Abstract
The coronavirus disease-2019 (COVID-19) pandemic is causing delayed ST-segment elevation myocardial infarction (STEMI) presentations associated with now unusual postinfarction complications. We describe a delayed (5-day) STEMI presentation because the patient feared contracting COVID-19 in the hospital. The patient experienced an extensive anterolateral STEMI complicated by subacute left ventricular free wall rupture that required a rapid surgical repair. (Level of Difficulty: Intermediate.)
Key Words: coronary angiography, coronavirus disease-2019, left ventricular aneurysm, LVFWR, myocardial infarction, percutaneous coronary intervention, pericardial effusion
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; CT, computed tomography; ECG, electrocardiogram; LAD, left anterior descending; LV, left ventricular; LVFWR, left ventricular free wall rupture; PCI, percutaneous coronary intervention; RV, right ventricular; STEMI, ST-segment elevation myocardial infarction; TTE, transthoracic echocardiogram
Graphical abstract
The COVID-19 pandemic is causing delayed STEMI presentations associated with now unusual postinfarction complications. We describe a delayed (5-…
A 72-year-old man came to emergency services on April 2020 after an episode of severe chest pain. He reported a similar episode 5 days before, and recurrent short similar episodes over the next 4 days. He never called emergency services for fear of contracting coronavirus disease-2019 (COVID-19). At first contact, his blood pressure was 85/50 mm Hg, and his heart rate 100 beats/min. Examinations of his heart and lungs were unremarkable.
Learning Objectives
-
•
COVID-19 may contribute to delayed presentations of acute myocardial infarction.
-
•
Delayed presentation with late reperfusion is associated with an increased risk of LVFWR.
-
•
When a high suspicion arises for an impending LVFWR in a patient recovering from extensive anterolateral transmural myocardial infarction, an early diagnosis and surgical repair initiated promptly can make a substantial difference in the outcome of this sometimes fatal complication.
Medical History
The patient had no cardiovascular risk factors, no individual history, and no family history of cardiovascular disease, and was not taking any medications or drugs.
Differential Diagnosis
No other diagnosis was possible, as the presentation was clearly an ST-segment elevation myocardial infarction (STEMI).
Investigations
The first contact electrocardiogram (ECG) was indicative of an extensive anterolateral STEMI (Figure 1A), therefore the patient was brought to the hospital to perform a primary percutaneous coronary intervention (PCI). Pertinent laboratory findings on admission and in the next 4 days are shown on Table 1. The transthoracic echocardiogram (TTE) on admission (Figure 2A, Video 1) demonstrated akinesia of the left ventricle (LV) anterolateral-apical wall, a large apical mural thrombus, severe LV dysfunction with an ejection fraction of 25%, mild-to-moderate circumferential pericardial effusion, diastolic right ventricle (RV) (Figure 2B) and systolic right atrium (RA) collapse (Figure 2A, Video 1). Coronary angiography showed proximal left anterior descending (LAD) artery occlusion (Figure 3A) and the remaining coronary arteries were normal.
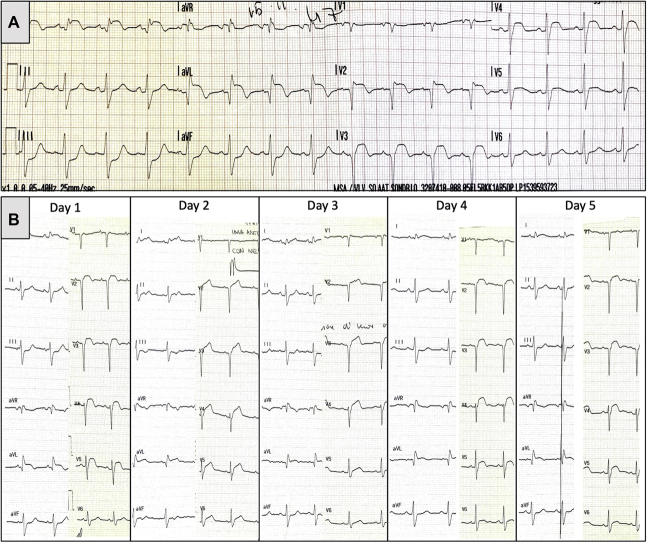
Figure 1.
Admission Electrocardiogram
(A) ST-segment elevation in leads V2 to V5 and I to aVL with specular ST-segment depression in leads III to aVF and Q waves in V1 to V3 and I to aVL. Daily electrocardiogram recorded after PCI (B) showing persistent ST-segment elevation.
Table 1.
Patient Laboratory Values on Admission and During Hospitalization
| Normal Value Range | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| AST, U/l | 3-40 | 840 | 522 | 208 | 125 | 66 |
| ALT, U/l | 3-37 | 1129 | 979 | 700 | 628 | 425 |
| LDH, U/l | 120-240 | 1549 | NR | 644 | NR | NR |
| ALT/LDH ratio | >1.50 | 0.73 | NR | 1.09 | NR | NR |
| TB, mg/dl | 0.30-1.00 | 1.60 | 1.03 | 1.01 | NR | NR |
| CK, U/l | 30-200 | 340 | NR | 131 | 97 | NR |
| Troponin I HS, ng/l | 0.0-34.2 | 32,906.0 | 26,710.8 | 20,079.3 | NR | NR |
| CRP, mg/l | 0.0-5.0 | 177.1 | 156.0 | 129.9 | 113.3 | 114.2 |
| WBC, 103/μl | 4.0-10.0 | 15.63 | 14.78 | 15.57 | 16.77 | 20.03 |
| Neutrophil number, 103/μl | 1.90-8.00 | 13.27 | 11.85 | 13.59 | 13.15 | 17.01 |
| Lymphocyte number, 103/μl | 1.00-4.00 | 1.11 | 1.92 | 1.88 | 2.25 | 1.28 |
| N/L ratio | <3.7 | 11.95 | 6.17 | 7.23 | 5.84 | 13.28 |
| aPTT ratio | 0.80-1.20 | 1.28 | 1.56 | 1.93 | 1.92 | 1.97 |
| Hemoglobin, g/dl | 13.0-17.0 | 13.5 | 11.4 | 12.3 | 13.4 | 14.1 |
| Creatinine, mg/dl | 0.73-1.18 | 1.07 | 0.99 | 0.91 | NR | NR |
ALT = alanine aminotransferase; aPTT = activated partial thromboplastin time; AST = aspartate transaminase; CK = creatine kinase; CRP C-reactive protein; HS = high sensitivity; LDH = lactate dehydrogenase; NR = no result; TB = total bilirubin; WBC = white blood cell count.
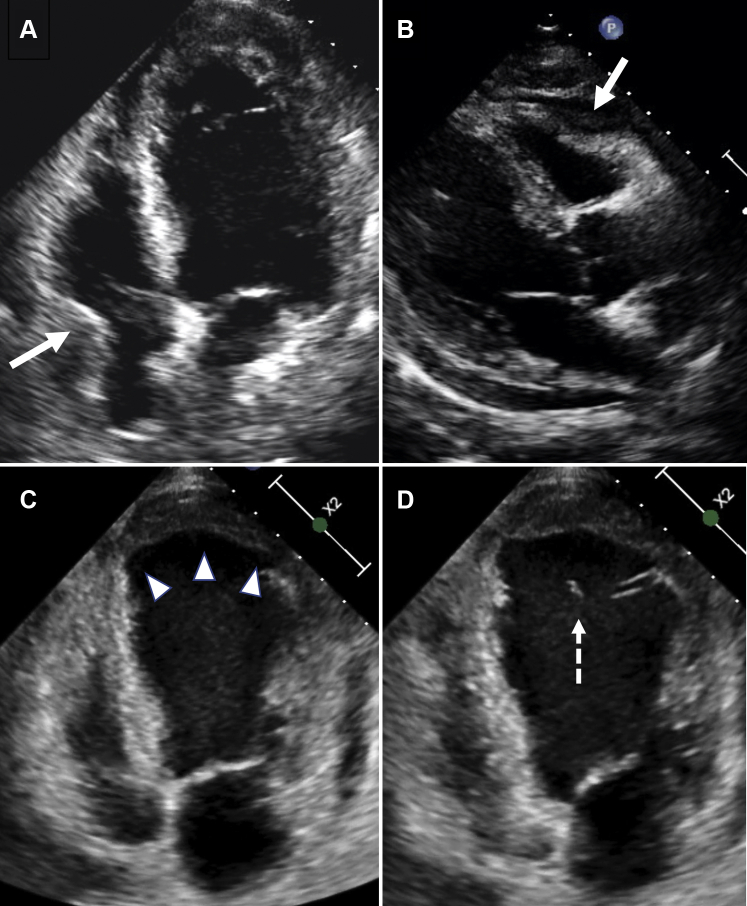
Figure 2.
Admission Echocardiogram
Admission echocardiogram in 4-chamber (A) and parasternal long-axis views (B) showing left ventricular (LV) apical mural thrombus, mild circumferential pericardial effusion, systolic right atrial (RA) (A, arrow) and diastolic right ventricular (RV) (B, arrow) collapse. Echocardiogram performed on day 4 shows (C) mild reduction in LV apical thrombus and pericardial effusion with expansion (arrowheads) of the anterolateral apical LV wall, and (D) a ruptured LV false tendon (dashed arrow).
Online Video 1.
Admission echocardiogram in 4-chamber view showing LV apical mural thrombus, mild circumferential pericardial effusion and systolic right (RA) atrial collapse.
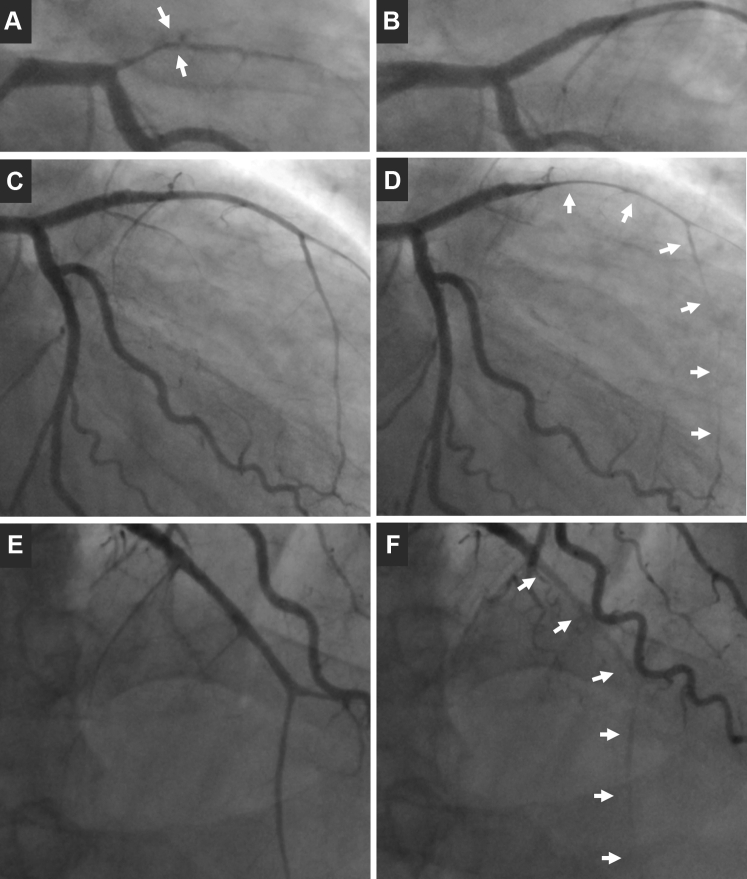
Figure 3.
Coronary Angiography at Baseline
Coronary angiography at baseline shows left anterior descending artery (LAD) occlusion (A) and final result (B) after stenting. Angiograms performed on day 5 in right anterior oblique (RAO)-caudal (C = diastole; D = systole) and RAO-cranial view (E = diastole; F = systole) show diffuse dynamic systolic severe compression of the mid-distal LAD (arrows).
Management
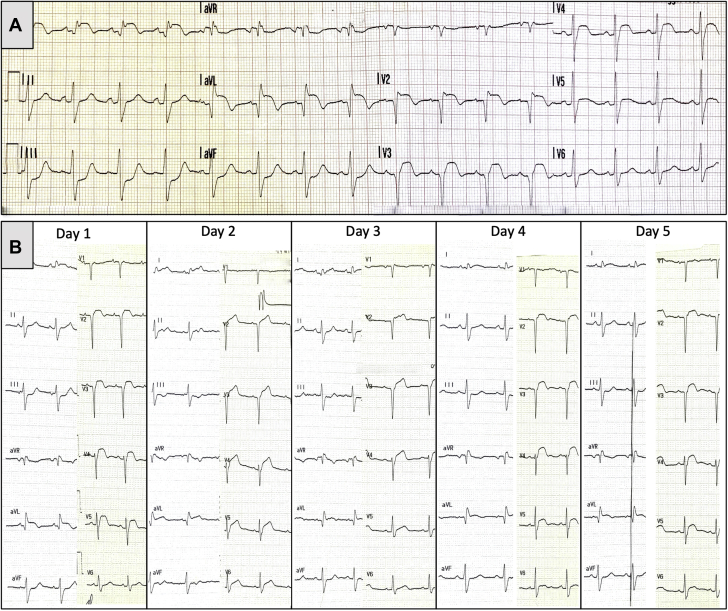
Primary PCI was performed. After manual thrombectomy, a stent was implanted at the LAD ostium with good final result (Figure 3B, Video 2). After PCI, the patient became asymptomatic. He remained asymptomatic, hemodynamically stable, with normal blood pressure and regular heart rate for the next 4 days, receiving medical therapy with aspirin, ticagrelor, intravenous (IV) heparin, diuretics, and a beta-blocker. As shown in Figure 1B, ECGs recorded after PCI from day 1 until day 5 demonstrated persistent ST-segment elevation, indicative of an aneurysmatic evolution. The TTE performed on day 4 (Figure 2C, Video 3) confirmed the presence of an aneurysmatic expansion of the anterolateral and apical LV walls. In addition, TTE showed mild worsening (ejection fraction ∼20%) of the already severely depressed LV function, mild reduction of both LV apical thrombosis and pericardial effusion, and the presence of an intracardiac mobile mass (Figure 2D, Video 3) referable to a ruptured LV false tendon attached to the neck of the aneurysm. On day 5, coronary angiography was performed to find clues to the presence of an impending left ventricular free wall rupture (LVFWR) (1) showing a diffuse dynamic systolic severe compression of the mid-distal LAD, sparing only its very apical segment (Figures 3C to 3F, Video 4). Cardiac CT scanning with IV contrast revealed the presence of an intramyocardial dissecting hematoma (2) into the aneurysmatic and thinner LV anterolateral wall (Figure 4, Videos 5 and 6). At that point, it was evident that the patient had an impending LVFWR. On day 5, the patient was transferred to the cardiac surgery department for rapid surgical repair (3). However, because the patient was asymptomatic and was receiving dual antiplatelet therapy and had minimal pericardial effusion and was a high surgical risk, the cardiac surgeons opted for initial short conservative management. In the meantime, a sudden cardiac arrest occurred on day 8 that required an emergency open-heart surgery repair. In the operating room, an “oozing-type” LVFWR was detected in the aneurysmatic anterolateral wall, which was very thin. It was repaired with a polyethylene terephthalate patch (Dacron, Dupont, Wilmington, Delaware) fixed onto the myocardial surface with surgical adhesive (BioGlue, CryoLife, Kennesaw, Georgia). Intra-aortic balloon pump (IABP) counterpulsation was started immediately after operation.
Online Video 2.
Coronary angiography in RAO-caudal view showing the good final result after stent implantation at the LAD ostium.
Online Video 3.
Echocardiogram in 4-chamber view performed on day 4 showing mild reduction in LV apical thrombus and pericardial effusion with expansion of the anterolateral-apical LV wall, and a ruptured left-ventricular false tendon.
Online Video 4.
Angiograms performed on day 5 in RAO-cranial view showing a diffuse dynamic systolic severe compression of the mid-distal LAD sparing only its very apical segment.
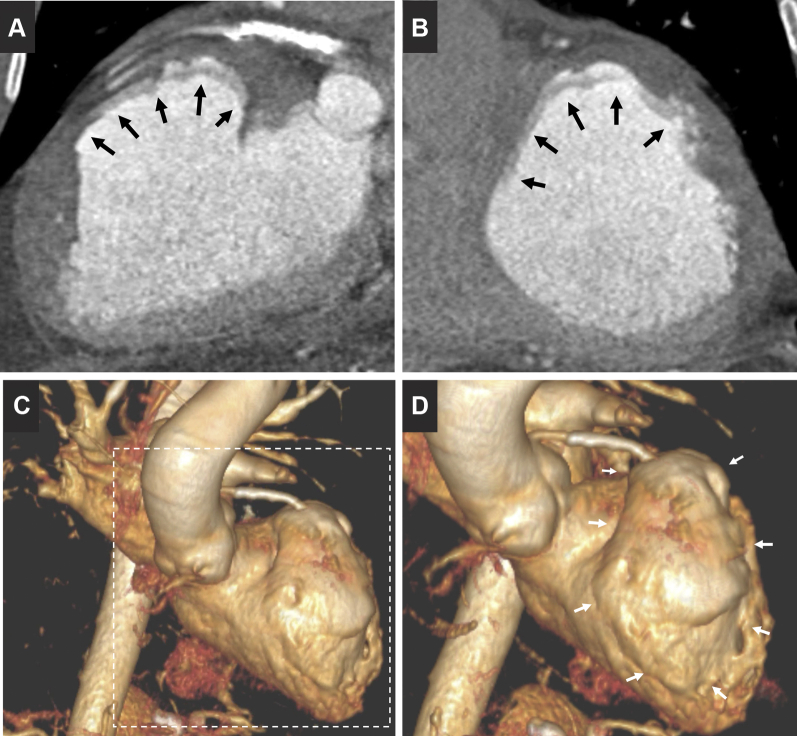
Figure 4.
Cardiac Computed Tomography Images
Cardiac computed tomography images in long-axis (A) and short-axis (B) views show the intramyocardial dissecting hematoma into the aneurysmatic and thinner left ventricular anterolateral wall (arrows). Three-dimensional reconstruction (C) shows, in detail (D), its position (arrows).
Online Video 5.
Cardiac CT images in long-axis showing the intramyocardial dissecting hematoma into the aneurysmatic and thinner LV anterolateral wall.
Online Video 6.
3D reconstruction of cardiac CT images showing the position of the intramyocardial dissecting hematoma into the aneurysmatic and thinner LV anterolateral wall.
Discussion
The coronavirus disease-2019 (COVID-19) pandemic may contribute to patients’ delayed presentations of STEMI for fear of contracting the infection in the hospital. A delayed presentation is associated with extensive myocardial necrosis and worse outcomes (4). In addition, a delayed presentation increases the probability of mechanical ventricular complications including LVFWR, ventricular septal defect, and acute papillary muscle rupture. These severe mechanical complications carry a high mortality. LVFWR occurs up to 10 times more frequently than septal or papillary muscle rupture. It usually occurs unexpectedly before the fifth day after STEMI (5) and is almost always fatal. Relatively few cases of LVFWR are diagnosed before death. In a multinational Global Registry of Acute Coronary Events registry, one of the largest studies, the incidence of LVFWR was 0.2% with an in-hospital mortality of 80% (6). Late patients with STEMI who present late face a worse risk profile and prognosis than patients who arrive <12 h from onset of symptoms. A 20-fold higher increase of myocardial rupture (4.1% vs. 0.2%, respectively; p < 0.001) has been reported in late-presenting STEMI patients by Cerrato et al. (4). The clinical suspicion of an impending LVFWR should be considered in patients recovering from a transmural myocardial infarction (MI) presenting with persistent ST-segment elevation, pericardial effusion, and an expanding LV aneurysm. LVFWR is an extreme form of infarct expansion during the early phase of STEMI. Defective infarct healing, as well as left ventricular wall stress, plays a major role in infarct expansion and may play an important role in the development of LVFWR. The myocardium is vulnerable to wall stress in the first week after infarction. In the present patient (Table 1), suspicion of LVFWR was supported by the disproportionate elevation of C-reactive protein levels, >20 mg/dl, and by the neutrophil-to-lymphocyte (N/L) ratio >3.7. Regarding the relationship between post-MI mechanical complications and inflammation, Anzai et al. (7) found C-reactive protein levels >20 mg/dl to be an independent predictor of cardiac rupture after the first STEMI (7). Pieck et al. (8) evaluated the levels of neutrophil and lymphocyte, 2 independent markers of inflammation, and showed that STEMI patients with LVFWR had increased N/L ratios. The use of these inflammatory markers has been proposed for predicting postinfarction LVFWR (8). Thus, STEMI patients with a disproportionate elevation of C-reactive protein levels >20 mg/dl and/or an elevated N/L ratio >3.7 should be followed more closely in terms of mechanical complications. In the present patient, the suspicion of impending LVFWR was reinforced by the angiographic presence of a diffuse dynamic systolic severe compression of the mid-distal LAD which was described as the first clue of a postinfarction mechanical complication and pseudoaneurysm formation by Kadavath et al. (1). The suspicion of LVFWR was finally confirmed by cardiac CT that detected an intramyocardial dissecting hematoma (2). In a percentage of STEMI patients, LVFWR does not occur as a sudden, explosive, and fatal episode but as an insidious, progressive entity. This “subacute” rupture, classified as an “oozing” type, is characterized by a small rupture or leakage through a friable aneurysm that may leak blood into the LV wall creating a subepicardial hematoma before massive rupture ensues (3). In this particular subgroup of patients, when diagnosis is suspected, surgical treatment is possible and must be initiated promptly to be lifesaving (3). In this patient, in whom the impending LVFWR was diagnosed on day 5 but surgery was delayed until day 8, an earlier surgery would have possibly decreased his risk of death.
Up to now, few data are available about the risk of LVFWR after revascularization by PCI in patients with late STEMI presentation. The indication to perform primary PCI in this patient was based on European guidelines (9) indicating that, in patients presenting days after the acute event with a completed MI, those with recurrent angina may be considered for revascularization when the infarct artery is occluded. The authors observed the presence of baseline Q waves in the admission ECG (Figure 1A), which is associated with adverse outcomes in patients with STEMI (10). Primary PCI of the occluded infarct-related artery may be less beneficial or even detrimental in STEMI patients with baseline Q waves or when performed 3 to 28 days after myocardial infarction, as demonstrated in the OAT (Occluded Artery Trial) (11). It is important to note, however that, even in the presence of Q waves, most patients have successful reperfusion as measured by ST-segment resolution after primary PCI (12). Moreover, the final myocardial salvage index in STEMI patients with Q waves was been reported as substantial (13). Our patient experienced recurrent episodes of chest pain after the initial episode, suggesting that the obstructed artery might have been spontaneously recanalized and that the obstruction might have been dynamic before the final episode and therefore the “full ischemic time” and the estimation of Q wave onset were indeterminable.
Follow-up
After undergoing surgical repair, the patient remained critically ill and died on day 9.
Conclusions
STEMI patients with a delayed presentation during COVID-19 pandemic due to fear of contracting the infection in the hospital will experience unnecessary morbidity and mortality. Delayed presentation with late reperfusion is often associated with an increased risk of LVFWR. Early diagnosis of an impending LVFWR and the surgical repair initiated promptly are crucial, even though mortality rates are still high.
Footnotes
Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Kadavath S., Ayan M., Al-Hawwas M. Dynamic systolic compression of the left anterior descending coronary artery as the first clue of postinfarction left ventricular pseudoaneurysm. Can J Cardiol. 2019;35:e9–e1419e11. doi: 10.1016/j.cjca.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Bekkers B.C., Prenger K., Waltenberger J. Images in cardiology: intramyocardial dissection after subacute anterior wall myocardial infarction. Heart. 2005;91:e54. doi: 10.1136/hrt.2004.052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matteucci M., Fina D., Jiritano F. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care. 2019;8:379–387. doi: 10.1177/2048872619840876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerrato E., Forno D., Ferro S., Chinaglia A. Characteristics, in-hospital management and outcome of late acute ST-elevation myocardial infarction presenters. J Cardiovasc Med (Hagerstown) 2017;18:567–571. doi: 10.2459/JCM.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 5.Figueras J., Cortadellas J., Soler-Soler J. Left ventricular free wall rupture: clinical presentation and management. Heart. 2000;83:499–504. doi: 10.1136/heart.83.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Sendon J., Gurfinkel E.P., Lopez de Sa E. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur Heart J. 2010;31:1449–1456. doi: 10.1093/eurheartj/ehq061. [DOI] [PubMed] [Google Scholar]
- 7.Anzai T., Yoshikawa T., Shiraki H. C-reactive protein as a predictor of infarct expansion and cardiac rupture after a first Q-wave acute myocardial infarction. Circulation. 1997;96:778–784. doi: 10.1161/01.cir.96.3.778. [DOI] [PubMed] [Google Scholar]
- 8.Ipek G., Onuk T., Karatas M.B. Relationship between neutrophil-to-lymphocyte ratio and left ventricular free wall rupture in acute myocardial infarction. Cardiology. 2015;132:105–110. doi: 10.1159/000431354. [DOI] [PubMed] [Google Scholar]
- 9.Neumann F.J., Sousa-Uva M., Ahlsson A. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y., Bainey K.R., Tyrrell B.D., Brass N., Armstrong P.W., Welsh R.C. Relationships between baseline Q Waves, time from symptom onset, and clinical outcomes in ST-segment-elevation myocardial infarction patients: insights from the vital heart response registry. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.005399. [DOI] [PubMed] [Google Scholar]
- 11.Hochman J.S., Lamas G.A., Buller C.E. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochar A., Granger C.B. Q waves at presentation in patients with ST-segment-elevation myocardial infarction: an underappreciated marker of risk. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.006085. [DOI] [PubMed] [Google Scholar]
- 13.Topal D.G., Lonborg J., Ahtarovski K.A. Association between early Q waves and reperfusion success in patients with ST-segment-elevation myocardial infarction treated with primary percutaneous coronary intervention: a cardiac magnetic resonance imaging study. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.116.004467. [DOI] [PubMed] [Google Scholar]