Abstract
Recent diffusion imaging studies using free-water (FW) elimination have shown increased FW in gray matter (GM) and white matter (WM) in first-episode psychosis (FEP) and lower corrected fractional anisotropy (FAt) in WM in chronic schizophrenia. However, little is known about the longitudinal stability and clinical significance of these findings.
To determine tissue-specific FW and FAt abnormalities in FEP, as part of a multicenter Spanish study, 132 FEP and 108 healthy controls (HC) were clinically characterized and underwent structural and diffusion-weighted MRI scanning. FEP subjects were classified as schizophrenia spectrum disorder (SSD) or non-SSD. Of these subjects, 45 FEP and 41 HC were longitudinally assessed and rescanned after 2 years. FA and FW tissue-specific measurements were cross-sectional and longitudinally compared between groups using voxel-wise analyses in the skeletonized WM and vertex-wise analyses in the GM surface.
SSD and non-SSD subjects showed (a) higher baseline FW in temporal regions and in whole GM average (P.adj(SSD vs HC) = .003, P.adj(Non-SSD vs HC) = .040) and (b) lower baseline FAt in several WM tracts. SSD, but not non-SSD, showed (a) higher FW in several WM tracts and in whole WM (P.adj(SSD vs HC)= .049) and (b) a significant FW decrease over time in temporal cortical regions and in whole GM average (P.adj = .011). Increased extracellular FW in the brain is a reliable finding in FEP, and in SSD appears to decrease over the early course of the illness. FAt abnormalities are stable during the first years of psychosis.
Introduction
A substantial number of studies using diffusion tensor imaging (DTI) techniques have shown white matter (WM) abnormalities in schizophrenia and in first-episode psychosis (FEP), which include widespread decreased fractional anisotropy (FA) in WM tracts.1,2 These findings have been replicated in samples of high-risk for psychosis subjects3 and never-medicated FEP.4 However, some of these results have been reported in regions that do not coincide between studies using different clinical samples, such as naive vs medicated subjects, and first-episode vs established schizophrenia.5–7 Moreover, potential confounding factors such as age,8 gender,9 and cannabis use10 have been reported in the interpretation of FA between-group differences. To better understand the biological role of WM abnormalities in schizophrenia, the literature emphasizes the need for additional studies using a longitudinal approach, the use of homogeneous samples, and more accurate and reproducible measurements.2,11
In this line, Pasternak et al12 published the results of an analysis using a bi-tensor model that allows an accurate estimation of the DTI tensor while mitigating partial volume effects, such as voxels containing WM tracts and water from cerebrospinal fluid (CSF) or edema surrounding the tracts. Following this method, the tensor is calculated without averaging the compartments, which could otherwise induce a biased estimation of the diffusion parameters.12 This method provides a novel measure of the presence of extracellular free-water (FW) in brain tissue, which can be measured in both WM and gray matter (GM). Elevated FW may suggest edema or neuroinflammation, as these processes involve an increased release of water to the extracellular space. However, other processes may also result in FW increases including edema or proximity to CSF, and FW imaging does not disambiguate these causes. Using this methodology, an initial small sample study in subjects with first-episode schizophrenia showed a significant increase in FW in both WM and GM), and, in contrast, a fairly limited number of regions with decrease in fractional anisotropy (FA) corrected by FW FAt.13 Further studies using this technique in patients with chronic schizophrenia found limited increases in FW and significant, albeit quite localized, decreases in FAt compared to controls.14,15 These findings were more localized than those reported in the literature measuring FA without FW correction.16 In summary, cross-sectional studies in different phases of psychosis suggest the presence of elevated FW with limited decreases in FAt in the early phases, while findings in more established illness include a more modest elevation of FW and a heightened decrease in FAt. The present study uses a longitudinal approach in subjects at the early phase of the illness to directly address the time course of changes in these measures during the early course of psychotic illness.
Moreover, FW increases in WM have been linked to symptoms such as delusions in chronic schizophrenia17 and, counterintuitively, to better cognitive functioning16. However, it remains unclear how FW increase is related to negative symptoms, illness severity, illness progression, or clinical outcomes, which could be better elucidated using longitudinal studies.
Furthermore, the biological underpinnings of these findings are not yet fully understood. Previous findings converge to suggest the evidence of a neuroimmune process in schizophrenia: Elevated pro-inflammatory markers have been reported in the serum of schizophrenia patients18; GM volume reductions in schizophrenia have been related to elevated neutrophil cell count19; and evidence of anti-inflammatory properties of antipsychotics has also been reported.20,21 In this regard, the excessive extracellular water in first-episode subjects has been hypothesized to rely on inflammatory processes,22 and, in this line, a recent study has shown an inverse correlation between increased FW in GM and glutathione measured by magnetic resonance spectroscopy in first-episode schizophrenia subjects.23 Notwithstanding the relation between FW and inflammation is not fully understood, and no studies have tested the correlation between FW elevation and other peripheral inflammatory markers such as neutrophil cell count. In this sense, previous to the search for a corresponding acute or chronic immune mechanism, it is crucial to determine the changes over time of FW and FAt abnormalities in psychosis. Surprisingly, no longitudinal studies have been published testing an FW change over time.
The goal of the present longitudinal multicenter study was to utilize the extracellular FW elimination model to determine the presence of WM and GM alterations in FEP, along with any changes in these measures that may occur during the first 2 years of the illness. Based upon the available literature, we hypothesize that patients with FEP will show increased FW in both GM and WM that may decrease across time (consistent with a shift from an acute neurimmune response to low-level chronic inflammation), as well as decreased FAt in WM (in agreement with previous studies) along with the evidence of possible anti-inflammatory effects of antipsychotic treatment. All of our participants were engaged in coordinated specialty care for early psychosis during the follow-up period, and most were treated with therapeutic doses of antipsychotics during this time. Additionally, we aimed to investigate the clinical significance of FW abnormalities by examining their relationship with symptomatology and neutrophil cell count.
Methods
Subjects
This study is a part of the multicenter PEPs study (“Phenotype–genotype and environmental interaction. Application of a predictive model in first psychotic episodes”) in which 335 FEP subjects and 253 healthy controls (HC) were recruited between January 2009 and December 2011, and prospectively followed for 2 years. The inclusion criteria and characteristics of the study have been previously described in detail.24,25 Briefly, subjects with FEP aged 7 to 35 years and who presented psychotic symptoms of less than 12 months duration were recruited from inpatient and outpatient units of 16 participating centers from the Center of Biomedical Research Network on Mental Health (CIBERSAM)26 in Spain. As in the primary analysis of structural neuroimaging of the same multicenter study (PEPs-Imaging),27 a maximum time of 6 months was established from inclusion to scan time as an additional inclusion criteria.
All centers received the approval of their respective Independent Ethics Committee before the study started. Written informed consent was obtained from all subjects prior to their participation in the study, and also from parents/legal guardians for children under 16 years of age (children gave assent).
From the total pool of subjects of the study, 132 FEP and 108 HC underwent an magnetic ressonance imaging (MRI) scan with the acquisition of diffusion images at baseline and 54 FEP and 49 HC at follow up (2 years from baseline). A consort diagram of the subjects analyzed in the study is shown in supplementary material (S1). The demographic and clinical assessments are described in supplementary material (S2).
To reduce sample heterogeneity, FEP subjects were grouped into (a) schizophrenia spectrum disorder (SSD) if they fulfill DSM IV diagnostic criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder, or (b) non-SSD, which included bipolar disorder with psychotic features, major depressive disorder with psychotic features, brief psychotic disorder, psychosis not-otherwise-specified, and drug-induced psychosis. A detailed list of diagnosis by group is shown in the supplementary material (S3).
Image Acquisition and Preprocessing
Although 6 different scanners were used in the study, only 2 sites included diffusion-weighted image (DWI) acquisitions, and thus only data from those subjects scanned in any of these two sites were used in this study. T1-weighted, T2-weighted, and DWI were acquired for each participant. The acquisition parameters of the DWI are available in supplementary material (S4), briefly: Siemens Trio-Trim 3T: matrix 128 × 128, 2 mm, 30 directions, 1 NEX, TR 8600 ms, TE 97 ms, single-shell b values of 1000, 1 b0 acquisition, voxel size 2 × 2 × 2 mm. Phillips Intera 1.5T: matrix 112 × 112, 2 mm, 15 directions, 2 NEX, TR 11884 ms, TE 82 ms, single-shell b values of 800, 2 b0 acquisitions, voxel sixe 2 × 2 × 2 mm. The proportion of subjects by group in each of the scanners is reported in table 1.
Table 1.
Sociodemographic and Clinical Characteristics at Baseline and Follow-Up in First-Episode of Psychosis Subjects and Healthy Controls
| Baseline | Follow-up (2 years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | Non-SSD | SSD | Stats | P-Value | HC | Non-SSD | SSD | Stats | P-Value | |
| n | 101 | 52 | 59 | 41 | 20 | 20 | ||||
| Mean age at baseline scan (SD) | 23.8 (6.4) | 23.3 (6.1) | 22.1(5.9) | F(2,109) = 1.406 | .248 | 23.9 (6.1) | 24.2 (5.1) | 22.9 (6.1) | F(1,79) = 0.287 | .594 |
| Age rangea (at baseline) | 10–36 | 12–35 | 12–35 | 14–36 | 16–33 | 16–35 | ||||
| Gender (% male) | 64.4% | 71.1% | 64.4% | X-sq(2) = 0.804 | .669 | 65.9% | 70.0% | 50.0% | X-sq(2) = 2.013 | .366 |
| Scanning site (% SIEMENS) | 76.2% | 88.5% | 83.1% | X-sq(2) = 3.547 | .170 | 75.6% | 85.0% | 95.0% | X-sq(2) = 3.632 | .163 |
| Ethnicity (% Caucassic) | 86.1% | 82.7% | 81.4% | X-sq(2) = 0.715 | .700 | 85.4% | 100% | 70% | X-sq(2) = 7.134 | .028 |
| Marital Status (%Single) | 84.2% | 84.6% | 93.2% | X-sq(2) = 2.953 | .228 | 83.0% | 80.0% | 90.0% | X-sq(2) = 0.807 | .668 |
| Employment (% Active, % Student, % Unemployed) | 43.6% 49.5% 6.9% | 15.4% 51.9% 32.7% | 10.5% 57.9% 31.6% | X-sq(4) = 35.273 | <.001 | 48.8% 48.8% 2.4% | 20.0% 50.0% 30.0% | 21.1% 63.2% 15.8% | X-sq(4) = 13.494 | .009 |
| SES, (% High-IH, % I, % LI-Low) | 41.0% 30.0% 29.0% | 28.8% 21.2% 50.0% | 31.6% 19.3% 49.1% | X-sq(4) = 9.356 | .053 | 55.0% 32.5% 12.5% | 25.0% 40.0% 35.0% | 50.0% 20.0% 30.0% | X-sq(4) = 7.662 | .105 |
| GAF (SD) | 93.3 (5.7) | 55.9 (22.2) | 46.0 (22.0) | F(2,208) = 182.1 | <.001 | 91.3 (3.7) | 83.9 (5.3) | 62.3 (13.5) | F(1,76) = 153.8) | <.001 |
| PANSS (SD) | At baseline | At follow-up | ||||||||
| Positive | NA | 17.4 (8.4) | 19.7 (7.9) | t(105.35) = −1.446 | .151 | NA | 8.5 (2.3) | 12.9 (4.5) | t(29.82) = −3.776 | <.001 |
| Negative | NA | 15.9 (6.8) | 22.3 (9.2) | t(106.1) = −4.187 | <.001 | NA | 11.9 (4.4) | 20.1 (6.5) | t(33.70) = −4.647 | <.001 |
| General | NA | 36.0 (10.6) | 40.4 (15.2) | t(106.6) = −1.768 | .080 | NA | 22.6 (5.5) | 31.2 (9.1) | t(31.616) = −3.590 | .001 |
| Total | NA | 69.3 (20.7) | 82.3 (27.6) | t(106.4) = −2.824 | .005 | NA | 43.0 (11.1) | 64.2 (17.3) | t(32.606) = −4.565 | <.001 |
| CGI (SD) | NA | 4.2 (1.0) | 4.8 (1.1) | t(105.2) = −3.078 | .003 | NA | 2.2 (1.4) | 3.7 (2.5) | t(37.918) = −4.454 | <.001 |
| Antipsychotic (CPZ eq) | NA | 439.7 (422.8) | 527.5 (409.4) | t(106.3) = −1.108 | .270 | NA | 178.8 (396.7) | 524.2 (228.3) | t(29.3) = 3.115 | .004 |
| Days from illness onset to MRI (SD) | NA | 141.2 (98.8) | 182.2 (120.4) | t(106.5) = 1.927 | .057 | NA | 847.5 | 880.4 | t(35.1) = 35.1 | .391 |
Note: Non-SSD: Psychosis other than schizophrenia spectrum disorder; SSD: Schizophrenia spectrum disorder, HC: Healthy controls; PANSS: Positive and Negative Symptoms Scale.
aFor the cross-sectional baseline analysis, only one SSD subject (1.6%), one non-SSD subject (1.9%), and three controls (3%) were younger than 14 years old (ages 12, 12, 10, 11, 11, respectively).
Diffusion images were initially visually inspected to discard errors during the acquisition, and were then preprocessed including quality control procedures [see details from the applied pipeline in the supplementary material (S5)].
The preprocessed images were entered in a computation bi-tensor model to calculate diffusion scalar images, with and without FW elimination. This model has been implemented in Mitk (http://www.mitk.org) by Maier-Hein et al,28 and it is based on a previously described process.12 All voxels containing an FW value higher than 0.7 were considered to be predominantly CSF and were removed from all the scalar images as suggested in commonly used pipelines.29
Image Analysis
To determine basal and longitudinal alterations in the diffusion parameters of WM and GM, using the extracellular FW elimination model, an initial analysis of the mean of the scalar measurements in each tissue was carried out, followed by a specific study of WM using tract-based spatial statistics (TBSS)30 and a specific study of GM analyzing FW on the GM surface. To minimize inter-site variability while maintaining variance related to group, age, gender, and time of acquisition, a batch-effect correction model (ComBat)31 was applied to those analysis involving spatial localization (WM in TBSS and the GM surface analysis), while only the addition of site as a covariate was used in the study of average volumes.
Average Scalar Measures
To determine between-group differences in tissue-specific measures, an average scalar analysis was implemented by applying WM and GM masks to the scalar images. In detail, T1 images were co-registered to the diffusion images (see supplementary material S6), and then segmented using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/) in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) to generate subject-specific WM and GM probabilistic images. The resulting images were then binarized using a probabilistic threshold of 0.7 to create a mask that ensure tissue-specific correspondence. WM and GM subject-specific masks were applied to extract FW averaged values for each subject across each of these tissues. To extract FAt and FA average values, only the WM mask was applied. For each scalar measure, the resulting values were compared between groups and the effect of the group × time interaction was tested using an ANOVA model of repeated measures. Post hoc comparisons were corrected using the Tukey-HSD method. For those scalar measures showing between-group significant differences, Pearson correlations with Positive and Negative Symptoms Scale (PANSS) total, PANSS negative subscore, PANSS positive subscore, daily and cumulative antipsychotic dose (chlorpromazine equivalents), neutrophil cell count (measured as in Nuñez et al19), and cannabis use were calculated at baseline and at 2 years. For those scalar measures showing statistically significant group × time interaction, the change over time (baseline scalar measure minus the baseline measure) was correlated with the percent change over time of the abovementioned clinical rating scales. Age, sex, and scanning site were entered as covariates in all the analyses.
Voxel-Wise Analysis in White Matter
To test the effect of the group and the effect of the group × time interaction on localized measurements of FW, FA, and FAt in WM, a voxel-wise analysis was performed using TBSS30 in FSL.32In this process, we added a procedure to harmonize the data between scanning sites using ComBat (see details in supplementary material (S7)). The results were thresholded using threshold-free cluster enhancement (TFCE) and p FWE-corrected <0.05. TFCE is a permutation method that avoids the arbitrary a priori selection of a cluster-forming threshold while preserving the sensitivity of a cluster-based analysis, and it has shown to increase results sensitivity in comparison to traditional cluster-based and voxel-based thresholding.33
Voxel-Wise Analysis in Gray Matter
To determine the spatial localization of the between-group differences in the scalar measures in GM, an analysis of FW in the GM surface was implemented.
To this purpose, for each subject, a GM surface was generated using CAT12 (http://dbm.neuro.uni-jena.de/cat/) by segmentation and surface reconstruction of the T1 images. Then, the FW images were mapped to the corresponding GM surface, resampled in a standardized space and corrected for the site using Combat. The details of this method are described in the supplementary material S8. Those resulting surface images of FW that had been acquired at baseline were entered in a one-way ANOVA model with three groups, and scanning site, age, and gender were introduced as covariates. Additionally, to test the effect of the group × time interaction, a two-way repeated measures ANOVA model with those subjects, whose follow-up images were available, was also implemented using a flexible factorial design in SPM. The results were log transformed and calculated using TFCE in SPM (http://dbm.neuro.uni-jena.de/tfce/). Finally, the results were corrected for multiple comparisons using family-wise-error correction (FWE) at P < .05 and displayed on a template surface. Each significant cluster was labeled using an overlay of the Desikan–Killiany DK40 atlas.34
Results
Subjects Characteristics
The flow chart of the participants at every imaging preprocessing step is shown in supplementary material (S1). The sociodemographics and main clinical characteristics of the total sample, separated by group and by time-point, are shown in table 1. FEP subjects with and without follow-up did not differ in age, sex, scanning site, duration of illness, PANSS scores/subscores, or proportion of SSD or affective psychosis (see details in supplementary material (S9)).
Average Free-Water and Fractional Anisotropy Tissue-Specific Study
Free-Water in Gray Matter and White Matter
At baseline, SSD and Non-SSD subjects had higher FW average values compared to healthy controls in GM (SSD = 0.288 +/− 0.015, Non-SSD = 0.286 +/− 0.014, controls = 0.281 +/− 0.014, F(2, 206) = 6.471, P = .002, P.adj(SSD vs. HC) = .003, P.adj(Non-SSD vs. HC) = .040). See also supplementary material (S10) showing details and plots controlling for confounding factors. SSD, but not Non-SSD, had higher FW average values than healthy controls in WM (SSD = 0.207 +/− 0.041, Non-SSD = 0.195 +/− 0.018, HC = 0.195 +/− 0.025, F(2, 206) = 3.262, P = .040, P.adj(SSD vs. HC) = .05 (rounded-up), P.adj(Non-SSD vs. HC) = .093).
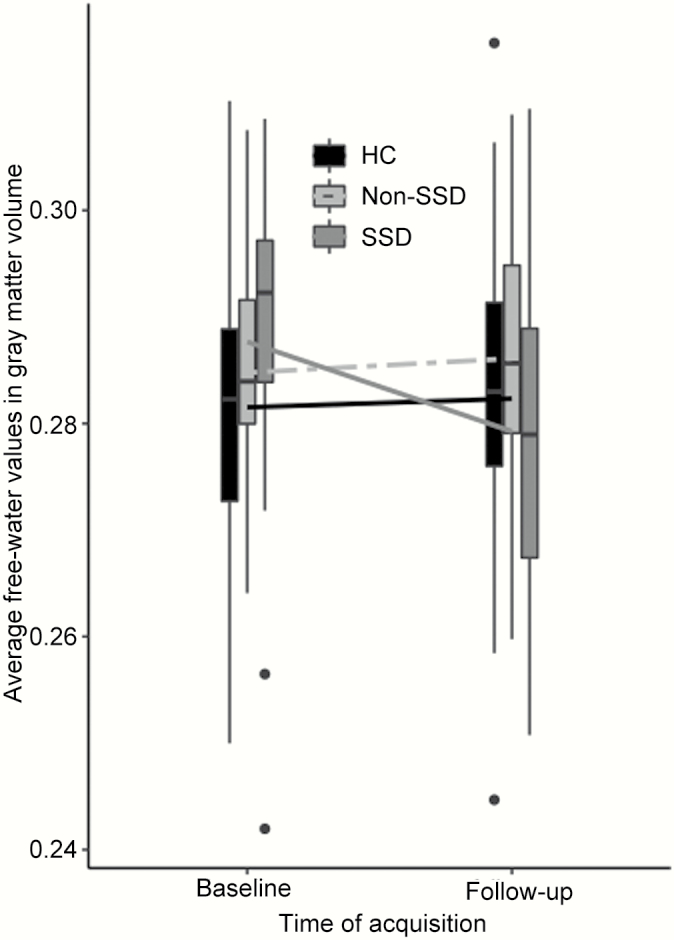
Only FW in GM showed a significant group × time interaction (F(2,78) = 5.088, P = .008). Post hoc comparisons showed a decrease in FW values in GM in the SSD group (mean increase = −0.008 +/− 0.003) in comparison to HC (mean increase = 0.001 +/− 0.002, P.adj = .011) and Non-SSD (mean increase = 0.001 +/− 0.003, P.adj = .023). See also figure 1.
Fig. 1.
Free-water average values by group in gray matter at baseline and follow-up.
Free-water values in this figure are average values corrected for age, gender, and site. Non-SSD: Non- schizophrenia spectrum disorder subjects, SSD: Schizophrenia spectrum disorder subjects; HC: Healthy controls.
No significant correlations were found between average FW values in GM or WM at baseline and clinical baseline measures, including PANSS total, positive, and negative subscores; neutrophil cell count at baseline; and cannabis use or daily/cumulative antipsychotic dose (chlorpromazine equivalents) at baseline. FW decrease in GM over time was positively correlated to an improvement in negative symptoms (t(34) = 2.677, P = .011). See supplementary material (S11) for details.
FA and FAt in White Matter
No significant between-group differences were found in FA (F(2,206) = 1.844, P = .161) or FAt (F(2,206) = 2.189, P = .115) at baseline. No significant group × time interactions were found for FA or FAt.
Voxel-Wise Analysis in White Matter (TBSS analysis)
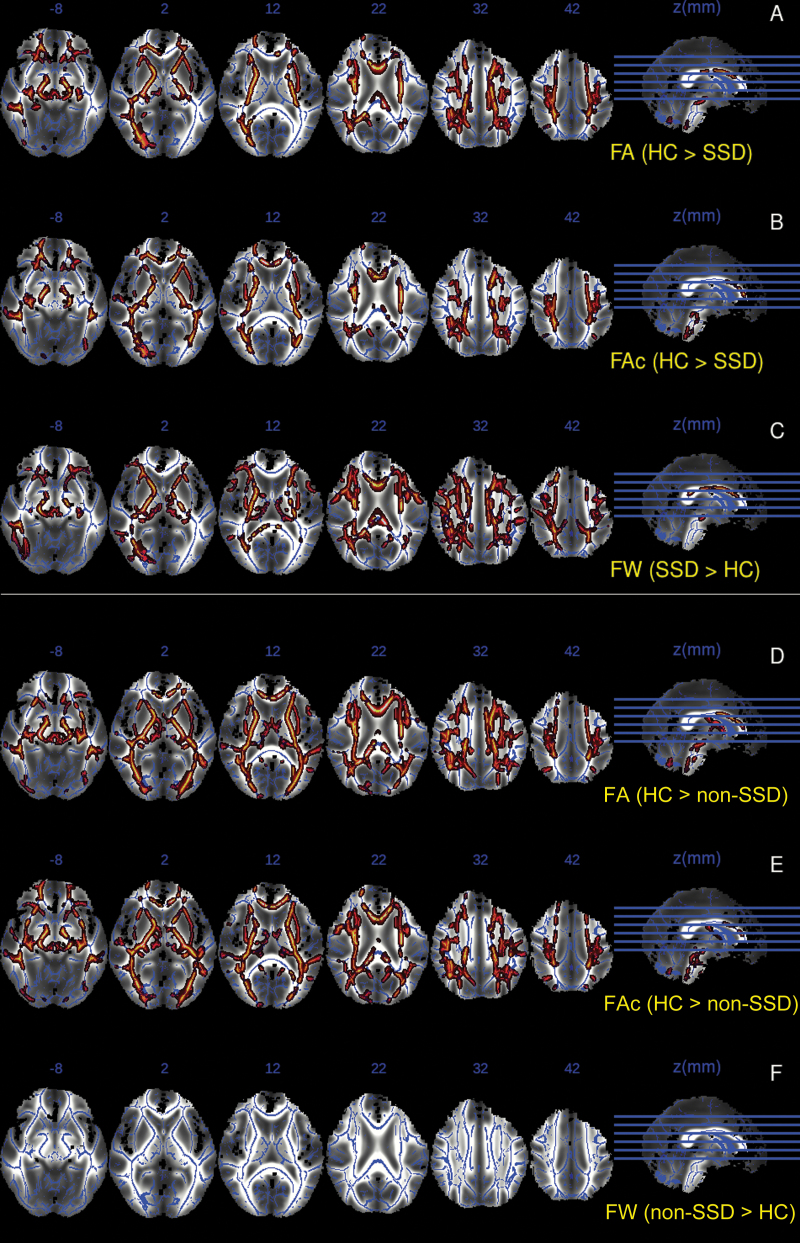
At baseline, FA values were significantly lower in both SSD and non-SSD compared to healthy controls, in several regions of the WM tracts, including bilateral Superior Corona Radiata, bilateral Internal and External Capsule, bilateral Superior Longitudinal Fasciculus, body of Corpus Callosum and Cerebral Peduncle, and bilateral Anterior and Posterior Thalamic Radiation (see figure 2A and 2C).
As regards to FAt at baseline, significant lower values in SSD and non-SSD, in relation to healthy controls, were found in a similar pattern of clusters to those of FA (see figure 2B and 2E). No suprathreshold clusters were found in the “SSD or non-SSD higher than controls” contrast for FAt or FA scalar values.
Fig. 2.
TBSS analysis results showing significant clusters with differences between groups in FA, FAt, and FW values projected onto a common white matter skeleton.
Composition image using mean fractional anisotropy generated during the TBSS process as background image, and overlaying the mean skeleton and the significant clusters for each of the following contrasts: A: Fractional anisotropy (FA) in healthy controls (HC) compared to schizophrenia spectrum disorders (SSD), B: Corrected fractional anisotropy (FAt) in HC compared to SSD, C: Free-water (FW) in SSD compared to HC, D: FA in HC compared to non-schizophrenia spectrum disorder subjects (non-SSD), E: FAt in HC compared to non-SSD. F: FW in non-SSD compared to HC.
SSD subjects, but no non-SSD subjects, showed significantly increased FW compared to healthy controls, in regions that partially overlapped with those showing reduced FA and FAt values, with significant clusters in Superior Corona Radiata, Internal Capsule, Superior Longitudinal Fasciculus, Inferior Longitudinal Fasciculus, and body of Corpus Callosum and Thalamic Radiation (see figure 2C and 2F).
No suprathreshold clusters reached significance in the group per time interaction for any of the scalar measures.Surface-Based Analysis in Gray Matter
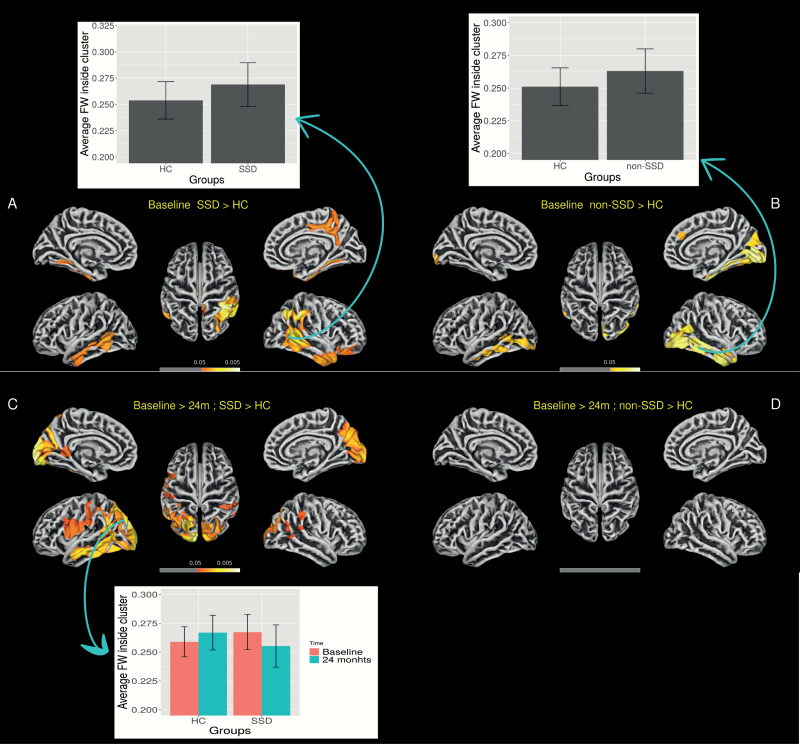
At baseline, SSD and non-SSD subjects had significantly higher FW values in GM surface mainly in regions of the left and right temporal lobes (see figure 3A and 3B, table 2). The interaction contrasts revealed a significant decrease over time in FW in the GM surface only in the SSD group in comparison to healthy controls (see figure 3C and 3D and table 2). See also supplementary material (S15) for an alternative multiple correction method using FDR.
Fig. 3.
Free-water in gray matter surface analysis showing between-group differences at baseline and decreases after 2 years.
Images represent free-water (FW) threshold-free-cluster enhancement results corrected for multiple comparison using family-wise error correction (FWE) and thresholded at P < 0.05. A: Baseline schizophrenia spectrum disorder subjects (SSD) compared to healthy controls. B: Baseline non-schizophrenia spectrum disorder subjects (non-SSD) compared to healthy controls. C: Decrease in free-water (baseline compared to 2 years) in SSD subjects compared to controls. D: Decrease in free-water (baseline compared to 2 years) in non-SSD subjects compared to controls. Bars represent average free-water inside the main clusters and are displayed to visualize the direction of the between-group differences. Error bars represent 1 standard deviation.
Table 2.
Significant Clusters of the Between-Group Contrasts of the Free-Water in Gray Matter Surface.
| P-FWE Value | Cluster Size (vortex) | Hemisphere | Overlap with Desikan–Killiany DK40 Atlas Regions. |
|---|---|---|---|
| Baseline SSD > HC | |||
| .005 | 3457 | Right | 28% inferior parietal, 22% supra marginal, 14% middle temporal, 11% superior parietal, 9% inferior temporal, 8% banks of the superior temporal sulcus |
| .031 | 1085 | Right | 47% precuneus, 22% paracentral, 20% posterior cingulate, 7% isthmus cingulate |
| .025 | 703 | Right | 48% inferior temporal, 34% middle temporal, 12% superior temporal |
| .037 | 443 | Right | 76% lateral orbitofrontal, 23% pars orbitalis |
| .024 | 258 | Right | 64% fusiform, 32% parahippocampal |
| .026 | 1347 | Left | 42% middle temporal, 30% inferior temporal, 11% fusiform, 11% banks of the superior temporal sulcus |
| .037 | 201 | Left | 73% fusiform, 20% parahippocampal |
| Baseline non-SSD > HC | |||
| .037 | 598 | Left | 50% lateral occipital, 19% middle temporal, 14% banks of the superior temporal sulcus, 12% inferior temporal |
| .038 | 348 | Left | 53% inferior temporal, 41% middle temporal |
| .013 | 3691 | Right | 19% interior temporal, 17% lateral occipital, 16% fusiform, 13% middle temporal, 11% lingual, 8 % inferior parietal |
| Decrease after 2 years, SSD > HC | |||
| .003 | 7275 | Left | 16% superior parietal, 13% inferior parietal, 12% lateral occipital, 9% middle temporal, 8% supramarginal, 6% postcentral, 4% pericalcarine, 4% inferior temporal, 4% precuneus, 4% precentral, 3% lingual, 3% ishmus cingulate, 3% banks of the superior temporal sulcus, 3% cuneus, 3% pars opercularis |
| .013 | 2146 | Right | 22% superior parietal, 18% precuneus, 17% lateral occipital, 14% inferior parietal, 12% cuneus, 9 % pericalcarine, 8 % lingual |
| .034 | 328 | Right | 81% supramarginal, 17% inferior parietal |
| .046 | 139 | Right | 100% inferior parietal |
| Decrease after 2 years, non-SSD > HC | |||
| – | – | – | – |
Note: Results obtained using log-transformation, threshold-free-cluster-enhancement, and thresholded using P-FWE < .05.
Discussion
In the present study, as predicted, we found elevated FW in GM and WM in FEP with schizophrenia spectrum disorders (SSD-FEP) compared to controls. In contrast, non-SSD-FEP showed higher FW in GM, but not in WM. Both groups of FEP, SSD and non-SSD, showed FA and (FAt values in WM in the voxel-wise analysis when compared with HC. However, differences in FA or FAt were not observed in the analyses of average values in WM volume, and no significant changes over time were found in WM. Importantly, in addition to these results, the present study is the first to report longitudinal changes in FW in SSD during the early course of the illness.
Based on the findings of widespread elevated FW in early phases of psychosis,13,16 and less pronounced differences in chronic schizophrenia,14,15,17 the possibility that FW may fluctuate over time and represent phases of clinical illness has been previously hypothesized.15 Our results are consistent with this hypothesis and, in addition, suggest that FW change over time may vary depending on the diagnosis, and it may be related to clinical recovery, at least in negative symptoms. However, the latter must be considered with caution as we did not find a significant correlation between symptoms and FW elevation at baseline.
With respect to WM, the present study revealed elevated FW in SSD but not in non-SSD. To our knowledge, no previous studies have compared FW in SSD and non-SSD separately, although one previous study did report significantly elevated FW in WM in chronic bipolar patients.35
The overall pattern of results suggests that, although the same neurobiological processes involving FW elevation and WM loss of integrity may underlay psychosis, both in SSD and in non-SSD subjects, its course over time may be different for each group, and explain different clinical outcomes.
Our findings emphasize the importance of elucidating the neurobiological mechanisms underlying FW elevation and its association with clinical outcomes in early psychosis patients. FW elevation in GM has been hypothetically related to an abnormal neuroimmune response in the brain.22 This hypothesis has received recent support by the finding of a negative association between FW and glutathione levels,23 a metabolite that decreases with inflammation and that can be measured by MR spectroscopy in the prefrontal cortex of FEP individuals with SSD.
In the present study, we did not find an association between FW elevations and a peripheral immune response marker, the neutrophil count. Such a negative result must be interpreted with caution, however, as the relationship between peripheral inflammatory markers and cellular and molecular processes in the CNS has not been well established.
In agreement with previous studies, we found large WM regions showing lower FAt and FA values both in SSD and in non-SSD compared to controls.36 However, our results do not show a progressive decrease over time in these values, and therefore, we did not find evidence of the WM neurodegeneration that Pasternak et al13 have hypothesized. The 2-year follow-up period of our study may not be long enough to capture signs of degeneration if this occurred during the chronic phase of the illness. Alternatively, FA decreases may occur even before the onset of the first psychotic episode, which is supported by findings of low FA in clinical high-risk patients3 and in unmedicated first-episode subjects.4 It is worth noting, however, that these studies did not use FW-corrected FA and may have overestimated the reduction of FA in WM.
A limitation of the present study was that antipsychotic-naïve status was not a requirement for inclusion in our study. Thus, although time from illness onset to the first evaluation was relatively short and no significant correlation between FW and antipsychotic dose at baseline was found, we cannot completely rule out the effects of antipsychotic drugs on baseline FW levels. Although we used specific methods to harmonize scanning procedures, the different MRI acquisition protocols in two different scanners and the single-shell DTI acquisition sequence also represent potential limitations of this work. Further studies using medication-naïve populations, using multi-shell diffusion protocols with higher b values, and using data collected on a single MRI system will be needed in order to address these limitations. Also, although we made a significant effort to obtain a good corregistration, some misattribution of CSF to GM could have occurred (see histograms in supplementary material (S13)), although less so in the central GM surface.
Conclusions and Future Directions
Our findings suggest that extracellular FW in the brain is elevated in FEP and show evidence of a decline over the first few years in GM only in subjects with a schizophrenia spectrum disorder. Although the cellular and molecular underpinnings of FW elevation are still unclear, the FW changes that we observed clearly during the first 2 years of the illness appear to be clinically meaningful as they are related to the changes in symptomatology. These changes could be related to the effects of antipsychotic medications, changes in underlying pathophysiology, or some combination of these factors. As such, our results suggest that FW is a potential marker of active pathological processes in the brain in early psychosis. On the other hand, fractional anisotropy seemed to be stable over the 2-year period of our study and was similarly altered cross-sectionally across both classes of psychotic disorders studied. Further studies on FW, systematically focusing on the effects of antipsychotic medications, examining the association of CNS FW, and using a diverse array of peripheral immune response markers, will increase our understanding of the neurobiological underpinnings of psychosis during the early stages of the illness.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Funding
This project received a grant from the ISCIII, Ministry of Economy and Competitiveness (Spain) on the 2008 call (ref. ISCIII 2009–2011: PEPs study PI080490), co-funded with European Union ERDF funds, and also supported by CIBSERAM. The first author received funds from the grant “Movilidad de profesionales sanitarios e investigadores del SNS (M-BAE)” from the ISCIII, and from the grant “Short-term visiting fellowships” from the Alicia Kolpowitz Foundation.
Conflict of Interest
Dr Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Abbott, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme, Horizon 2020, and the Stanley Medical Research Institute. Dr Bergé has received funds from Otsuka and Lundbeck for congress registration expenses.
-PEPs group (collaborators): Gisela Mezquida (Hospital Clínic Barcelona), Silvia Amoretti (Hospital Clínic Barcelona), Laura Pina-Camacho (Hospital Gregorio Marañon), Celso Arango (Hospital Gregorio Marañon), I. González-Ortega (Hospital Santiago Apóstol), S.García (Hospital Santiago Apóstol), C. De-la-Cámara (Instituto Aragonés Ciencias de la Salud), N. Fayed (Instituto Aragonés Ciencias de la Salud), Julio Sanjuan (Hospital Clínico Valencia), EJ Aguilar (Hospital Clínico Valencia), Joyce Y. Guo (UCDAVIS), Purificación Salgado (Hospital del Mar Medical Research Institute), Joquim Raduà (Hospital Clínic Barcelona), J.Sánchez-Moreno (Hospital Clínic Barcelona), Elena de la Serna (Hospital Clínic Barcelona), Ima Baeza (Hospital Clínic Barcelona), Fernando Contreras-Fernández (Hospital Universitari de Bellvitge), C.Saiz-Masvidal (Hospital Universitari de Bellvitge), L. González-Blanco (Universidad de Oviedo), L. Jiménez-Treviño (Universidad de Oviedo), M. Dompablo (Hospital 12 de Octubre), I. Torío (Hospital 12 de Octubre), A. Butjosa (Hospital Sant Joan de Déu), E. Rubio-Abadel (Hospital Sant Joan de Déu), S. Sarró (FIDMAG), E. Pomarol-Clotet (FIDMAG)
Contributor Information
PEPs group (collaborators):
Gisela Mezquida, Silvia Amoretti, Laura Pina-Camacho, Celso Arango, I González-Ortega, S García, C De-la-Cámara, N Fayed, Julio Sanjuan, E J Aguilar, Joyce Y Guo, Purificación Salgado, Joquim Raduà, J Sánchez-Moreno, Elena de la Serna, Ima Baeza, Fernando Contreras-Fernández, C Saiz-Masvidal, L González-Blanco, L Jiménez-Treviño, M Dompablo, I Torío, A Butjosa, E Rubio-Abadel, S Sarró, and E Pomarol-Clotet
References
- 1. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24(2):101–110. [DOI] [PubMed] [Google Scholar]
- 3. Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106(2–3):115–124. [DOI] [PubMed] [Google Scholar]
- 4. Cheung V, Cheung C, McAlonan GM, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38(6):877–885. [DOI] [PubMed] [Google Scholar]
- 5. Guo W, Liu F, Liu Z, et al. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci Lett. 2012;531(1):5–9. [DOI] [PubMed] [Google Scholar]
- 6. Karlsgodt KH. Diffusion imaging of white matter in schizophrenia: progress and future directions. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X, Lai Y, Wang X, et al. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav Brain Res. 2013;252:157–163. [DOI] [PubMed] [Google Scholar]
- 8. Yang X, Cao D, Liang X, Zhao J. Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology. 2017;59(7):699–708. [DOI] [PubMed] [Google Scholar]
- 9. Shahab S, Stefanik L, Foussias G, Lai MC, Anderson KK, Voineskos AN. Sex and diffusion tensor imaging of white matter in schizophrenia: a systematic review plus meta-analysis of the Corpus Callosum. Schizophr Bull. 2018;44(1):203–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cookey J, Bernier D, Tibbo PG. White matter changes in early phase schizophrenia and cannabis use: an update and systematic review of diffusion tensor imaging studies. Schizophr Res. 2014;156(2–3):137–142. [DOI] [PubMed] [Google Scholar]
- 11. Kubicki M, Shenton ME. Diffusion tensor imaging findings and their implications in schizophrenia. Curr Opin Psychiatry. 2014;27(3):179–184. [DOI] [PubMed] [Google Scholar]
- 12. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 13. Pasternak O, Westin C-F, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oestreich LKL, Lyall AE, Pasternak O, et al. ; Australian Schizophrenia Research Bank Characterizing white matter changes in chronic schizophrenia: a free-water imaging multi-site study. Schizophr Res. 2017;189:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasternak O, Westin CF, Dahlben B, Bouix S, Kubicki M. The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophr Res. 2015;161(1):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyall AE, Pasternak O, Robinson DG, et al. Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatry. 2018;23(3):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oestreich LK, Pasternak O, Shenton ME, et al. ; Australian Schizophrenia Research Bank Abnormal white matter microstructure and increased extracellular free-water in the cingulum bundle associated with delusions in chronic schizophrenia. Neuroimage Clin. 2016;12:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García-Bueno B, Bioque M, Mac-Dowell KS, et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40(2): 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Núñez C, Stephan-Otto C, Usall J, et al. Neutrophil count is associated with reduced gray matter and enlarged ventricles in first-episode psychosis. Schizophr Bull. 2019;45(4):846–858. doi: 10.1093/schbul/sby113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stapel B, Sieve I, Falk CS, Bleich S, Hilfiker-Kleiner D, Kahl KG. Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. J Psychiatr Res. 2018;105:95–102. [DOI] [PubMed] [Google Scholar]
- 21. Venugopal D, Shivakumar V, Subbanna M, et al. Impact of antipsychotic treatment on methylation status of Interleukin-6 [IL-6] gene in Schizophrenia. J Psychiatr Res. 2018;104:88–95. [DOI] [PubMed] [Google Scholar]
- 22. Pasternak O, Kubicki M, Shenton ME. In vivo imaging of neuroinflammation in schizophrenia. Schizophr Res. 2016;173(3):200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesh TA, Maddock RJ, Howell A, et al. Extracellular free water and glutathione in first-episode psychosis-a multimodal investigation of an inflammatory model for psychosis [published online ahead of print May 28, 2019]. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernardo M, Bioque M, Parellada M, et al. ; PEPs Group Assessing clinical and functional outcomes in a gene-environment interaction study in first episode of psychosis (PEPs). Rev Psiquiatr Salud Ment. 2013;6(1):4–16. [DOI] [PubMed] [Google Scholar]
- 25. Cuesta MJ, Sánchez-Torres AM, Cabrera B, et al. ; PEPs Group Premorbid adjustment and clinical correlates of cognitive impairment in first-episode psychosis. The PEPsCog Study. Schizophr Res. 2015;164(1-3):65–73. [DOI] [PubMed] [Google Scholar]
- 26. Salagre E, Arango C, Artigas F, et al. CIBERSAM: ten years of collaborative translational research in mental disorders. Rev Psiquiatr Salud Ment. 2019;12(1):1–8. [DOI] [PubMed] [Google Scholar]
- 27. Pina-Camacho L, Del Rey-Mejías Á, Janssen J, et al. ; PEPs Group Age at first episode modulates diagnosis-related structural brain abnormalities in psychosis. Schizophr Bull. 2016;42(2):344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maier-Hein KH, Westin CF, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement. 2015;11(5):485–493.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garyfallidis E, Brett M, Amirbekian B, et al. ; Dipy Contributors Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 31. Fortin JP, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(suppl 1):S208–219. [DOI] [PubMed] [Google Scholar]
- 33. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 34. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 35. Tuozzo C, Lyall AE, Pasternak O, James ACD, Crow TJ, Kubicki M. Patients with chronic bipolar disorder exhibit widespread increases in extracellular free water. Bipolar Disord. 2018;20(6):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.