Abstract
A recent conceptual development in schizophrenia is to view its manifestations as interactive networks rather than individual symptoms. Negative symptoms, which are associated with poor functional outcome and reduced rates of recovery, represent a critical need in schizophrenia therapeutics. MIN101 (roluperidone), a compound in development, demonstrated efficacy in the treatment of negative symptoms in schizophrenia. However, it is unclear how the drug achieved its effect from a network perspective. The current study evaluated the efficacy of roluperidone from a network perspective. In this randomized clinical trial, participants with schizophrenia and moderate to severe negative symptoms were randomly assigned to roluperidone 32 mg (n = 78), 64 mg (n = 83), or placebo (N = 83). Macroscopic network properties were evaluated to determine whether roluperidone altered the overall density of the interconnections among symptoms. Microscopic properties were evaluated to examine which individual symptoms were most influential (ie, interconnected) on other symptoms in the network and are responsible for successful treatment effects. Participants receiving roluperidone did not differ from those randomized to placebo on macroscopic properties. However, microscopic properties (degree and closeness centrality) indicated that avolition was highly central in patients receiving placebo and that roluperidone reduced this level of centrality. These findings suggest that decoupling the influence of motivational processes from other negative symptom domains is essential for producing global improvements. The search for pathophysiological mechanisms and targeted treatment development should be focused on avolition, with the expectation of improvement in the entire constellation of negative symptoms if avolition is effectively treated.
Keywords: amotivation, psychosis, treatment, negative symptoms
Introduction
Results of a phase 2b trial indicate that roluperidone, a novel cyclic amide derivative with high affinities for 5-hydroxytryptamine 2A, sigma2, and alpha-1 adrenergic receptors, exerts a significant improvement on negative symptoms in schizophrenia patients.1 Although these results provide evidence for efficacy, they do not explain how the drug achieved its effect. Recently, there has been increasing interest in taking a network approach to evaluating psychiatric symptoms to help explain how treatments are effective.2 The underlying theory behind this approach is that mental illnesses arise from the interactions among symptoms in a network, whereby the occurrence of one symptom increases the probability that an interrelated set of symptoms also manifest.3 Symptom networks are described in terms of their density. Dense networks are highly interconnected and co-activate once symptom exacerbation occurs, forming closely joined clusters of psychopathology that maintain each other and become self-sustaining. Often, a specific symptom will be more “central” within a network than others, with strong interconnections to other symptoms in the network that cause those symptoms to emerge whenever the central symptom is present. In strongly connected networks, the activation of a central symptom may lead to the continued activation of other symptoms, even after the factors which triggered that symptom have disappeared. This process may be how symptoms become chronic, whereby self-sustaining feedback loops are triggered by a central symptom that leads to the activation of other symptoms, resulting in a global worsening in psychopathology.4
It is unclear whether more or less densely connected symptom networks are most responsive to treatment, as there is evidence for both possibilities.5,6 Densely connected networks may be difficult to treat because symptoms are closely coupled. In such a network, the treatment must effectively target the most central symptom and produce a spreading effect on the global symptom network that yields improvement across other domains. Alternatively, less densely connected networks may be very difficult to treat because symptoms have little interaction with one another. In this instance, even if a central symptom is successfully impacted, the lack of interconnection among symptoms makes it difficult for spreading effects to occur because symptoms function as their own islands of psychopathology with little interaction.
Determining which domains are most central and driving global negative symptom exacerbation has critical treatment implications. If this notion is correct, regardless of which individual symptoms are most central, it would suggest that the search for pathophysiological mechanisms and targeted treatment development should be focused on those key symptoms, with the expectation of improvement in the entire constellation of negative symptoms if they are effectively treated. Historically, there is precedent to expect avolition (ie, reductions in the desire for and initiation of motivated behavior) to be the most central of the 5 negative symptom domains identified in the 2005 NIMH Consensus Conference (the others being alogia, blunted affect, asociality, and anhedonia). For example, Kraepelin7 attributed considerable importance to avolition, describing schizophrenia patients as having: “A weakening of those emotional activities which permanently form the mainsprings of volition ….” Bleuler8 also proposed a central role for avolition, positing that: “Indifference seems to be the external sign of their state …. The will (is) . . . disturbed in a number of ways . . . The patients appear lazy and negligent because they no longer have the urge to do anything either of their own initiative or at the bidding of another.” In more modern conceptualizations, avolition is also thought to be key. For example, in their seminal review, Foussias and Remington9 drew upon the principle of “Occam’s Razor” and proposed that avolition led to the emergence of all other negative symptoms. Specifically, reductions in motivation were proposed to underlie decreased speech output (alogia), reductions in facial and vocal expression of emotion (blunted affect), diminished pursuit of pleasurable activities (anhedonia), and limited engagement in social interactions (asociality). Notably, the capacity to engage in these behaviors may be preserved, but the level of motivation needed for behavioral initiation was not sufficient to execute them. In a study using network analysis, Strauss et al.10 recently found support for this notion, with evidence that avolition was a central negative symptom of schizophrenia. This finding may suggest that treatments successfully targeting avolition could have a cascading effect, producing global improvements in the entire negative symptom cluster once motivation is effectively enhanced. Historical difficulties in successfully treating negative symptoms have made the exploration of such possibilities infeasible; however, the recent success of roluperidone offers a unique opportunity to evaluate how successful treatment of negative symptoms occurs from a network perspective.
In the current analysis, we took a network approach to evaluating negative symptoms to determine whether roluperidone achieved its effect by impacting a specific negative symptom domain or the overall density of symptom connections. We adopted a standard approach to evaluating symptom networks by calculating macroscopic and microscopic network properties.11 Macroscopic properties (eg, network density, average clustering coefficient, and average shortest path length) provide information about the overall connectedness of the network as a whole (see table 1). Networks with a higher density, average clustering coefficient, and lower average shortest path length are tightly connected (ie, symptoms are highly interdependent). In contrast, microscopic properties (eg, degree centrality, closeness centrality) provide information about which individual symptoms are most influential and interconnected with other symptoms in the network. Highly central symptoms are tightly coupled with other symptoms in the network and interact with those symptoms more directly.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Placebo (n = 83) | Roluperidone 32 mg/d (n = 78) | Roluperidone 64 mg/d (n = 83) | Roluperidone All (32 + 64 mg/d) (n = 161) | |
|---|---|---|---|---|
| Age | 40.0 (10.2) | 39.8 (10.2) | 40.6 (10.6) | 40.2 (10.4) |
| Male % | 57.8% | 52.6% | 57.8% | 55.3% |
| PANSS total at baseline | 80.2 (10.7) | 81.2 (9.8) | 79.7 (11.1) | 80.4 (10.5) |
| BNSS total at baseline | 47.3 (9.0) | 47.3 (9.4) | 47.1 (9.6) | 47.2 (9.5) |
| CGI score at baseline | 4.1 (0.7) | 4.2 (0.6) | 4.1 (0.7) | 4.2 (0.6) |
Note: PANSS = Positive and Negative Syndrome Scale; CGI = Clinical Global Impression; BNSS = Brief Negative Symptom Scale.
Based on historical clinical impression,7,8 the avolition and Occam’s Razor theory,9 and results of a recent network analysis,10 we hypothesized that avolition would be highly central among patients on placebo and that roluperidone would reduce this level of centrality. We also examined network density using macroscopic properties. Based on evidence indicating that highly dense networks are more difficult to treat,2 we hypothesized that roluperidone may achieve its effect by decreasing the global density of negative symptom networks. Exploratory analyses were also conducted to evaluate sex differences in the effect of roluperidone on macroscopic and microscopic properties given that men and women with schizophrenia display differences in both density and centrality of negative symptoms.10
Methods
Participants
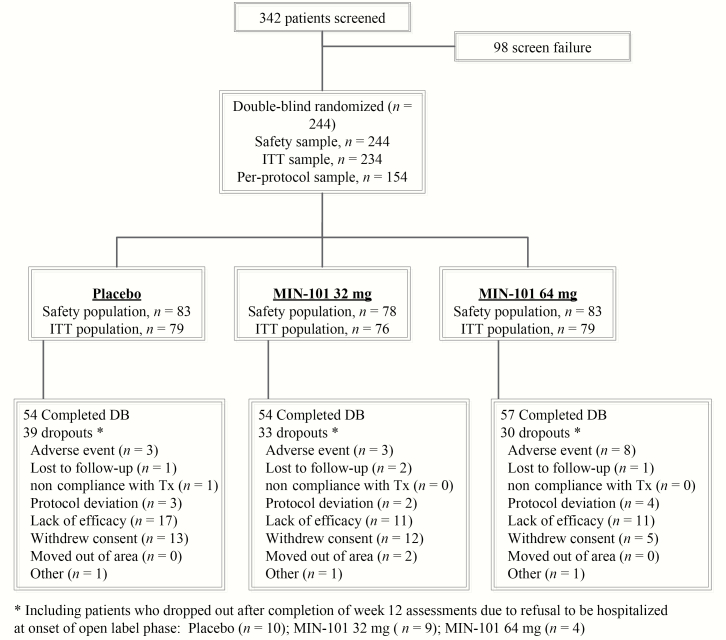
Participants included individuals with schizophrenia enrolled in an international, multicenter, double-blind, randomized controlled trial with 3 parallel arms: roluperidone with a daily dose of 32 mg (n = 78) or 64 mg (n = 83), and placebo (N = 83) administered as monotherapy (see figure 1). The study was registered as EudraCT Number: 2014-004878-42). See table 1 for basic demographics and Davidson et al.1 and Kirkpatrick et al.12 for full details and results of primary and secondary outcome measures.
Fig. 1.
Clinical trial flow diagram.
Two hundred forty-four patients between the ages of 18 and 60 entered the trial. Entry criteria included (1) a DSM-5 diagnosis of schizophrenia, (2) clinically stable and exhibiting negative symptoms for 3 months prior to entering the study, as determined by their treating psychiatrist, and (3) on the Positive and Negative Syndrome Scale, a total score ≥ 20 on the PANSS negative syndrome subscale (items N1-N7), and scores <4 on the PANSS excitement, hyperactivity, hostility, suspiciousness, uncooperativeness, and poor impulse control items. Exclusions were a diagnosis of another mental disorder, a significant risk of suicide, a positive urine test for illicit drugs, a history of substance abuse, or an unstable medical disorder. There were also exclusion criteria related to QT values, and for poor and intermediate metabolizers for P450 CYP2D6.1
Study Design
Eligible patients were withdrawn from depot antipsychotics, if any, for ≥1 month. All patients were then hospitalized and withdrawn from all psychotropic drugs for ≥5 days prior to randomization to oral roluperidone 32 mg/day, 64 mg/day, or placebo, in a 1:1:1 ratio. They remained hospitalized for at least 36 h after randomization, or longer at the discretion of the investigator if clinically indicated. Study treatment lasted for 12 weeks. No psychotropic medications were allowed during the trial, other than (1) oral lorazepam, oral zolpidem, or injectable sodium amytal for insomnia or agitation, or (2) anticholinergic medications for any extrapyramidal symptoms that emerged during the study.
Data Analysis
Analyses were performed with the NetworkX package in Python using data from the secondary outcome measure, the Brief Negative Symptom Scale (BNSS).13 The symptom network was constructed using the association between BNSS items calculated with cosine similarity measure. Cosine similarity measures the similarity of 2 vectors X and Y (ie, symptom change over time) by calculating the angle between them as follows:
Details regarding macroscopic and microscopic properties are summarized in table 2. To evaluate hypothesis 1, a 2 Group (roluperidone, Placebo) × 13 BNSS Item repeated-measures ANOVA was performed separately on degree centrality and closeness centrality. Significant interactions were followed-up by independent samples t-tests to determine which BNSS items were most central to treatment effects. To evaluate hypothesis 2, independent samples t-tests were run on the 3 macroscopic properties: density, average shortest path length, and clustering coefficient. Exploratory analyses were conducted to evaluate sex differences by repeating the analytic approach used for hypotheses 1 and 2, using sex as an additional between-subjects factor. Furthermore, additional analyses evaluating dose (32 mg, 64 mg, Placebo) were conducted using one-way ANOVAs. All exploratory analyses indicated nonsignificant effects of dose and sex. As such, primary analyses were reported that collapsed across doses and sexes.
Table 2.
Summary of Network Measures
| Type | Measure | Definition | Clinical Meaning | Equation |
|---|---|---|---|---|
| Macroscopic | Density | Avg. of all the edge weights in the network | To what extent symptoms in the network are interconnected | |
| Harmonic mean shortest path length | Avg. shortest path length between all nodes | Level of information efficiency in the network | ||
| Avg. clustering coefficient | Overall clustering in the network | To what extent symptoms tend to cluster together | ||
| Microscopic | Degree centrality | Sum of the edge weights connected to a node | Level of connectivity of a symptom in the network | |
| Harmonic mean closeness centrality | Distance of a node to all other nodes in the network | How quickly one symptom reaches other symptoms |
Note: i, j, u,v = Node (BNSS Symptom) Index; N = total number of nodes; V is the set of all nodes in the network; wij, wiu, wiv = weight between nodes i and j, i and u, i and v; d(i,j) = shortest path between nodes i and j calculated based on inverse of their weight (1/wij) ; ki and kj = degrees of nodes i and j.
Results
Microscopic Analyses
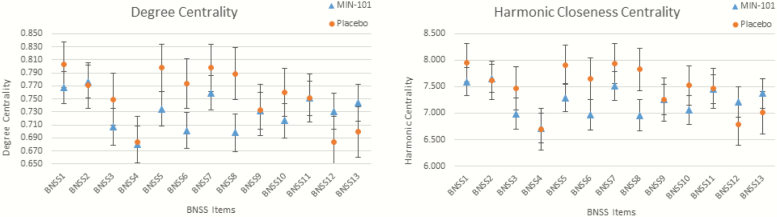
Mixed-models ANOVA revealed a significant Group X BNSS item interaction for both degree centrality, F (12, 2892) = 1.91, P = .028, and closeness centrality, F (12, 2892) = 1.89, P = .032. Follow-up independent samples t-tests indicated that for both centrality measures, BNSS item 8 (avolition internal experience) distinguished treatment and placebo groups, with roluperidone showing lower centrality than Placebo (degree centrality t = −1.93, P = .05; closeness centrality t = −1.84, P = .06). All other items were nonsignificant (see figures 2 and 3; analyses were rerun after randomly removing 10% of the data as a consistency test and t-test results held significance. Changes in centrality were proportional to change in covariance structure). To confirm the reliability of these results, we conducted a “leave one out” analysis, where network analysis was repeated with item 8 excluded. The results confirmed the reliability of the original analyses. Specifically, there were no statistically significant differences between roluperidone and placebo for closeness or degree centrality on any of the other 12 items when item 8 was left out. Notably, there was a trend for item 7 for degree (P = .075) and closeness (P = .080) centrality. Although only a trend, this is noteworthy because item 7 assesses avolition: behavior, thereby providing further confirmation that avolition is indeed the core domain that is most central to treatment response.
Fig. 2.
Microscopic centrality results. Note: BNSS Items: 1 = intensity of pleasure during activities, 2 = frequency of pleasurable activities; 3 = intensity of expected pleasure from future activities; 4 = lack of normal distress; 5 = asociality behavior; 6 = asociality internal experience; 7 = avolition behavior; 8 = avolition internal experience; 9 = facial expression; 10 = vocal expression; 11 = expressive gestures; 12 = quantity of speech; 13 = spontaneous elaboration.
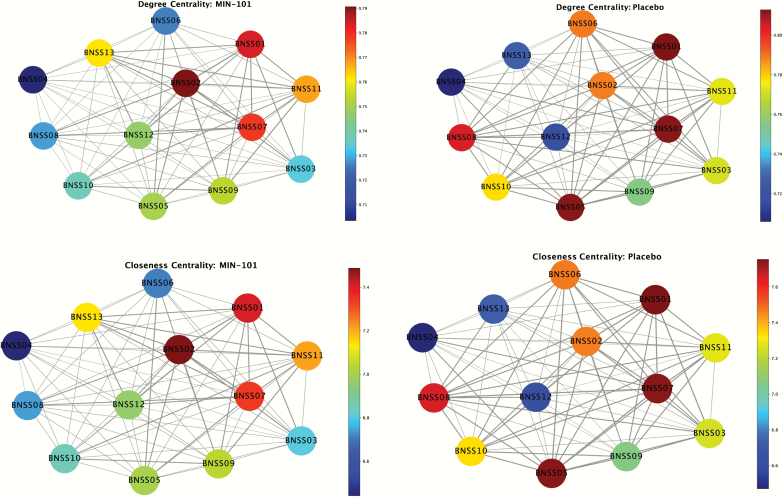
Fig. 3.
Topographic map of closeness and degree centrality. Note: Nodes/circles represent BNSS items; edge/line weight represents strength of association. Warmer (darker) node color (lighter) reflects greater magnitude of degree or closeness centrality. BNSS Items: 1 = intensity of pleasure during activities, 2 = frequency of pleasurable activities; 3 = intensity of expected pleasure from future activities; 4 = lack of normal distress; 5 = asociality behavior; 6 = asociality internal experience; 7 = avolition behavior; 8 = avolition internal experience; 9 = facial expression; 10 = vocal expression; 11 = expressive gestures; 12 = quantity of speech; 13 = spontaneous elaboration.
Macroscopic Analyses
Independent samples t-tests examining the effects of treatment on density (t = −.63, P = .53), average shortest path length (t = −.70, P = .49), and clustering coefficient (t = −.59, P = .56) were nonsignificant.
Discussion
The current study examined the nature of successful negative symptom treatment from a network perspective using data from the roluperidone trial. Macroscopic properties, which evaluate the overall connectedness of the network, did not distinguish between active drug and placebo. However, as hypothesized, microscopic properties indicated that avolition was highly central in patients on placebo (ie, tightly coupled and directly interacting with other negative symptoms) and roluperidone reduced this level of centrality.
These findings have several important implications. First, the lack of a macroscopic effect suggests that future drug development need not focus on targeting mechanisms that have relevance to all 5 negative symptom domains (which may be vastly different). Rather, the specificity of an effect on a single domain within the microscopic analyses indicates that targeted treatment development should focus on that domain, with the expectation that the entire symptom constellation will improve if the domain is effectively treated. Second, the specificity of the microscopic analysis effects to avolition internal experience is important. This suggests that decoupling the influence of motivational processes on other aspects of negative symptoms should be the goal of pharmacological treatment. Third, the observation that the internal experience avolition item was most central to successful treatment provides key insight into the processes underlying negative symptoms. These findings are consistent with historical views offered by Bleuler and Krapelin,7,8 as well as the Occam’s Razor conceptualization posited by Foussias and Remmington9 that emphasized the centrality of avolition. How this network-level symptom effect is realized at the neural level is unclear. Modern conceptualizations of the pathophysiology of avolition propose that dysfunctional cortico-striatal interactions lead to impairments in a range of reward processing mechanisms that impede the initiation of goal-directed behavior.14,15 It is possible that dysfunction in these circuits and reward processes may have an under-recognized effect on the other negative symptom domains as well. Via its effects on 5HT2A and Sigma2 receptor functioning, roluperidone may produce beneficial effects on these reward circuits that have a direct impact on the negative symptom that is most central to global improvements—avolition.
Certain limitations should be considered when interpreting these findings. Network analysis was run on only a single negative symptom measure: the BNSS. Although the PANSS was collected as the primary outcome measure in the trial,1 its items do not assess negative symptoms according to the most current conceptualizations in the field and it does not include items relevant to all 5 constructs identified in the 2005 NIMH consensus conference.16 As such, only the BNSS was evaluated for the purpose of evaluating macroscopic and microscopic structures; however, it is possible that alternate measures would produce different results. The BNSS data were analyzed at the level of individual items to capture potential meaningful differences within negative symptom domains (eg, dissociations between avolition behavior and internal experience). However, the BNSS data are not agnostic to latent variables and potential latent confounding. Additionally, we were unable to evaluate what Borsboom2 referred to as “environmental factors” that act on symptom networks. These are variables external to the symptom network that can be either internal aspects relevant to the participant (eg, inflammation, neural circuitry, genetics) or factors from the environment itself (eg, early life stress). If such environmental factors exist, they would also become relevant treatment targets.
Despite these limitations, findings indicate that avolition plays a central role in global negative symptom pathology. Motivational deficits may be at the core of blunted affect, alogia, anhedonia, and asociality. Effectively treating motivational deficits is key to successful global negative symptom improvements. Animal models that closely map onto how avolition manifests in schizophrenia, such as the D2 receptor overexpression mouse model,17 may be useful for targeted treatment development. Network analysis may be a promising analytic tool for determining how psychiatric medications achieve efficacy. Traditional univariate analyses provide an indication of whether a drug holds efficacy compared to placebo, but not how that effect comes about. As evidenced by the current microscopic analyses supporting a role for avolition, network analysis is capable of indicating whether certain symptoms are driving treatment response by having dynamic influences on the entire symptom constellation. Future studies could consider evaluating microscopic properties at baseline to determine which domain is most central, and using this information to take a personalized medicine approach to targeting specific symptoms. For example, once multiple medications and psychosocial treatments are developed that are efficacious for negative symptoms, baseline network scores indicating whether a patient has avolition vs anhedonia vs blunted affect as the most central negative symptom could be used to direct the course of treatment based on mechanism of actions. Using these “computational phenotypes” to guide clinical decision-making would be a novel approach to personalized medicine.
Funding
The phase 2b clinical trial was funded by Minerva Neurosciences. The authors were not paid to generate or review this manuscript.
Acknowledgments
Drs Strauss and Kirkpatrick are original developers of the Brief Negative Symptom Scale (BNSS) and receive royalties and consultation fees from ProPhase LLC in connection with commercial use of the BNSS and other professional activities; these fees are donated to the Brain and Behavior Research Foundation. GPS and BK have received honoraria and travel support from ProPhase LLC for training pharmaceutical company raters on the BNSS. Drs Strauss received consulting fees and travel support from Minerva Neurosciences and Lundbeck. Dr Kirkpatrick received consulting fees and travel support from Minerva Neurosciences and ProPhase LLC, consulting fees from an anonymized pharmaceutical company through Decision Resources, Inc., from an anonymized investment capital company through Guideposts, and from Wockhardt Bio AG (through Sterne, Kessler, Goldstein, & Fox) for consulting on a legal issue. Dr Kirkpatrick also receives fees from Walsh Medical Media for editorial services. Dr Opler is an employee of Medavante-Prophase LLC. Dr Saoud, Dr Davidson, and Dr Luthringer are employees of Minerva Neurosciences and own stock options in Minerva Neurosciences. Drs Sayama and Esfahlani have no conflicts to report. European Clinical Trials Database (EudraCT) identifier: 2014-004878-42.
Contributions
The study was designed by Minerva Neurosciences. Analyses were conducted by Drs Esfahlani and Strauss. Dr Strauss created the first draft of the manuscript which was subsequently edited by all authors.
References
- 1. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;174(12):1195–1202. [DOI] [PubMed] [Google Scholar]
- 2. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. [DOI] [PubMed] [Google Scholar]
- 4. Borsboom D, Robinaugh DJ, Rhemtulla M, Cramer AOJ; Psychosystems Group Robustness and replicability of psychopathology networks. World Psychiatry. 2018;17(2):143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esfahlani FZ, Visser KF, Strauss GP, Sayama H. A network-based classification framework for predicting treatment response of schizophrenia patients. Expert Syst Appl. 2018;109:152–161. [Google Scholar]
- 6. Lutz W, Schwartz B, Hofmann SG, Fisher AJ, Husen K, Rubel JA. Using network analysis for the prediction of treatment dropout in patients with mood and anxiety disorders: a methodological proof-of-concept study. Sci Rep. 2018;8(1):7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kraepelin E. Dementia Praecox and Paraphrenia. New York, NY: Robert E Krieger; 1919. [Google Scholar]
- 8. Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1911. [Google Scholar]
- 9. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strauss GP, Esfahlani FZ, Kirkpatrick B, et al. Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull. 2019;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sayama H. Introduction to the Modeling and Analysis of Complex Systems: Open SUNY Textbooks; 2015. [Google Scholar]
- 12. Kirkpatrick B, Saoud JB, Strauss GP, et al. The brief negative symptom scale (BNSS): sensitivity to treatment effects. Schizophr Res. 2018;197:269–273. [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(suppl 2):S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward RD, Simpson EH, Richards VL, et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37(7):1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]