Abstract
As every country in the world struggles with the ongoing COVID-19 pandemic, it is essential that as many people as possible understand the epidemic containment, elimination and exclusion strategies required to tackle it. Simplified arithmetic models of COVID-19 transmission, control and elimination are presented in user-friendly Shiny and Excel formats that allow non-specialists to explore, query, critique and understand the containment decisions facing their country and the world at large. Although the predictive model is broadly applicable, the simulations presented are based on parameter values representative of the United Republic of Tanzania, which is still early enough in its epidemic cycle and response to avert a national catastrophe. The predictions of these models illustrate (1) why ambitious lock-down interventions to crush the curve represent the only realistic way for individual countries to contain their national-level epidemics before they turn into outright catastrophes, (2) why these need to be implemented so early, so stringently and for such extended periods, (3) why high prevalence of other pathogens causing similar symptoms to mild COVID-19 precludes the use of contact tracing as a substitute for lock down interventions to contain and eliminate epidemics, (4) why partial containment strategies intended to merely flatten the curve, by maintaining epidemics at manageably low levels, are grossly unrealistic, and (5) why local elimination may only be sustained after lock down ends if imported cases are comprehensively excluded, so international co-operation to conditionally re-open trade and travel between countries certified as free of COVID-19 represents the best strategy for motivating progress towards pandemic eradication at global level. The three sequential goals that every country needs to emphatically embrace are contain, eliminate and exclude. As recently emphasized by the World Health Organization, success will require widespread genuine national unity and unprecedented global solidarity.
Keywords: Coronavirus, COVID-19, SARS2, Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, Model, Epidemiology, Outbreak, Zoonosis, Emerging infection
Abbreviations: COVID-19, 2019 Novel Coronavirus 2
Introduction
Currently, half the world’s population is already under lock-down of some kind, meaning vertically enforced and severe restrictions of movement, and these measures may well need to be extended or re-imposed if the ongoing 2019 novel coronavirus (COVID-19) pandemic is to be contained (Chowdhury et al., 2020; Ferguson et al., 2020; Kissler, Tedijanto, Goldstein, Grad, & Lipsitch, 2020; Walker et al., 2020). Faced with such brutally difficult decisions, it is essential for policy-makers, health professionals, journalists and the general public that as many people as possible understand the stark consequences of the choices ahead of them.
Methods
Here we introduce a simplified arithmetic modelling tool for predicting COVID-19 transmission dynamics and how it is likely to respond to different containment, delay or mitigation strategies. We coin the term arithmetic modelling, as distinct from the ubiquitously used term mathematical modelling, to convey the fact that it uses only addition, subtraction, multiplication, division, rounding off, a few conditional statements (eg. if, less than/greater than, and/or), and two unavoidable power terms, to make the necessary calculations. This tool includes no differential equations, calculus, limits, distributions, stochastic simulations or agent-based approaches that would render it opaque to most non-specialist readers, such as medical and public health practitioners, decision-makers, journalists and the general public. The model is presented in user-friendly Excel® and Shiny® formats that allow non-specialists to explore, query, critique and understand the containment decisions facing their country and the world at large (https://skiware.shinyapps.io/COVID19/). For those who wish to satisfy themselves that the calculations make intuitive sense, the Excel® version provides a complementary spreadsheet format in which the formula for each cell can be critically examined. For those content to accept the underlying arithmetic, the Shiny® format provides a convenient interactive web application that can be used on any device. While a formal mathematical description of this model has been critically reviewed by specialist experts, it is provided only as an online supplement because none of the principles, assumptions or predictions are entirely new (Anderson, Heesterbeek, Klinkenberg, & Hollingsworth, 2020; Boldog et al., 2020; Chinazzi et al., 2020; Choi & Ki, 2020; De Salazar, Niehus, Taylor, Buckee, & Lipsitch, 2020; Fang, Nie, & Penny, 2020; Ferguson et al., 2020; Karako, Song, Chen, & Tang, 2020; Koo et al., 2020; Kucharski et al., 2020; Kuniya, 2020; R.; Li, Ji, et al., 2020; Lin et al., 2020; Mandal et al., 2020; Neher, Dyrdak, Druelle, Hodcroft, & Albert, 2020; Prem et al., 2020; Roosa et al., 2020b; Tang et al., 2020, Tang et al., 2020, Tang et al., 2020; Tariq et al., 2020; Walker et al., 2020; H.; Wang, Liu, et al., 2020; Wu, Leung, & Leung, 2020; Zhao & Chen, 2020), and because in our experience nothing deters non-specialists from reading an article faster than equations do.
We caution readers not to expect too much from any predictive model in terms of exact numerical reliability (Briggs, Sabel, & Lee, 2009; Christley et al., 2013; Enserink & Kupferschmidt, 2020) and note that this one is no different. We specifically advise against interpreting the exact numbers this tool generates at face value: Any predictive model is, by definition, a deliberately simplified representation of complex real-world processes, the usefulness of which is largely subjective (Briggs et al., 2009; Christley et al., 2013; Enserink & Kupferschmidt, 2020). The exact numerical predictions should therefore not be used to confidently define precise operational timelines for introducing and sustaining interventions, or set effectiveness thresholds required of specific containment measures. Instead, the purpose of this tool is to help users broadly understand the inevitable consequences of an uncontained epidemic, explore the likely outcomes a wide range of different possible containment strategies, identify those which could plausibly succeed and understand the failures of those which seem unlikely to do so.
Any users finding themselves forced to use numerical predictions from this model to make programmatic intervention decisions, presumably for want of a more reliable alternative in their specific context, should therefore assume sizable imprecisions that will not be possible to quantify prospectively and will be too late to quantify retrospectively. They should therefore factor such unknown levels of uncertainty into their response plans by allowing for wide margins for error when planning the timing, intensity and duration of new interventions, always being more ambitious and cautious whenever in any doubt.
Tanzania as an illustrative example of national containment options and requirements
Assumed input parameters values were chosen to be representative of the United Republic of Tanzania (Table 1), because it has experienced relatively modest inbound air traffic from China (Gilbert et al., 2020; Haider et al., 2020) and may still early enough in its epidemic cycle and response for a national catastrophe to be averted. Tanzania has also had more opportunity to learn from ongoing experiences in Asia, Europe and north America, and prepare by establishing testing capacity at the outset of the national epidemic, more consistent with that simplifying assumption of the model than Asian and European countries affected earlier in the pandemic would be. Tanzania is also a typically vulnerable, low-income African country (Agyeman, Laar, & Ofori-Asenso, 2020; Gilbert et al., 2020; Lloyd-Sherlock, Ebrahim, Geffen, & McKee, 2020; Makoni, 2020; Nkengasong & Mankoula, 2020; J.; Wang, Liu, et al., 2020), which had only 38 ICU beds in all four national referral hospitals combined in 2019 (Engdahl Mtango, Lugazia, Baker, Johansson, & Baker, 2019) and is representative of the pandemic that is now imminent all across Africa (Nkengasong & Mankoula, 2020; Pearson, Van Schalkwyk, Foss, O’Reilly, & Pulliam, 2020).
Table 1.
Assumed values for input parameters of the arithmetic model as intended to be representative of COVID-19 transmission and successful epidemic containment in the United Republic of Tanzania (Fig. 1). A detailed formal description of how the model calculations are made, the underlying assumptions are provided in the online methodological supplement to this paper.
| Input parameter description | Assumed value | References |
|---|---|---|
| Basic reproductive number (Average number of new infections arising from a single existing infection over its full duration if allowed to do so in a fully susceptible, immunologically naïve population in the absence of any control measures) | 4.0 | Anastassopoulou, Russo, Tsakris, & Siettos, 2020; Chen et al., 2020; Choi & Ki, 2020; Kucharski et al., 2020; Mizumoto, Kagaya, & Chowell, 2020; Read, Bridgen, Cummings, Ho, & Jewell, 2020; Roosa et al., 2020b, 2020a; Sanche et al., 2020; Shim, Tariq, Choi, Lee, & Chowell, 2020; B. Tang et al., 2020, Tang et al., 2020, Tang et al., 2020, X.; Wang et al., 2020, Wang et al., 2020; Wu et al., 2020 |
| Duration of infection (Average number of weeks an infection lasts in a human before it is eliminated by the immune system). | 3 | Hu et al., 2020; Kam et al., 2020; Linton et al., 2020; Rothe et al., 2020; A.; Tang, Tong, et al., 2020; Xing et al., 2020; Zou et al., 2020 |
| Human population size | 57 million | National Bureau of Statistics & Ministry of Finance and Planning Dar es Salaam and the Office of the Chief Government Statistician & Ministry of Finance and Planning Zanzibar, 2018 |
| Baseline incidence of unrelated similar symptoms (Proportion of population per week experiencing similar symptoms to COVID-19 but caused by other common pathogens like the common cold, influenza, malaria, etc) | 1% | BMJ Best Practice, 2020; Ghinai et al., 2020 |
| Initial importation rate (Number of new primary cases arriving into the country each week) | 5 | Assumed |
| Time to initiation of importation containment intervention at border posts, airports and ports of entry (Number of weeks since the first imported cases before inbound travellers to the country are isolated on arrival) | 2 | Assumed |
| Time to initiation of lock-down intervention (Number of weeks since the first imported cases before population-wide restrictions are introduced to prevent personal exposure behaviours) | 5 | Assumed |
| Duration of lock-down intervention (Number of weeks since initiation of population-wide restrictions to prevent personal exposure behaviours until these restrictions are lifted) | 15 | Assumed |
| Asymptomatic proportion of cases (Proportion of all cases who lack, don’t notice or don’t report any overt symptoms associated with the infection) | 50% | Gostic, Gomez, Mummah, Kucharski, & Lloyd-Smith, 2020; Li et al., 2020; Mizumoto et al., 2020; Nishiura et al., 2020, Nishiura et al., 2020; Nishiura, Linton, & Akhmetzhanov, 2020; Qiu et al., 2020; Su et al., 2020; C.; Wang, Liu, et al., 2020 |
| Proportion of symptomatic cases which are clinically severe (Percentage of all cases exhibiting and reporting with severe symptoms, all of whom are assumed to be tested unless the limits of testing capacity are exceeded) | 20% | Guan et al., 2020; C.; Wang, Liu, et al., 2020 |
| Proportion of mild and severe symptomatic cases requiring critical care (Percentage of all cases exhibiting and reporting with any mild or severe symptoms who need intensive care, which can also be described as ) | 4% | Guan et al., 2020; C.; Wang, Liu, et al., 2020; Yang et al., 2020 |
| Intensive care unit (ICU) capacity (Maximum achievable percentage of the population that could be admitted to an ICU at a given time, allowing for maximum emergency expansion of capacity at short notice) | 0.0002% | Engdahl Mtango et al. (2019) |
| Case fatality rate in ICUs (Percentage of cases needing intensive care who access it but die nevertheless) | 20% | Guan et al. (2020) |
| Case fatality rate outside ICUs (Percentage of cases needing intensive care but who cannot access it and die subsequently) | 50% | Assumed |
| Maximum achievable diagnostic testing rate (Percentage of entire population per week) | 0.02% | Assumed |
| Proportional containment of imported cases (Percentage of secondary cases arising from primary imported cases which are prevented by travel restrictions and isolation of inbound travellers from affected countries on arrival) | 100% | Assumed |
| Proportional containment of contact clusters of confirmed cases (Percentage of secondary cases arising from diagnostic-confirmed primary cases which are prevented by contact tracing and isolation) | 90% | Ghinai et al., 2020; Pung et al., 2020 |
| Proportional lock down effectiveness (Percentage reduction of exposure behaviours behaviours, eg. close personal contact, sharing venues, transport, goods and other objects, among the fraction of the population included in and compliant with the lock down interventions) | 90% | C. Wang, Liu, et al., 2020 |
| Proportional lock down coverage (Percentage of entire population included in and compliant with interventions to reduce exposure behaviours, inclusive of staying indoors, avoiding other people, wearing face masks, and frequent hand washing) | 90% | C. Wang, Liu, et al., 2020 |
Having said that, most parameter values had to be obtained from elsewhere, particularly China, where the greatest volume of formal scientific investigation thus far originates from. Parameter values were selected to approximately match reasonable median values based on the widest variety of primary literature available at the time, all of which is cited in Table 1. Literature considered for informing parameter values assumed in Table 1, and for supporting the narrative generally, were identified by searching PubMed using the search terms “coronavirus” and “epidemiology”, “transmission”, “model” or “asymptomatic” up to April 2, 2020. Only articles related to novel coronavirus-2019 and published in English were included.
Public involvement in modelling tool development
These modelling tools were initially piloted and refined by providing with minimal instruction to one banker, one human resource manager, three general practitioners, one data scientist, one media officer, one epidemiologist, one paediatric oncologist, one medical statistician and one print and web journalist, all of whom reached conclusions overlapping with those of the authors. The modelling tools and simulation results were then shared and discussed with governmental decision makers, funding partners and health sector colleagues in Tanzania and Ireland. The modelling tools provided have since been used by the journalist end-user participant to generate graphs included in published articles advocating for consideration of crush the curve national strategies to contain, eliminate and exclude COVID-19. Both authors are actively engaged in dissemination to interested stakeholders and end users in Tanzania and Ireland.
Results and discussion
COVID-19 may be eliminated and excluded by ambitious national containment campaigns
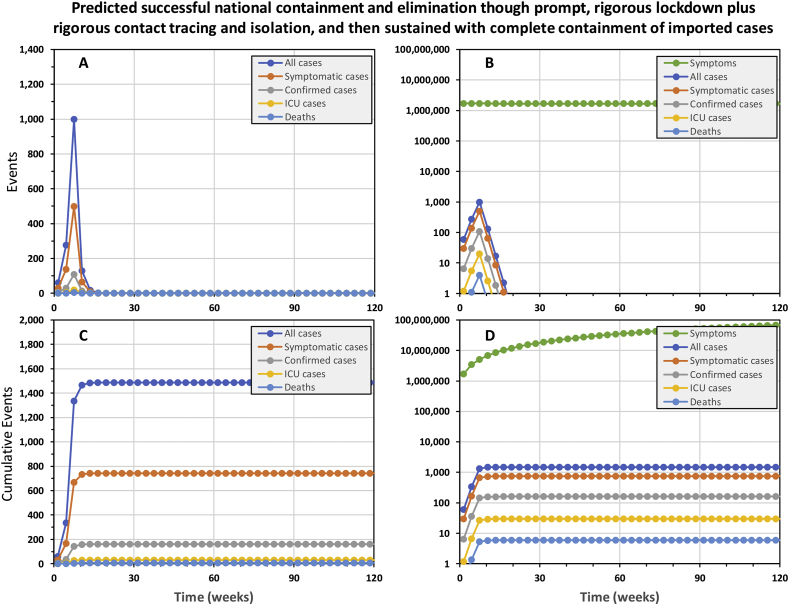
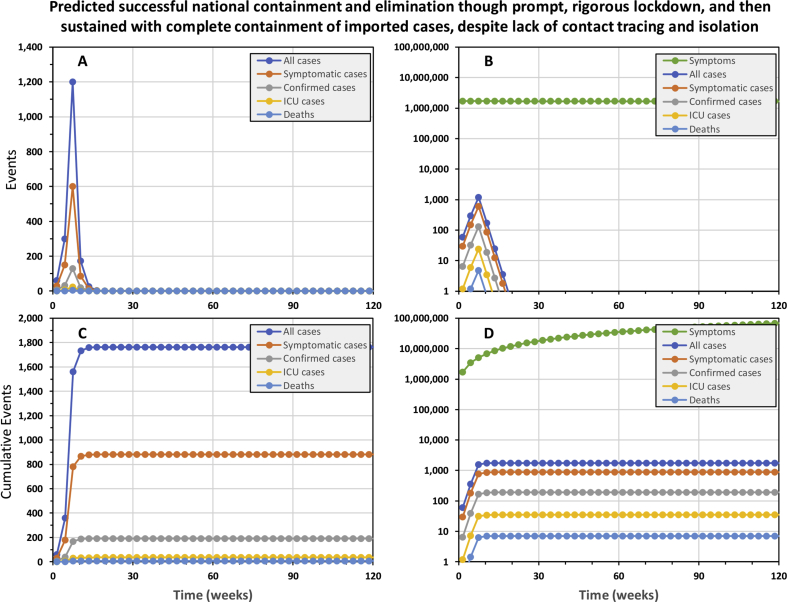
The simplified model predicts that national containment and elimination may be achieved and sustained, without ever exceeding national ICU capacity, by using a full, timely package of interventions. The national epidemic may be contained with only 1486 cases and 6 deaths by highly rigorous 15-week lockdown (90% effective exposure prevention behaviours by 90% of the population) as soon the first cases are confirmed, 5 weeks into the epidemic, complemented by 90% effective tracing and isolation of all contacts for confirmed cases (Fig. 1). National containment and elimination may indeed be achievable in principle if highly rigorous lock down can be sustained for approximately 4 months, so recently suggested strategies to crush the curve (Fineberg, 2020) appear plausible.
Fig. 1.
The predicted trajectory of a successfully contained national COVID-19 epidemic in the United Republic of Tanzania. In this simulation, a rigorous 15-week lock down was initiated from week 5 onwards and complemented by complete containment of imported cases, as well as contact tracing and isolation of confirmed cases. Rigorous lock down was assumed to achieve 90% reduction of exposure behaviours by 90% of the population. Complete 100% containment of imported cases assumes that all inbound international visitors are fully isolated for three weeks (Hu et al., 2020; Kam et al., 2020; Ling et al., 2020; Linton et al., 2020; Rothe et al., 2020; Xing et al., 2020; Zou et al., 2020), except those coming from countries that may be certified as free of local transmission by WHO in the future. Contact tracing and isolation follow up from confirmed cases was assumed to be 90% effective at preventing onward transmission from entire contact clusters.
As points of reference against which ongoing national containment campaigns may benchmark themselves, the epidemic was predicted to grow 59% bigger each week at the outset and shrink by 48% each week once rigorous lock down had been in place for several weeks. Note, however, that even these alarming projections for the rate of expansion of the epidemic and the rate of contraction required to contain it may under-represent the scale of the challenge in real epidemics. For example, at the outset of the epidemic in China, numbers of confirmed cases doubled every week (Li et al., 2020; Wu et al., 2020). Furthermore, subsequent analyses allowing for frequent carriage without overt symptoms indicate much higher viral reproduction rates than assumed in Table 1, and suggest true doubling time for all cases may be less than 3 days (Mizumoto, Kagaya, & Chowell, 2020; Sanche et al., 2020).
Interesting, almost exactly the same containment trajectory is predicted even if contact tracing and isolation is completely removed from the intervention package (Supplementary Fig. 1), resulting in only 276 more cases and one more death. The explanation for this becomes apparent when one examines the trajectories of confirmed versus all cases: Even though the number of real cases never approaches an optimistically-assumed full testing capacity of 11,400 patients per week, half of all cases are never tested because they are asymptomatic and most of the remainder are only mildly symptomatic, so they get lost in the mass of other people who appear equally sick for unrelated to COVID-19. As illustrated in Fig. 1D, the background noise of similar mild symptoms caused by other common pathogens dwarfs the mild COID-19 cases, so almost all of them go untested and undetected. Less than one in every 4000 tests is conducted on a mildly symptomatic case of COVID-19, so even though we assume all severe cases are tested, only 11% of cases predicted to occur were confirmed. With contact tracing and isolation only being possible for this very small fraction of cases, there are obvious limits to how much it can achieve as a containment intervention in its own right.
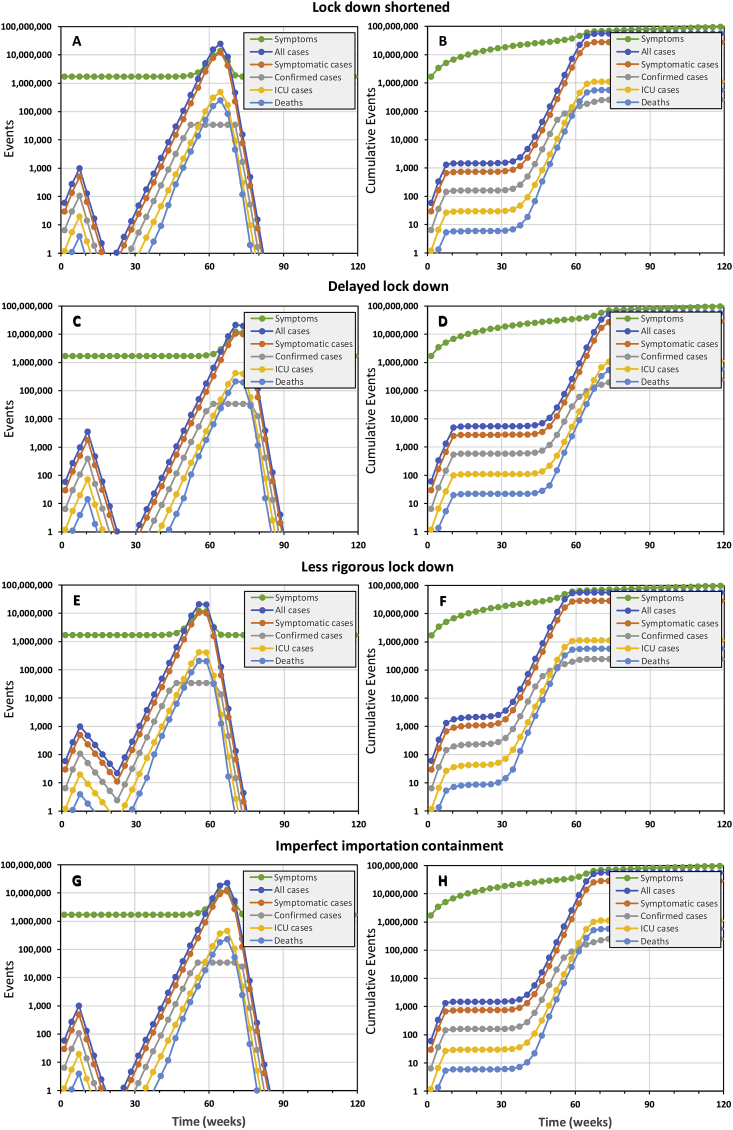
Even the slightest relaxation of lock down or importation controls cause containment failure
However, successful containment (Fig. 1) does requires that the lock down intervention is maintained for the full 15 weeks (Fig. 2A and B) to eliminate the virus. Delaying a 15-week lock-down by only 3 weeks, the duration of one generation of viral infection, also allows the virus to persist and the epidemic resumes soon afterwards (Fig. 2C and D). A slightly less rigorous lock down of the same duration, which nevertheless achieves 80% coverage with 80% reductions of personal exposure behaviours, also fails to eliminate the epidemic with tragic consequences (Fig. 2E and F).
Fig. 2.
The simulated epidemic trajectories for slightly less robust national COVID-19 epidemic containment responses in the United Republic of Tanzania than that illustrated inFig. 1, all of which are predicted to fail and result in a catastrophic rebound of transmission, morbidity and mortality. All these simulations have identical input parameters to Fig. 1 except for (1) shortening the lock down period by 3 weeks, from 15 to 12 weeks (Panels A and B), (2) delaying the lock down by 3 weeks, starting on week 8 rather than week 5 (Panels C and D), (3) reducing the coverage and protective effectiveness of exposure behaviour reduction from 90% to 80% (Panels E and F), and (4) reducing importation containment from 100% to 90% (Panels G and H).
Furthermore, elimination may only be sustained by comprehensively containing case importation from outside the country (Fig. 2G and H). Preventing reintroduction requires isolation of all incoming travellers, except those coming from countries that may be certified as free of local transmission by WHO in the future, to achieve 100% prevention of onward local transmission (Fig. 1). Even 90% containment of imported cases seems unlikely to protect the country against reintroduction of the virus and re-initiation of the epidemic (Fig. 2G and H). Tanzania therefore did the right thing by isolating all inbound travelers since March 23rd for two weeks following their arrival). However, for such importation containment measures to effectively exclude new cases from a COVID-free Tanzania in the future, isolation periods may need to be extended to three weeks (Hu et al., 2020; Kam et al., 2020; Ling et al., 2020; Linton et al., 2020; Rothe et al., 2020; Xing et al., 2020; Zou et al., 2020). However, it is also notable that all the scenarios in figure two, except for panels G and H, assume 100% effective containment of imported cases. It is therefore clear that local transmission must be eliminated before such rigorous control of inbound travellers can usefully protect the country against reintroduction.
All these delays, truncations or inadequacies of lock down, or imperfections of importation containment, result in failure to eliminate local transmission that then rebounds and rapidly spirals out of control without a second full containment campaign (Fig. 2). The implications of such an uncontained rebound scenario are essentially identical to doing nothing in the first place: In all cases, 99% of the population is expected to become infected over about a year, resulting in approximately 540,000 deaths and ICU demand exceeding capacity about 800 times over. It is also worth noting that total national hospital inpatient capacity of approximately 50,000 beds (Ministry of Health Community Development Gender Elderly and Children, 2018, p. 24) would be overwhelmed by cases of severe COID-19 disease peaking at 2.3 million over a three-week period. Under such conditions of a full-blown public health catastrophe, the mitigating effect of stronger health systems in high income countries are largely negated, so our predictions of over half a million deaths in Tanzania compare well with those of others for the United Kingdom (Ferguson et al., 2020), which has a similar population size. Considering also the travel distances and household costs of hospital attendance in Tanzania (Lyimo & Mosha, 2019; Mhalu et al., 2019; Ngowi, Kamazima, Kibusi, Gesase, & Bali, 2017; Nyamuryekung’e, Lahti, & Tuominen, 2019), it also raises the question as to whether severe COVID patients should be cared for in hospitals and other health facilities (Bryson-Cahn et al., 2020; Glauser, 2020; Mahase, 2020, 2020b) which are already 52% understaffed (Ministry of Health Community Development Gender Elderly and Children, 2019) or at home (Bryson-Cahn et al., 2020; Glauser, 2020; Mahase, 2020, 2020b) with support from a rapidly mobilized cadre of Community Health Workers, for which well-characterized curricula and training platforms already exist (Baynes et al., 2017, 2018; Ngilangwa & Mgomella, 2018).
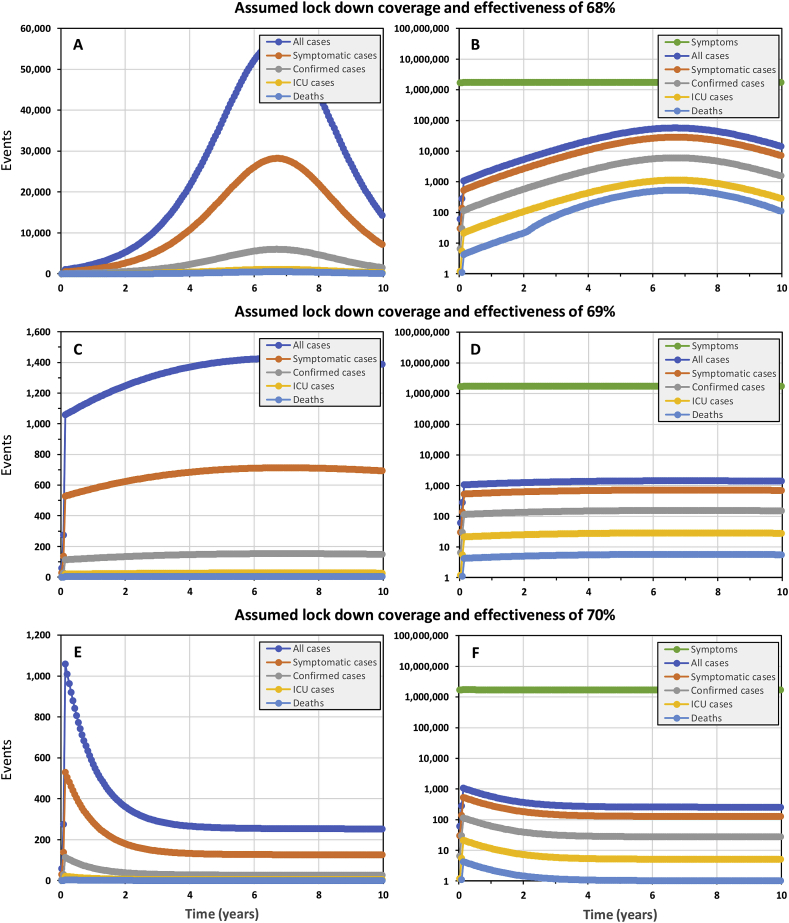
The mirage of flattening the curve to steadily acquire population-wide herd immunity
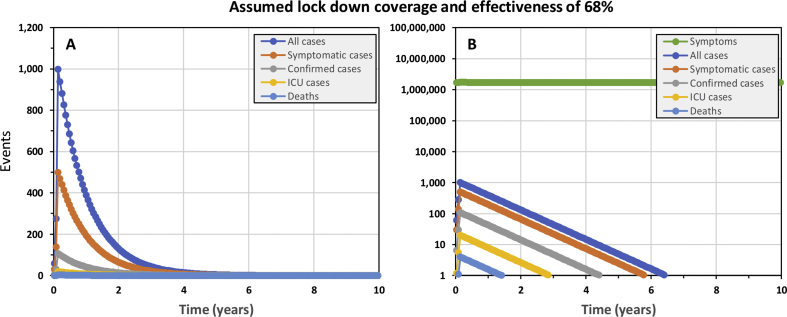
The best-case scenario we could identify for “flattening the curve”, as advocated by many national and international authorities, required removing all importation controls to ensure steady re-seeding of the epidemic with a small number of cases and relaxing lock down assumptions to exactly 69% effective reduction of exposure behaviours among 69% of the population (Fig. 3C and D). Under such precisely assumed conditions, the epidemic proceeds steadily with between 7 and 9 ICU cases per week over a decade, at the end of which national ICU capacity has never been exceeded and only 1085 deaths will have occurred. However, at the end of such a 10-year campaign, with no end in sight for at least several decades, only 0.5% of the population would have acquired hard-won immunity through prior infection, so the remainder of the population would remain just as vulnerable to a resurgent epidemic.
Fig. 3.
The simulated epidemic trajectories for a less robust national COVID-19 epidemic containment responses in the United Republic of Tanzania than that illustrated inFig. 1, intended to flatten the curve enough for national health system capacity to cope while herd immunity is acquired over the long term. All these simulations have identical input parameters to Fig. 1 except that no importation containment is assumed and the coverage and protective effectiveness of exposure behaviour reduction is assumed to be lower, at 68% (Panels A and B), 69% (Panels C and D) or 70% (Panels E and F).
However, such precise control over real epidemics, with such sensitive and extremely curved trajectories, will be unachievable in practice. Even lowering the assumed lock down coverage and effectiveness parameters for a simulated epidemic by only 1% to 68% results in a long drawn out peak that completely overwhelms ICU capacity within 3 years and continues to do so after a decade (Fig. 3A and B), nevertheless leaving 91% of the population lacking acquired immunity. On the other hand, raising assumed lock down coverage and effectiveness by only 1% to 70% results in a long drawn out containment trajectory that never reaches the elimination end game (Fig. 3E and F) because the steady trickle of imported cases sustains transmission. Re-introducing complete containment of imported cases merely results in a more extended version of Fig. 2E and F, with elimination taking over 6 years to achieve (Supplementary Fig. 2).
Perhaps more to the point, simply expressing ICU capacity as a proportion of overall population size pragmatically puts suggestions that countries should aim to merely slow and mitigate their COVID-19 epidemics into stark perspective. Even if Tanzania can build its ICU capacity from 38 to 114 beds in the coming weeks, and even if the whole population could be somehow perfectly queued up for COVID-19 exposure to make full sequential use of that capacity, assuming each patient needs only 1 week in the ICU and all regular causes of ICU admission magically disappeared, it would take almost two centuries to care for the 1.14 million COVID-19 cases expected. Readjusting such hypothetical calculations to represent higher capacity countries like Ireland or the UK shortens these timeline to decades rather than years, so “flattening the curve” to achieve population-wide “herd immunity” is clearly an infeasible and unwise choice.
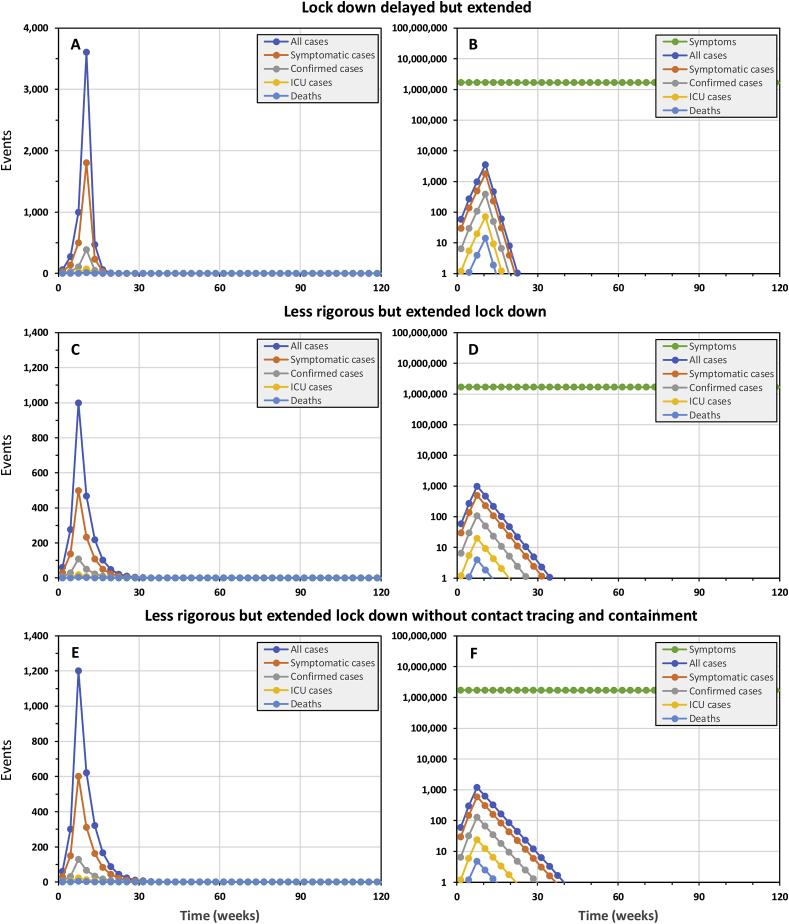
The spiralling costs of catching up on lost time to implement a lock down
If a lock down is delayed by three weeks, approximately the duration of one viral infection, the epidemic may still be contained be extending it by the same length of time, from 15 weeks to 18 weeks (Fig. 4A and B). Note, however, that the epidemic peaks at an almost four-fold higher incidence of cases, resulting in 5485 cases and 22 deaths overall. Although ICU capacity is not expected to be overwhelmed, timely access will clearly represent a challenge for many patients in country with a surface area of almost a million square kilometres and only four national referral hospitals. Longer delays of 6, 9 and 12 weeks necessitate prolonged lock downs (21 weeks for the latter) to contain epidemics of rapidly expanding scale: 19,925, 77,055 and then 260,103 cases, exceeding ICU capacity by 151, 994 and 4597 patients, and resulting in 125, 586 and 2420 fatalities, respectively.
Fig. 4.
The simulated epidemic trajectories for national COVID-19 epidemic containment responses in the United Republic of Tanzania with slightly delayed or less rigorous lock down than illustrated inFig. 1, all of which necessitated extension of the lock down period to achieve successful containment. All these simulations have identical input parameters to Fig. 1 except for (1) delaying the lock down by 3 weeks, starting on week 8 rather than week 5 (Panels A and B), (2) reducing the coverage and protective effectiveness of exposure behaviour reduction from 90% to 80% (Panels C, D, E and F), (3) removing the contact tracing and isolation component (Panels E and F), and (4) necessarily extending the lock down period from 15 to 18 weeks (Panels A and B) or from 15 to 40 weeks (Panels C, D, E and F).
The hidden dangers of stealthy epidemics
Note, however, that none of this will be obvious during the silent early phase of the epidemic, during which time the number of undetected cases snowballs: Even if the lock down response is initiated after only 5 weeks post-initiation, immediately after the first 6 cases are confirmed in this simulation, the epidemic has already quietly progressed much further than most members of the public would guess. Indeed, far enough that another 271 people are already actively infected and almost 1000 new cases are predicted to occur in the subsequent 3-week period, out of which only 108 (11%) will be detected.
Infectious carriers who exhibit little or no symptoms at the time (COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, & Korea Centers for Disease Control and Prevention, 2020; Dong et al., 2020; Du et al., 2020; Hoehl et al., 2020; Hu et al., 2020; Kam et al., 2020; Ki and Task Force for 2019-nCoV, 2020; Lai et al., 2020; C. Li, Ji, et al., 2020; Li et al., 2020; Lu et al., 2020; Mizumoto, Kagaya, Zarebski, et al., 2020; Nishiura, Kobayashi, et al., 2020; Nishiura, Kobayashi, et al., 2020; Qiu et al., 2020; Rothe et al., 2020; Shi et al., 2020; Su et al., 2020; A. Tang, Tong, et al., 2020; Tian et al., 2020; C. Wang, Liu, et al., 2020; Zhang et al., 2020, Zhu, Zhang, Han, & Huang, 2020; Zou et al., 2020) clearly contribute to the cryptic nature of an early-stage COVID-19 epidemic: In this case we assumed this accounts for 50% of cases lacking symptoms overt enough to consider self-reporting and seeking a test (Table 1). However, a much more important factor is the sheer volume of background noise arising from similar symptoms caused by more common pathogens, such as the common cold and malaria. Even though these simulations assume that capacity for conducting 11,400 COVID-19 tests per week would have been established in Tanzania before the outbreak began, total confirmed cases are only expected to exceed 100 about 3 weeks after the lock down is introduced. Most of these confirmed cases are accounted for by the clinically severe fraction we assume will all be tested. Only 4% (8/899) of predicted mild or asymptomatic cases are expected to be confirmed because the relatively small number of COVID-19 cases are so easy to miss in a population of 57 million people, out of whom we assume 1% or 570,000 will experience a fever, cough or stomach pains in any given week for unrelated reasons (Fig. 1B and D). Note, however, that even this is a very conservative assumption about background rates of illness with similar symptoms to COVID-19: In the first contact-tracing study in the USA, over 12% of all carefully-followed contacts became symptomatic within 2 weeks, even though none of them became infected with COVID-19 (Ghinai et al., 2020).
These simulations are nevertheless useful in that they illustrate how no perceptible increase in the incidence of such common symptoms may be obvious to the general population unless containment efforts fail and a full-scale, resurgent epidemic sweeps through the country (Fig. 2). Tanzania therefore did exactly the right thing by reacting fast during the silent earliest phase of the epidemic, announcing school closures within a day of the first confirmed case report and introducing additional restrictions immediately afterwards.
Note, however, that the quiet tail of a fading epidemic may be just as dangerous as the it’s silent onset. Three of the four scenarios in Fig. 2 include periods of two month or more when few if any confirmed cases are expected, but some mild cases persist that can re-seed the whole epidemic afresh. The predicted persistence of the epidemic despite total predicted cases dropping below zero is an artefact of the simplified deterministic form of the model, which calculates case numbers as a continuous decimal outcome until it drops below 0.1, at which point it is set to 0 because the probability of elimination is 90% or better. The take-home message is nevertheless clear: persist with rigorous lock-down until one can be sure that elimination has been achieved, drawing on statistical approaches used by veterinary epidemiologists to certify elimination with imperfect surveillance systems (A. Cameron, Njeumi, Chibeu, & Martin, 2014; A. R. Cameron & Baldock, 1998a, 1998b; Martin et al., 2007, Martin et al., 2007; Stresman, Cameron, & Drakeley, 2017).
The vital importance of ambition and rigour to lockdown outcome: who dares loses least!
As illustrated by Fig. 4C and D, it is now crucial that Tanzania urgently builds on that early momentum to ramp up lock down efforts to the most rigorous level practically attainable. The implications of even a slightly less rigorous lock down appear less daunting in epidemiological terms but far more severe in practical and economic terms, because it greatly prolongs the lockdown period required: Even reducing coverage and effectiveness of personal protection measures by only 10%, from 90% to 80% requires that the lock down period is extended by more than 150%, from 15 weeks to 40 weeks (Fig. 4C and D). The practical social and economic sustainability of such a protracted lock down period is very questionable, but much can be learned from the predicted benefits of getting such an imperfect lock down started in good time: The 1108 cases and 11 deaths predicted over the course of such a “slow burn” containment campaign are only marginally higher than for the best case scenario illustrated in Fig. 1. It is therefore important to get some form of reasonably rigorous lock down in place as early as possible, and then intensify it as rapidly as possible. Like any race, it is critical to get an early head-start by any means possible, but then build up speed towards a strong finish.
While compliance and enforcement is of great importance to lock down effectiveness, so is acceptability and socio-economic feasibility. While high income countries move to facilitate population-wide compliance with direct financial support and augmented social services, different tactics will be required in low income countries like Tanzania. Although Tanzania is urbanizing very rapidly, most of the population still resides in rural areas (National Bureau of Statistics, 2018a, National Bureau of Statistics, 2018) where propagation of directly-transmitted diseases like COVID-19 is always less intense. Fortunately, Tanzania is currently in the midst of this year’s farming season, during which many rural families are out in the fields where social distancing is relatively easy. While farming season also conveniently brings a lull in trading activity at commercial hubs in rural towns and villages, it also represents a seasonal low point in the domestic food reserves of many rural households, so selective food support may be invaluable for enabling the most vulnerable families to comply effectively with self-quarantine and self-isolation directives. However, the growing urban population represents a much larger challenge, because far fewer people rely on farming for their livelihoods. Many live in crowded informal settlements where they lack shelter, water, sanitation and space, relying on unreliable, informal sources of income to survive on a day-to-day basis. Informal livelihoods and settlements in the busiest urban centres of the country will therefore require particularly urgent attention and creativity, to support daily food, water and hygiene needs. It may also be useful to consider providing safe transport with managed social distancing (to be followed by self-isolation) for those with options to sit out the epidemic with family and friends in rural areas.
As is the case for elimination of other diseases, such as malaria for example (Killeen et al., 2013), it may be more useful to think about gaps in coverage and effectiveness to understand how such apparently minor deficiencies can make all the difference between success and failure: While a shift from 90% to 80% lock down coverage and effectiveness might seems small in relative terms, a 20% shortfall relative to perfect containment is twice as big as 10%. And the difference between 100% prevention of onward local transmission from imported cases contrasts starkly with even such high targets as 90%: when you need to achieve zero new cases in a country, any other number simply isn’t good enough.
In practical terms, we should first think of most the vulnerable, such those lacking homes, shelter, security, citizenship or family support (Ahmed, Ahmed, Pissarides, & Stiglitz, 2020; Liem, Wang, Wariyanti, Latkin, & Hall, 2020; Poole, Escudero, Gostin, Leblang, & Talbot, 2020), especially those in the low income countries at greatest risk (Agyeman et al., 2020; Ahmed et al., 2020; Gilbert et al., 2020; Lloyd-Sherlock, Ebrahim, Geffen, & McKee, 2020; J.; Wang, Liu, et al., 2020). Beyond these long-neglected population groups, the most important lock down coverage and effectiveness gaps will be accounted for by the most important exceptions to restrictions and those exceptional individuals most determined to evade them.
Unfortunately, the most obvious exceptions to lock down restrictions, who will facilitate continued transmission, will be health service personnel (Bedford et al., 2020; Bhadelia, 2020; Rose, 2020; Z.; Zhang et al., 2020, Wang et al., 2020), notably those caring for those most vulnerable to the disease. However, all other essential workers in shops, markets, kitchens, food processing facilities, factories, banks, post offices, transport services and law enforcement agencies will also inevitably mediate more transmission than they would if they stayed at home. Indeed, it was the crew that enabled self-sustaining levels of COVID-19 transmission to persist aboard the quarantined Diamond Princess cruise ship (Mizumoto & Chowell, 2020). It is also worth remembering that the anti-hero of infectious disease epidemiology, the infamous Typhoid Mary, worked in the service industry and might be classified as an essential worker today (Box 1). This is not to say that such essential services should necessarily be suspended, but rather that the roles and working practices of these personnel should be scrutinized particularly carefully. How essential is essential? What is the minimum level of service needed to facilitate extended lock down while mitigating indirect effects on health, well-being and economic welfare that are even worse than COVID-19? What procedures, behaviours and protective equipment could most effectively minimize persistent workplace transmission?
Box 1. Typhoid Mary as an historical example of the kind of extremely non-compliant, asymptomatic, super-spreader individual who might evade lock down as an essential worker today.
Typhoid Mary (real name Mary Mallon, from Tyrone in Northern Ireland) was a cook by profession who infected at least 53 people, three of whom died (Soper, 1939). Like many COVID-19 cases (COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, & Korea Centers for Disease Control and Prevention, 2020; Dong et al., 2020; Du et al., 2020; Hoehl et al., 2020; Hu et al., 2020; Kam et al., 2020; Ki and Task Force for 2019-nCoV, 2020; Lai et al., 2020; C. Li, Ji, et al., 2020; P. Li, Ji, et al., 2020; Lu et al., 2020; Mizumoto, Kagaya, Zarebski, et al., 2020; Nishiura, Kobayashi, et al., 2020; Nishiura, Kobayashi, et al., 2020; Qiu et al., 2020; Rothe et al., 2020; Shi et al., 2020; Su et al., 2020; A. Tang, Tong, et al., 2020; Tian et al., 2020; C. Wang, Liu, et al., 2020; Zhu, Zhang, Han, & Huang, 2020; Zou et al., 2020), Mary was a silent carrier of the disease: She herself lived to a ripe old age and died of a stroke rather than typhoid. Mary repeatedly returned to working as a cook because it paid better and frequently changed jobs as people fell ill around her, even changing her name to evade more than 30 years of quarantines imposed on her. It took 4 policemen over 3 h to apprehend her despite a stealthy approach and forced entry to her home. Eventually Mary was found hiding in an outside closet at the rear of a neighbour’s house, and things remained spicy following her arrest:
“She fought and struggled and cursed. I tried to explain to her that I only wanted the specimens and that then she could go back home. She again refused and I told the policemen to pick her up and put her in the ambulance. This we did and the ride down to the hospital was quite a wild one.” (Soper, 1939)
When we read about the ongoing “Coronavirus challenge” game mediated through social media, or of groups of grown adults meeting to share a few drinks in public places during official lock down periods, we are inclined to think the spirit of Mary Mallon is alive and well and will need to be curbed. The experiences of those who knew Mary Mallon seem extreme but are difficult to disregard completely in the context of a pandemic threatening a global population of over 8 billion people with more eccentric characters, miscreants and outright criminals than we would wish in the circumstances:
“Mary was now about forty-eight years of age and a good deal heavier than she was when she slipped through a kitchen full of servants, jumped the back fence and put up a fight with strong young policemen. She was as strong as ever, but she had lost something of that remarkable energy and activity which had characterized her young days and urged her forward to meet undaunted whatever situation the world presented to her. In these eight years since she was first arrested, she had learned what it was to yield to other wills than her own and to know pain.” (Soper, 1939)
Alt-text: Box 1
And with so many people’s livelihoods on the lines, we may be asking too much of human nature by expecting everyone to do the right thing voluntarily. Many of the greatest public health campaigns in history have necessitated an authoritarian style, and it may be necessary for people all over the world to temporarily embrace and accept new restriction measures they would otherwise justifiably describe as draconian. Perhaps the single most important take-home message of the widely-accepted 80–20 rule of epidemiology (less than 20% of people cause more than 80% of transmission) (Woolhouse et al., 1997) is that the extremes of human circumstances and behaviour, especially during mass gatherings and population movements, are more important to the survival of pathogens than the average. It inevitably follows that such exceptions are vitally important to target if one wishes to eliminate COVID-19 (S. Chen, Yang, Yang, Wang, & Barnighausen, 2020; Ebrahim & Memish, 2020; Frieden & Lee, 2020; Liu, Eggo, & Kucharski, 2020). Again, it is worth remembering Typhoid Mary (Soper, 1939), who resisted repeated efforts to get her out of the kitchen and did nothing to disprove stereotypes about the stubborn Irish (Box 1).
Contact tracing as an epidemiological surveillance platform, rather than an intervention per se
As for the full, timely intervention package simulated in Fig. 1 (Compare with Fig. S1), removing contact tracing from the less rigorous but extended lock down intervention package has only a modest effect on the overall containment trajectory (Fig. 4E and F), with only 797 more cases and 3 additional fatalities. Under the far more extreme conditions of a failed containment campaign, followed by a resurgent, full-blown epidemic, contact tracing becomes a rather pointless exercise, even for targeting clinical disease management. At the peak of the epidemic, over 4 million new symptomatic cases may occur per week and even mortality rate may outstrip testing capacity (Fig. 2), so case confirmation success rates may plummet to below 0.2%.
However, such spectacular containment failures are to be avoided at all costs and this simplified model only accounts for the direct preventative effects of follow up on subsequent transmission, so none of these simulations should be used to in any way imply that contact tracing and isolation should be de-prioritized. In particular, it does not account for the invaluable functions of contact tracing for monitoring and characterizing an epidemic (Ghinai et al., 2020; C.; Li, Ji, et al., 2020; P.; Li, Ji, et al., 2020; Lu et al., 2020; Pung et al., 2020; Zhu, Zhang, Han, & Huang, 2020), and for understanding the influence of interventions on transmission dynamics. For example, the tracing of transmission to a relatively small number of clusters in Korea, and especially the incrimination of venues like the Shincheonji Church provide invaluable insights that guide more rigorous, effective follow up on lock down measures (Shim et al., 2020) In Ireland, early observations that mean size of close contact clusters had shrunk from 20 to 5 were reported to the public as an encouraging early sign that behavioural interventions were impacting risks of onward transmission. Without such essential detailed information about how transmission persists, as well as the strengths and weakness of ongoing intervention efforts, national containment programmes would be flying blind.
It should be noted, however, that testing, contact tracing and isolation of known contacts is only useful as part of a deliberate containment strategy that keeps an epidemic manageably small, and may be particularly useful for extinguishing the remaining embers of an effectively-contained epidemic (B. Tang, F. Xia et al., 2020). While testing is always useful for clinical management of severe cases, once 1% or more of the population has been infected even this important subset of cases alone overwhelms testing capacity (Fig. 2B, D, E and F) and the fraction of non-severe cases confirmed plummets to negligible levels. In any case, population-wide testing of mildly symptomatic cases becomes unhelpful as a guide to targeting containment measures: How does one selectively target those at immediate risk when that means everyone? And how would we attempt contract tracing if we allowed the epidemic to grow to tens or hundreds of thousands of new cases each week? Note, however, that the expected failure of contact tracing, and indeed testing generally, is just one more good reason to contain national COVID-19 epidemics before they progress from emergencies (Fig. 1, Fig. 4) into outright catastrophes (Fig. 2).
Limitations, caveats and comparisons with other models
Like other recent models of COVID-19, our simplified formulation does not attempt to predict complex indirect effects of the pandemic upon morbidity and mortality from other causes that will be exacerbated by the expected pressures on a health system that is already overstretched (Enserink & Kupferschmidt, 2020). Nor does it attempt to anticipate the extent of economic and social damage that will arise from different epidemic containment scenarios (Enserink & Kupferschmidt, 2020), partly because doing so would defeat the purpose of developing a simplified arithmetic formulation.
Despite its limitations as a relatively simple and untested model, the predictions described above are consistent with those of most other process-explicit models using more sophisticated mathematical formulations and specialist software (Anderson et al., 2020; Boldog et al., 2020; Chinazzi et al., 2020; Choi & Ki, 2020; Chowdhury et al., 2020; De Salazar et al., 2020; Fang et al., 2020; Ferguson et al., 2020; Karako et al., 2020; Koo et al., 2020; Kucharski et al., 2020; Kuniya, 2020; R.; Li, Ji, et al., 2020; Lin et al., 2020; Mandal et al., 2020; Neher et al., 2020; Prem et al., 2020; Roosa et al., 2020b; B. Tang et al., 2020, Tang et al., 2020, Tang et al., 2020; Tariq et al., 2020; Walker et al., 2020, p. 19; H.; Wang, Liu, et al., 2020; Wu et al., 2020; Zhao & Chen, 2020), as well as recent reports of success from China (Gong et al., 2020; Prem et al., 2020; Tang et al., 2020; C.; Wang, Liu, et al., 2020; S.; Zhang et al., 2020) In fact, perhaps the most useful new lessons to be learned come from a few studies that reach substantively different conclusions based on markedly different underlying assumptions.
Our predictions that contact tracing and isolation will play only a minor role in successful containment contrast with those of others (Hellewell et al., 2020) which assumed asymptomatic carriage by only 10% of cases or less, and which do not account for the detection dilution effect of similar mild symptoms caused by other common pathogens (Fig. 2B, D, F, H).
On the other hand, the predictions presented here appear relatively optimistic when compared with recent reports suggesting viral reproduction rates are higher than generally thought (Mizumoto, Kagaya, & Chowell, 2020; Sanche et al., 2020) because previous analyses failed to consider the likelihood that large fractions of cases may go undetected (Dong et al., 2020; Mizumoto, Kagaya, Zarebski, et al., 2020; Nishiura, Kobayashi, et al., 2020; Nishiura, Kobayashi, et al., 2020; Qiu et al., 2020; Su et al., 2020; C.; Wang, Liu, et al., 2020) because they exhibit only mild, non-specific symptoms, if any (COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, & Korea Centers for Disease Control and Prevention, 2020; Hoehl et al., 2020; Hu et al., 2020; Kam et al., 2020; Ki and Task Force for 2019-nCoV, 2020; Lai et al., 2020; C. Li, Ji, et al., 2020; Lu et al., 2020; Nishiura, Kobayashi, et al., 2020; Qiu et al., 2020; Rothe et al., 2020; Shi et al., 2020; Su et al., 2020; A. Tang, Tong, et al., 2020; Tian et al., 2020; Zhu, Zhang, Han, & Huang, 2020; Zou et al., 2020). As underlined right at the outset of the global response (Q. Li, Ji, et al., 2020; Munster, Koopmans, van Doremalen, van Riel, & de Wit, 2020), the most important remaining question that needs to be answered to reduce the uncertainties of model predictions is the extent of asymptomatic carriage and infectiousness. Learning lessons from other diseases like endemic malaria, which is primarily a chronic illness transmitted by semi-immune adult carriers (Ross, Killeen, & Smith, 2006), the term asymptomatic may well be a misnomer, not only because some individuals become infectious before (COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, & Korea Centers for Disease Control and Prevention, 2020; Du et al., 2020; Hoehl et al., 2020; Hu et al., 2020; P. Li, Ji, et al., 2020; Nishiura, Linton, et al., 2020; Rothe et al., 2020; Zhu, Zhang, Han, & Huang, 2020) or after (Hu et al., 2020; Kam et al., 2020; Ling et al., 2020; Linton et al., 2020; Rothe et al., 2020; Xing et al., 2020; Zou et al., 2020) exhibiting symptoms but also because it is often applied to those who are paucisymptomatic and shrug off mild symptoms to get on with their daily lives (I. Chen et al., 2016; Sifft et al., 2016; C. Wang, Liu, et al., 2020).
A particularly important caveat arising from current uncertainty about the role of cryptic carriers is that it also has a major influence on estimation of fatality rate for infections rather than clinical cases. The latest analyses allowing for this phenomenon suggest that fatality rates are may be 10 to 40 times lower per infection than per confirmed case (Anastassopoulou et al., 2020; Mizumoto, Kagaya, & Chowell, 2020), consistent with our conclusion that the vast majority of cases are never confirmed. While fatality rates are difficult to estimate directly (Battegay et al., 2020; Kobayashi et al., 2020), these modelling analyses support the conclusions of the most controlled empirical epidemiological studies, indicating that the COVID-19 fatality rates may be comfortably below 1% (Nishiura, Kobayashi, et al., 2020). The surge of severe cases and fatalities in an uncontained epidemic may therefore peak at a far lower level than those predicted in Fig. 2. However, much of the variation between fatality rate estimates appears related to geographic differences in health system capacity and burden (Battegay et al., 2020; Ji, Ma, Peppelenbosch, & Pan, 2020), so low income countries will be much more vulnerable. Even if the best worst-case scenario proves to be less catastrophic than previously projected (Anastassopoulou et al., 2020; Mizumoto, Kagaya, & Chowell, 2020), it will nevertheless overwhelm critical care capacity several times over and should be avoided if at all possible.
Conclusions
The current global health emergency demands immediate, bold, pre-emptive decisions in the absence of unambiguous evidence (Horton, 2018; Smith & Pell, 2003), based on our best understanding of COVID-19 epidemiology as it stands today (Anderson et al., 2020; Fauci, Lane, & Redfield, 2020; Horton, 2020). The three key sequential goals every country needs to embrace as early and emphatically as possible are contain, eliminate and exclude. Even when faced with the prospect of lock downs lasting 4 months or more, there is no place for more timid terms like slow, flatten or mitigate when faced with an epidemic capable of overwhelming ICU capacity hundreds of times over or taking several years of restrictions to slowly burn through an entire population at rates that ICUs can cope with.
And tackling this pandemic will rely overwhelmingly upon widespread understanding and mass participation by the entire global public, rather than just the health professionals and high-level decision makers who will lead the response. Currently, half the world’s population is already under lock down of some kind, meaning vertically enforced and severe restrictions of movement and physical interaction, and the remainder will have to follow if the ongoing COVID-19 virus pandemic is to be contained. Faced with such brutally difficult decisions, it is essential to policy-makers, health professionals and the general public that as many people as possible understand why stringent lock down interventions represent the only realistic way for individual countries to contain their national-level epidemics before they turn into public health catastrophes. It also vital for as many people as possible to understand why these need to be implemented so early, so aggressively and for such extended periods, to deliberately crush the curve (Fineberg, 2020) of epidemic trajectories.
Over the medium-to-long term, it will also be vital for us all to understand why widespread decisive national action and international co-operation (Gong et al., 2020; S.; Zhang et al., 2020) will be required to conditionally re-open trade and travel between countries that have successfully eliminated local transmission. As explained by the simplified simulations presented here, this appears to be the only means by which national elimination efforts can be sustained, following which pandemic eradication may be pursued at global level. At a time when so many decision-makers are considering tempting justifications for relaxing unpopular lock down restrictions, it is now vital that the governments and citizens of every country instead embrace intensified containment, elimination and exclusion efforts. Unless we all respond constructively to the recent World Health Organization appeal for genuine national unity and global solidarity (World Health Organization, 2020), it appears unlikely that we can collectively defeat the COVID-19 pandemic.
Funding
No funding was received from any source for the preparation of this article but GFK is supported by an AXA Research Chair award and Irish Aid (Department of Foreign Affairs and Trade, Government of Ireland) supported the open access publication costs of the study through the Embassy of Ireland in Tanzania (Award number AI-TAN/2020/086). The ideas, opinions and comments of the authors are entirely their own responsibility and do not necessarily represent or reflect Irish Aid policy.
Author contribution
GFK wrote the first draft of the model in Excel format and the manuscript. SSK edited the model and the manuscript, drafted the formal mathematical description of the model, and coded the Shiny format of the model. Both authors reviewed and edited the final version of the manuscript and agree to its submission and publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Kim Mwalemo, Isaac Lyatuu, Jerry Hella, Eveline Geubbels, Bilal Aziz, Adrian Fitzgerald, Patricia Scanlan, David Hanley, Aisling Eddy-Mooney, Aoife Cuddy, Anthony Fitzgerald and Rachel Lavin for participating as end users and advisers.
Handling editor:
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.idm.2020.06.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
The predicted trajectory of a successfully contained national COVID-19 epidemic in the United Republic of Tanzania, achieved without any contact tracing and isolation. In this simulation, a rigorous 15-week lock down was initiated from week 5 onwards and complemented by complete containment of imported cases, as well as contact tracing and isolation of confirmed cases. Rigorous lock down was assumed to achieve 90% reduction of exposure behaviours by 90% of the population. Complete 100% containment of imported cases assumes that all inbound international visitors are fully isolated for three weeks (Hu et al., 2020; Kam et al., 2020; Ling et al., 2020; Linton et al., 2020; Rothe et al., 2020; Xing et al., 2020; Zou et al., 2020), except those coming from countries that may be certified as free of local transmission by WHO in the future. However, these simulations differ from Fig. 1 in that absolutely no contact tracing and isolation was assumed.
Supplementary Fig. 2.
The simulated epidemic trajectory for a less robust national COVID-19 epidemic containment responses in the United Republic of Tanzania than that illustrated inFig. 1, intended to flatten the curve enough for national health system capacity to cope while also excluding importation of new cases. This simulation has identical input parameters to Fig. 3E and F except that complete importation containment is assumed.
References
- Agyeman A.A., Laar A., Ofori-Asenso R. Will COVID-19 be a litmus test for post-Ebola sub-Saharan Africa? Journal of Medical Virology. 2020 doi: 10.1002/jmv.25780. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Ahmed N., Pissarides C., Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5 doi: 10.1016/S2468-2667(20)30085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassopoulou C., Russo L., Tsakris A., Siettos C. Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PloS One. 2020;15 doi: 10.1371/journal.pone.0230405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay M., Kuehl R., Tschudin-Sutter S., Hirsch H.H., Widmer A.F., Neher R.A. 2019-novel coronavirus (2019-nCoV): Estimating the case fatality rate - a word of caution. Swiss Medical Weekly. 2020;150:w20203. doi: 10.4414/smw.2020.20203. [DOI] [PubMed] [Google Scholar]
- Baynes C., Mboya D., Likasi S., Maganga D., Pemba S., Baraka J. Quality of sick child-care delivered by community health workers in Tanzania. International Journal of Health Policy and Management. 2018;7:1097–1109. doi: 10.15171/ijhpm.2018.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes C., Semu H., Baraka J., Mushi H., Ramsey K., Kante A.M. An exploration of the feasibility, acceptability, and effectiveness of professional, multitasked community health workers in Tanzania. Global Public Health. 2017;12:1018–1032. doi: 10.1080/17441692.2015.1080750. [DOI] [PubMed] [Google Scholar]
- Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G. COVID-19: Towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadelia N. Coronavirus: Hospitals must learn from past pandemics. Nature. 2020;578:193. doi: 10.1038/d41586-020-00354-4. [DOI] [PubMed] [Google Scholar]
- BMJ Best Practice Common cold. British Medical Journal. 2020 [Google Scholar]
- Boldog P., Tekeli T., Vizi Z., Denes A., Bartha F.A., Rost G. Risk assessment of novel coronavirus COVID-19 outbreaks outside China. Journal of Clinical Medicine. 2020;9:571. doi: 10.3390/jcm9020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs D.J., Sabel C.E., Lee K. Uncertainty in epidemiology and health risk and impact assessment. Environmental Geochemistry and Health. 2009;31:189–203. doi: 10.1007/s10653-008-9214-5. [DOI] [PubMed] [Google Scholar]
- Bryson-Cahn C., Duchin J., Makarewicz V.A., Kay M., Rietberg K., Napolitano N. A novel approach for a novel pathogen: Using a home assessment team to evaluate patients for 2019 novel coronavirus (SARS-CoV-2) Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa256. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A.R., Baldock F.C. A new probability formula for surveys to substantiate freedom from disease. Preventive Veterinary Medicine. 1998;34:1–17. doi: 10.1016/s0167-5877(97)00081-0. [DOI] [PubMed] [Google Scholar]
- Cameron A.R., Baldock F.C. Two-stage sampling in surveys to substantiate freedom from disease. Preventive Veterinary Medicine. 1998;34:19–30. doi: 10.1016/s0167-5877(97)00073-1. [DOI] [PubMed] [Google Scholar]
- Cameron A., Njeumi F., Chibeu D., Martin T. Food and Agriculture Organization of the United Nations; 2014. Risk-based disease surveillance: A manual for veterinarians on the design and analysis of surveillance for demonstration of freedom from disease. [Google Scholar]
- Chen I., Clarke S.E., Gosling R., Hamainza B., Killeen G., Magill A. “Asymptomatic” malaria: A chronic and debilitating infection that should be treated. PLoS Medicine. 2016;13 doi: 10.1371/journal.pmed.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.M., Rui J., Wang Q.P., Zhao Z.Y., Cui J.A., Yin L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect Dis Poverty. 2020;9:24. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yang J., Yang W., Wang C., Barnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395:764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.C., Ki M. Estimating the reproductive number and the outbreak size of Novel Coronavirus disease (COVID-19) using mathematical model in Republic of Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Heng K., Shawon M.S.R., Goh G., Okonofua D., Ochoa-Rosales C. Dynamic interventions to control COVID-19 pandemic: A multivariate prediction modelling study comparing 16 worldwide countries. European Journal of Epidemiology. 2020;35:389–399. doi: 10.1007/s10654-020-00649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christley R.M., Mort M., Wynne B., Wastling J.M., Heathwaite A.L., Pickup R. Wrong, but useful": Negotiating uncertainty in infectious disease modelling. PloS One. 2013;8 doi: 10.1371/journal.pone.0076277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020;11:8–14. doi: 10.24171/j.phrp.2020.11.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Salazar P.M., Niehus R., Taylor A., Buckee C.O., Lipsitch M. Identifying locations with possible undetected imported severe acute respiratory syndrome coronavirus 2 cases by using importation predictions. Emerging Infectious Diseases. 2020;26:1465–1469. doi: 10.3201/eid2607.200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Cao Y.Y., Lu X.X., Zhang J.J., Du H., Yan Y.Q. Eleven faces of coronavirus disease 2019. Allergy. 2020 doi: 10.1111/all.14289. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Xu X., Wu Y., Wang L., Cowling B.J., Meyers L.A. Serial interval of COVID-19 among publicly reported confirmed cases. Emerging Infectious Diseases. 2020;26:1341–1343. doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S.H., Memish Z.A. COVID-19: Preparing for superspreader potential among umrah pilgrims to Saudi arabia. Lancet. 2020;395:e48. doi: 10.1016/S0140-6736(20)30466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl Mtango S., Lugazia E., Baker U., Johansson Y., Baker T. Referral and admission to intensive care: A qualitative study of doctors’ practices in a Tanzanian university hospital. PloS One. 2019;14 doi: 10.1371/journal.pone.0224355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M., Kupferschmidt K. With COVID-19, modeling takes on life and death importance. Science. 2020;367:1414–1415. doi: 10.1126/science.367.6485.1414-b. [DOI] [PubMed] [Google Scholar]
- Fang Y., Nie Y., Penny M. Transmission dynamics of the COVID-19 outbreak and effectiveness of government interventions: A data-driven analysis. Journal of Medical Virology. 2020;92:645–659. doi: 10.1002/jmv.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. New England Journal of Medicine. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Laydon D., Nedjati-Gilani G., Imai N., Ainslie K., Baguelin M. Abdul Latif Jameel Institute for Disease and Emergency Analytics & Imperial College London; 2020. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand (pp. 20): WHO collaborating centre for infectious disease modelling, MRC centre for global infectious disease analysis. [Google Scholar]
- Fineberg H.V. Ten weeks to crush the curve. New England Journal of Medicine. 2020;382:e37. doi: 10.1056/NEJMe2007263. [DOI] [PubMed] [Google Scholar]
- Frieden T.R., Lee C.T. Identifying and interrupting superspreading events-implications for control of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases. 2020;26:1059–1066. doi: 10.3201/eid2606.200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M., Pullano G., Pinotti F., Valdano E., Poletto C., Boelle P.Y. Preparedness and vulnerability of African countries against importations of COVID-19: A modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser W. Proposed protocol to keep COVID-19 out of hospitals. Canadian Medical Association Journal. 2020;192:E264–E265. doi: 10.1503/cmaj.1095852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F., Xiong Y., Xiao J., Lin L., Liu X., Wang D. China’s local governments are combating COVID-19 with unprecedented responses - from a Wenzhou governance perspective. Frontiers of Medicine. 2020;14:220–224. doi: 10.1007/s11684-020-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostic K., Gomez A.C., Mummah R.O., Kucharski A.J., Lloyd-Smith J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;99 doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider N., Yavlinsky A., Simons D., Osman A.Y., Ntoumi F., Zumla A. Passengers’ destinations from China: Low risk of novel coronavirus (2019-nCoV) transmission into Africa and south America. Epidemiology and Infection. 2020;148:e41. doi: 10.1017/S0950268820000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. New England Journal of Medicine. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. Offline: Apostasy against the public health elites. Lancet. 2018;391:643. doi: 10.1016/S0140-6736(18)30304-0. [DOI] [PubMed] [Google Scholar]
- Horton R. Offline: COVID-19 and the NHS-a national scandal. Lancet. 2020;395:1022. doi: 10.1016/S0140-6736(20)30727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Science China Life Sciences. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8 doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K.Q., Yung C.F., Cui L., Lin Tzer Pin R., Mak T.M., Maiwald M. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa201. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karako K., Song P., Chen Y., Tang W. Analysis of COVID-19 infection spread in Japan based on stochastic transition model. Biosci Trends. 2020;14:134–138. doi: 10.5582/bst.2020.01482. [DOI] [PubMed] [Google Scholar]
- Killeen G.F., Seyoum A., Sikaala C.H., Zomboko A.S., Gimnig J.E., Govella N.J. Eliminating malaria vectors. Parasites & Vectors. 2013;6:172. doi: 10.1186/1756-3305-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki M., Task Force for 2019-nCoV Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Jung S.M., Linton N.M., Kinoshita R., Hayashi K., Miyama T. Communicating the risk of death from novel coronavirus disease (COVID-19) Journal of Clinical Medicine. 2020;9:538. doi: 10.3390/jcm9020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.R., Cook A.R., Park M., Sun Y., Sun H., Lim J.T. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: A modelling study. The Lancet Infectious Diseases. 2020;20:678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Russell T.W., Diamond C., Liu Y., Edmunds J., Funk S. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. The Lancet Infectious Diseases. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniya T. Prediction of the epidemic peak of coronavirus disease in Japan, 2020. Journal of Clinical Medicine. 2020;9:789. doi: 10.3390/jcm9030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. Journal of Microbiology, Immunology, and Infection. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem A., Wang C., Wariyanti Y., Latkin C.A., Hall B.J. The neglected health of international migrant workers in the COVID-19 epidemic. Lancet Psychiatry. 2020;7 doi: 10.1016/S2215-0366(20)30076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Fu J.B., Li K.F., Chen Y., Wang H.L., Liu L.J. Transmission of COVID-19 in the terminal stage of incubation period: A familial cluster. International Journal of Infectious Diseases. 2020;96:452–453. doi: 10.1016/j.ijid.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New England Journal of Medicine. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ji F., Wang L., Wang L., Hao J., Dai M. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, xuzhou, China. Emerging Infectious Diseases. 2020;26 doi: 10.3201/eid2607.200718. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chinese Medical Journal. 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.M. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. Journal of Clinical Medicine. 2020;9:538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Zhao S., Gao D., Lou Y., Yang S., Musa S.S. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. International Journal of Infectious Diseases. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Eggo R.M., Kucharski A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395:e47. doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Sherlock P., Ebrahim S., Geffen L., McKee M. Bearing the brunt of COVID-19: Older people in low and middle income countries. BMJ. 2020;368:m1052. doi: 10.1136/bmj.m1052. [DOI] [PubMed] [Google Scholar]
- Lu S., Lin J., Zhang Z., Xiao L., Jiang Z., Chen J. Alert for non-respiratory symptoms of coronavirus disease 2019 (COVID-19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients. Journal of Medical Virology. 2020 doi: 10.1002/jmv.25776. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- Lyimo M.A., Mosha I.H. Reasons for delay in seeking treatment among women with obstetric fistula in Tanzania: A qualitative study. BMC Women’s Health. 2019;19:93. doi: 10.1186/s12905-019-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Coronavirus: Home testing pilot launched in London to cut hospital visits and ambulance use. BMJ. 2020;368:m621. doi: 10.1136/bmj.m621. [DOI] [PubMed] [Google Scholar]
- Mahase E. Coronavirus: Wales tests 90% of suspected patients in their own home. BMJ. 2020;368:m698. doi: 10.1136/bmj.m698. [DOI] [PubMed] [Google Scholar]
- Makoni M. Africa prepares for coronavirus. Lancet. 2020;395:483. doi: 10.1016/S0140-6736(20)30355-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S., Bhatnagar T., Arinaminpathy N., Agarwal A., Chowdhury A., Murhekar M. Prudent public health intervention strategies to control the coronavirus disease 2019 transmission in India: A mathematical model-based approach. Indian Journal of Medical Research. 2020;151:190–199. doi: 10.4103/ijmr.IJMR_504_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.A., Cameron A.R., Barfod K., Sergeant E.S., Greiner M. Demonstrating freedom from disease using multiple complex data sources 2: Case study--classical swine fever in Denmark. Preventive Veterinary Medicine. 2007;79:98–115. doi: 10.1016/j.prevetmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Martin P.A., Cameron A.R., Greiner M. Demonstrating freedom from disease using multiple complex data sources 1: A new methodology based on scenario trees. Preventive Veterinary Medicine. 2007;79:71–97. doi: 10.1016/j.prevetmed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Mhalu G., Hella J., Mhimbira F., Said K., Mosabi T., Mlacha Y.P. Pathways and associated costs of care in patients with confirmed and presumptive tuberculosis in Tanzania: A cross-sectional study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Community Development Gender Elderly and Children . 2018. Health statistical tables and figures for 2017. (United Republic of Tanzania) [Google Scholar]
- Ministry of Health Community Development Gender Elderly and Children . 2019. Mid term review of the health sector strategic plan IV for 2015 - 2020: Main report; p. 106. (United Republic of Tanzania) [Google Scholar]
- Mizumoto K., Chowell G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect Dis Model. 2020;5:264–270. doi: 10.1016/j.idm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Chowell G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in wuhan city: China, January-February, 2020. MedRXiv. 2020 doi: 10.1101/2020.02.12.20022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveillance. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. New England Journal of Medicine. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics . 2018. Tanzania in figures. (Dodoma: United Republic of Tanzania) [Google Scholar]
- National Bureau of Statistics . National Population Projections; United Republic of Tanzania: 2018. Ministry of Finance and Planning, Dar es Salaam and the Office of the Chief Government Statistician & Ministry of Finance and Planning, Zanzibar. [Google Scholar]
- Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Medical Weekly. 2020;150:w20224. doi: 10.4414/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- Ngilangwa D.P., Mgomella G.S. Factors associated with retention of community health workers in maternal, newborn and child health programme in Simiyu Region, Tanzania. Afr J Prim Health Care Fam Med. 2018;10:e1–e8. doi: 10.4102/phcfm.v10i1.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngowi A.F., Kamazima S.R., Kibusi S., Gesase A., Bali T. Women’s determinant factors for preferred place of delivery in dodoma region Tanzania: A cross sectional study. Reproductive Health. 2017;14:112. doi: 10.1186/s12978-017-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Suzuki A., Jung S.-M., Hayashi K., Kinoshita R. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) International Journal of Infectious Diseases. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Yang Y., Hayashi K., Miyama T., Kinoshita R. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: Estimation using Japanese passengers data on evacuation flights. Journal of Clinical Medicine. 2020;9:419. doi: 10.3390/jcm9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. International Journal of Infectious Diseases. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Mankoula W. Looming threat of COVID-19 infection in Africa: Act collectively, and fast. Lancet. 2020;395:841–842. doi: 10.1016/S0140-6736(20)30464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamuryekung’e K.K., Lahti S., Tuominen R. Costs of dental care and its financial impacts on patients in a population with low availability of services. Community Dental Health. 2019;36:131–136. doi: 10.1922/CDH_4389Nyamuryekung’e06. [DOI] [PubMed] [Google Scholar]
- Pearson C.A.B., Van Schalkwyk C., Foss A., O’Reilly K., Pulliam J. London School of Hygiene & Tropical Medicine; 2020. Projection of early spread of COVID19 in Africa as of 25 March 2020. [Google Scholar]
- Poole D.N., Escudero D.J., Gostin L.O., Leblang D., Talbot E.A. Responding to the COVID-19 pandemic in complex humanitarian crises. International Journal for Equity in Health. 2020;19:41. doi: 10.1186/s12939-020-01162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem K., Liu Y., Russell T.W., Kucharski A.J., Eggo R.M., Davies N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in wuhan, China: A modelling study. Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I., Clapham H.E. Investigation of three clusters of COVID-19 in Singapore: Implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infectious Diseases. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J.M., Bridgen J.R., Cummings D.A., Ho A., Jewell C.P. Novel coronavirus 2019-nCoV: Early estimation of epidemiological parameters and epidemic predictions. MedRXiv. 2020 doi: 10.1101/2020.01.23.20018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosa K., Lee Y., Luo R., Kirpich A., Rothenberg R., Hyman J.M. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect Dis Model. 2020;5:256–263. doi: 10.1016/j.idm.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosa K., Lee Y., Luo R., Kirpich A., Rothenberg R., Hyman J.M. Short-term forecasts of the COVID-19 epidemic in Guangdong and Zhejiang, China: February 13-23, 2020. Journal of Clinical Medicine. 2020;9:596. doi: 10.3390/jcm9020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C. Am I part of the cure or Am I part of the disease? Keeping coronavirus out when a doctor comes home. New England Journal of Medicine. 2020;382:1684–1685. doi: 10.1056/NEJMp2004768. [DOI] [PubMed] [Google Scholar]
- Ross A., Killeen G.F., Smith T.A. Relationships of host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene. 2006;75(Suppl. 2):32–37. doi: 10.4269/ajtmh.2006.75.32. [DOI] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. The Lancet Infectious Diseases. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. International Journal of Infectious Diseases. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifft K.C., Geus D., Mukampunga C., Mugisha J.C., Habarugira F., Fraundorfer K. Asymptomatic only at first sight: Malaria infection among schoolchildren in highland Rwanda. Malaria Journal. 2016;15:553. doi: 10.1186/s12936-016-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.C., Pell J.P. Parachute use to prevent death and major trauma related to gravitational challenge: Systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461. doi: 10.1136/bmj.327.7429.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper G.A. The curious career of Typhoid Mary. Bulletin of the New York Academy of Medicine. 1939;15:698–712. [PMC free article] [PubMed] [Google Scholar]
- Stresman G., Cameron A., Drakeley C. Freedom from infection: Confirming interruption of malaria transmission. Trends in Parasitology. 2017;33:345–352. doi: 10.1016/j.pt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Su L., Ma X., Yu H., Zhang Z., Bian P., Han Y. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerging Microbes & Infections. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infect Dis Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerging Infectious Diseases. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B., Wang X., Li Q., Bragazzi N.L., Tang S., Xiao Y. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. Journal of Clinical Medicine. 2020;9:462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B., Xia F., Tang S., Bragazzi N.L., Li Q., Sun X. The effectiveness of quarantine and isolation determine the trend of the COVID-19 epidemics in the final phase of the current outbreak in China. International Journal of Infectious Diseases. 2020;95:288–293. doi: 10.1016/j.ijid.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq A., Lee Y., Roosa K., Blumberg S., Yan P., Ma S. Real-time monitoring the transmission potential of COVID-19 in Singapore. BMC Medicine. 2020;18:166. doi: 10.1101/2020.02.21.20026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. Journal of Infection. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P.G.T., Whittaker C., Watson O., Baguelin M., Djaafara A., Ainslie K.E.C. WHO collaborating centre for infectious disease modelling. MRC Centre for Global Infectious Disease Analysis, Abdul Latif Jameel Institute for Disease and Emergency Analytics and Imperial College London; 2020. The global impact of COVID-19 and strategies for mitigation and suppression. [Google Scholar]
- Wang C., Liu L., Hao X., Guo H., Wang Q., Huang J. Evolving epidemiology and impact of non-pharmaceutical interventions on the outbreak of coronavirus disease 2019 in wuhan, China. MedRXiv. 2020 doi: 10.1101/2020.03.03.20030593. [DOI] [Google Scholar]
- Wang H., Wang Z., Dong Y., Chang R., Xu C., Yu X. Phase-adjusted estimation of the number of coronavirus disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10. doi: 10.1038/s41421-020-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xu C., Wong Y.K., He Y., Adegnika A.A., Kremsner P.G. Preparedness is essential for malaria-endemic regions during the COVID-19 pandemic. Lancet. 2020;395:1094–1096. doi: 10.1016/S0140-6736(20)30561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Dye C., Etard J.F., Smith T., Charlwood J.D., Garnett G.P. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. COVID-19 virtual press conference-20 April, 2020. [Google Scholar]
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in wuhan, China: A modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Mo P., Xiao Y., Zhao O., Zhang Y., Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveillance. 2020;25:2000191. doi: 10.2807/1560-7917.ES.2020.25.10.2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Cao P., Du P., Wu Z., Zhuang Z., Yang L. Early estimation of the case fatality rate of COVID-19 in mainland China: A data-driven analysis. Annals of Translational Medicine. 2020;8:128. doi: 10.21037/atm.2020.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Liu S., Xiang M., Li S., Zhao D., Huang C. Protecting healthcare personnel from 2019-nCoV infection risks: Lessons and suggestions. Frontiers of Medicine. 2020;14:229–231. doi: 10.1007/s11684-020-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang Z., Chang R., Wang H., Xu C., Yu X. COVID-19 containment: China provides important lessons for global response. Frontiers of Medicine. 2020;14:215–219. doi: 10.1007/s11684-020-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Chen H. Modeling the epidemic dynamics and control of COVID-19 outbreak in China. Quant Biol. 2020;8:11–19. doi: 10.1007/s40484-020-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. Journal of Infectious Diseases. 2020;221:1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England Journal of Medicine. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.