Abstract
Introduction
Chinese medicine (CM) has been used to treat Novel Coronavirus 2019 (COVID-19) pneumonia in China. This meta-analysis was conducted to evaluate the clinical efficacy and safety of CM in the treatment of COVID-19 pneumonia.
Methods
Randomized controlled trials (RCTs) involving CM in the treatment of COVID-19 pneumonia were identified from Cochrane Central Register of Controlled Trials, PubMed, EMBASE, Chinese National Knowledge Infrastructure, Chinese Biomedical Database, Wanfang Database and VIP Information Database. The methodological quality of trials was evaluated with Cochrane Hanadbook criteria, and the Cochrane Collaboration's Review Manager 5.3 software was used for meta-analysis.
Results
A total of 7 valid studies involving 681 patients were included. The meta-analysis exhibited in comparison to conventional treatment, CM combined with conventional treatment significantly improved clinical efficacy (RR = 1.21, 95% CI [1.08,1.36]), and significantly increased viral nucleic acid negative conversion rate (RR = 1.49, 95% CI [1.13,1.97]). CM also prominently reduced pulmonary inflammation (RR = 1.27, 95% CI [1.12,1.44]), and improved host immune function (WBC, MD = 0.92, 95% CI [0.07,1.76]; LYM, MD = 0.33, 95% CI [0.08,0.57]; LYM%, MD = 2.90, 95% CI [2.09,3.71]; CRP, MD = −12.66, 95% CI [−24.40, −0.92]). Meanwhile, CM did not increase the incidence of adverse reactions (RR = 1.17, 95% CI [0.39,3.52]).
Conclusion
According to the allocated data, CM has demonstrated clinical efficacy and safety on COVID-19 pneumonia, which need to be confirmed by high quality, multiple-center, large sample randomized controlled trials.
Keywords: Chinese medicine, COVID-19 pneumonia, Meta-analysis
1. Introduction
Novel Coronavirus disease 2019 (COVID-19) pneumonia is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which firstly appeared in Wuhan, China [1,2]. It is highly infectious and spreads through respiratory droplets and contact [3]. It is characterized by acute onset, severe symptoms, such as dyspnea and multi-organ dysfunction [4]. The World Health Organization listed this pneumonia epidemic of Wuhan, China, as a public health emergency of international concern [5]. The world is now facing a pandemic of COVID-19, for which no proven specific therapies are available, other than supportive care.
On January 20, 2020, National Health Commission of the People's Republic of China announced that COVID-19 would be classified as category A infectious disease [6]. At the same time, Chinese medicine experts quickly reached a consensus on Chinese medicine (CM) therapy. As medical therapy, CM treatment was written in “Diagnosis and Treatment of Pneumonia Infected by 2019-nCoV (trial implementation 7th Edition)” published by National Health Commission of the People's Republic of China [7]. However, compared with conventional treatment, there is no high-level evidence to support the effectiveness of CM treatment. Therefore, the meta-analysis method will be used to systematically review the clinical efficacy and safety of CM for COVID-19 pneumonia. This analysis is expected to obtain meaningful conclusions and provide a high level of evidence-based medicine evidence.
2. Methods
2.1. Inclusion and exclusion criteria
Studies meeting the following criteria were included: (1) randomized controlled trials (RCTs) using CM (including Chinese herbal medicine, Chinese patent medicine and Chinese medicine injections) to treat COVID-19 pneumonia regardless of blinding, allocation concealment. (2) patients enrolled were diagnosed with COVID-19 pneumonia. There are no restrictions on gender, course of disease, course of treatment. (3) the treatment group received CM for treating COVID-19 pneumonia, and the control group received conventional treatment. (4) the primary outcomes for this meta-analysis were clinical effective rate and incidence of adverse reactions. Besides, viral nucleic acid negative conversion rate, remission rate of pulmonary inflammation (chest CT) and biochemical markers were also evaluated. The following studies were excluded: (1) animal experiment; (2) review; (3) wrong patient population.
2.2. Search strategy
Allocation of data was systematically conducted in both English and Chinese databases from 01 December 2019 to 31 March 2020: Cochrane Central Register of Controlled Trials, PubMed, EMBASE, Chinese National Knowledge Infrastructure, Chinese Biomedical Database, Wanfang database, VIP information database. There were no language restrictions, meanwhile the medical subject headings (MeSH) and free text words were applied. The following three terms were used as search strategy and modified to suit each database: health condition (“COVID-19 pneumonia” OR “COVID-19” OR “SARS-CoV-2” OR “2019 nCoV” OR “novel coronavirus”), intervention (“Chinese medicine” OR “Chinese herbal medicine” OR “Chinese patent medicine” OR “Chinese medicine injections”), study type (randomized controlled trials). In addition, reference lists of credited published articles were verified.
2.3. Study selection and data extraction
We used NoteExpress 3.0 software to manage the retrieved articles. After the duplicate literature were removed, two review authors independently read the tittles and abstracts to exclude wrong literature (e.g., review, animal experiment). The rest literature were read full text to sort out the eligible. The main components of extracted data included the following: first author, publication date, sample size, diagnosis standard, CM (e.g., Chinese herbal medicine, Chinese patent medicine and Chinese medicine injections), conventional treatment, course of treatment, clinical effective rate, incidence of adverse reactions, viral nucleic acid negative conversion rate, remission rate of pulmonary inflammation (chest CT), and biochemical markers.
2.4. Risk of bias assessment
The methodological quality of the eligible RCTs was evaluated independently, according to the Cochrane Handbook for Systematic Reviews of Interventions [8]. The details included: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias. Reference this statement each entry was assessed as “low risk”, “unclear risk” or “high risk”. Any disagreement was resolved by discussion with a third review author.
2.5. Statistical analysis
Cochrane Collaboration's Review Manager 5.3 software was used for meta-analysis. If two or more homogeneous studies are available, we will use aggregated data for meta-analysis. If the data are not available for quantitative analysis, we will report result by qualitative description. For dichotomous outcomes, we calculated the risk ratio (RR), 95% confidence intervals (CI) and P values. For continuous variable, we calculated the mean difference (MD), 95% CI and P values. We used the inverse variance method to calculate pooled MD values and the Mantel-Haenszel estimator to calculate RR. Studies will be evaluated for heterogeneity using I squared and chi-squared test. If I2 > 50%, or P < .05, the studies will be considered heterogeneous, and the pooling model will choose a random effects model, otherwise fixed effects model will be used. The funnel plot will be used to evaluate publication bias.
3. Results
3.1. Characteristics of included studies
716 unique citations were identified from electronic database. After duplicates removed, 697 literature remained. By reading titles and abstracts, 42 articles were downloaded. A total of 42 articles were retrieved for further assessment, among which 35 were further excluded, for the reasons: wrong patient population; self-control; retrospective studies. In total, seven [[9], [10], [11], [12], [13], [14], [15]] RCTs met the inclusion criteria and were subjected to data extraction (Fig. 1 ).
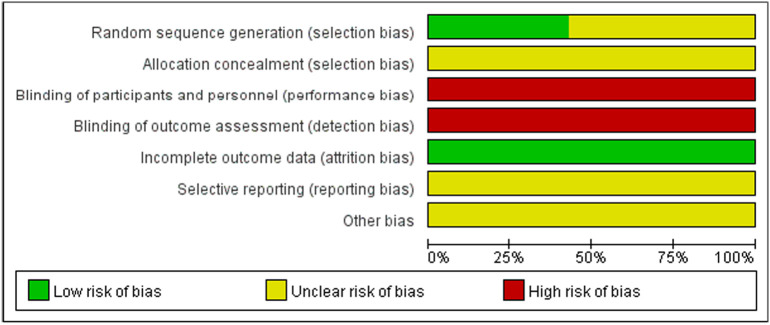
Fig. 1.
Details about the risk of bias.
All studies included involved seven RCTs with 681 patients. Of the seven RCTs, two RCTs [9,13] reported clinical effective rate, seven RCTs [[9], [10], [11], [12], [13], [14], [15]] recorded incidence of adverse reactions, three RCTs [10,11,14] reported viral nucleic acid negative conversion rate, four RCTs [10,[12], [13], [14]] recorded remission rate of pulmonary inflammation (chest CT).
There were three RCTs [9,13,14] reported white blood cell count (WBC), three RCTs [9,10,14] reported lymphocyte count (LYM), two RCTs [9,13] reported lymphocyte ratio (LYM%), four RCTs [9,10,12,14] reported C-reactive proteins, and two RCTs [12,14] reported interleukin-6. The comparison of baseline characteristics showed there were no significant differences in gender, age, or disease duration between the treatment and control groups (P > .05). The characteristics of the studies are illustrated in Table 1 .
Table 1.
Characteristics of the included randomized controlled trials.
| Studies | Sample size (T/C) | Patients enrolled condition | Diagnosis standard | Treatment group | Control group | Course of treatment (d) | Outcomes |
|---|---|---|---|---|---|---|---|
| Fu et al. [9] | 37/36 | MOCP | 6th Edition | TouxieQuwen prescription (2 dose/d) + Control group | Arbidol (0.2 g, tid) and ambroxol (30 mg, tid) | 15 | 1/2/5/6/7/8 |
| Yang et al. [10] | 26/23 | MOCP | 3/4/5th Edition | Reyanning mixture (10–20 ml, bid-q6h) + Control group | Lopinavir-ritonavir (400–100 mg, bid) or recombinant human interferon α1b for injection (5 million U, bid) or ribavirin (0.5 g, bid) or arbidol (0.2 g, tid) | 7 | 2/3/4/6/8 |
| Qu et al. [11] | 40/30 | MICP and MOCP | 4/5th Edition | Shufengjiedu capsule (2.08 g, tid) + Control group | Arbidol (0.2 g, tid) | 10 | 2/3 |
| Ding et al. [12] | 51/49 | T:MICP:10; MOCP:36; SCP and CCP: 5; C:MICP:11; MOCP:34; SCP and CCP: 4 | 5th Edition | Qingfeitouxiefuzheng prescription (1 dose/d) + Control group | Recombinant human interferon α1b for injection (5 million U, bid) or ribavirin (0.5 g, bid) | 10 | 2/4/8/9 |
| Xiao et al. [13] | 100/100 | MICP | 5th Edition | Shufengjiedu capsule (2.08 g, tid) + Control group | Arbidol (0.2 g, tid) | 14 | 1/2/4/5/7 |
| Huang et al. [14] | 52/14 | T:MOCP:44; SCP:9; C:MOCP:12; SCP:3 | 7th Edition | Feiyanyihao prescription or feiyanerhao prescription (1 dose/d) + Control group | Lopinavir-ritonavir or recombinant human interferon α1b or ribavirin or arbidol (regular recommended dosing) | 10 | 2/3/4/5/6/8/9 |
| Duan et al. [15] | 82/41 | MICP | 5th Edition | Jinhuaqinggan granule (10 g, tid) + Control group | Lopinavir-ritonavir (400–100 mg, bid) or recombinant human interferon α1b (5 million U, bid) or ribavirin (1.2 g, tid) | 5 | 2 |
Abbreviation: T: treatment group; C: control group; d: day; mild case patients (MICP); moderate case patients (MOCP); severe case patients (SCP); critical case patients (CCP); 3/4/5/6/7th Edition: “Diagnosis and Treatment of Pneumonia Infected by 2019-nCoV (trial implementation 3/4/5/67th Edition)” published by the “National Health Commission of the People's Republic of China.”; Outcomes: 1: clinical effective rate; 2: incidence of adverse reactions; 3: viral nucleic acid negative conversion rate; 4: remission rate of pulmonary inflammation (chest CT); 5: white blood cell count (WBC); 6: lymphocyte count (LYM); 7: lymphocyte ratio (LYM%); 8: C-reactive protein (CRP); 9: interleukin 6 (IL-6).
3.2. Quality evaluation of the included studies
For the eligible studies, three studies [9,12,15] mentioned the use of random distribution. None of the studies provided useful information to estimate allocation concealment. All studies failed to report the use of blinding, thus the item was regarded as “High risk of bias”. The incomplete outcome data item was appraised as “Low risk of bias” since none of the included studies had incomplete data. Since there had no information available in the included studies about selective reporting or any factors that might lead to risk, the items of selective reporting and other bias were evaluated as “Unclear risk of bias”. Details about the risk of bias are presented in Fig. 1.
3.3. Meta-analysis results
3.3.1. Clinical effective rate
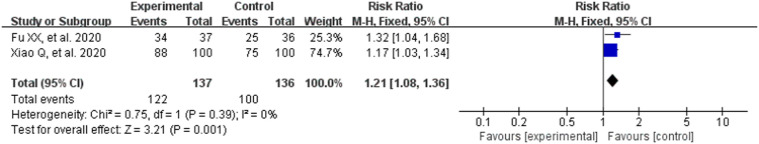
The clinical effective rate was tested in two RCTs involved 273 patients. The efficacy criteria were predominantly based on reduction of clinical symptoms and could be divided into three grades: cured, remarkable recovery, unrecovered. The rate calculated by this formula: (number of cured patients + number of remarkable recovery patients)/total number × 100% [16].
Heterogeneity test results (P = .39, I2 = 0%) indicated that there were no statistical significant difference between the studies, and the fixed effect model was selected for meta-analysis. As can be observes in Fig. 2 , CM combined with conventional treatment had a higher clinical effective rate compared with conventional treatment (RR = 1.21, 95% CI [1.08,1.36], Z = 3.21, P = .001; Fig. 2).
Fig. 2.
Analysis of treatment group and control group with clinical effective rate.
3.3.2. Incidence of adverse reactions
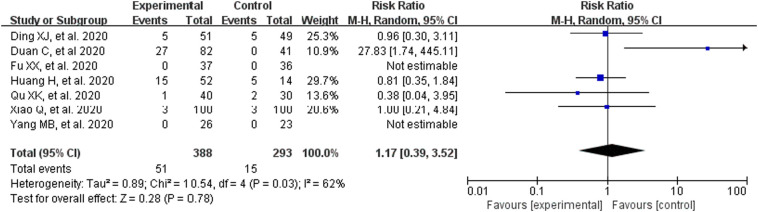
Taking incidence of adverse reactions into consideration (including seven RCTs, 681patients), we noted there is high heterogeneity between studies (P = .03, I2 = 62%). Therefore, it was decided to choose the random effect model for meta-analysis. Fig. 3 demonstrates, CM had no significant effect on the incidence of adverse reactions (RR = 1.17, 95% CI [0.39,3.52], Z = 0.28, P = .78; Fig. 3).
Fig. 3.
Analysis of treatment group and control group with the incidence of adverse reactions.
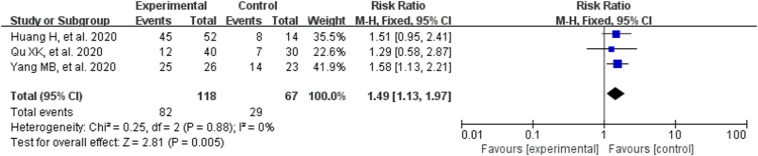
3.3.3. Viral nucleic acid negative conversion rate
Since high homogeneity was observed in the result (P = .88, I2 = 0%), a fixed effect model was used to calculate estimation. Significant differences were observed on viral nucleic acid negative conversion rate in favor of CM combined with conventional treatment (RR = 1.49, 95% CI [1.13,1.97], Z = 2.81, P = .005; Fig. 4 ). Compared with conventional treatment alone, CM combined with conventional treatment can significantly increase viral nucleic acid negative conversion rate.
Fig. 4.
Analysis of treatment group and control group with the viral nucleic acid negative conversion rate.
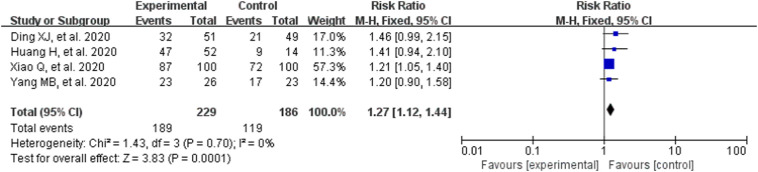
3.3.4. Remission rate of pulmonary inflammation (chest CT)
The chest CT findings were typical of findings for COVID-19 pneumonia [17,18]. In the included RCTs, four RCTs compared CM combined with conventional treatment alone. The heterogeneity test result is: P = .79, I2 = 0%, so the fixed effect model was adopted. We observed significant differences on remission rate of pulmonary inflammation (RR = 1.27, 95% CI [1.12,1.44], Z = 3.83, P = .001; Fig. 5 ). The results indicated that, compared with conventional treatment alone, combined therapy with CM can significantly reduce pulmonary inflammation (chest CT).
Fig. 5.
Analysis of treatment group and control group with the remission rate of pulmonary inflammation (chest CT).
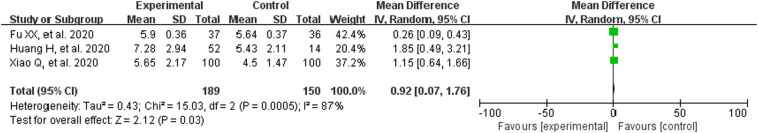
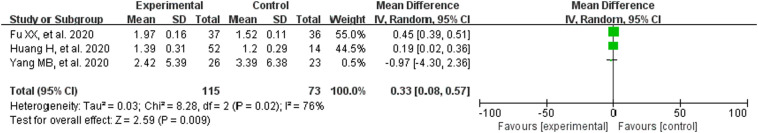
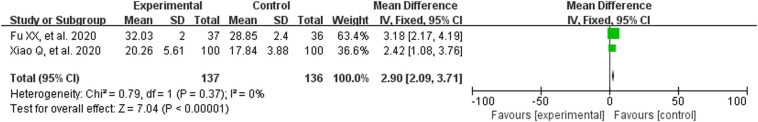
3.3.5. Biochemical markers
The heterogeneity test results are: WBC (P = .0005, I2 = 87%), LYM (P = .02, I2 = 76%), CRP (P < .00001, I2 = 97%), IL-6 (P = .06, I2 = 73%), so the random effect model was utilized. LYM% (P = .37, I2 = 0%), the fixed effect model was adopted. The results suggested that CM combined with conventional treatment significantly increased the white blood cell count (MD = 0.92, 95% CI [0.07,1.76]; Fig. 6 ), lymphocyte count (MD = 0.33, 95% CI [0.08,0.57]; Fig. 7 ), lymphocyte ratio (MD = 2.90, 95% CI [2.09,3.71]; Fig. 8 ). Meanwhile, it can also reduce the amount of CRP (MD = −12.66, 95% CI [−24.40,−0.92]; Fig. 9 ). These results meant that the participants receiving CM can more effectively improve immune function than those with conventional treatment alone.
Fig. 6.
Analysis of treatment group and control group with WBC.
Fig. 7.
Analysis of treatment group and control group with LYM.
Fig. 8.
Analysis of treatment group and control group with LYM%.
Fig. 9.
Analysis of treatment group and control group with CRP.
However, although two RCTs showed that CM treatment can reduce IL-6 levels, meta-analysis showed no significant difference (MD = −8.17, 95% CI [−22.40,6.06]; Fig. 10 ).
Fig. 10.
Analysis of treatment group and control group with IL-6.
3.3.6. Funnel plot characteristics
A funnel plot for the remission rate of pulmonary inflammation was presented in Fig. 11 . The funnel plot was considered visually asymmetry, this creates a possible publication bias.
Fig. 11.
Funnel plot.
4. Discussion
Since December 2019, many cases of “a novel coronavirus pneumonia” have emerged from the city of Wuhan in China's Hubei Province. A previously unknown beta-coronavirus was discovered through the use of unbiased sequencing in samples from patients with pneumonia of unknown cause [19]. On January 6, 2020, the 2019 novel coronavirus (2019-nCoV) was confirmed as the cause of these reported cases, and the outbreak was subsequently named COVID-19 [20]. The novel coronavirus was speculated to be linked to the Huanan Seafood Market, Wuhan [21]. However, there is no consensus on the actual origin of the COVID-19 so far. As of May 9, 2020, widespread human-to-human transmission has resulted in 3,855,788 cases in 213 countries and regions, with 265,862 deaths [22].
COVID-19 possesses particularly powerful pathogenicity and infectivity, and being infected within a very short exposure time is possible [23]. A majority of patients developed COVID-19 pneumonia, and more severely, infections causing pneumonia may lead to severe acute respiratory syndrome and even death [24].
The main diagnostic methods for COVID-19 pneumonia are physical examination, chest CT imaging examination, and viral nucleic acid detection. In general, CT findings and nucleic acid detection results are concordant. However, some studies have shown that some patients with initially negative real-time RT-PCR screening results who had typical CT findings of COVID-19 pneumonia had their RT-PCR results became positive after a few days [25]. Therefore, published clinical guidelines strongly recommend chest CT for patients with suspected COVID-19 [26]. COVID-19 can also affect the body's immune response; for example, there are relatively lower levels of WBC and LYM, markedly higher levels of CRP and IL-6, etc. [[27], [28], [29]]. These immunological markers may be of importance due to their correlation with disease severity in COVID-19.
There are no specific pharmacological interventions discovered for treatment of COVID-19 pneumonia so far. The World Health Organization recommended that potential antiviral medicines should be developed [30]. However, the development of potential antiviral medicines may take months, even years. In consideration of these limitations, the application of Chinese medicine could be promoted. The Chinese government has advised doctors to combine antiviral drugs with CM remedies in combating COVID-19 pneumonia [7]. To date, there have been some reports on the role of CM for COVID-19 pneumonia, and clinical practice results showed that CM has excellent outcomes in the treatment of COVID-19, bringing new hope for the control of COVID-19 pneumonia [[9], [10], [11], [12], [13], [14], [15]].
4.1. The effectiveness of CM in treating COVID-19 pneumonia
The results of this meta-analysis are encouraging. In terms of clinical effective rate, viral nucleic acid negative conversion rate, remission rate of pulmonary inflammation, and biochemical markers, CM exhibited superior performance. Meanwhile, CM has therapeutic safety.
4.2. Comparison with previous reviews
As the first meta-analysis evaluating the efficacy and safety of CM for the treatment of COVID-19 pneumonia, the wide extent of literature screening and the introduction of statistical analysis methods have ensured the validity of this review, as well as providing a rational conclusion. It is critical for clinicians to accurately determine the severity of a patients condition. At the same time, clinicians follow the theory of Chinese medicine, and determine the appropriate prescription to ensure effective treatment. Especially, in the absence of Chinese herbal medicine, a variety of Chinese patent medicines (such as Shufengjiedu capsule, Jinhuaqinggan granule, Reyanning mixture, etc.) are also effective. These findings will be valuable for clinicians selecting medical interventions for COVID-19 pneumonia treatment.
4.3. Limitations and future directions
Several limitations exist in this meta-analysis and the findings must be carefully explained. Trials were conducted in China, which limited the evaluation of results to consider other ethnicities living in different countries. The inadequate quality of the publications also affects the credibility of the conclusion of this analysis. The authors will continue to monitor the literature, and this review will be updated when new evidence emerges.
5. Conclusions
A total of seven studies encompassing 681 patients were included in this meta-analysis. According to the findings, CM combined with conventional treatment was the better treatment choice for COVID-19 pneumonia. This result provides a high level of evidence for the CM treatment of COVID-19 pneumonia. In terms of limitations, large, multicentre clinical trials are needed to verify the credibility of the conclusion.
Funding
This study was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX19_1211).
Author contributions
CY Sun contributed to Conceptualization and Data curation. YL Sun and CY Sun contributed to Formal analysis and Funding acquisition. CY Sun, YL Sun and XM Li contributed to Resources and Software. YL Sun and XM Li contributed to Writing - original draft, and YL Sun contributed to Writing - review & editing. The final submitted version has been confirmed by all authors.
Ethical approval
Ethical approval is not necessary in the meta-analysis because our analysis only gather RCTs from a literature database.
Declaration of Competing Interest
None.
References
- 1.World Health Organization WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China. https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china (Published Jan 9 2020, accessed May 10, 2020)
- 2.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J. Med. Virol. 2020;92:639–644. doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization WHO Director-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV) https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov) (Published Jan 30 2020, accessed May 10, 2020)
- 6.National Health Commission of the People'’s Republic of China Pneumonia Infected by COVID-19 Included in the Management of Legal Infectious Diseases. http://www.nhc.gov.cn/xcs/zhengcwj/202001/44a3b8245e8049d2837a4f27529cd386.shtml (Published Jan 20 2020, accessed May 10, 2020)
- 7.National Health Commission of the People'’s Republic of China Diagnosis and Treatment of Pneumonia Infected by 2019-nCoV (Trial Implementation 7th Edition) http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (Published Mar 4 2020, accessed May 10, 2020)
- 8.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane DB Syst Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu X.X., Lin L.P., Tan X.H. Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020;31:600–604. http://kns.cnki.net/kcms/detail/44.1308.R.20200319.1644.002.html [Google Scholar]
- 10.Yang M.B., Dang S.S., Huang S., Li Y.J., Guo Y.L. Multi-center clinical observation of Reyanning mixture in treatment of novel coronavirus pneumonia. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:7–12. http://kns.cnki.net/kcms/detail/44.1308.R.20200319.1644.002.html [Google Scholar]
- 11.Qu X.K., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Tang C., et al. Observation on clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsule in treatment of COVID-19. Chin. Tradit. Herb. Drug. 2020;51:1167–1170. https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2020&filename=ZCYO202005012&uid=WEEvREcwSlJHSldRa1FhcEFLUmViU1hLQ3BYVitNNVB0VlBzSUwwRG94UT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MjE5OTJUM3FUcldNMUZyQ1VSN3FmWXVkdUZ5amxVN3ZLUHk3U1liRzRITkhNcW85RVpvUjhlWDFMdXhZUzdEaDE= [Google Scholar]
- 12.Ding X.J., Zhang Y., He D.C., Zhang M.Y., Tan Y.J., Yu A.R., et al. Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of novel coronavirus pneumonia. Herald Med. 2020;39:640–644. https://kns.cnki.net/KCMS/detail/42.1293.R.20200302.1615.002.html?uid=WEEvREcwSlJHSldRa1FhcEFLUmViU1hLQ3BYVitNNVB0VlBzSUwwRG94UT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MjQ2MTJxV00wQ0xMN1I3cWRaK1pxRnlqbFU3ekpKRm89UERUUGJMRzRITkhNclkxTVpPc1BZdzlNem1SbjZqNTdUM2Zs [Google Scholar]
- 13.Xiao Q., Jiang Y.J., Wu S.S., Wang Y., An J., Xu W.P., et al. Analysis of the value of Shufeng Jiedu capsules combined with Abidol in the treatment of mild novel coronavirus pneumonia. J. Emerg. Tradit. Chin. Med. 2020;29:756–758. https://kns.cnki.net/KCMS/detail/50.1102.R.20200309.1528.004.html?uid=WEEvREcwSlJHSldRa1FhcEFLUmViU1hLQ3BYVitNNVB0VlBzSUwwRG94UT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MTM3NTk9UHpUQmRMRzRITkhNckk5RFpPc1BZdzlNem1SbjZqNTdUM2ZscVdNMENMTDdSN3FkWitacUZ5amxVN3pBSkZZ [Google Scholar]
- 14.Huang H., Zhao Y., Zuo X.H., Jin J., Guo Y. Treatment of COVID-19 by pneumonia no. 1 prescription and pneumonia no. 2 prescription. Acta Chinese Medicine. 2020 https://kns.cnki.net/KCMS/detail/41.1411.R.20200323.1016.002.html?uid=WEEvREcwSlJHSldRa1FhcEFLUmViU1hLQ3BYVitNNVB0VlBzSUwwRG94UT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MTk5ODl3OU16bVJuNmo1N1QzZmxxV00wQ0xMN1I3cWRaK1pxRnlqbFU3elBJbDA9TFNQUlpiRzRITkhNckk1TVpPc1BZ published-ahead-of-print. [Google Scholar]
- 15.Duan C., Xia W.G., Zheng C.J., Sun G.B., Li Z.L., Li Q.L., et al. Clinical observation of Jinhua Qinggan granule in treating pneumonia infected by novel coronavirus. J. Tradit. Chin. Med. 2020 https://kns.cnki.net/KCMS/detail/11.2166.R.20200323.0853.002.html?uid=WEEvREcwSlJHSldRa1FhcEFLUmViU1hLQ3BYVitNNVB0VlBzSUwwRG94UT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MDU1OTRSbjZqNTdUM2ZscVdNMENMTDdSN3FkWitacUZ5amxVN3pCSkZrPVB6ZlNkTEc0SE5ITXJJMUVaT3NQWXc5TXpt [Google Scholar]
- 16.Zheng X.Y. 1st ed. China Medical Science and Technology Press; Beijing, China: 2002. Guiding Principles for Clinical Research of New Chinese Medicines. [Google Scholar]
- 17.Huang P., Liu T., Huang L., Liu H., Lei M., Xu W., et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295:22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed May 10, 2020)
- 21.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report-110. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (Published Mar 30 2020, accessed May 10, 2020), 2019.
- 23.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;12:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y., Cai L., Cheng Z., Cheng H., Deng T., Fan Y., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Di. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.X., Wu W., Yang T., Zhou W., Fu Y.M., Feng Q.M., et al. Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19. Chin. J. Int. Med. 2020;59:E003. DOI:3760.10/cma.j.cn112138-20200221-00114. [PubMed] [Google Scholar]
- 29.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Investig. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan Z., Karatas Y., Rahman H. Anti COVID-19 drugs: need for more clinical evidence and global action. Adv. Ther. 2020 doi: 10.1007/s12325-020-01351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]