent study reported a high rate of pancreatic injury in COVID-19 infection (11). It is important to note that in our case series, all patients had a relatively high hemoglobin A1c, ranging from 9.5% to 11.9%. This may suggest that suboptimal glucose control predisposes to more severe forms of COVID-19 or to the precipitation of DKA. We also note that 3 of our 5 patients were Hispanic. Although there is no pathophysiologic basis to suspect that Hispanic patients would be at higher risk for COVID-19–associated DKA, this is notable in that our hospital population is only approximately 10% Hispanic. Additional study is needed to help elucidate risk factors for the development of DKA among diabetic patients with COVID-19.
Although the definitive pathophysiology behind SARS-CoV-2 acting as a precipitant for DKA in people with DM2 is unknown, this illness pattern does have important implications. For instance, patients with DKA often rely on compensatory tachypnea for the regulation of acidosis, but this may be difficult to manage in COVID-19 patients. Due to aerosolization concerns with COVID-19 and constraints of negative pressure room supply, many centers are moving away from noninvasive positive pressure ventilation and instead using high-flow nasal cannula (HFNC) or early intubation strategies. HFNC allows providers to either avoid intubation or to optimize preoxygenation for early intubation and mechanical ventilation (12). This clinical predicament may have contributed to the poor outcome in Case 5 of our series, as shortly after intubation, the patient spiraled into profound metabolic acidosis, hemodynamic instability, and ultimately, multi-organ system failure. Another important implication of co-existing DKA and COVID-19 infection is how to manage fluid balance. Intravenous crystalloid is a staple in the resuscitation of patients with DKA, but its use has been drawn into question for patients with COVID-19 pneumonia for fear of worsening respiratory status and oxygenation. Further studies on the optimal management of concurrent acidosis and respiratory insufficiency from pneumonia in patients with DKA precipitated by COVID-19 would be of great use.
Why Should an Emergency Physician Be Aware of This?
In the midst of the current pandemic, COVID-19 infection should be considered as a possible precipitant in patients with DKA. Minute ventilation matching and judicious fluid resuscitation are essential to the management of the combined DKA and COVID-19 disease processes. It is paramount to identify patients in this cohort to protect health care workers while intervening aggressively to optimize patient outcomes.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;127:104371. [Google Scholar]
- 3.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 4.Kitabchi A.E., Umpierrez G.E., Murphy M.B., Kreisberg R.A. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739–2748. doi: 10.2337/dc06-9916. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez G.E., Kitabchi A.E. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi: 10.2165/00024677-200302020-00003. [DOI] [PubMed] [Google Scholar]
- 6.COVID-19 National Emergency Response Center Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Heal Res Perspect. 2020;11:85–90. doi: 10.24171/j.phrp.2020.11.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghadami M., Honarvar B., Sabaeian B. H1N1 influenza infection complicated with diabetic ketoacidosis. Arch Iran Med. 2012;15:55–58. [PubMed] [Google Scholar]
- 8.Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2(3):a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehman K., Akash M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J Biomed Sci. 2016;23(1) doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poston J.T., Patel B.K., Davis A.M. Management of critically ill adults with COVID-19. JAMA. 2020:E1–E3. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]