Abstract
Aim:
The primary intent of the study is to analyze the prescribing pattern and to identify the various drug-related problems (DRPs) associated with the therapy in chronic kidney disease (CKD) patients.
Subjects and Methods:
A prospective observational study was conducted in 160 patients diagnosed with any stages of CKD. The prescribing pattern was studied and DRPs were identified, reported, and categorized as per the Pharmaceutical Care Network Europe classification V 5.01. The association between categorical variables was analyzed using the Chi-square test. The predictors of DRPs were identified using binary logistic regression analysis.
Results:
The mean age of the study population was 50.08 ± 15.32 years with male predominance (71%). The average number of drugs per prescription was found to be 9.16 ± 3.01. The most prescribed drug category was antihypertensives and the most commonly prescribed drugs were diuretics. A total of 337 DRPs were identified, out of which the most common DRP was drug interactions (60%), followed by frequency errors (11.6%). Logistic regression analysis identified comorbidities more than three (odds ratio 2.09), antihypertensives more than two (odds ratio 1.9), alcoholism (odds ratio 1.5), and polypharmacy (odds ratio 1.2) as the predictors of DRPs even though they were not statistically significant at P = 0.01.
Conclusion:
DRPs increase the risk of deterioration of the disease state and increase the length of hospital stay. Identification and resolving of the DRPs will lead to better patient care and proper treatment. Early identification and modification of the above-mentioned predictors could possibly prevent/reduce DRPs.
Keywords: Chronic kidney disease, comorbidities, drug-related problems, polypharmacy, prescribing pattern
INTRODUCTION
The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as either kidney damage or a decreased glomerular filtration rate of <60 mL/min/1.73 m2 for 3 or more months.[1] A recent systematic review and meta-analysis disclosed the huge burden of CKD in India, which is as high as 17.2%.[2] The reason for India becoming a leading reservoir of CKD can be attributed to the growing prevalence of diabetes and hypertension.[3] In the early stages of CKD, the treatment is essential only for the causal conditions (diabetes, hypertension, and other primary risk factors) and to alleviate the advancement of CKD. However, as kidney function deteriorates, a number of medications are administered to manage associated conditions such as mineral and bone disorders, anemia, hyperlipidemia, and cardiovascular complications. Once the patient reaches end-stage renal disease, they may require 10–12 medications.[4] Cost of treatment at this point is exorbitant and out of reach for more than 90% of patients in India. This administration of multiple medications as well as abysmal compliance with drug regimens may contribute to drug-related problems (DRPs).[3]
A DRP is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. These could avert or impede patients from achieving desired therapeutic goals.[5] Medication errors and adverse drug reactions (ADRs) form the major part of DRPs. Hospitalized patients are more prone to DRPs leading to increased adverse drug effects, which can be reflected by long hospital stays, increased health-care utilization, and costs.[6] Awareness of DRPs may aid in identifying, resolving, and preventing potential DRPs and is a prerequisite for better patient care.[7]
Prescribing patterns change with different treating physicians, disease conditions, and population being treated, which makes it important to study the drug utilization over a period of time.[8] Prescribing pattern studies in chronic kidney disease (CKD) can help propose alterations in prescribing practices to make medical care sagacious. Appropriate selection of treatment thus guarantees utmost benefits to the patients while minimizing the DRPs.[9] However, in India, there is no depiction of overall medication profile in CKD patients. In this context, the present study was aimed to assess the physician's prescribing patterns and to identify and categorize the various DRPs associated with the therapy in CKD patients.
SUBJECTS AND METHODS
The work entitled “Evaluation of prescribing practices and drug-related problems in chronic kidney disease patients: A cross-sectional study” was performed over a 6-month duration at a tertiary care teaching hospital in South India. The inclusion criteria were patients who were inpatients diagnosed with CKD and exclusion criteria were patients attending the outpatient department and pregnant and lactating women. The study was performed with the approval of the Institutional Ethics Committee and the consent of the study participants. Regular ward rounds were carried out and patient profiles were reviewed. Data obtained from patient profiles and nursing charts were documented and analyzed for prescribing pattern and DRPs. The collected data were analyzed using IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp.). Descriptive statistics were used to summarize the data. The association between categorical variables was analyzed using the Chi-square test. The predictors of DRPs were identified using binary logistic regression analysis. Any P < 0.05 was considered statistically significant. The various DRPs associated with treatment were found by analyzing the prescription and categorized according to the Pharmaceutical Care Network Europe (PCNE) Classification V 5.01 [Table 1].
Table 1.
Pharmaceutical Care Network Europe classification V 5.01
| Primary domain | Code | Sub-classes |
|---|---|---|
| ADR | P1.1 | Hypoglycemia |
| Gastrointestinal disturbances | ||
| Cough | ||
| Muscle ache and cramps | ||
| Dizziness | ||
| Others | ||
| Drug choice problems | P2.1 | Inappropriate drug |
| P2.3 | Inappropriate duplication | |
| P2.4 | Contraindication | |
| P2.5 | Drug use without indication | |
| P2.6 | No drug prescribed but clear indication | |
| P2.7 | Inadequate regimen | |
| P2.8 | Drug not required. Repeated | |
| Dosing problem | P3.1 | Dose too low or frequency not enough |
| P3.2 | Dose too high or frequency too often | |
| P3.3 | Duration of treatment too short | |
| P3.4 | Duration of treatment too long | |
| P3.5 | Inappropriate dosing | |
| Drug use problems | P4.1 | Drug not taken or administered at all |
| P4.3 | Incorrect administration | |
| P4.4 | Nonadherence to medication | |
| Drug interaction | P5.1 | Potential interaction |
| Others | P6.2 | Insufficient awareness/knowledge |
| P6.3 | Unclear complaints | |
| P6.4 | Therapy failure | |
| P6.5 | Worries about complications/ADR | |
| P6.6 | Dispensing issues | |
| P6.7 | Lifestyle modifications | |
| P6.8 | Monitoring for side effects | |
| P6.9 | Technical issues |
ADR=Adverse drug reactions
RESULTS
During the study, 160 patients were included as per the inclusion and exclusion criteria, of which 114 (71.25%) were male and 46 (28.75%) were female. The mean age of the study population was 50.08 ± 15.32 years, the eldest 86 years, and youngest 10 years [Table 2]. On the analysis of comorbid conditions in the study population, all the patients had hypertension, 47 (29.31%) had diabetes mellitus, and 18 (11%) had anemia [Table 3]. Average comorbidity in the study population was 1.7 ± 1.86 with a maximum of four and a minimum of one. The number of drugs prescribed in the study population was analyzed [Table 4]. It was observed that none of the patients were prescribed only one drug. About 91.25% of patients were prescribed more than five drugs. The mean number of drugs prescribed was 9.16 ± 3.01 and the highest prescribed to a patient was 17. An assessment of the prescribing pattern in the study population indicated diuretics as the most commonly used drugs (77.50%) in the antihypertensive class, followed by alpha agonists (32.50%), alpha blockers (25.62%), and beta blockers (11.87%). Insulin for glycemic control was being prescribed to 25.62% patients. The utilization of phosphate binders and iron supplements was analyzed which showed sevelamer (8.75%) and erythropoietin (23.12%) to be the most used phosphate binder and iron supplement, respectively. Calcium carbonate (8.75%) and Vitamin D3 (5%) were the most used calcium and Vitamin D supplements [Table 5].
Table 2.
Age variation in the study population
| Age (years) | Number of patients (n=160), n (%) | |
|---|---|---|
| Males | Females | |
| <20 | 4 (2.5) | 3 (1.88) |
| 21-40 | 31 (19.38) | 8 (5) |
| 41-60 | 53 (33.13) | 22 (13.75) |
| 61-80 | 23 (14.38) | 13 (8.13) |
| >80 | 3 (1.88) | 0 |
Table 3.
Comorbidities in the study population
| Comorbidities | Number of patients (n=160), n (%) |
|---|---|
| Hypertension | 160 (100) |
| Diabetes mellitus | 47 (29.38) |
| Anemia | 18 (11.25) |
| Ischemic heart disease | 15 (9.38) |
| Hypothyroidism | 7 (4.38) |
| Lower respiratory tract infections | 5 (3.13) |
| Others | 28 (17.5) |
Table 4.
Number of drugs prescribed in the study population
| Number of drugs | Number of patients (n=160), n (%) |
|---|---|
| 1 | 0 |
| 2-5 | 14 (8.7) |
| >5 | 146 (91.25) |
Table 5.
Prescribing pattern of the study population
| Drug category | Number of patients (n=160), n (%) |
|---|---|
| Antihypertensives | 160 (100) |
| Antidiabetics | 44 (27.52) |
| Phosphate binders | 14 (8.71) |
| Iron supplements | 56 (35.0) |
| Vitamin D supplements | 22 (13.78) |
| Statins | 15 (9.38) |
| Antiplatelets | 33 (20.66) |
| Antibiotics | 35 (21.85) |
| Others | 25 (15.6) |
DRPs in the study population were studied [Table 6]. A total of 337 DRPs were identified among 160 patients. The most common DRP was drug interactions (59.94%), followed by frequency error (11.57%) and indication without drug (11.28%). Pharmacokinetic interactions (82.67%) were found to be higher versus pharmacodynamic interactions (17.32%). Severe drug interactions were comparatively lower (28.21%) than moderate (41.58%) and minor (30.19%). Among 160 prescriptions, 35 (21.87%) were free from any DRPs, 38 (23.75%) prescriptions included only one DRP, and 37 (23.12%) prescriptions included two DRPs [Figure 1]. The average number of DRPs was 2.08 ± 1.62 with a maximum of 38 and a minimum of 1.
Table 6.
Drug-related problems in the study population
| Drug-related problems | Number of prescriptions (%) |
|---|---|
| ADR | 4 (1.19) |
| Inappropriate drug | 4 (1.19) |
| Drug without indication | 3 (0.89) |
| Indication without drug | 38 (11.28) |
| Frequency error | 39 (11.57) |
| Contraindications | 11 (3.26) |
| Too high dose | 14 (4.15) |
| Dose adjustment | 10 (2.97) |
| Drug interactions | 202 (59.94) |
| Drug duplication | 4 (1.19) |
| Insufficient awareness | 4 (1.19) |
| Worries about ADR | 4 (1.19) |
ADR=Adverse drug reactions
Figure 1.
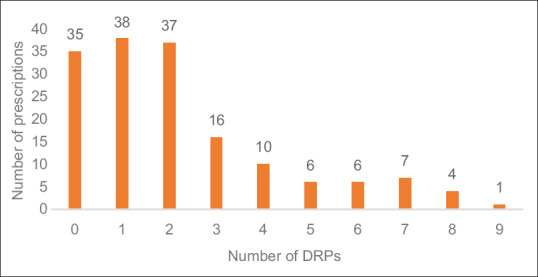
Drug-related problems per prescription
DISCUSSION
In this study of 160 patients, one-third belonged to the 51–60 years age group. This is on par with the study conducted by Rani et al.,[10] in which majority of patients belonged to the 41–60 years age group. This is because the prevalence of CKD is expected to increase with age. This can also be explained by the reduced life expectancy in the CKD population resulting in a lower number of patients aged 80 years and above.[10,11,12] In this study, the major comorbidities were hypertension, type 2 diabetes mellitus, and anemia. This is in accordance with the results of a study conducted by Rani et al.,[10] in which the major comorbidities were found to be hypertension, diabetes mellitus, and coronary artery disease. It is known that the prevalence of CKD is high in patients with these comorbidities.[9] Hypertension and diabetes are considered as diseases that led to the initiation and progression of CKD which support the prevalence of these conditions in the study population.[13] The multiple comorbidities and complications in CKD increase the risk of polypharmacy. Almost all the CKD patients have hypertension as the most common comorbidity. The relationship between hypertension and CKD is cyclic. Uncontrolled hypertension, a risk factor for developing CKD, is associated with a more rapid progression of CKD. Meanwhile, progressive renal disease can exacerbate uncontrolled hypertension due to volume expansion and increased systemic vascular resistance.[10] Diuretics were found to be more used in the study population, followed by calcium-channel blockers (CCBs), alpha-agonists, and alpha-blockers. This was similar to a study conducted by Bajait et al.,[1] where diuretics are more used than CCBs. Diuretics are necessary in CKD for the control of extracellular fluid volume expansion and their associated effects on blood pressure. Fluid retention is one of the complications of hypertension in CKD patients.[1] Patients require diuretics to reduce blood pressure and to control fluid overload. Loop diuretics (torsemide and furosemide) are more used than thiazide diuretics. Former is recommended in the final stages (Stages 4 and 5) and latter is recommended in the initial stages (Stages 1–3) according to KDOQI guideline. All thiazide diuretics except metolazone are ineffective in CKD Stages 4 and 5 due to thiazide's inability to reach the site of action. Torsemide, furosemide, and metolazone are the diuretics used in the CKD patients in this study. Metoprolol is the only prescribed beta-blocker. It is lipid soluble and does not require dosage adjustment and therefore preferable in CKD.[14] Nevertheless, beta-blockers are least preferred in CKD patients with diabetes since it masks the symptoms of hypoglycemia. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which are preferred drugs in the initial stages of CKD, can cause hyperkalemia in the end stages of CKD. Dose adjustment is required for ACEIs and ARBs in chronic renal failure due to the altered pharmacokinetic parameters. Hence, these drugs are least preferred in the end stages of CKD. However, to reduce proteinuria, ACEIs and ARBs are preferred over other drugs since they can reduce intra-osmotic pressure in Bowman's capsule.[1] In this study, 27.5% of patients were prescribed antidiabetic medications. Insulin was the most prescribed antidiabetic versus oral hypoglycemic drugs. This was similar to a study conducted by Bajait et al.[1] In diabetic patients, rigorous glycemic control decreases the rate and progression of microalbuminuria. However, during the phase of deteriorating renal function, insulin requirement falls because the kidney is a site of insulin degradation, and this might be the reason for the remarkable number of patients not receiving any antidiabetic agent. The most prescribed phosphate binder and iron supplement included sevelamer and erythropoietin, respectively. In a study conducted by Slatopolsky et al.,[15] in CKD patients, calcium carbonate was the most preferred phosphate binder. Erythropoietin was the most commonly used iron supplement in a study by Collins.[16]
DRPs in this study population were found to be 337 out of 160 prescriptions. The number of inappropriate drug interactions was highest, followed by frequency error and indication without drug. This was contrary to the study conducted by Zaman Huri and Fun Wee[17] where insufficient awareness of health and diseases was highest, followed by inappropriate drug interactions. In this study, there was polypharmacy in almost all patients, which might be the reason for increased events of drug interactions. The busy schedule of nursing staff and patient's noncompliance to medications are two main factors that contribute to increased frequency errors. Often, some of the underlying comorbidities or complications do not show any overt signs or symptoms. Hence, those conditions are left untreated leading to increase in indications without drugs. The effect of some drugs also leads to masking of symptoms of diseases present. Since the number of CKD patients is steadily increasing, the care given to individual patients has decreased which has led to errors in dose adjustments and proper dosing of drugs. In this study, the problems due to insufficient awareness in patients, ADR, inappropriate drugs, contraindications, and worries about ADR are negligible and no DRPs were found in 38 prescriptions according to PCNE classification. This indicates that effective patient education was given to the study population, and the provision of pharmaceutical services is improving. The active participation of a complete pharmaceutical care team, including doctors, pharmacists, nursing staffs, and other paramedical staffs can resolve DRPs and can improve overall patient care.[11,18,19]
CONCLUSION
In the present study, DRPs were found to be directly proportional to polypharmacy. One-fourth of the population was free from DRPs. The most prominent DRP was found to be drug interaction, followed by frequency error and indication without drug. Continual identification and resolution of DRPs by a complete pharmaceutical care team can help to improve the health status and quality of life of these patients and could play a vital role in achieving better clinical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bajait CS, Pimpalkhute SA, Sontakke SD, Jaiswal KM, Dawri AV. Prescribing pattern of medicines in chronic kidney disease with emphasis on phosphate binders. Indian J Pharmacol. 2014;46:35–9. doi: 10.4103/0253-7613.125163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh AK, Farag YM, Mittal BV, Subramanian KK, Reddy SR, Acharya VN, et al. Epidemiology and risk factors of chronic kidney disease in India – Results from the SEEK (Screening and early evaluation of kidney disease) study. BMC Nephrol. 2013;14:114. doi: 10.1186/1471-2369-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty S, Ghosh S, Banerjea A, De RR, Hazra A, Mandal SK, et al. Prescribing patterns of medicines in chronic kidney disease patients on maintenance hemodialysis. Indian J Pharmacol. 2016;48:586–90. doi: 10.4103/0253-7613.190760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason NA. Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:492–7. doi: 10.1097/MNH.0b013e328349c261. [DOI] [PubMed] [Google Scholar]
- 5.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: Their structure and function. DICP. 1990;24:1093–7. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 6.Hassan Y, Al-Ramahi RJ, Aziz NA, Ghazali R. Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother. 2009;43:1598–605. doi: 10.1345/aph.1M187. [DOI] [PubMed] [Google Scholar]
- 7.van den Bemt PM, Egberts TC, de Jong-van den Berg LT, Brouwers JR. Drug-related problems in hospitalised patients. Drug Saf. 2000;22:321–33. doi: 10.2165/00002018-200022040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ahlawat R, D’Cruz S, Tiwari P. Drug utilization pattern in chronic kidney disease patients at a tertiary care public teaching hospital: Evidence from a cross-sectional study. J Pharm Care Health Syst. 2015;3:2376–419. [Google Scholar]
- 9.Minutolo R, De Nicola L, Mazzaglia G, Postorino M, Cricelli C, Mantovani LG, et al. Detection and awareness of moderate to advanced CKD by primary care practitioners: A cross-sectional study from Italy. Am J Kidney Dis. 2008;52:444–53. doi: 10.1053/j.ajkd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Rani NV, Thomas R, Rohini E. A study on DRPS in chronic kidney disease patients of a tertiary care teaching hospital in South India. World J Pharm Res. 2014;3:1403–17. [Google Scholar]
- 11.Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, et al. Medication prescribing patterns in CKD patients: Comparisons of USRDS to a large non-for-profit dialysis provider. Nephrol Dial Transplant. 2001;17:1543–7. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 12.Pronovost P, Weast B, Schwarz M, Wyskiel RM, Prow D, Milanovich SN, et al. Medication reconciliation: A practical tool to reduce the risk of medication errors. J Crit Care. 2003;18:201–5. doi: 10.1016/j.jcrc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Dipiro JT, Robert LT, Gary CY, Gary RM, Barbara GW, Michael LP. Chronic kidney disease Pharmacotherapy: A Pathophysiologic Approach. 7th ed. USA: MaGraw-Hill Companies, Inc; 2008. Ch 46 Sec 5. [Google Scholar]
- 14.Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75:1487–96. [PubMed] [Google Scholar]
- 15.Slatopolsky E, Weerts C, Lopez-Hilker S, Norwood K, Zink M, Windus D, et al. Calcium carbonate as a phosphate binder in patients with chronic renal failure undergoing dialysis. N Engl J Med. 1986;315:157–61. doi: 10.1056/NEJM198607173150304. [DOI] [PubMed] [Google Scholar]
- 16.Collins AJ. Anaemia management prior to dialysis: Cardiovascular and cost-benefit observations. Nephrol Dial Transplant. 2003;18(Suppl 2):ii2–6. [PubMed] [Google Scholar]
- 17.Zaman Huri H, Fun Wee H. Drug related problems in type 2 diabetes patients with hypertension: A cross-sectional retrospective study. BMC Endocr Disord. 2013;13:2. doi: 10.1186/1472-6823-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang I, Vrahnos D, Hatoum H, Lau A. Effectiveness of clinical pharmacist interventions in a hemodialysis unit. Clin Ther. 1993;15:459–64. [PubMed] [Google Scholar]
- 19.Skoutakis VA, Acchiardo SR, Martinez DR, Lorisch D, Wood GC. Role-effectiveness of the pharmacist in the treatment of hemodialysis patients. Am J Hosp Pharm. 1978;35:62–5. [PubMed] [Google Scholar]


