Abstract
Anti-inflammatory drugs such as corticosteroids may beneficially modulate the host inflammatory response to coronavirus disease 2019 (COVID-19) pneumonia. The aim of this study was to evaluate the impact of addition of corticosteroids to the hospital protocol for treatment of suspected or confirmed COVID-19 pneumonia on rates of death or intensive care unit (ICU) admission. A before–after study was performed to evaluate the effect of addition of corticosteroids to our institution's COVID-19 treatment protocol on hospital mortality. A total of 257 patients with a COVID-19 diagnosis were included in this study between 3 March 2020 and 14 April 2020. As corticosteroids were widely used after 27 March 2020, two periods were considered for the purposes of this study: the ‘before’ period from 3–20 March 2020 (n = 85); and the ‘after’ period from 26 March–14 April 2020 (n = 172). The ‘after’ period was associated with a lower risk of death [adjusted hazard ratio (aHR) = 0.47, 95% confidence interval (CI) 0.23–0.97; P = 0.04] and a lower risk of ICU admission or of death before ICU admission (aHR = 0.37, 95% CI 0.21–0.64; P = 0.0005) by multivariate analysis adjusted for age, National Early Warning score and institutionalisation status. In conclusion, addition of corticosteroids to our institution's COVID-19 treatment protocol was associated with a significant reduction in hospital mortality in the ‘after’ period.
Keywords: COVID-19, SARS-CoV-2, COVID-19 pneumonia, Corticosteroid
1. Introduction
There is evidence that severe coronavirus disease 2019 (COVID-19) patients present overwhelming inflammatory reactions with high levels of cytokines and inflammatory biomarkers, leading to lung injury [1,2]. Anti-inflammatory drugs such as corticosteroids may beneficially modulate the host immune response to COVID-19 pneumonia. With an average of 2–3 days between the occurrence of dyspnoea and intensive care unit (ICU) admission, we postulate that corticosteroid treatment initiated as soon as the patient has shortness of breath or requires oxygen therapy might be effective in preventing acute respiratory distress syndrome and death [3]. Therefore, since 27 March 2020, we have systematically included corticosteroids in the treatment of patients with COVID-19 pneumonia. Prednisone or methylprednisolone at a dose of 1 mg/kg equivalent per day (0.5 mg/kg for patients also receiving antiviral therapy with ritonavir, as co-administration of a corticosteroid and ritonavir leads to an increased corticosteroid plasma concentration and half-life) for 3–4 weeks according to the severity of pneumonia, with dose tapering over the last week, was added to our initial therapeutic protocol for hospitalised COVID-19 patients [4]. This protocol included antiviral therapy [lopinavir plus ritonavir (LPV/r) or darunavir plus ritonavir (DRV/r)] and/or hydroxychloroquine, empirical broad-spectrum antibiotic treatment for 14 days and preventive anticoagulation for 14–21 days. The long duration of corticosteroid treatment was chosen by analogy with that recommended for severe pneumocystis pneumonia in order to additionally prevent pulmonary fibrosis [5]. As LPV/r treatment was not available after mid March owing to a drug shortage in our hospital, most of the patients received another anti-HIV protease inhibitor (DRV/r) after this date.
To evaluate the impact of addition of corticosteroids to the hospital protocol for treatment of suspected or confirmed COVID-19 pneumonia, rates of death (primary outcome) or of intensive care unit (ICU) admission and/or death before ICU admission (secondary outcome) were compared in a before-and-after study, with the introduction of corticosteroids in the therapeutic protocol as the event defining the start of the ‘after’ period.
2. Patients and methods
Between 3 March and 14 April 2020, a total of 319 patients with a COVID-19 diagnosis, defined as a positive result by reverse transcriptase (RT)-PCR testing of a nasopharyngeal sample or the presence of characteristic findings on chest computed tomography (CT) scan, were followed in the University Hospital of Reims (Reims, France) [6]. Two periods were considered for the purposes of this study: the first, from 3–20 March 2020, corresponded to the ‘before’ period and the admission of the first cases to our centre. During the ‘before’ period, corticosteroid therapy was not recommended. The second (‘after’) period comprised 26 March–14 April 2020, with wide use of corticosteroid therapy in this period following our decision to introduce it systematically owing to the biological rationale of its use in the inflammatory phase. Patients with initiation of corticosteroid therapy during the transition period (from 21–25 March 2020) were not included in the before–after analysis. Patients with <7 days between symptom onset and 14 April 2020, which was the endpoint date of follow-up, were not included.
Individual follow-up was defined as the time from first symptoms to death during hospitalisation for the primary outcome and to ICU admission or death before ICU admission for the secondary outcome. ICU admission alone was not considered as an outcome since we did not exclude patients aged >80 years and/or with co-morbidities who were less likely to have access to ICU care. Data are expressed as the mean ± standard deviation or number (%) as appropriate. Quantitative variables were compared between the two periods using Student's t-test, and qualitative variables were compared using the χ2 test or Fisher's exact test as appropriate. For the impact of the period on death and on ICU admission and/or death, Kaplan–Meier curves were constructed and were compared using the log-rank test. For multivariate analysis, Cox proportional hazard models systematically adjusted for age, National Early Warning score (NEWS) [7] and institutionalisation status at hospital admission were used.
3. Results
At the time of data extraction, a total of 319 patients were included in the cohort, namely 85 patients in the ‘before’ period (until 20 March 2020), 62 patients in the transition period (21–25 March 2020) and 172 patients in the ‘after’ period (26 March–14 April 2020). Of the included patients, 11 patients (12.9%) received corticosteroid therapy in the ‘before’ period, 20 (32.3%) in the transition period and 119 (69.2%) in the ‘after’ period. The main characteristics of the 257 patients in the ‘before’ and ‘after’ periods are summarised in Table 1 . Patients in the ‘after’ period were significantly more frequently nursing home residents, had a higher prevalence of dementia, had a longer time from symptom onset to hospitalisation, less frequently received LPV and/or hydroxychloroquine, and more often required oxygen therapy than in the ‘before’ period. Patients in the ‘after’ period also had higher serum creatinine levels. The mean duration of follow-up was 16.0 ± 7.0 days and was similar between periods (16.0 ± 8.7 vs. 16.1 ± 6.2; P = 0.92). Of note, deceased patients hospitalised in the medical ward were older than those who were transferred to the ICU (mean age, 83.9 ± 11.3 years vs. 69.6 ± 7.2 years, respectively).
Table 1.
Main characteristics of patients in the periods before and after the introduction of corticosteroids for COVID-19 pneumonia in Reims University Hospital (Reims, France) a
| Characteristic | Before (n = 85) | After (n = 172) | P-value |
|---|---|---|---|
| Received corticosteroids | 11 (12.9) | 119 (69.2) | <0.0001 |
| Age (years) | 70.1 ± 15.1 | 71.8 ± 16.4 | 0.44 |
| Male sex | 46 (54.1) | 89 (51.7) | 0.72 |
| Charlson comorbidity index | 1.8 ± 2.0 | 2.05 ± 1.94 | 0.42 |
| Dementia | 8 (9.4) | 33 (19.2) | 0.04 |
| Nursing home resident | 8 (9.4) | 47 (27.3) | 0.001 |
| Time from symptom onset to hospitalisation (days) | 5.8 ± 4.2 | 7.5 ± 4.9 | 0.009 |
| Diagnosis by positive RT-PCR | 80 (94.1) | 143 (83.1) | 0.01 |
| Diagnosis by chest CT scan | 5 (5.9) | 29 (16.9) | |
| Risk factor for severity | 71 (83.5) | 156 (90.7) | 0.09 |
| Immunocompromised | 11 (12.9) | 22 (12.8) | 0.97 |
| Cardiovascular disease | 41 (48.2) | 94 (54.7) | 0.33 |
| Complicated diabetes | 7 (8.2) | 23 (13.4) | 0.23 |
| Cirrhosis | 1 (1.2) | 3 (1.7) | 0.99 |
| Chronic respiratory disease | 22 (25.9) | 32 (18.6) | 0.18 |
| Chronic renal disease | 4 (4.7) | 16 (9.3) | 0.20 |
| Cancer | 7 (8.2) | 10 (5.8) | 0.46 |
| BMI > 40 | 2 (2.4) | 13 (7.6) | 0.15 |
| National Early Warning score | 6.2 ± 3.8 | 6.9 ± 3.2 | 0.12 |
| Biological characteristics | |||
| Lymphocyte count (× 109/L) | 1.1 ± 0.56 | 1.1 ± 1.0 | 0.73 |
| Lymphopenia <1 × 109/L | 43 (51.2) | 91 (53.9) | 0.69 |
| Neutrophil count (× 109/L) | 5.0 ± 3.6 | 5.6 ± 4.0 | 0.26 |
| C-reactive protein (mg/L) | 98.0 ± 90.2 | 89.9 ± 77.1 | 0.46 |
| Serum creatinine (μmol/L) | 103.5 ± 106.2 | 137.7 ± 174.3 | 0.05 |
| Treatment use with expected antiviral activity | 63 (75.0) | 135 (79.4) | 0.43 |
| Lopinavir | 46 (54.8) | 13 (7.9) | <0.0001 |
| Darunavir | 27 (32.5) | 133 (78.2) | <0.0001 |
| Hydroxychloroquine | 11 (13.3) | 10 (6.0) | 0.049 |
| Antibiotic therapy | 80 (95.2) | 162 (95.9) | 0.99 |
| Evolution | |||
| Required oxygen therapy | 52 (61.9) | 125 (76.7) | 0.01 |
| Maximum oxygen flow in medical ward b | 5.0 ± 7.6 | 5.7 ± 5.2 | 0.48 |
| Death | 17 (20.0) | 31 (18.0) | 0.70 |
| ICU admission and/or death | 29 (34.1) | 40 (23.6) | 0.07 |
RT, reverse transcriptase; CT, computed tomography; BMI, body mass index; ICU, intensive care unit.
Data are n (%) or mean ± standard deviation.
Among those who received oxygen therapy.
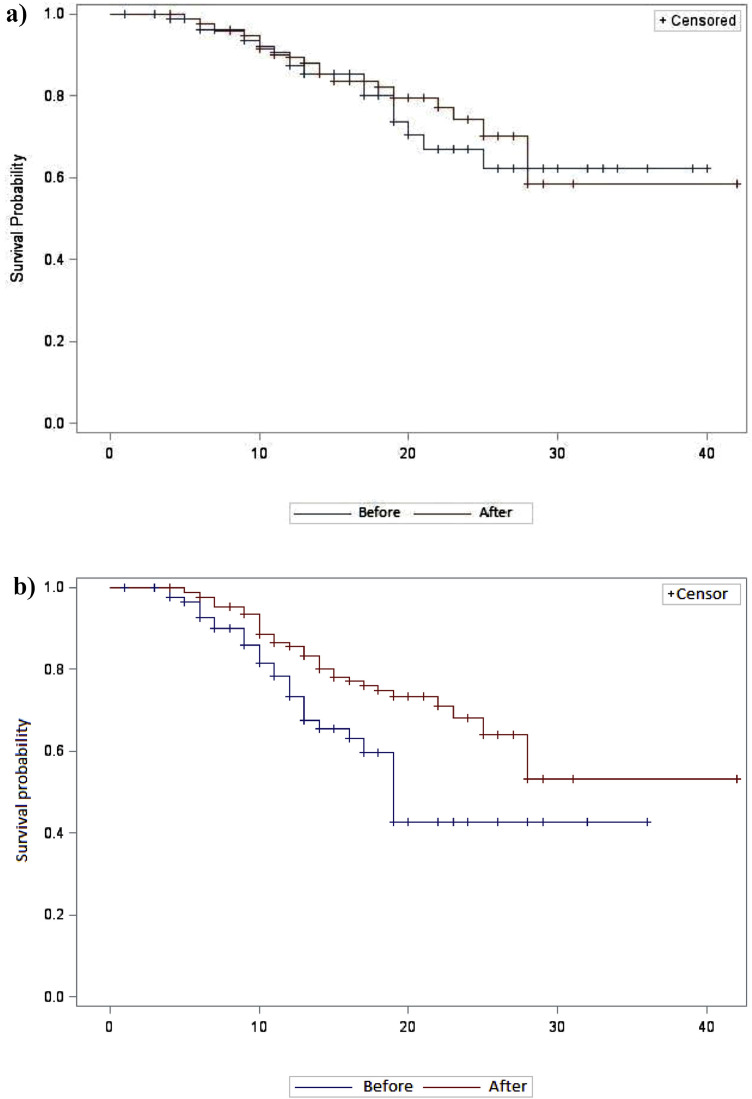
The ‘after’ period was not associated with a lower risk of death [hazard ratio (HR) = 0.86, 95% confidence interval (CI) 0.47–1.56; P = 0.62] by bivariate analysis (Fig. 1 a) but was associated by multivariate analysis adjusted for age, NEWS and institutionalisation status [adjusted HR (aHR) = 0.47, 95% CI 0.23–0.97; P = 0.04].
Fig. 1.
Kaplan–Meier curves for (a) death during hospitalisation and (b) ICU admission and/or death before ICU admission between patients ‘before’ and ‘after’ implementation of corticosteroids for COVID-19 pneumonia in Reims University Hospital (Reims, France). The ‘before’ period was 3–20 March 2020 and the ‘after’ period was 26 March–14 April 2020. P = 0.006, log-rank test.
The ‘after’ period was associated with a lower risk of ICU admission and/or death before ICU admission by bivariate analysis (HR = 0.25, 95% CI 0.11–0.55) (Fig. 1b) and by multivariate analysis adjusted for age, NEWS and institutionalisation status (aHR = 0.37, 95% CI 0.21–0.64; P = 0.0005).
4. Discussion
In this before-and-after study of 319 hospitalised COVID-19 patients, after adjustment for age, NEWS and institutionalisation status, the ‘after’ period (n = 172 patients), during which corticosteroids were routinely recommended for patients presenting with COVID-19 pneumonia at our institution, was associated with a lower risk of death (aHR = 0.47, 95% CI 0.23–0.97; P = 0.04) and a lower risk of ICU admission and/or death before ICU admission (aHR = 0.37, 95% CI 0.21–0.64; P = 0.0005).
To this day, corticosteroids are not recommended by the World Health Organization (WHO) for the treatment of COVID-19 pneumonia owing to their potential adverse effects such as secondary infections and prolonged virus shedding [8]. However, with our improving knowledge of the role played by overwhelming inflammation in severe COVID-19 patients, immunomodulatory drugs such as interleukin-6 (IL-6) or IL-1 blockade or anti-tumour necrosis factor (anti-TNF) therapy are being evaluated and all are in favour of a beneficial effect of immunomodulatory drugs during the inflammatory phase of COVID-19 infection [1,9]. Corticosteroids are old medicines that are inexpensive and accessible to the whole world. In the current study, corticosteroids were associated with a decrease of over 50% in mortality and in the rate of death and/or ICU admission, even though patients were more dependent and more often required oxygen in the ‘after’ period at the censoring date (although the follow-up duration was similar between the two groups).
We acknowledge that a before-and-after study yields a low level of evidence, the difference may be the result of overall better patient care with improvements in thrombosis prophylaxis, and some of these patients remained hospitalised at the end of follow-up and were thus censored for outcomes. Furthermore, the favourable outcome observed with corticosteroids may be partly due to the use of concurrent antiviral drugs in these patients. Another limitation of this study is that the COVID-19 pneumonia diagnosis was more often performed by chest CT scan in patients who received corticosteroids than in patients who did not. Positive RT-PCR is the gold standard for confirming the diagnosis of COVID-19 but its performance presents variable sensitivities ranging from 37–71% [10]. Although chest CT scan is highly sensitive for detecting COVID-19 pneumonia, overlapping CT image features with other viral pneumonias and other respiratory diseases make an exclusion diagnosis difficult and could be therefore a source of bias in this study [10]. Finally, the unavailability of safety data should be acknowledged as a limitation.
Nevertheless, these preliminary data support the initiation of clinical trials testing corticosteroids during the inflammatory phase of COVID-19 and may potentially lead to a change in treatment recommendations.
5. Reims COVID Study Group
The Reims COVID Study Group includes: Ailsa Robbins, Kévin Didier, Pauline Orquevaux, Violaine Noel, Paola Marianetti, Juliette Romaru, Dorothée Lambert, Jean Luc Berger, Sandra Dury, Maxime Dewolf, Jean Hugues Salmon, Jérôme Costa, Julia Simon, Natacha Noel, Sara Barraud, Marion Barrois, Hédia Brixi, Quentin Laurent-Badr, Manuelle Viguier, Clélia Vanhaecke, Laurence Gusdorf, Isabelle Quatresous, Aline Carsin-Vu, Véronique Brodard, Antoine Huguenin, Morgane Bonnet and Aurore Thierry.
Acknowledgments
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Editor: Jean-Marc Rolain
References
- 1.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse K.H., Formentini E., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of antiretroviral drugs on the pharmacokinetics of prednisolone in HIV-infected individuals. J Acquir Immune Defic Syndr. 2008;48:561–566. doi: 10.1097/QAI.0b013e31817bebeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzette S.A., Sattler F.R., Chiu J., Wu A.W., Gluckstein D., Kemper C., et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323:1451–1457. doi: 10.1056/NEJM199011223232104. [DOI] [PubMed] [Google Scholar]
- 6.Xu J., Wu R., Huang H., Zheng W., Ren X., Wu N., et al. Computed tomographic imaging of 3 patients with coronavirus disease 2019 pneumonia with negative virus real-time reverse-transcription polymerase chain reaction test. Clin Infect Dis. 2020 Mar 31 doi: 10.1093/cid/ciaa207. ciaa207 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams B., Alberti G., Ball C., Ball D., Binks R., Durham L.National Early Warning Score (NEWS). Standardising the assessment of acute-illness severity in the NHS. London, UK: Royal College of Physicians.
- 8.World Health Organization (WHO) WHO; 2020. Clinical management of COVID-19.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-%28ncov%29-infection-is-suspected [accessed 6 July 2020] [PubMed] [Google Scholar]
- 9.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin G.D., Ryerson C.J., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020;158:10–16. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]