Abstract
Background and Objective
Tools for the non-invasive assessment of colorectal cancer (CRC) prognosis have profound significance. Although plasma coagulation tests have been investigated in a variety of tumours, the prognostic value of the prothrombin time (PT) and activated partial thromboplastin time (APTT) in CRC has not been discussed. Our study objective was to explore the prognostic significance of preoperative PT and APTT in CRC patients.
Patients and Methods
A retrospective analysis of preoperative coagulation indexes including PT, PTA, INR, APTT, FIB, TT, PLT, NLR and PLR in 250 patients with CRC was performed. Kaplan–Meier and multivariate Cox regression analysis were used to demonstrate the prognostic value of these preoperative coagulation indexes.
Results
The overall survival (OS, p<0.05) and disease-free survival (DFS, p<0.05) of CRC patients with lower PT and APTT levels were significantly prolonged. Based on univariate analysis, PT levels (p<0.001, p<0.001), PTA levels (p=0.001, p=0.001), APTT levels (p=0.001, p<0.001), INR levels (p<0.001, p<0.001), fibrinogen levels (p=0.032, p=0.036), tumour status (p=0.005, p=0.003), nodal status (p<0.001, p<0.001), metastasis status (p<0.001, p<0.001) and TNM stages (p<0.001, p<0.001) were remarkably associated with DFS and OS. Multivariate Cox regression analysis suggested that the levels of PT (HR: 2.699, p=0.006) and APTT (HR: 1.942, p=0.015), metastasis status (HR: 2.091, p= 0.015) and TNM stage (HR: 7.086, p=0.006) were independent predictors of survival in CRC. In the whole cohort, the enrolled patients were then divided into three groups according to their PT and APTT levels. The OS and DFS differed notably among the low-risk (PT<11.85 sec and APTT<25.85 sec), medium-risk (PT≥11.85 sec or APTT≥25.85 sec), and high-risk (PT≥11.85 sec and APTT≥25.85 sec) groups.
Conclusion
Elevated levels of preoperative PT and APTT were predictors of poor outcomes in CRC patients. Moreover, the combination of preoperative PT and APTT can be a new prognostic stratification approach for more precise clinical staging of CRC.
Keywords: PT, APTT, CRC, prognosis
Introduction
Colorectal cancer (CRC) is a commonly diagnosed cancer with a poor prognosis. In China, the morbidity and mortality of CRC are ranked second and third for women and third and fourth for men, respectively.1 Although there are many reports that have shown that the survival of patients with CRC can be predicted by many coagulation indexes, the specificity and accuracy of these tests are controversial and limited.2,3 Therefore, there is an urgent need to look for accurate indicators for CRC to assess prognosis and guide treatment for patients.
Patients with malignant solid tumours usually present with aberrant activation of the blood coagulation system, which results in an elevated incidence of thromboembolism.4–7 Patients who suffer from cancer can be frequently found to have haemostatic system changes and indications of chronic haemostatic activation, such as DIC.8,9 Above all, tumour-induced turbulence in haemostatic activity has been proven to enhance tumour progression, dissemination, angiogenesis, and metastasis as well as the inflammatory cell response.10,11 In addition, massive coagulopathy including hyperfibrinogenaemia, thrombocytosis, and elevated levels of von Willebrand factor (vWF), D-dimer and fibrin degradation products (FDP) have been proven to have a close association with the progression and the unfavourable prognosis of various types of solid malignant tumours, such as gastric, oesophageal, pulmonary, vesical, colorectal and ovarian cancer.12–17
Recently, many novel biomarkers (especially haematological indicators) have been increasingly used to predict the outcome of some cancers, including nine coagulation indicators: activated partial thromboplastin time (APTT), prothrombin time activity (PTA), prothrombin time (PT), international normalized ratio, fibrinogen (FIB), thrombin time (TT), platelet count (PLT), and platelet-to-lymphocyte ratio (PLR).18–23 Importantly, these markers can be easily monitored through relatively non-invasive procedures.
In our study, the predictive values of nine blood coagulation markers (PT, APTT, FIB, TT, PTA, PLT, INR and PLR) were investigated. In fact, PT and APTT are the two most commonly applied coagulation tests in laboratory examinations, and their prognostic value has been reported in some human tumours, such as hepatocellular carcinoma and lung cancer.24–26 However, the prognostic value of PT and APTT in CRC remains unclear. Therefore, our present retrospective study explored the prognostic significance of PT and APTT for DFS and OS among CRC patients, and the association between their levels and clinical features was evaluated. Moreover, the levels of PT and APTT were combined to serve as a prognostic grouping system for CRC, which was beneficial to producing a significant prognostic classification of the population in our study.
Patients and Methods
Patient Selection
The medical records of 250 patients (144 men and 106 women; age range from 26 to 79 years; median age, 58) were analysed in a retrospective study. These patients were all treated with surgery at Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China). Surgery was conducted between January 2008 and December 2010 as the primary treatment for CRC. Patients with one of the following conditions were excluded: (1) patients who had received previous chemotherapy and/or radiotherapy treatment; (2) patients with previous or coexisting cancer; (3) patients who received blood transfusions, procoagulants or anticoagulant therapy within one month before investigation initiation; (4) patients who died of postoperative complications; and (5) patients diagnosed with a thromboembolism or disseminated intravascular coagulation (DIC) within one month of investigation initiation or during the subsequent therapy.
We examined nine coagulation marker levels within one week prior to surgery as a routine preoperative assessment. Detailed clinical and pathological characteristics were available from all patients, including smoking history, demographic variables, alcohol history, disease-free survival, pathological tumour node metastasis (pTNM) stage, and overall survival. The overall survival (OS) of the subjects was defined as the time from surgery to death or to the end of follow-up, taking whichever happened first. OS was taken as an index to define the primary patient outcome. Sixty-eight of the 250 enrolled subjects died by the end of follow-up.
Laboratory Measurements
All data on nine coagulation markers (PT, PTA, INR, APTT, FIB, TT and PLR from plasma, except PLT from total blood) of the studied patients were collected from preoperative medical records. Peripheral venous blood specimens were collected from patients within 1 week before surgery. Blood samples were collected using tubes with sodium citrate.The blood specimens were instantly centrifuged and detected within 2 hours on the basis of the manufacturer’s protocols. PT, INR, APTT, FIB and TT were determined using reagents (Siemens AG, Munich, Germany) supplied by the kinetic nephelometric detecting system with Diagon Dia-Timer 4 (Diagon Ltd, Budapest, Hungary) and detected by a CA-7000 automatic coagulation analyser (Sysmex Corporation, Kobe, Japan) in the laboratory of SYSUCC. PTA was used to calibrate PT and INR and PTA were calculated according to PT. An XE-5000 automatic blood-cell counter (Sysmex Corporation, Kobe, Japan) was used to detect the complete blood cell counts, PLT, neutrophil counts, lymphocyte counts and leukocyte differential counts of all venous blood samples. The platelet-to-lymphocyte ratio was calculated.
Ethical Statement
The study was carried out in accordance with the Helsinki Declaration. The Institutional Research Ethics Committee of SYSUCC (Guangzhou, China) approved the study, and each patient signed informed consent for genetic analysis of their biological specimens. In our institution, patients were followed up every 3 months during the first year, every 6 months in the following 2 years, and then once a year for those without diagnosis of relapse. The follow-up was ended in March 2016. The Medical Information Unit in Cancer Center verified the informed consent and patient survival status by contacting the patient or their family directly through telecommunication.
Statistical Analysis
The results are presented as the mean or median values according to the distribution pattern. Median values were the cut-off points for categorizing continuous variables (PT, PTA, INR, APTT, TT, FIB, PLT and PLR) according to the mean ± SD. Independent t-tests and χ2 tests were applied to analyse the difference, and the Mann–Whitney U-test was applied to analyse the relationship between the coagulation tests and the clinical characteristics. Parameters found to be significant by the univariate analysis were then analysed by multivariate Cox regression. The Kaplan–Meier method was adopted to analyse actuarial survival rates plotted against time. The differences between the curves were compared using a Log rank test. SPSS version 17.0 (SPSS, Inc., Chicago, USA) was used for statistical analysis. All calculated P values were two-sided. P values <0.05 were considered to indicate statistically significant.
Results
Correlation Analysis of Coagulation Indexes and Clinical Characteristics
Table 1 summarizes the correlation between clinical characteristics and the coagulation indexes. Specifically, we observed higher INR scores (p=0.033) in older patients (58 years old). Moreover, higher values of PLT (p=0.040) and PLR (p=0.017) were found among female patients. Values of PLR rose significantly (P=0.039) with alcohol history. Furthermore, concentrations of the clotting markers (TT) rose significantly (p<0.01) in those with hypertension. In patients who suffered with advanced T stage classification of colorectal cancer, higher PLT concentrations (p =0.045) were observed. The value of PTA was dramatically elevated in patients with metastases. None of these clinical features were significantly associated with the levels of APTT, PT or FIB.
Table 1.
Main Clinical Characteristics of Patients Group According to Coagulation Parameter Levels
| Variables | Coagulation Parameters (Median and Range) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | PT | PTA | INR | APTT (sec) | Fibrinogen (g/L) | TT | PLT | PLR | |
| (sec) | (%) | (sec) | (103/mm3) | ||||||
| Gender | |||||||||
| Male | 144 | 11.15 | 103.5 | 1.02 | 25.9 | 3.01 | 17.75 | 241.5 | 133.94 |
| −57.6 | (9–14) | (57–158) | (0.78–1.34) | (21.0–60.9) | (1.66–6.32) | (14.8–65.0) | (89.0–594.0) | (47.82–571.11) | |
| Female | 106 | 11.1 | 106.35 | 1.02 | 25.82 | 3.01 | 17.85 | 254.5 | 152.5 |
| −42.4 | (10–50) | (13–160) | (0.76–4.73) | (20.2–38.6) | (1.24–6.23) | (13.6–25.4) | (30.0–528.0) | (10.34–718.0) | |
| p=0.470 | p=0.382 | p=0.539 | p=0.365 | p=0.482 | p=0.321 | p=0.040 | p=0.017 | ||
| Age | |||||||||
| <58 years | 119 | 11.2 | 103.5 | 1.03 | 26.4 | 3.02 | 17.9 | 248 | 143.83 |
| −47.6 | (10–14) | (57–130) | (0.78–1.29) | (20.6–60.9) | (1.24–6.32) | (14.0–65.0) | (38.0–594.0) | (19.0–662.25) | |
| ≥58years | 131 | 11.1 | 105.4 | 1 | 25.5 | 2.99 | 17.7 | 244 | 139.69 |
| −52.4 | (9–50) | (13–158) | (0.76–4.73) | (20.2–45.8) | (1.66–6.00) | (13.6–25.4) | (30.0–514.0) | (10.34–718.0) | |
| p=0.142 | p=0.186 | p=0.033 | p=0.068 | p=0.920 | p=0.935 | p=0.924 | p=0.809 | ||
| Tobacco history | |||||||||
| No | 180 | 11.1 | 105.4 | 1.02 | 25.85 | 3.02 | 17.8 | 239.5 | 139.69 |
| −72 | (10–50) | (13–160) | (0.76–4.73) | (20.2–60.9) | (1.24–6.23) | (13.6–65.0) | (30–528) | (10.34–718.0) | |
| Yes | 70 | 11.15 | 104.35 | 1.02 | 26.05 | 3.01 | 17.85 | 255.5 | 145.94 |
| −28 | (9–14) | (63–158) | (0.79–1.34) | (21.0–45.8) | (1.66–6.32) | (15.1–23.0) | (131–594) | (47.82–571.11) | |
| p=0.624 | p=0.914 | p=0.502 | p=0.613 | p=0.811 | p=0.946 | p=0.123 | p=0.763 | ||
| Alcohol history | |||||||||
| No | 217 | 11.1 | 105.4 | 1.02 | 11.15 | 2.99 | 17.8 | 243 | 136.09 |
| −86.8 | (9–50) | (13–160) | (0.76–4.73) | (9–14) | (1.24–6.32) | (13.6–15.0) | (30–528) | (10.34–718.0) | |
| Yes | 33 | 11.05 | 106.35 | 1.02 | 11.1 | 3.24 | 17.65 | 258 | 160.45 |
| −13.2 | (10–12) | (92–153) | (0.85–1.10) | (10–50) | (2.37–5.15) | (15.7–23.6) | (161–594) | (47.82–412.5) | |
| p=0.476 | p=0.362 | p=0.680 | p=0.341 | p=0.150 | p=0.867 | p=0.067 | p=0.039 | ||
| Family history of cancer | |||||||||
| No | 215 | 11.1 | 105.4 | 1.02 | 25.9 | 3.02 | 17.75 | 244 | 137.73 |
| −86 | (9–50) | (13–160) | (0.76–4.73) | (20.2–60.9) | (1.24–6.23) | (13.6–65.0) | (30–528) | (10.34–626.25) | |
| Yes | 35 | 11.2 | 110.83 | 1.04 | 25.4 | 2.86 | 17.9 | 256 | 150.59 |
| −14 | (10–14) | (78–153) | (0.88–1.20) | (20.7–35.7) | (2.05–5.78) | (15.1–21.4) | (120–594) | (53.16–718.0) | |
| p=0.615 | p=0.853 | p=0.778 | p=0.662 | p=0.534 | p=0.510 | p=0.179 | p=0.179 | ||
| Hypertension | |||||||||
| No | 210 | 11.1 | 105.4 | 1.02 | 26.15 | 2.99 | 17.6 | 254.53 | 139.68 |
| −84 | (9–50) | (13–160) | (0.76–4.73) | (20.2–60.9) | (1.24–6.32) | (13.6–65.0) | (30–594) | (10.34–718.0) | |
| Yes | 40 | 11.15 | 105.4 | 1.04 | 25.5 | 3.07 | 18 | 240.5 | 146.82 |
| −16 | (10–14) | (67–145) | (0.85–1.29) | (20.5–33.4) | (1.94–5.20) | (14.7–22.8) | (91–421) | (72.86–300.71) | |
| p=0.940 | p=0.879 | p=0.667 | p=0.274 | p=0.600 | p=0.008 | p=0.616 | p=0.069 | ||
| Diabetes | |||||||||
| No | 235 | 11.1 | 105.4 | 1.02 | 25.9 | 3.12 | 17.8 | 244 | 140 |
| −94 | (9–50) | (13–160) | (0.76–4.73) | (20.2–60.9) | (1.24–6.32) | (13.6–65.0) | (30–594) | (10.34–718.0) | |
| Yes | 15 | 11.1 | 105.4 | 1.02 | 25.7 | 3.38 | 17.8 | 276 | 160.91 |
| −6 | (10–13) | (83–120) | (0.87–1.16) | (20.5–29.8) | (2.40–4.99) | (15.9–22.1) | (177–352) | (86–293.33) | |
| p=0.438 | p=0.725 | p=0.845 | p=0.718 | p=0.325 | p=0.956 | p=0.616 | p=0.614 | ||
| T classification | |||||||||
| T1-2 | 43 | 11.1 | 105.4 | 1 | 25.5 | 2.81 | 17.9 | 231 | 129.5 |
| −17.2 | (10–14) | (67–155) | (0.76–1.29) | (20.2–35.7) | (1.94–5.78) | (15.5–20.6) | (118–393) | (64.17–296) | |
| T3-4 | 207 | 11.2 | 104.7 | 1.02 | 26 | 3.07 | 17.8 | 248 | 142.67 |
| −82.8 | (9–50) | (13–160) | (0.78–4.73) | (20.6–60.9) | (1.24–6.32) | (13.6–65.0) | (30–594) | (10.34–718) | |
| p=0.152 | p=0.360 | p=0.420 | p=0.409 | p=0.058 | p=0.642 | p=0.045 | p=0.150 | ||
| N classification | |||||||||
| No | 180 | 11.1 | 105.4 | 1.02 | 25.75 | 2.99 | 17.8 | 243 | 142.67 |
| −72 | (9–15) | (13–160) | (0.78–1.39) | (20.6–45.8) | (1.66–6.32) | (13.6–23.6) | (63–594) | (27.05–718.00) | |
| N1-2 | 70 | 11.2 | 103.5 | 1.03 | 26.3 | 3.07 | 17.6 | 255 | 128 |
| −38 | (10–50) | (15–155) | (0.76–4.73) | (20.2–60.9) | (1.24–6.23) | (14.0–65.0) | (30–528) | (10.34–626.25) | |
| p=0.265 | p=0.129 | p=0.379 | p=0.481 | p=0.526 | p=0.977 | p=0.117 | p=0.638 | ||
| Metastasis | |||||||||
| M0 | 190 | 11.1 | 105.4 | 1.02 | 25.6 | 2.96 | 17.9 | 243 | 140 |
| −76 | (9–50) | (13–160) | (0.76–4.73) | (20.6–60.9) | (1.24–6.32) | (13.6–65.0) | (38–594) | (19.00–718.00) | |
| M1 | 60 | 11.25 | 100.7 | 1.04 | 26.3 | 3.17 | 17.25 | 252.5 | 145 |
| −24 | (10–15) | (59–153) | (0.88–1.39) | (20.2–38.6) | (1.98–6.23) | (14–25.4) | (30–528) | (10.34–626.25) | |
| p=0.081 | p=0.025 | p=0.074 | p=0.318 | p=0.115 | p=0.094 | p=0.102 | p=0.226 | ||
| pTNM stage | |||||||||
| I–II | 113 | 11.1 | 105.4 | 1.02 | 25.5 | 2.98 | 17.9 | 238 | 142.5 |
| −45.2 | (10–50) | (15–153) | (0.78–4.73) | (20.5–35.8) | (1.24–6.32) | (14.5–23.6) | (38–594) | (19–613.33) | |
| III–IV | 137 | 11.1 | 105.4 | 1.02 | 26.1 | 3.07 | 17.6 | 250 | 139.04 |
| −54.8 | (9–15) | (13–160) | (0.76–1.39) | (20.2–60.9) | (1.66–6.23) | (13.6–65.0) | (30–528) | (10.34–718) | |
| p=0.404 | p=0.484 | p=0.882 | p=0.464 | p=0.872 | p=0.134 | p=0.135 | p=0.904 | ||
Notes: Mann–Whitney U-test, independent t-tests, and Kruskal–Wallis test were used in Table 1. TNM denotes tumor-node-metastasis. Bold values indicate significant differences (p<0.05).
Abbreviations: PT, prothrombin time; PTA%, prothrombin time activity; INR, international normalized ratio; APTT, activated partial thromboplastin time; TT, thrombin time; PLT, platelets; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; T, tumor; N, node; M, metastasis.
Prognostic Value of the Clotting Markers in Colorectal Cancer
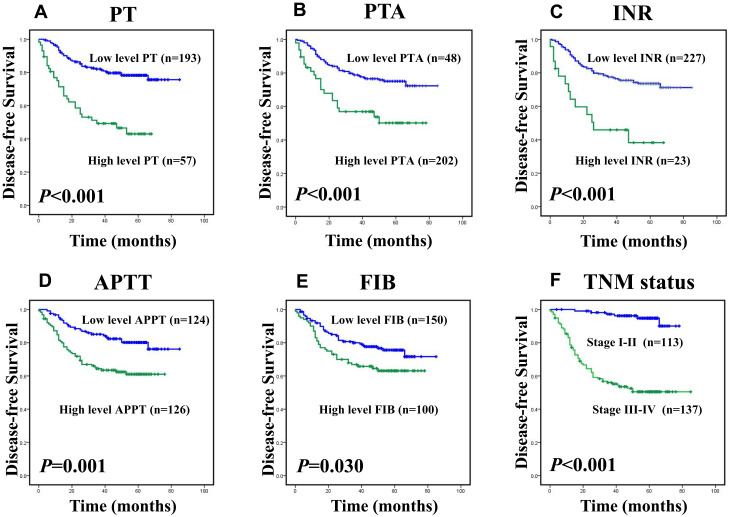
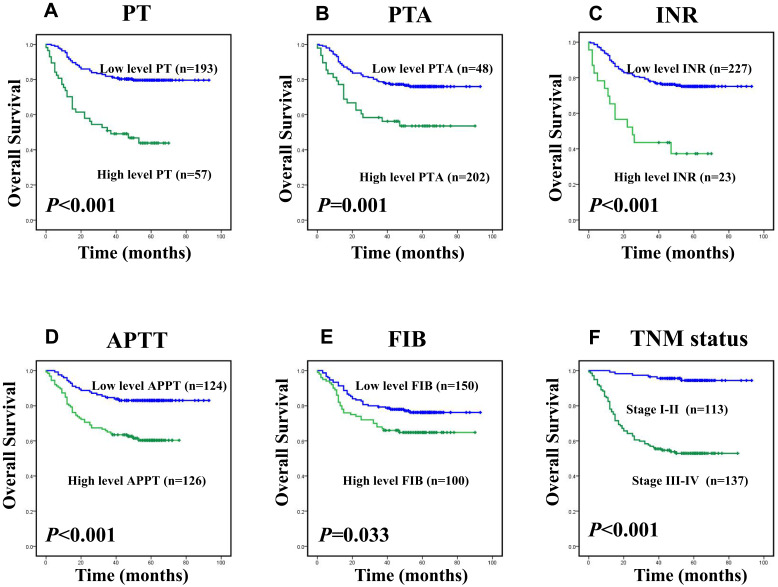
Among 250 colorectal cancer patients, the median OS and DFS, respectively, were 55 and 50 months. The cumulative DFS and OS rates of the enrolled patients were 71% and 72%, respectively (data not shown). The Kaplan–Meier survival analysis revealed that the levels of PT, PTA, INR, APTT, FIB and TNM stages had strong correlations with overall survival and disease-free survival, as shown in Figure 1A–F and Figure 2A–F (p< 0.05).However, the levels of the other clotting markers (TT, PLT and PLR) were only weakly correlated with overall survival and disease-free survival (data not shown). The univariate analysis indicated that elevated levels of PT (p<0.001 and p<0.001), PTA (p=0.001, p=0.001), INR (p<0.001 and p<0.001), APTT (p=0.001 and p<0.001), and FIB (p=0.032, p=0.036) were significantly associated with decreased DFS and OS, as shown in Table 2. Table 2 shows that clinicopathological parameters such as tumour status, nodal status, and metastasis status as well as TNM stage all have prognostic value. In addition to classic prognostic indicators such as metastasis status (p=0.008, p=0.015) and TNM stage (p<0.001, p=0.006), the levels of PT (p=0.021, p=0.006) and APTT (p=0.046, p=0.015) were found to be independent predictors of DFS and OS according to the multivariate Cox model analysis, as shown in Table 3.
Figure 1.
Kaplan–Meier survival analysis of patients with CRC, disease-free survival (DFS) curves for the patients according to (A) the PT level (Low level:<11.85sec, n=193; high level: ≥11.85sec, n=57); (B) the PTA% level (low level:<91.75%, n=48; high level: ≥91.75%, n=202); (C) the INR level (low level:<1.15, n=227; high level: ≥1.15, n=23); (D) the APTT level (low level:<25.85sec, n=124; high level: ≥25.85sec, n=126); (E) the FIB level (Low level:<3.245g/L, n=150; high level: ≥3.245g/L, n=100); (F) TNM stage (I–II, n=113; III–IV, n=137).
Figure 2.
Kaplan–Meier survival analysis of patients with CRC, overall survival (OS) curves for the patients according to (A) the PT level (low level:<11.85sec, n=193; high level: ≥11.85sec, n=57); (B) the PTA% level (low level:<91.75%, n=48; high level: ≥91.75%, n=202); (C) the INR level (low level:<1.15, n=227; high level: ≥1.15, n=23); (D) the APTT level (low level:<25.85sec, n=124; high level: ≥25.85sec, n=126); (E) the FIB level (low level:<3.245g/L, n=150; high level: ≥3.245g/L, n=100); (F) TNM stage (I–II, n=113; III–IV, n=137).
Table 2.
Univariate Cox Regression Analysis for Disease-Free Survival and Overall Survival in 250 Patients with Colorectal Cancer
| Factors | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p value* | HR (95% CI) | p value* | |
| Gender (male/female) | 1.222 (0.766–1.949) | 0.399 | 1.193 (0.745–1.909) | 0.463 |
| Age, years (≤58>58) | 0.984 (0.618–1.567) | 0.945 | 0.998 (0.624–1.594) | 0.992 |
| Tobacco history (No/Yes) | 1.088 (0.654–1.812) | 0.745 | 1.112 (0.667–1.853) | 0.685 |
| Alcohol history (No/Yes) | 1.070 (0.548–2.089) | 0.843 | 1.114 (0.570–2.176) | 0.751 |
| Family history of cancer (No/Yes) | 0.979 (0.501–1.194) | 0.951 | 1.036 (0.529–2.026) | 0.919 |
| Hypertension (No/Yes) | 0.766 (0.381–1.542) | 0.445 | 0.877 (0.449–1.173) | 0.700 |
| Diabetes (No/Yes) | 1.670 (0.723–3.858) | 0.230 | 1.667 (0.722–3.852) | 0.231 |
| Tumor status (1–2/3-4) | 16.61 (2.308–119.625) | 0.005* | 8.275 (2.028–33.770) | 0.003* |
| Nodal status (0/1) | 2.972 (1.864–4.738) | <0.001* | 2.899 (1.805–4.622) | <0.001* |
| Metastasis status (0/1) | 4.796 (3.002–7.633) | <0.001* | 4.602 (2.871–7.377) | <0.001* |
| TNM stages (I–II/III–IV) | 12.13 (5.250–28.025) | <0.001* | 11.688 (5.507–27.015) | <0.001* |
| PT (≥11.85/<11.85) | 3.457 (2.153–5.550) | <0.001* | 3.614 (2.252–5.801) | <0.001* |
| PTA% (≥91.75/<91.75) | 0.418 (0.252–0.691) | 0.001 | 0.424 (0.256–0.703) | 0.001 |
| INR (≥1.15/<1.15) | 3.225 (1.765–5.895) | <0.001* | 3.472 (1.930–6.246) | <0.001* |
| APTT (≥25.85/<25.85) | 2.281 (1.394–3.731) | 0.001* | 2.687 (1.611–4.483) | <0.001* |
| Fibrinogen (≥3.245/<3.245) | 1.622 (1.043–2.646) | 0.032 | 1.653 (1.035–2.641) | 0.036 |
| TT (≥20.70/<20.70) | 1.562 (0.716–0.341) | 0.263 | 1.575 (0.721–3.439) | 0.254 |
| PLT (≥239.5/<239.5) | 1.633 (1.003–2.659) | 0.051 | 1.414 (0.874–2.289) | 0.158 |
| PLR (≥113/<113) | 1.699 (0.985–2.931) | 0.057 | 1.707 (0.988–2.948) | 0.055 |
Notes: *Univariate analysis, p<0.05 considered as statistically significant. Bold values indicate significant differences (p<0.05).
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Table 3.
Multivariate Cox Regression Analysis for Disease-Free Survival and Overall Survival in 250 Patients with Colorectal Cancer
| Factors | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p value** | HR (95% CI) | p value** | |
| Tumor status (1–2/3-4) | 7.159 (0.969–52.863) | 0.054 | 3.255 (0.769–13.768) | 0.109 |
| Nodal status (0/1) | 0.910 (0.510–1.624) | 0.751 | 0.934 (0.515–1.694) | 0.822 |
| Metastasis status (0/1) | 2.185 (1.224–3.898) | 0.008** | 2.091 (1.156–3.783) | 0.015** |
| TNM stages(I–II/III–IV) | 7.381 (3.044–17.897) | <0.001** | 7.086 (2.923–17.178) | 0.006** |
| PT (≥11.85/<11.85) | 2.361 (1.140–4.893) | 0.021** | 2.699 (1.333–5.463) | 0.006** |
| PTA% (≥91.75/<91.75) | 1.173 (0.466–2.952) | 0.735 | 1.606 (0.629–4.103) | 0.322 |
| INR (≥1.15/<1.15) | 1.277 (0.528–3.085) | 0.588 | 1.545 (0.631–3.782) | 0.341 |
| APTT (≥25.85/<25.85) | 1.649 (0.987–2.755) | 0.046** | 1.942 (1.140–3.309) | 0.015** |
| Fibrinogen (≥3.245/<3.245) | 1.240 (0.756–2.035) | 0.394 | 1.250 (0.760–2.056) | 0.380 |
Notes: **Cox proportional hazards model, p<0.05 considered as statistically significant. Bold values indicate significant differences (p<0.05).
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Correlation Analysis Between PT, APTT and Clinical Features in 250 CRC Patients
The relationships between PT, APTT and clinical features of these patients are shown in Table 4. PT had no association with age, sex, alcohol history, tobacco history, family history of cancer, hypertension, diabetes, Tumor status, N classification and metastasis or TNM stages. There was also no association between APTT and sex, tobacco history, alcohol history, family history of cancer, hypertension, diabetes, histological differentiation, N classification or metastasis. Additionally, the patient cohort was split into four groups: the low and high PT groups and the low and high APTT groups, by the mean PT (11.85 sec) and the mean APTT (25.85sec). Compared with patients in the high group, patients with a lower PT tended to have an earlier stage of TNM stages (p= 0.023). Patients with a lower APTT were younger than those in the high group (p= 0.012).
Table 4.
Association of the Expression of PT, APTT and Clinicopathologic Parameters in 250 Patients with Colorectal Cancer
| Clinicopathologic Parameter | Case | PT Expression (%) | APTT Expression (%) | ||||
|---|---|---|---|---|---|---|---|
| Low | High | p value* | Low | High | p value* | ||
| Age, years | |||||||
| Median (35–90) | |||||||
| ≤58 | 119 | 87 (73.1) | 32 (26.9) | 0.174 | 49 (41.2) | 70 (58.8) | 0.012* |
| >58 | 131 | 106 (80.9) | 25 (19.1) | 75 (57.3) | 56 (42.7) | ||
| Gender | |||||||
| Male | 144 | 112 (77.8) | 32 (22.2) | 0.879 | 71 (49.3) | 73 (50.7) | 1.000 |
| Female | 106 | 81 (76.4) | 25 (23.6) | 53 (50.0) | 53 (50.0) | ||
| Tobacco history | |||||||
| No | 180 | 136 (75.6) | 44 (24.4) | 0.402 | 90 (50.0) | 90 (50.0) | 0.888 |
| Yes | 70 | 57 (81.4) | 13 (18.6) | 34 (48.6) | 36 (51.4) | ||
| Alcohol history | |||||||
| No | 217 | 164 (75.6) | 53 (24.4) | 0.177 | 104 (47.9) | 113 (52.1) | 0.259 |
| Yes | 33 | 28 (84.8) | 5 (15.2) | 19 (57.5) | 14 (42.5) | ||
| Family history of cancer | |||||||
| No | 212 | 161 (75.9) | 51 (24.1) | 0.516 | 104 (49.1) | 108 (50.9) | 0.856 |
| Yes | 38 | 29 (76.3) | 11 (23.7) | 20 (52.6) | 18 (47.4) | ||
| Hypertension | |||||||
| No | 210 | 162 (77.1) | 48 (22.9) | 1.000 | 100 (47.6) | 110 (52.4) | 0.170 |
| Yes | 40 | 31 (77.5) | 9 (22.5) | 24 (60.0) | 16 (40.0) | ||
| Diabetes | |||||||
| No | 235 | 183 (77.9) | 52 (22.1) | 0.343 | 116 (49.4) | 119 (50.6) | 0.796 |
| Yes | 15 | 10 (66.7) | 5 (33.3) | 8 (53.3) | 7 (46.7) | ||
| Tumor status | |||||||
| T1-2 | 43 | 37 (86.0) | 6 (14.0) | 0.163 | 25 (58.1) | 18 (41.9) | 0.243 |
| T3-4 | 207 | 156 (77.2) | 51 (22.8) | 99 (47.8) | 108 (52.2) | ||
| Nodal status | |||||||
| N0 | 180 | 144 (80.0) | 36 (20.0) | 0.096 | 92 (51.1) | 88 (48.9) | 0.236 |
| N1/2 | 70 | 49 (70.0) | 21 (30.0) | 32 (45.7) | 38 (54.3) | ||
| Metastasis status | |||||||
| M0 | 190 | 152 (80.0) | 38 (20.0) | 0.077 | 100 (52.6) | 90 (47.4) | 0.104 |
| M1 | 60 | 41 (68.3) | 19 (31.7) | 24 (40.0) | 36 (60.0) | ||
| TNM stages | |||||||
| I–II | 113 | 95 (84.1) | 18 (15.9) | 0.023* | 61 (54.0) | 52 (46.0) | 0.253 |
| III–IV | 137 | 98 (71.5) | 39 (28.5) | 63 (46.0) | 74 (54.0) | ||
Notes: *p values were calculated by the chi-square test (χ2 test), p<0.05 considered as statistically significant. Bold values indicate significant differences (p<0.05).
Abbreviations: T, tumor; N, node; M, metastasis; TNM, tumor-node-metastasis.
A Novel Prognostic Grouping System Based on the Combination of PT and APTT
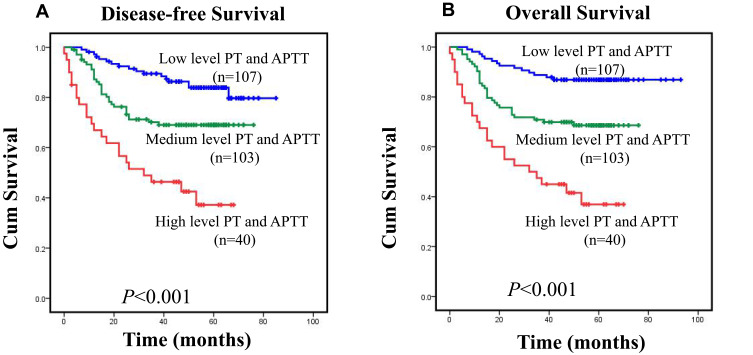
In this analysis, PT and APTT were independent risk factors for CRC patients, and the actual outcomes of patients with CRC were reflected more accurately by the further subsets shown in Figure 3A and B. These patients were classified as follows: low risk (PT<11.85sec and APTT <25.85 sec), medium risk (PT≥11.85sec or APTT ≥25.85 sec), and high risk (PT≥11.85sec and APTT≥25.85 sec). In the entire CRC cohort, disease-free survival of patients with CRC differed significantly according to group (Figure 3A; p<0.001), with mean survival times as follows: low risk, 51.7 months (95% CI: 48.3–55.08; n= 107); medium risk, 36.7 months (95% CI: 37.7–46.0; n=103); and high risk, 29.8 months (95% CI: 22.7–36.9; n=40). The overall survival of patients differed significantly in each group (Figure 3B; p<0.001). The mean survival times were as follows: low risk, 55.5 months (95% CI: 52.3–58.7; n=107); medium risk, 44.9 months (95% CI: 40.7–49.1; n=103); and high risk, 31.3 months (95% CI: 24.0–38.6; n=40). These data indicated that combining PT and APTT results could produce a new prognostic grouping system, in which the further subsets more accurately reflected the actual outcomes of patients with CRC, as shown in Figure 3A and B.
Figure 3.
(A) Disease-free survival (DFS) and (B) overall survival (OS) curves for the three-tiered stratification of patients with colorectal cancer, incorporating two independent prognostic variables (PT and APTT). Survival in low-risk (PT<11.85 sec and APTT<25.85 sec, n=107), medium-risk (PT≥11.85 sec or APTT≥25.85 sec, n=103), and high-risk (PT≥11.85 sec and APTT≥25.85 sec, n=40) groups differed significantly (p<0.05 for both variables as determined by Log rank significance tests).
Discussion
In our study, the prognostic values of eight clotting markers in 250 patients with CRC were evaluated and, for the first time, we identified PT and APTT as independent prognostic factors for CRC. Despite the 5-year survival rates of colorectal cancer being generally favourable the prognosis of some patients is still poor.27
The relationship between prognosis and various clinicopathologic characteristics, including patient age, clinical stage, histologic type, and lymphatic vascular space invasion, has been well-studied previously.28–31 Current efforts increasingly focus on molecular biomarkers, which may make the histologic classification of tumours more accurate and facilitate us in identifying more aggressive cancers, promoting staging revisions, and creating personalized therapy and better clinical outcomes.32–36 The goal of this study was to identify the prognostic value of certain clotting markers for patients with colorectal cancer.
Our evidence showed that INR was significantly aligned with patient age. Additionally, PLT and PLR levels were significantly correlated with sex. Levels of the clotting markers PLR, TT and INR were significantly related to age, sex, alcohol history and hypertension, which are important risk factors. The findings of this study about the risk factors for colorectal cancer are consistent with other studies.37–39 In addition, this study found that PLT had strong associations with histologic subtypes of colorectal cancer, and these differences may be caused by severe systemic inflammation in T3-4 colorectal cancer.40 Furthermore, the levels of PTA increased significantly in metastatic patients. The above evidence suggested that the coagulation markers were closely related to clinical characteristics. The prognostic significance of the clotting markers was analysed by the Kaplan–Meier method and Cox proportional hazards regression. In univariate analyses, PT, APTT, PTA, INR, FIB and all of the pathological stages were significantly correlated with OS and DFS. With multivariate analyses, PT, APTT, metastasis status and TNM stage were shown to be independently correlated with prognosis in colorectal cancer.
The prothrombin time test is a simple, inexpensive and widely used laboratory test for detecting coagulation, anticoagulation and fibrinolysis function.41 The PT test is a kind of monitoring indicator for the exogenous coagulation system, related to fibrinogen deficiency, the primary fibrinolytic system and disseminated intravascular coagulation (DIC). Haemorrhage is a common complication of malignant digestive tract tumours, owing to the tumour increasing FIB and inducing a hypercoagulative state. The disorder of coagulation and anticoagulation would then lead to bleeding through the plasminogen activator system. Coagulation in patients with cancer can also be promoted by suppressed fibrinolytic activity and/or decreased levels of anticoagulant factors. In cancer patients, thromboembolic complications occur irrespective of the levels of anticoagulant factors, suggesting that those parameters are not predictors of thrombotic disease. Interestingly, PT and APTT can predict mortality.42 In this study, the PT level in patients with advanced stage colorectal cancer was increased significantly compared with those in an early stage, which indicated that PT could be used as a marker for evaluating prognosis in patients with CRC.
The activated partial thromboplastin time (APTT) test is used to monitor the endogenous clotting system. This study has shown that OS and DFS rates differed significantly between CRC patients with low or high APTT scores. Furthermore, in multivariate analyses, the prominent elevation of APTT scores was sustained, coupled with a larger hazard ratio. The significance of increased APTT score was maintained in multivariate analysis with larger hazard ratios. Many recent studies have shown that APTT could be used as a reference index for patients with early stage malignant gastrointestinal tumours who are in a critical condition and have a poor prognosis.43 This study also showed that APTT increased gradually in patients with CRC, which could cause decreases in blood coagulation function and render patients prone to colorectal bleeding.
Increases in APTT in cancer patients could be due to malignant tumour growth, weakened immunity, the consumption of the body by the tumour, physical failure and liver function damage induced by vitamin K synthetic obstacles, all of which affect blood coagulation factor synthesis.44,45 Therefore, improvement of the nutritional status, such as adding clotting factors and vitamin K, could help prevent or reduce bleeding tendencies in patients with CRC. Moreover, coagulopathy is closely related to tumour proliferation and metastasis. After applying laboratory testing, the discovery of clotting abnormalities followed by proper anticoagulant therapy could prevent thrombosis and improve the prognosis of patients with CRC. Thus, detection of thromboembolism preoperatively is a crucial aspect of postoperative care and therapeutic strategies.46 In fact, the PT and APTT tests are the primary tests used for surgical pre-screening and oral anticoagulation.
In this study, we also developed a new grouping using PT and APTT cut-off points and found that survival time differed significantly among the three tiers based on the PT and APTT levels, which could be defined as useful tools for preoperative assessment of patients with colorectal cancer. In addition, this method may provide a stronger biological basis for improving the existing clinical staging system. However, although our study used a relatively large sample size with a long follow-up period, it is still inevitably subject to multiple biases similar to all other retrospective studies.
In summary, for the first time,the prognostic values of preoperative coagulation indexes in 250 CRC patients were evaluated. In the whole cohort, PT and APTT were independent prognostic factors for patients with CRC. Prognostic indicators for CRC are currently unavailable, but PT and APTT levels can be measured by a highly reproducible assay that can be easily performed in most clinical laboratories. Therefore, the combination of PT and APTT may be used as a novel prognostic grouping system for predicting the overall survival and disease-free survival of patients with colorectal cancer, and this finding may prompt the development of a novel treatment stratification system for CRC.
Acknowledgments
We thank the staff of the biochemical lab of Sun Yat-sen University Cancer Center who provided various biochemical markers and all the staff who supported our study.
Funding Statement
This paper is supported by the Natural Science Foundation of Guangdong Province, China (grant no. 2018A030310260), and the Science and Technology Planning Project of Guangdong Province of China (grant no. 2017ZC0029).
Abbreviations
PT, prothrombin time; APTT, activated partial thromboplastin time; CRC, colorectal cancer; PTA, prothrombin time activity; INR, international normalized ratio; FIB, fibrinogen; TT, thrombin time; PLR, platelet-to-lymphocyte ratio; PLT, platelet.
Data Sharing Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2020001491.
Author Contributions
LZ and JY contributed equally. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Song Y, Liu M, Yang FG, Cui LH, Lu XY, Chen C. Dietary fibre and the risk of colorectal cancer: a case- control study. Asian Pac J Cancer Prev. 2015;16(9):3747–3752. doi: 10.7314/APJCP.2015.16.9.3747 [DOI] [PubMed] [Google Scholar]
- 2.Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44(5):797–803. doi: 10.1007/s00595-013-0606-5 [DOI] [PubMed] [Google Scholar]
- 3.Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us? Platelets. 2015;26(2):114–118. doi: 10.3109/09537104.2014.894969 [DOI] [PubMed] [Google Scholar]
- 4.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715 [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24(3):484–490. doi: 10.1200/JCO.2005.03.8877 [DOI] [PubMed] [Google Scholar]
- 6.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27(29):4902–4911. doi: 10.1200/JCO.2009.22.4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi S, Sidana S, Elson P, Khorana AA, McCrae KR. Symptomatic and incidental venous thromboembolic disease are both associated with mortality in patients with prostate cancer. PLoS One. 2014;9(8):e94048. doi: 10.1371/journal.pone.0094048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg N, Kahn SR, Solymoss S. Markers of coagulation and angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol. 2003;21(22):4194–4199. doi: 10.1200/JCO.2003.05.165 [DOI] [PubMed] [Google Scholar]
- 9.Sallah S, Husain A, Sigounas V, et al. Plasma coagulation markers in patients with solid tumors and venous thromboembolic disease receiving oral anticoagulation therapy. Clin Cancer Res. 2004;10(21):7238–7243. doi: 10.1158/1078-0432.CCR-04-0445 [DOI] [PubMed] [Google Scholar]
- 10.Amirkhosravi A, Meyer T, Amaya M, et al. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin Thromb Hemost. 2007;33(7):643–652. doi: 10.1055/s-2007-991531 [DOI] [PubMed] [Google Scholar]
- 11.Langer F. [Haemostatic aspects in clinical oncology]. Hamostaseologie. 2015;35(2):152–164; quiz 165. German. doi: 10.5482/HAMO-14-11-0057 [DOI] [PubMed] [Google Scholar]
- 12.Tsihlias J, Grossman HB. The utility of fibrin/fibrinogen degradation products in superficial bladder cancer. Urol Clin North Am. 2000;27(1):39–46. doi: 10.1016/S0094-0143(05)70232-9 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147. doi: 10.1186/1471-2407-6-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aminian A, Karimian F, Mirsharifi R, et al. Significance of platelet count in esophageal carcinomas. Saudi J Gastroenterol. 2011;17(2):134–137. doi: 10.4103/1319-3767.77245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schellerer VS, Mueller-Bergh L, Merkel S, et al. The clinical value of von Willebrand factor in colorectal carcinomas. Am J Transl Res. 2011;3(5):445–453. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Huang X, Chen Y, Jin X, Li Q, Yi TN. Prognostic value of TP/PD-ECGF and thrombocytosis in gastric carcinoma. Eur J Surg Oncol. 2012;38(7):568–573. doi: 10.1016/j.ejso.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Tas F, Kilic L, Bilgin E, et al. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23(2):276–281. doi: 10.1097/IGC.0b013e31827b8796 [DOI] [PubMed] [Google Scholar]
- 18.Ustuner Z, Akay OM, Keskin M, Kus E, Bal C, Gulbas Z. Evaluating coagulation disorders in the use of bevacizumab for metastatic colorectal cancer by thrombelastography. Med Oncol. 2012;29(5):3125–3128. doi: 10.1007/s12032-012-0274-0 [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Yang JX, Cao DY, et al. Preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. Onco Targets Ther. 2013;6:211–216. doi: 10.2147/OTT.S41711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119. doi: 10.1371/journal.pone.0101119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93(6):468–474. [PubMed] [Google Scholar]
- 22.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(5):699–707. doi: 10.1373/clinchem.2015.248625 [DOI] [PubMed] [Google Scholar]
- 23.Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107(3):451–457. doi: 10.1016/j.rmed.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Haruki K, Shiba H, Saito N, et al. Risk stratification using a novel liver functional reserve score of combination prothrombin time-international normalized ratio to albumin ratio and albumin in patients with hepatocellular carcinoma. Surgery. 2018;164(3):404–410. doi: 10.1016/j.surg.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Bie F, Wang Y, Wang W, Du J. Prognostic effect of a risk index model in postoperative non-small-cell lung cancer patients. Future Oncol. 2019;15(24):2829–2840. doi: 10.2217/fon-2019-0048 [DOI] [PubMed] [Google Scholar]
- 26.Zhu M, Dai Y, Gao F, et al. Correlations of coagulation indexes and inflammatory changes with the prognosis of lung cancer complicated with thromboembolic disease. J BUON. 2019;24(2):585–590. [PubMed] [Google Scholar]
- 27.Kavanagh DO, Carter MC, Keegan D, et al. Management of colorectal cancer in patients with inflammatory bowel disease. Tech Coloproctol. 2014;18(1):23–28. doi: 10.1007/s10151-013-0981-3 [DOI] [PubMed] [Google Scholar]
- 28.Bae JM, Kim JH, Rhee YY, Cho NY, Kim TY, Kang GH. Annexin A10 expression in colorectal cancers with emphasis on the serrated neoplasia pathway. World J Gastroenterol. 2015;21(33):9749–9757. doi: 10.3748/wjg.v21.i33.9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YP, Guo PT, Zhu Z, et al. Macroscopic serosal classification of colorectal cancer and its clinical significance. Int J Clin Exp Med. 2015;8(11):20123–20134. [PMC free article] [PubMed] [Google Scholar]
- 30.Bian Z, Feng Y, Xue Y, et al. Down-regulation of SNX1 predicts poor prognosis and contributes to drug resistance in colorectal cancer. Tumour Biol. 2016;37(5):6619–6625. doi: 10.1007/s13277-015-3814-3 [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29(9):1104–1112. doi: 10.1038/modpathol.2016.95 [DOI] [PubMed] [Google Scholar]
- 32.Rink M, Chun FK, Robinson B, et al. Tissue-based molecular markers for renal cell carcinoma. Minerva Urol Nefrol. 2011;63(4):293–308. [PubMed] [Google Scholar]
- 33.Neubauer E, Wirtz RM, Kaemmerer D, et al. Comparative evaluation of three proliferation markers, Ki-67, TOP2A, and RacGAP1, in bronchopulmonary neuroendocrine neoplasms: issues and prospects. Oncotarget. 2016;7(27):41959–41973. doi: 10.18632/oncotarget.9747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Yuan L. Predictive biomarkers for targeted and cytotoxic agents in gastric cancer for personalized medicine. Biosci Trends. 2016;10(3):171–180. doi: 10.5582/bst.2016.01078 [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Yu H, Yi S, et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7(29):45370–45384. doi: 10.18632/oncotarget.9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valverde LF, de Freitas RD, Pereira TA, et al. MCM3: a novel proliferation marker in oral squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2018;26(2):120–125. doi: 10.1097/PAI.0000000000000397 [DOI] [PubMed] [Google Scholar]
- 37.Dickneite G, Seiffge D, Diehl KH, et al. Pharmacological characterization of a new 4-amidinophenyl-alanine thrombin-inhibitor (CRC 220). Thromb Res. 1995;77(4):357–368. [DOI] [PubMed] [Google Scholar]
- 38.Jia J, Zheng X, Chen Y, et al. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol. 2015;36(12):9319–9325. doi: 10.1007/s13277-015-3667-9 [DOI] [PubMed] [Google Scholar]
- 39.Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi: 10.3892/ol.2016.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Ye GY, Qin SL, et al. High expression of Rab3D predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. 2016;75:53–62. doi: 10.1016/j.biocel.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 41.Senthil M, Chaudhary P, Smith DD, et al. A shortened activated partial thromboplastin time predicts the risk of catheter-associated venous thrombosis in cancer patients. Thromb Res. 2014;134(1):165–168. doi: 10.1016/j.thromres.2014.04.022 [DOI] [PubMed] [Google Scholar]
- 42.Vavilala MS, Dunbar PJ, Rivara FP, Lam AM. Coagulopathy predicts poor outcome following head injury in children less than 16 years of age. J Neurosurg Anesthesiol. 2001;13(1):13–18. doi: 10.1097/00008506-200101000-00003 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Shimada H, Nanami T, et al. Hyperfibrinogenemia is associated with inflammatory mediators and poor prognosis in patients with gastric cancer. Surg Today. 2016;46(12):1394–1401. doi: 10.1007/s00595-016-1339-z [DOI] [PubMed] [Google Scholar]
- 44.Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inal T, Anar C, Polat G, Unsal I, Halilcolar H. The prognostic value of D-dimer in lung cancer. Clin Respir J. 2015;9(3):305–313. doi: 10.1111/crj.12144 [DOI] [PubMed] [Google Scholar]
- 46.Ghezzi F, Cromi A, Siesto G, et al. Prognostic significance of preoperative plasma fibrinogen in endometrial cancer. Gynecol Oncol. 2010;119(2):309–313. doi: 10.1016/j.ygyno.2010.07.014 [DOI] [PubMed] [Google Scholar]