Abstract
Introduction
Gastric cancer (GC) is one of the most malignancies leading to human mortality due to its development, progress, metastasis and poor prognosis, despite the development of remarkable chemotherapy and surgery. The 5-fluorouracil (5-Fu) is an effective anti-gastric cancer agent. However, a fraction of GC patients acquire 5-Fu chemoresistance.
Methods
In this study, the CagA protein was detected from CagA-positive gastric cancer patients by qRT-PCR and immunohistochemistry. The 5-Fu resistant gastric cancer cell line was generated from MKN45-CagA cells which was transfected with CagA overexpression vector. Cellular glucose metabolism was determined by measurements of glucose uptake, lactate product and glycolysis enzymes.
Results
We report that the Helicobacter pylori (H. pylori)-secreted Cytotoxin-associated gene A (CagA) oncoprotein is positively correlated with 5-Fu resistance of gastric cancer. From totally 72 CagA-positive gastric cancer patients, CagA high-expressed patients showed more resistance to 5-Fu than CagA low-expressed patients. Moreover, statistical analysis revealed that CagA mRNA and protein expressions were significantly upregulated in 5-Fu resistant gastric cancer patients. We observed that CagA protein is upregulated in 5-Fu resistant gastric cancer cells compared with sensitive cells. Interestingly, cellular glucose metabolism was upregulated; the glucose uptake and lactate production were significantly higher in 5-Fu resistant gastric cancer cells. The Akt phosphorylation and expressions of glycolysis key enzymes, Hexokinase 2 and LDHA, were significantly upregulated in 5-Fu resistant gastric cancer cells. On the other way, inhibition of glycolysis or Akt pathway effectively overcame 5-Fu resistance from both in vitro and in vivo models. Finally, we report that the combination of Akt or glycolysis inhibitor with 5-Fu could synergistically enhance the cytotoxicity of 5-Fu to CagA-overexpressed gastric cancer cells.
Discussion
In summary, our study demonstrated a CagA-Akt-glycolysis-5-Fu resistance axis, contributing to the development of new therapeutic agents against chemoresistant human gastric cancer.
Keywords: aerobic glycolysis, gastric cancer, 5-Fu resistance, CagA, Helicobacter pylori infection, Warburg effect, chemoresistance
Introduction
Gastric cancer (GC), one of the most common cause of cancer-related death tumors, is the second leading cause of malignant mortality due to its development, progress, metastasis and poor prognosis.1,2 GC patients with advanced tumor stage generally have a disappointing prognosis, despite the development of remarkable chemotherapy and surgery.3,4 Among multiple therapeutic agents applied to GC treatment, the 5-fluorouracil (5-Fu) is one of adjuvant and palliative therapeutic strategies.5 The mechanism for inhibiting cancer cells by 5-Fu is to elicit cytotoxicity through interfering the nucleotide synthetic enzyme and incorporating fluoronucleotides into RNA and DNA.5,6 However, although an effective response to gastric cancer patients initially, a fraction of GC patients acquire refractory to 5-Fu chemotherapy,7 making it a major challenge for the 5-Fu-based chemotherapy. Therefore, investigating the underlying mechanisms of 5-Fu resistance and developing potential anti-cancer agents against 5-Fu resistance is an urgent task.
Helicobacter pylori (H. pylori) was firstly discovered as a gastric pathogen which causes peptic ulcer disease and affect around 50–60% of the world’s population.8 In addition, Helicobacter pylori was frequently colonized the human stomach to be a critical risk factor for gastric cancer by injecting the Cytotoxin-associated gene A (CagA) oncoprotein into host gastric epithelial cells to induce cellular transformation.9 Meanwhile, the hosts which are CagA-positive strains demonstrated an increased risk of gastric cancer,9,10 suggests that CagA is positively associated with the diagnosis of gastric cancer. However, the precise roles for CagA in pathogenesis or development of gastric tumor have not been illustrated.
The metabolism of glucose allows generating ATP and other metabolic intermediates through the oxidation of glucose, a process is essential for sustaining mammalian life.11 In tumors and other proliferating cells, the rate of glucose metabolism is dramatically increased accompanying increased lactate production, even in the presence of enough oxygen and healthy mitochondria.12 This phenomenon, is known as the “Warburg Effect”.13 In addition, the Warburg Effect is linked to chemoresistance of cancer cells.14 Studies demonstrated the Warburg Effect of cancer cells is frequently associated with cellular resistance to conventional chemotherapies.14,15 Recent reports revealed that 5-Fu resistant colon cancer cells displayed upregulated glycolysis rate and could be re-sensitized by inhibiting glycolysis enzyme, PKM2.16 Furthermore, another study reported inhibition of glycolysis of ovarian cancer cells significantly enhanced cisplatin-induced cell death,17 suggesting reversing Warburg effect of cancer cells could undoubtedly enhance the therapeutic effects of anti-cancer agents.
In this study, we demonstrated that CagA expression is positively correlated with 5-Fu resistance in Helicobacter pylori-infected gastric cancer patients. Moreover, 5-Fu resistant gastric cancer cells displayed higher glycolytic rate than parental gastric cancer cells. The molecular mechanisms for the CagA-mediated glycolysis and 5-Fu resistance will be investigated using in vitro and in vivo models. Our findings contribute to developing innovative therapeutic strategies for 5-Fu resistant gastric cancer patients.
Patients and Methods
Patient Samples
A total of 72 Cag-A positive gastric cancer patients, including specimens from 63 patients who had early gastric cancer and 9 who had advanced gastric cancer were evaluated in this study. The low-CagA expression patients were 33 and high-CagA expression patients were 39. Gastric cancer tissues were obtained from patients who underwent surgery at the Department of General Surgery, China-Japan Union Hospital of Jilin University, China between 2013 and 2018. Patient samples obtained surgically were immediately frozen by liquid nitrogen and stored at −80°C. This study was approved by the Institutional Review Board of the China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, P. R. China. Patients did not receive radio- or/and chemo- therapy before surgery. All participants gave written informed consent. Immunohistochemical analysis of CagA protein expressions from paraffin embedded specimens was performed according to previous description.18
Cell Culture and Reagents
Gastric cancer cell lines MKN45 and AGS were obtained from American Type Culture Collection (ATCC). Cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2. The anti-CagA antibody (HPP-5003-9) was from ASTRAL Biologicals (San Ramon, CA, USA). Rabbit anti-Hexokinase 2 (#2867); Rabbit anti-LDHA (#3582) and Rabbit anti-β-actin (#4970) antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). 5-Fu and Oxamate were purchased from Sigma-Aldrich (Shanghai, China). Control siRNA and siLDHA were purchased from Ambion (Austin, TX, USA).
Plasmid DNA Transfection
The pCDNA3.1-CagA overexpression plasmid was constructed according to previous description.19 Control empty pCDNA3.1 vector or pCDNA3.1-CagA were transfected into gastric cancer cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Forty-eight hours after transfection, cells were collected for downstream transient CagA overexpression assays. For stably transfection of CagA, cells were transfected with the CagA overexpression vector for 48 hours. Cells were then treated with Geneticin for 72 hours. The survival cells were cultured with Geneticin to generate resistant cell colonies, followed by individual colony expanding in 6-well plate. Stably CagA overexpression was verified in twenty colonies by Western blotting and three of the highest CagA overexpression colonies were mixed and used in this study.
Total RNA Isolation and qRT-PCR
Total RNA was isolated from surgically resected gastric tumors or cultured gastric cancer cells using the RNeasy Mini Kit (#74,104, Qiagen, Shanghai, China) according to the manufacturers’ protocols. First-strand cDNA was synthesized using 0.5 ug total RNA using a SuperScript First-Standard Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. qRT-PCR experiments were performed using the SYBR Green qPCR Master Mix (ThermoFisher Scientific, Shanghai, China). Samples were analyzed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, NJ, USA). Primers for qRT-PCR were: CagA: Forward: 5ʹ-ATAATGCTAAATTAGACAACTTGAGCGA-3ʹ, Reverse: 5ʹ-TTAGAATAATCAACAAACATCACGCCAT-3ʹ; HK2: Forward: 5ʹ-TACACTCAATGACATCCGAACTG-3ʹ, Reverse: 5ʹ-CGTCCTTATCGTCTTCAATATCC-3ʹ; LDHA: Forward: 5ʹ-ATGAAGGACTTGGCGGATGA-3ʹ, Reverse: 5ʹ-ATCTCGCCCTTGAGTTTGTCTT-3ʹ; β-actin: Forward: 5ʹ-CTGAGAGGGAAATCGTGCGT-3ʹ, Reverse: 5ʹ-CCACAGGATTCCATACCCAAGA-3ʹ. The expression of β-actin was used to normalize the relative expression levels. The thermal profile was set as follows: 95°C for 1 min and 40 cycles at 95°C for 15 s, 58°C for 20 s, and 72°C for 20 s. The results were analyzed using the 2−ΔΔCt method.
Cell Viability Assay
Cell viability rate was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Sigma-Aldrich, Shanghai, China). Briefly, cells (5x103 cells/well) were seeded into 96-well plates at 80% confluence. Cells were aspirated and washed with PBS, followed by adding MTT solution at 37 °C for 2 hours. Samples in each well were incubated with 0.1 mL 10% SDS (dissolved by 0.01 MHCl) overnight at 37°C to solubilize the blue crystals. The optical density (OD) of formazan concentrations was determined at 540 nm then normalized by cell numbers. Relative viability was obtained from the absorbance at 540 nm of drug-treated cells/the absorbance at 540 nm of untreated cells. Experiments were performed in triplicate and repeated three times.
Colony Formation Assay
Cells (500 cells/well) were seeded in 6-well plates and cultured for 3 weeks. Cells were examined every three days, and cell culture medium was refreshed every three days. Cell colonies were then fixed with 4% paraformaldehyde (Sangon Ltd., China) for 20 min and stained with 0.5% crystal violet (Beyotime Ltd., China) for 30 min at room temperature. Colonies that grew beyond 30 mm diameter were counted under the microscope. Each experiment was performed in triplicates.
Glucose Uptake and Lactate Production
Gastric cancer cells were seeded on a 12-well plate (1x105 per well). The glucose uptake experiments were carried out using the glucose test kit (Applygen Technologies, Beijing, China) according to the manufacturer’s instruction. The lactate production detection was performed using the L-lactate assay kit (BioVision, Milpitas, CA, USA) according to the previous descriptions.20 Experiments were repeated three time. Results were normalized by the ratio of the cell number of treated cells to that of control cells.
Immunohistochemistry
The paraffin-embedded human gastric tumor specimens CagA staining was performed according to previous report.18 Briefly, samples were de-paraffinized with xylene followed by dehydration with a graded series of alcohol. Antigen retrieval were performed by heat treatment performed in 0.1 M citrate buffer. After blocking with IHC blocking buffer (5% BSA), slides were incubated with antibodies. Experiments were repeated three times.
Western Blot
Total proteins were extracted from gastric cancer cells using RIPA lysis buffer (Beyotime Ltd., China) supplemented with 1x phosphatase inhibitors and 1x protease inhibitors (Sigma Chemical Ltd., USA). Lysates were incubated on ice for 30 minutes then collected and centrifugated for 10 min at 4 °C. Protein concentrations were measured by Bradford method. Equal amounts of proteins were applied to a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in room temperature for 1 hour. After complete washing by PBST, membranes were incubated with primary antibodies (1:1000 dilution) with gentle shaking at 4°C overnight. After washing, membranes were incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit antibody (1:3000 dilution) at room temperature for 1 hour. The detection of antibody-bound protein signals was performed using the enhanced chemiluminescence kit (Bio-Rad Ltd., USA). Experiments were repeated three times.
Gastric Cancer Cell Xenograft Model
Totally eighty-six eight-week-old nude mice were used in this study. Mice were kept on a regular 12/12 hr light-dark cycle cages with access to food and water. Nude mice were separated to two groups (40 each) then were injected subcutaneously with AGS-CagA or MKN45-CagA gastric cancer cells (5x106) to establish xenograft models. Tumor progress was monitored every three day until tumors reached a size of greater than 100 mm3. Mice from each xenograft group were then randomly divided into 4 groups (10 mice per group) and treated as follows: PBS control; 5-Fu alone [40 mg/kg intraperitoneal (i.p.), 2 times/wk]; Oxamate alone [500 mg/kg, i.p., 2 times/wk] and 5-Fu plus Oxamate. Mice mortality was monitored daily. After 80 days the mice were euthanized by CO2 method and the xenograft tumor tissues were dissected and immediately frozen in liquid nitrogen and stored at −80°C for downstream analysis. All of the xenograft experiments were complied with the guidelines of the Institutional Animal Care and Use Committee of the China-Japan Union Hospital of Jilin University. Experimental protocol was carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by an institutional review committee from Institutional Animal Care and Use Committee of the China-Japan Union Hospital of Jilin University.
Statistical Analysis
Statistical difference was analyzed using the GraphPad Prism 6.0 software. Results are presented as the mean ± SEM. The unpaired Student’s t-test was used for the data analysis between two groups and significance among three or more groups was analyzed by one-way ANOVA followed by Bonferroni corrections. All experiments were performed in triplicate and repeated three times. A statistical difference of p < 0.05 was considered significant.
Results
CagA Expression Is Correlated with 5-Fu Sensitivity in Helicobacter pylori-Infected Gastric Cancer Patients
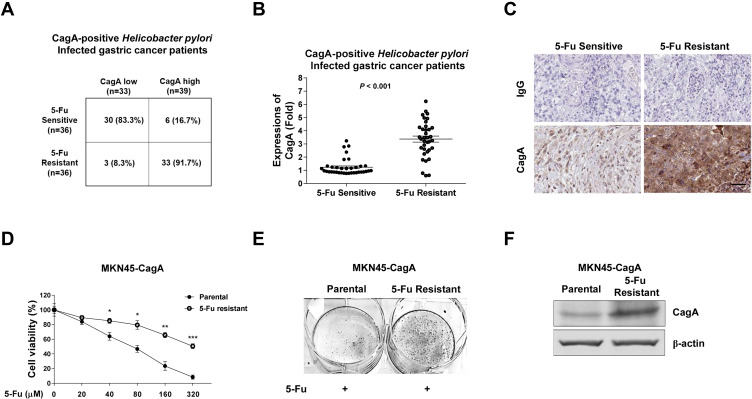
It is well-known that H. pylori infection is the primary risk factor of gastric cancer.8,9 Patients infected with CagA-positive H. pylori strains demonstrated that CagA is positively associated with an increased risk of gastric cancer.9,10 To investigate the roles of CagA protein in the chemo-sensitivity of human gastric cancer, the correlation between 5-Fu sensitivity and CagA expressions in CagA-positive Helicobacter pylori-infected gastric cancer patients was assessed. From totally 72 Cag-A positive gastric cancer patients, 63 patients had early gastric cancer and 9 had advanced gastric cancer. The low-CagA expression patients were 33 and high-CagA expression patients were 39. Among them, 5-Fu sensitive patients were 36 and resistant patients were 36. From the 5-Fu sensitive patients, we found thirty of them (83.3%) displayed low-CagA expressions, while only six patient (16.7%) was found to be associated with high CagA expression (Figure 1A). One the other way, among the totally 36 5-Fu resistant patients, only three (8.3%) of them were associated with low CagA expression, but the majority of them (33 cases, 91.7%) were CagA-high phenotypes (Figure 1A). Statistical analysis showed CagA mRNA expressions were significantly upregulated in 5-Fu resistant gastric cancer patients (Figure 1B). Furthermore, CagA protein was consistently upregulated in 5-Fu resistant human gastric cancer patients who were H. pylori-infected (Figure 1C). Taken together, the above clinical statistics revealed a strong positive correlation between H. pylori-infected CagA protein and 5-Fu sensitivity in gastric cancer.
Figure 1.
Positive correlation between CagA and 5-Fu resistance. (A) Correlation between CagA positive H. pylori-infected gastric patients and 5-Fu resistance. (B) CagA mRNA and (C) CagA protein expressions were upregulated in CagA positive H. pylori-infected, 5-Fu resistant gastric patients. (D) Selection of 5-Fu resistant MKN45-CagA cell line. MKN45 cells were stably transfected with CagA plasmid then treated with gradually elevated concentrations of 5-Fu to select resistant cells. (E) Colonegenesis assay showed much more colon formation from 5-Fu resistant MKN45-CagA cells under 5-Fu treatment compared with parental cells. (F) Protein expressions of CagA in MKN45-CagA parental and 5-Fu resistant cells. Proteins were detected by Western blot. β-actin was a loading control. Columns, mean of three independent experiments; bars, SE. *p < 0.05; **p < 0.01; ***p < 0.001.
CagA Protein Is Upregulated in 5-Fu Resistant Gastric Cancer Cells
To further explore the cellular mechanisms for the CagA-mediated chemoresistance, we stably overexpressed CagA protein in human gastric cancer cell line, MKN45 by transfection of ectopic CagA overexpression plasmid. Cells were subjected to 5-Fu selection under gradually increased 5-Fu treatments to establish the 5-Fu resistant cell line.21 Cell viability assay demonstrated MKN45-CagA 5-Fu resistant cells could tolerate higher concentrations of 5-Fu than parental cells (Figure 1D and E). The concentration of 5-Fu causing 50% of the cell death (IC50) after 48 hours of treatment was 77.6 uM for MKN45-CagA parental cells, which was significantly lower than IC50 of MKN45-CagA 5-Fu resistant cells (322.3 uM) (Figure 1D). Expectedly, CagA protein expression was significantly upregulated in 5-Fu resistant gastric cancer cells (Figure 1F). In summary, the above in vitro results supported CagA protein contributes to 5-Fu resistance of gastric cancer cells.
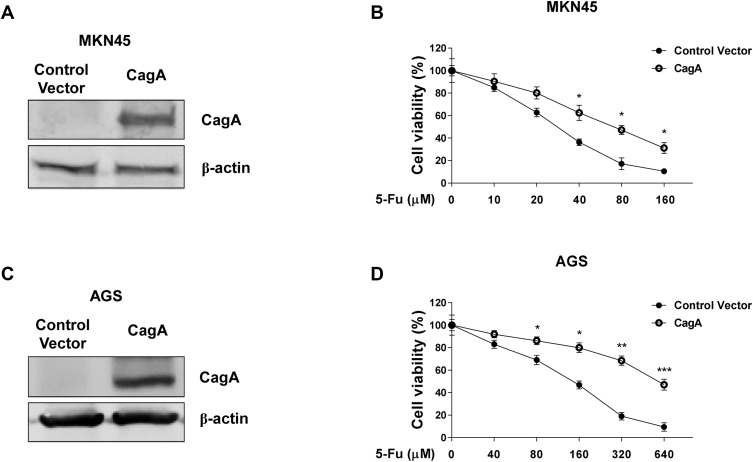
Overexpression of CagA Desensitizes Gastric Cancer Cells to 5-Fu
Given the correlations of H. pylori CagA and 5-Fu resistance in clinical gastric cancer samples and in vitro gastric cancer cells, we sought to determine whether the CagA positive H. pylori infection directly drive gastric cancer cells resistant to 5-Fu. We stably overexpressed CagA protein in two gastric cancer cell lines, MKN45 and AGS (Figure 2A and C). To test our hypothesis that CagA could desensitize gastric cancer cells to 5-Fu, cells without or with CagA overexpression were subjected to multiple concentrations of 5-Fu treatments for 48 hours. Expectedly, MKN45 and AGS CagA overexpressing cells were more resistant to 5-Fu (Figure 2B and D). The IC50 of MKN45 control vector transfected cells were 27.5 uM. Meanwhile the IC50 of MKN45 CagA overexpressing cells were 75.6 uM (Figure 2B). The similar results were consistently found in AGS cells that the CagA expressing AGS cells treated with 5-Fu resulted in significantly lower the level of cell death in comparison with AGS vector control cells (Figure 2D).
Figure 2.
Overexpression of CagA desensitizes gastric cancer cells to 5-Fu. (A) MKN45 cells were transfected with control vector or CagA overexpression plasmid for 48 hours. The expressions of CagA were detected by Western blot. β-actin was a loading control. (B) MKN45 cells with or without CagA overexpression were treated with 5-Fu at 0, 10, 20, 40, 80 or 160 uM for 48 hours. Cell viabilities were measured by MTT assay. (C) AGS cells were transfected with control vector or CagA overexpression plasmid for 48 hours. The expressions of CagA were detected by Western blot. β-actin was a loading control. (D) AGS cells with or without CagA overexpression were treated with 5-Fu at 0, 40, 80, 160, 320 or 640 uM for 48 hours. Cell viabilities were measured by MTT assay. Columns, mean of three independent experiments; bars, SE. *p < 0.05; **p < 0.01; ***p < 0.001.
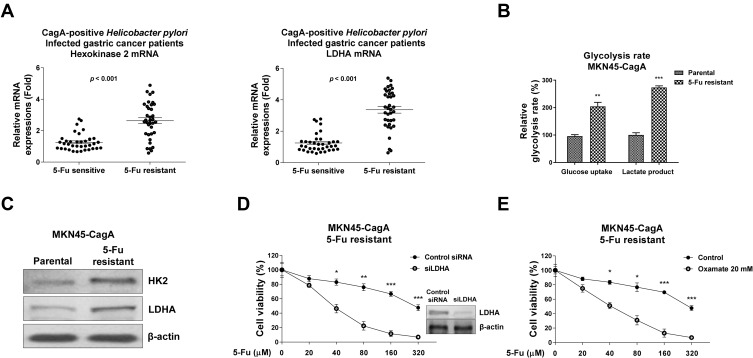
Cellular Glucose Metabolism Is Upregulated in 5-Fu Resistant Gastric Cancer Cells
Recent studies revealed the dysregulated cellular glucose metabolism affected cancer aggressiveness, including resistance to various processes such as hypoxia, apoptosis and cytotoxic drugs.13–15 To further investigate the chemoresistant phenomenon induced by CagA on gastric cancer cells, we examined the cellular glucose metabolism of CagA-positive H. pylori-infected gastric cancer patients. Two glucose metabolism key enzymes, HK2 and LDHA were significantly upregulated in 5-Fu resistant patients compared with normal 5-Fu response patients (Figure 3A), suggesting the upregulated glucose metabolism was correlated with the CagA-mediated 5-Fu resistance in gastric cancer patients. Furthermore, in vitro model demonstrated two well-studied glucose metabolism readouts, glucose uptake and lactate production were apparently elevated in the established MKN45-CagA 5-Fu resistant cells compared with their parental cells (Figure 3B). Consistently, two glycolysis key enzymes, HK2 which catalyzes the first committed step of glucose metabolism by phosphorylating glucose12 and LDHA which catalyzes the interconversion of pyruvate and lactate12 were significantly upregulated in 5-Fu resistant MKN45-CagA cells (Figure 3C). To obtain evidence of the CagA-mediated 5-Fu resistance was through promoting glycolysis, we transiently knocked-down LDHA in MKN45-CagA 5-Fu resistant cells to examine the 5-Fu sensitivity. Cell viability assays showed knocked-down LDHA obviously sensitized 5-Fu resistant cells (Figure 3D). Consistently, 5-Fu resistant cells were more vulnerable to 5-Fu under glycolysis inhibitor, Oxamate, a specific LDHA inhibitor (Figure 3E). Taken together, the above results from clinical patients and in vitro 5-Fu resistant gastric cancer cells demonstrated CagA promoted 5-Fu resistance through upregulation of glucose metabolism.
Figure 3.
Glycolysis is promoted in CagA overexpressed gastric cancer cells and patients. (A) HK2 and LDHA mRNA expressions were detected in CagA positive H. pylori-infected 5-Fu sensitive and resistant gastric patients. (B) Glucose uptake and lactate product were measured in MKN45-CagA parental or 5-Fu resistant cells. (C) Protein expressions of HK2 and LHDA were measured by Western blot in MKN45-CagA parental or 5-Fu resistant cells. β-actin was a loading control. (D) MKN45-CagA 5-Fu resistant cells were transfected with control siRNA or siLDHA for 48 hours, followed by 5-Fu treatments at 0, 20, 40, 80, 160 or 320 uM for 48 hours. Cell viability was measured by MTT assay. (E) MKN45-CagA 5-Fu resistant cells were co-treated by control or Oxamate with 5-Fu at 0, 20, 40, 80, 160 or 320 uM for 48 hours. Cell viability was measured by MTT assay. Columns, mean of three independent experiments; bars, SE. *p < 0.05; **p < 0.01; ***p < 0.001.
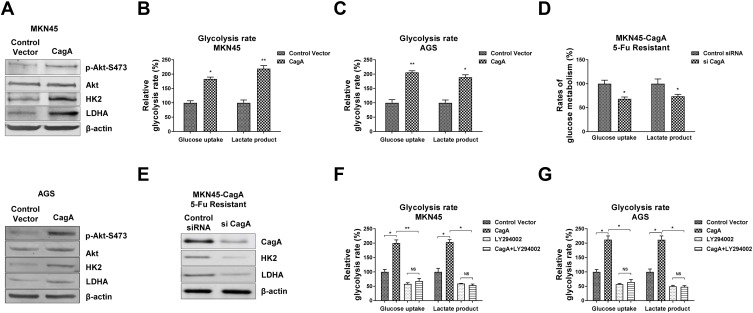
Overexpression of CagA Promotes Glycolysis Rate of Gastric Cancer Cells Through Akt Pathway
Recently studies illustrated the Helicobacter pylori CagA protein activates Akt pathway and desensitizes etoposide, another cytotoxic agent for treatment of advanced gastric cancer,22 indicating CagA promotes glycolysis could through activation of Akt. To further investigate the underlying molecular mechanism of the CagA-promoted glucose metabolism, we examined Akt pathway in CagA positive gastric cancer cells. As we expected, MKN45 cells with CagA overexpression demonstrated significantly upregulated Akt phosphorylation (Figure 4A). Consequently, HK2 and LDHA protein expressions were increased in CagA overexpressed gastric cancer cells compared with control transfected cells (Figure 4A). The same phenotypes were observed in another gastric cancer cell line, AGS. To assess whether CagA confers the promotion of glycolysis, we first verified the CagA-upregulated glycolysis rates in MKN45 and AGS cells. Results in Figure 4B and C demonstrated overexpression of CagA significantly stimulated glucose uptake and lactate production in MKN45 and AGS cells. On the contrary, silencing CagA in 5-Fu resistant MKN45-CagA cells successfully blocked the glucose metabolism and enzyme expressions (Figure 4D and E). Furthermore, we examined the effect of Akt inhibitor LY294002 on glycolysis rates of MKN45 and AGS cells without or with CagA over expressions. Expectedly, inhibition of Akt activity significantly attenuated the CagA-promoted glucose uptake and lactate product (Figure 4F and G). In summary, these results further confirmed that Akt signaling pathway participated in the CagA-modulated glycolysis promotion in CagA overexpressed gastric cancer cells.
Figure 4.
CagA stimulates gastric cancer cell glycolysis through activating Akt. (A) MKN45 cells (upper) and AGS cells (lower) were transfected with control vector or CagA overexpression plasmid for 48 hours. The phosphorylation of Akt, total Akt, KH2 and LDHA were examined by Western blot. β-actin was a loading control. (B) MKN45 cells and (C) AGS cells were transfected with control vector or CagA overexpression plasmid for 48 hours. The glucose uptake and lactate product were measured. (D) MKN45-CagA 5-Fu resistant cells were transfected with control siRNA or siCagA for 48 hours, the glucose uptake, lactate product and (E) protein expressions of HK2 and LDHA were measured. (F) MKN45 cells and (G) AGS without or with CagA overexpression were treated with control or Akt inhibitor for 24 hours. The glucose uptake and lactate product were measured. Columns, mean of three independent experiments; bars, SE. NS, no significance; *p < 0.05; **p < 0.01.
The CagA-Mediated 5-Fu Resistance Is Through Activating Akt Glycolysis of Gastric Cancer Cells in vitro and in vivo
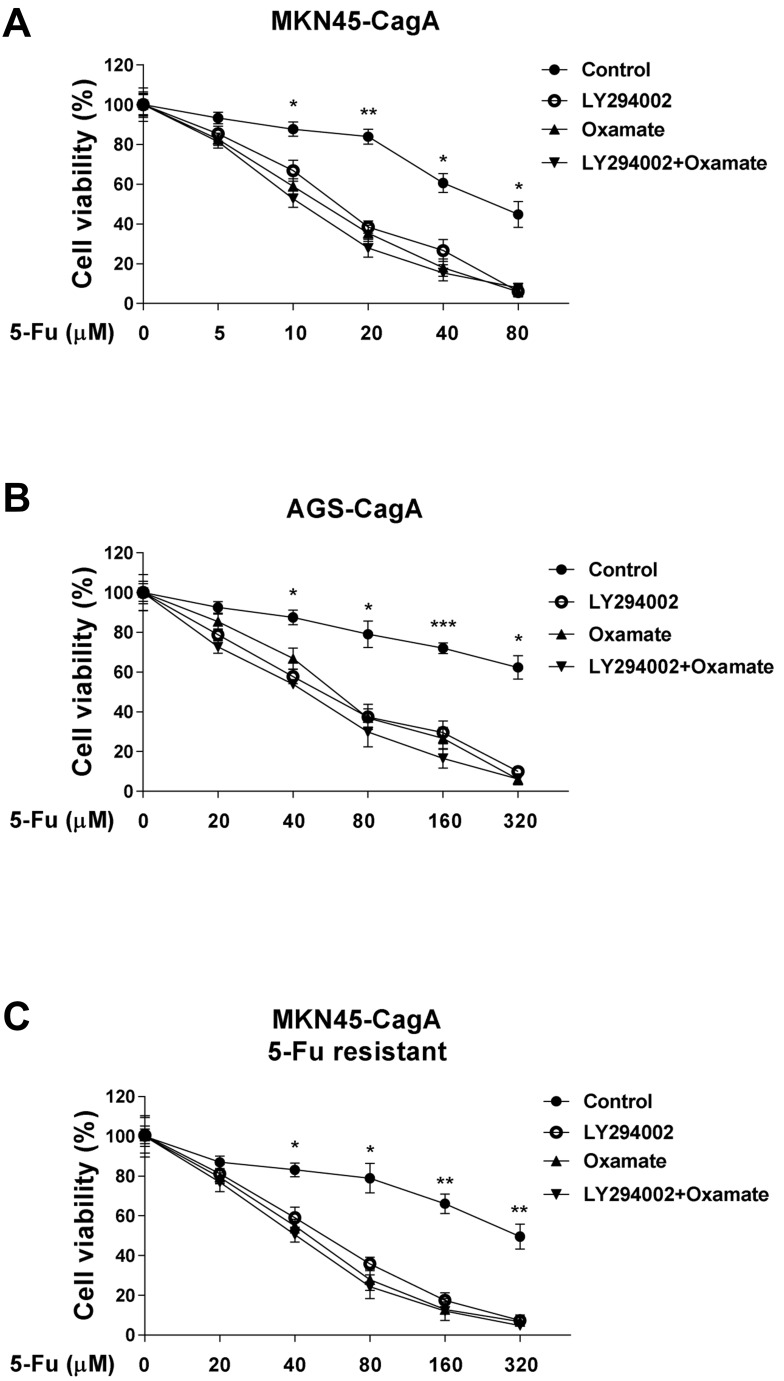
We next addressed the questions that whether the combination of Akt inhibitor or glycolysis inhibitor with 5-Fu could synergistically enhance the cytotoxicity of 5-Fu to CagA overexpressed gastric cancer cells. MKN45-CagA and AGS-CagA cells were co-treated with control, 5-Fu and Akt inhibitor LY294002 and/or glycolysis inhibitor, Oxamate for 48 hours. Results in Figure 5A and B demonstrated co-treatments with 5-Fu and LY294002 or Oxamate alone or LY294002 plus Oxamate showed synergistically inhibitory effects on gastric cancer cells, suggesting the combination of 5-Fu with glycolysis inhibitor could contribute to the development of therapeutic agents against Helicobacter pylori-infected gastric cancer. To verify whether CagA confers the 5-Fu resistance via Akt-glycolysis activation, we examined the cytotoxicity effects of co-treatment with Akt inhibitor LY294002 and/or glycolysis inhibitor, Oxamate and 5-Fu on MKN45-CagA 5-Fu resistant cells. The addition of LY294002 or Oxamate alone significantly enhanced 5-Fu sensitivity in 5-Fu resistant cells (Figure 5C), and the combination of Akt inhibitor and glycolysis inhibitor resulted in a slightly increased cytotoxicity in the 5-Fu resistant MKN45-CagA cells with the presence of 5-Fu (Figure 5C), suggesting no other signal pathway was involved in the CagA-Akt-glycolysis axis.
Figure 5.
Glycolysis inhibition sensitizes gastric cancer cells to 5-Fu. (A) MKN45 CagA overexpression cells and (B) AGS CagA overexpression cells were co-treated with 5-Fu and control, LY294002 alone, Oxamate alone or LY294002 plus Oxamate for 24 hours. The cell viability was measured by MTT assay. (C) MKN45-CagA 5-Fu resistant cells were co-treated with 5-Fu and control, LY294002 alone, Oxamate alone or LY294002 plus Oxamate for 24 hours. The cell viability was measured by MTT assay. Columns, mean of three independent experiments; bars, SE. *p < 0.05; **p < 0.01; ***p < 0.001.
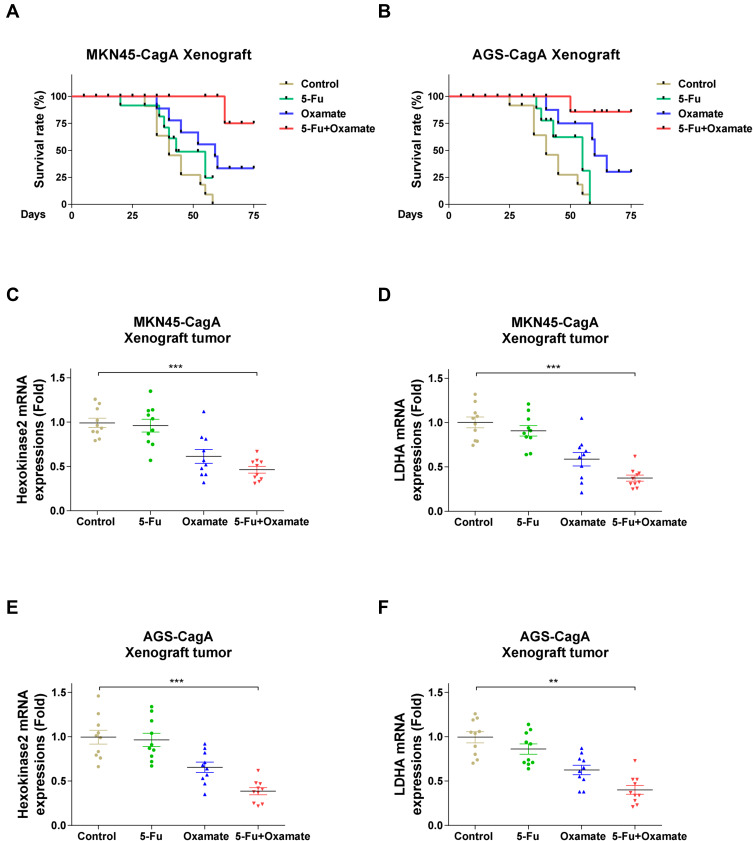
To confirm the above results from in vitro gastric cancer cells, we performed in vivo experiments using xenograft mouse model. AGS-CagA or MKN45-CagA cells were injected into nude mice and when the tumor established, mice were treated with control saline, 5-Fu alone, Oxamate alone or 5-Fu plus glycolysis inhibitor via intraperitoneal injection twice a week, for ten consecutive weeks. Most of mice received treatment with 5-Fu alone as well as control treatment died within 2 months. Although Oxamate alone did not dramatically increase the survival rate with the inoculation of CagA overexpressed gastric cancer cells, treatment with the combination of 5-Fu and glycolysis inhibitor achieved a significantly prolonged survival outcome (Figure 6A and B). We examined the mRNA expressions of HK2 and LDHA in xenograft tumors from the above gastric cancer cells inoculated mice. Consistently, HK2 and LDHA mRNAs were significantly repressed by 5-Fu plus Oxamate treatments (Figure 6C–F), although 5-Fu alone treatment only slightly inhibit the glycolysis enzymes. In summary, our results demonstrated that the combination of 5-Fu and glycolysis inhibitor could achieve a significantly improved xenograft mice survival rate.
Figure 6.
5-Fu treatment combined with glycolysis inhibitor therapy prolonged xenograft mice survival. (A) MKN45-CagA and (B) AGS-CagA Xenograft mice were treated with control saline, 5-Fu alone, Oxamate alone or 5-Fu plus glycolysis inhibitor via intraperitoneal injection twice a week, for ten consecutive weeks. Mice survival rates were examined. (C–F) MKN45-CagA and AGS-CagA Xenograft tumors were isolated and the mRNA expressions of HK2 and LDHA were examined by qRT-PCR. GAPDH was an internal control. Bars, SE. **p < 0.01; ***p < 0.001.
Discussion
Helicobacter pylori infects about 50% of the world’s population, resulting in gastritis or gastric cancers.8,9 In addition, the Helicobacter pylori is classified as a group I carcinogen through delivering CagA protein into gastric epithelial cells,10 indicating that CagA is associated with the risk of gastric cancer. Nevertheless, the acquired chemoresistance is a key barrier to the efficacy of gastric cancer treatment. In this study, we assessed the correlation between 5-Fu sensitivity and CagA expressions in CagA-positive Helicobacter pylori-infected gastric cancer patients. Consistent with the previous reports that CagA played an oncogenic role in gastric cancers,23 statistic results illustrated CagA mRNA and protein were consistently upregulated in 5-Fu resistant human gastric cancer patients who were H. pylori-infected. Using in vitro gastric cancer cell model, we observed significantly upregulated CagA proteins in 5-Fu resistant gastric cancer cells. Furthermore, overexpression of CagA desensitized gastric cancer cells to 5-Fu treatments. Taken together, the above results indicated targeting CagA protein could sensitize gastric cancer cells to 5-Fu.
We investigated the cellular mechanisms for the CagA-mediated 5-Fu resistance in gastric cancer cells. Although aerobic glycolysis is a widely studied new hallmark of cancer cells, the roles of cellular metabolic switch in the CagA oncoprotein-mediated chemoresistant remain elusive. Previous studies demonstrated a correlation between CagA overexpression and a Snail-mediated epithelial-mesenchymal transition (EMT) of gastric cancer cells.18 In addition, the oncoprotein-promoted EMT process is correlated with cellular glucose metabolic status,24 suggesting the CagA might regulate EMT as well as chemosensitivity of gastric cancer cells through activating cellular glucose metabolism. Consistently, our results clearly revealed that: (i) glycolysis was significantly upregulated in CagA-positive H. pylori-infected gastric cancer patients and 5-Fu gastric cancer cells; (ii) overexpression of CagA promoted cellular glucose metabolism of gastric cancer cells. We therefore hypothesized that CagA contributes to 5-Fu resistance through promoting glycolysis of gastric cancer cells. By treating of glycolysis inhibitor Oxamate for 48 hours, CagA overexpressed-gastric cancer cells showed significantly inhibitory effects under 5-Fu compared with gastric cancer cells without CagA overexpression, suggesting the CagA-mediated 5-Fu resistance was through stimulating glucose metabolism of gastric cancer cells.
Currently, elucidating the underlying mechanisms is imperative for developing therapeutic strategies against chemoresistant gastric cancers. To investigate the molecular mechanisms of the CagA-promoted glycolysis, we examined the phosphorylation of Akt since it has been reported that activation of Akt pathway contributed to the glycolysis upregulation.22 Expectedly, overexpression of CagA promoted the phosphorylation of Akt. Thus, we treated gastric cancer cells with Akt inhibitor and found inhibition of Akt significantly blocked the CagA-upregulated cellular glycolysis, suggesting blocking glycolysis by either Akt inhibitor or glycolysis inhibitor could enhance the cytotoxicity of 5-Fu. Results demonstrated co-treatments with 5-Fu and Akt inhibitor or glycolysis inhibitor showed synergistically inhibitory effects on gastric cancer cells from in vitro and in vivo models. The xenograft data strongly supported our conclusions. Although further investigation is required to address the detailed molecular mechanisms for the CagA-mediated glycolysis and chemoresistance, our study contributes to the discovering new therapeutic targets against 5-Fu resistant gastric cancers.
Acknowledgment
The authors would sincerely thank all the doctors, research faculties and staffs from the Department of General Surgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin. The authors also thank the reviewers and editors for critically and thoughtful comments for this paper.
Funding Statement
This work was supported by the Natural Science 545 Foundation of Science and Technology Development Plan in Jilin Province, China (No. 20180101303JC).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biagioni A, Skalamera I, Peri S, et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38(3):537–548. []. doi: 10.1007/s10555-019-09803-7 [DOI] [PubMed] [Google Scholar]
- 3.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. doi: 10.1177/1010428317714626 [DOI] [PubMed] [Google Scholar]
- 4.Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403–2414. doi: 10.3748/wjg.v22.i8.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilson DH. Advances in the treatment of gastric cancer: 2019. Curr Opin Gastroenterol. 2019;35(6):551–554. doi: 10.1097/MOG.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 6.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074 [DOI] [PubMed] [Google Scholar]
- 7.Shi WJ, Gao JB. Molecular mechanisms of chemoresistance in gastric cancer. World J Gastrointest Oncol. 2016;8(9):673–681. doi: 10.4251/wjgo.v8.i9.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makristathis A, Hirschl AM, Mégraud F, Bessède E. Review: diagnosis of Helicobacter pylori infection. Helicobacter. 2019;24(Suppl 1):e12641. doi: 10.1111/hel.12641 [DOI] [PubMed] [Google Scholar]
- 9.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150(1):64–78. doi: 10.1053/j.gastro.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi: 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 11.Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53(6):667–682. doi: 10.1080/10409238.2018.1556578 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang Z, Wang J, et al. Metabolic reprogramming results in abnormal glycolysis in gastric cancer: a review. Onco Targets Ther. 2019;12:1195–1204. doi: 10.2147/OTT.S189687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz L, Supuran CT, Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anticancer Agents Med Chem. 2017;17(2):164–170. doi: 10.2174/1871520616666161031143301 [DOI] [PubMed] [Google Scholar]
- 14.Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J, Tan M. Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013;73(9):2709–2717. doi: 10.1158/0008-5472.CAN-12-3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Liu S, Yin S, et al. The reverse warburg effect is likely to be an Achilles‘ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813–57825. doi: 10.18632/oncotarget.18175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Xie G, Tong J, et al. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70(2):1343–1350. doi: 10.1007/s12013-014-0062-x [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty PK, Mustafi SB, Xiong X, et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017;8(1):14634. doi: 10.1038/ncomms14634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DG, Kim HS, Lee YS, et al. Helicobacter pylori CagA promotes snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat Commun. 2014;5(1):4423. doi: 10.1038/ncomms5423 [DOI] [PubMed] [Google Scholar]
- 19.Shi D, Liu Y, Wu D, Hu X. Transfection of the Helicobacter pylori CagA gene alters MUC5AC expression in human gastric cancer cells. Oncol Lett. 2018;15(4):5208–5212. doi: 10.3892/ol.2018.7960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Cheng C, Lu H, Wang Y. miR-4458 suppresses glycolysis and lactate production by directly targeting hexokinase2 in colon cancer cells. Biochem Biophys Res Commun. 2016;469(1):37–43. doi: 10.1016/j.bbrc.2015.11.066 [DOI] [PubMed] [Google Scholar]
- 21.Kim HM, Kim SA, Park SB, Cho JH, Song SY. Sorafenib inhibits 5-fluorouracil-resistant gastric cancer cell growth. Scand J Gastroenterol. 2017;52(5):577–584. doi: 10.1080/00365521.2017.1278786 [DOI] [PubMed] [Google Scholar]
- 22.Lan KH, Lee WP, Wang YS, Liao SX, Lan KH. Helicobacter pylori CagA protein activates Akt and attenuates chemotherapeutics-induced apoptosis in gastric cancer cells. Oncotarget. 2017;8(69):113460–113471. doi: 10.18632/oncotarget.23050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15(3):306–316. doi: 10.1016/j.chom.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Duan Q, Zhang Z, et al. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21(9):2055–2067. doi: 10.1111/jcmm.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]