Abstract
Introduction
Tuberculosis (TB) case finding strategies are recommended to increase yield for TB in key populations. Several key populations are identified in the literature, but techniques for estimating yield and prioritising interventions are needed.
Methods
We conducted a scoping review of existing evidence on TB burden to assess contribution of key populations to the TB epidemic in South Africa. Reports, articles and conference abstracts from January 2000 to December 2016 were reviewed to determine TB incidence, prevalence and size of key populations in South Africa. Meta-analysis summarised prevalence and incidence rates of TB in selected key populations assessed for heterogeneity. TB risk was calculated for each key population. Number needed to screen (NNS) to diagnose one case of TB disease was computed. Population attributable fraction estimated the potential impact of interventions on TB cases per population.
Results
The search yielded 140 citations, of which 49 were included in the review and a final 32 were included in the meta-analysis. A high prevalence of TB disease was observed in HIV-infected patients with an estimated effect size (ES=0.25, 95% CI 0.20 to 0.30). Heterogeneity was high in this population (I2=94.8%, p value=0.000). The highest incidence rate of TB disease was observed in the HIV-infected population (ES=6.07, 95% CI 4.90 to 7.51). The risk of TB disease in South Africa was high in informal settlements (RR=5.8), HIV-infected (RR=5.4) and inmates (RR=5.0). Most cases of TB would be found in inmates (NNS=26) and household contacts of patients with TB (NNS=25). A larger impact would be observed if interventions are directed towards inmates (31%), people living with HIV (PLHIV (37%) and informal settlements (43%).
Conclusions
Our findings illustrate the of value using available epidemiological evidence to inform targeted TB interventions. This review suggests that targeting interventions towards inmates, PLHIV and informal settlements would have a bigger impact on TB burden in South Africa.
Keywords: epidemiology, tuberculosis, review
Key questions.
What is already known?
Active case finding enhances tuberculosis (TB) case detection by finding, diagnosing and treating missing patients with TB.
What are the new findings?
There is limited TB research conducted in key populations such as diabetes, migrants/refugees and the elderly. This may lead to overestimation or underestimation of estimates in South Africa.
Focusing active case finding in populations such as household contacts, inmates, informal settlements, HIV-infected and migrants/refugees, as they are likely to have higher TB yields.
What do the new findings imply?
Though more research is needed to provide accurate estimates, existing epidemiological information can provide evidence that can inform TB control efforts in South Africa.
Introduction
Tuberculosis (TB) is still a major public health challenge contributing to high morbidity and mortality rates globally. Early diagnosis and prompt linkage into care are essential for successful TB programmes. It is estimated that in 2018, nearly four million cases globally were unreported or undiagnosed,1 including approximately 160 000 ‘missing’ in South Africa. Since these patients are likely to perpetuate the spread of infection,2 it is important to find and link them to quality healthcare, as emphasised by the End TB strategy.3,4 In order to focus on innovative approaches to TB control, one could use epidemiological methods to estimate the size of TB epidemics in key populations and to adopt an integrated differentiated model of TB service delivery in vulnerable populations.
South Africa’s National Strategic Plan (NSP) for HIV, TB and STIs 2017–2022 has highlighted healthcare workers (HCWs), inmates, people living with HIV (PLHIV) and in informal settlements, pregnant women, children under 5 years, household contacts for index patients with TB, diabetics and mineworkers and perimining communities as key populations for TB (box 1).5 The 90−(90)−90 targets of the Global Plan require tuberculosis programmes to increase their efforts in scaling up the coverage of TB care. This includes targeting and accessing key populations for tuberculosis control (second 90). The first step decision makers at the national level need to do is to determine the true burden of TB by understanding the local epidemiology, size estimation and contribution of key populations to the TB epidemic in South Africa. This information will support mobilising resources while taking into account the unique implementation challenges for each population. This review therefore uses the existing epidemiological information and readily available epidemiological tools to estimate the yield of TB and the potential impact of interventions on TB cases in each key population in South Africa.
Box 1. Key populations outlined in the National Strategic Plan 2017–2022.
Key populations at risk of exposure to or transmitting tuberculosis (TB) disease
Children.
People living with HIV.
Diabetics.
Smokers.
Alcohol and substance users.
People who are malnourished or have silicosis.
Mobile, migrant and refugee populations.
People living and working in poorly ventilated environments, including informal settlements.
Key populations at risk of TB infection and reinfection
Healthcare workers.
Mineworkers.
Inmates.
Correctional officers.
Household contacts of patients with confirmed TB.
Methods
We chose to perform a scoping review to answer our research question, given the broad nature of the topic, and to identify the nuances in the different key populations and gaps in the literature. The methods used in this review are in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR).6 The PRISMA-ScR checklist was completed as the protocol for literature reviews.
Search strategy
Two independent researchers (LAC and NM) conducted a review of peer-reviewed literature published between January 2000 and December 2016 from PubMed. The references of included articles were scanned to identify additional articles of interest. Grey literature from websites of national departments (ie, Health, Chamber of Mines, Correctional Services and Statistics South Africa) and international organisations (ie, WHO, United Nations High Commission for Refugees, Global Diabetes Scorecard and Stop TB partnership) were also searched using Google and Google Scholar. A comprehensive search strategy using the following Medical Subject Headings terms (table 1) was adopted. For each key population, we performed a separate search using the three-keyword combinations. A Boolean operator ‘AND’ was used to refine the search by excluding articles outside South Africa. No authors were contacted and no additional ongoing research was included.
Table 1.
Detailed search strategy conducted with PubMed and Google Scholar
| ID number | Search |
| 1 | MeSH descriptor (tuberculosis) |
| 2 | MeSH descriptor (South Africa) |
| 3 | 1 and 2 |
| 4 | “Healthcare workers” or “Nurses” or “Doctors” or “Mineworkers” or “Miners” or “Inmates” or “prisoners” or “prisons” or “correctional facilities” or “Informal settlements” or “townships” or “peri-urban” or “resource-limited settings” or “tuberculosis contacts” or “Household tuberculosis contacts” or “HIV-infected” or “HIV-positive “or “Diabetics” or “diabetes” or “Paediatric” or “children” or “Pregnant women” or “antenatal” |
| 5 | 3 and 4 |
MeSH, Medical Subject Headings.
Study selection
A three-pronged approach was used to analyse the search results. First, articles were included or excluded based on their title. Second, abstracts were reviewed and articles were either selected or dropped. Lastly, full-text versions were obtained for the remaining articles. LAC and NM scanned all articles by title, abstract and full-text independently. Where there was no agreement on the study selection or methodology, discussions were held to resolve so as not to exclude any article based on strict scientific criteria. We included English language articles that reported on TB prevalence and/or incidence rates and/or case notifications. The studies had to use direct measurement of prevalence or incident rates. Articles that did not meet the aforementioned criteria were excluded. We included observational studies with cross-sectional and cohort designs and experimental studies with randomised trial designs. Duplicates and articles published outside the defined review period were excluded. The selected studies and their characteristics are summarised in table 2.
Table 2.
Characteristics of included articles
| Risk group | Type of study and setting | Author and year (study period) |
Sample size | Diagnosis method | Prevalence per 100 000 | Incidence per 100 000 | Estimate from meta-analysis per 100 | Study included in review and reason |
| HCWs | Cross-sectional on community health workers in Western Cape | Kranzer et al, 2010 (2008–2009) |
215 | Smear and culture | 5000 | – | Prevalence: 0.01 Incidence: 0.88 |
Prevalence: 1400 Grobler et al systematic review.* Incidence: 1470 from Grobler et al systematic review.* |
| Cross-sectional among medical doctors in KZN | Naidoo et al, 2013 (2007–2009) |
40 | Sputum microscopy, culture and chest X-ray | 8000 | – | |||
| Retrospective record review in eight public sector hospitals, KZN | Naidoo and Jinabhai, 2006 (1999–2004) | 583 | Smear and culture | 7620 | – | |||
| Retrospective cohort in three district hospitals, KZN | Tudor et al, 2014 (2006–2010) |
1427 | Not specified | 900 | 1958 | |||
| Review of 31 studies conducted in SA (EVISAT) | Nicol et al, 2014 (1966–2013) |
Not specified | Sputum microscopy, culture | 1400 | 1958 in KZN 900 in WC |
|||
| Systematic review of the epidemiology of TB in HCWs, South Africa | Grobler et al, 2016 (1966–2013) |
3677 | Smears, cultures, microscopies, chest X-ray | 1400 | 1470 | |||
| Cross sectional study of TB among HCWs in a hospital in the Western Cape | Ayuk, 2013 (2008–2011) |
249 | Not specified | – | 397 | |||
| Mineworkersrs | Cluster randomised study in three gold mining companies in SA | Churchyard et al, 2014 (not specified) |
27 126 | Sputum microscopy, culture and chest X-ray | – | 2230 | Prevalence: 0.03 Incidence: 1.81 |
Prevalence: 1300 from EVISAT systematic review* Incidence: 3000 from the EVISAT systematic review* |
| Review of 61 studies conducted in SA (EVISAT) | Machingaidze et al, 2014 (1966–2013) |
Not specified | Sputum microscopy, culture | 1300 | 3000 | |||
| Prospective cohort study among SA gold miners | Hermans et al, 2016 (2006–2010) |
18 520 | Symptoms screening and chest X-ray | – | 1900 | |||
| Cross-sectional survey of a community-wide nurse delivered IPT intervention and TB screening | Lewis et al, 2013 | 27 126 | Smear and culture | 2600 | ||||
| Retrospective cohort at four gold mines in Gauteng province using data from routine sources | Sonnenberg et al, 2005 | 23 874 | Sputum smear | – | 1390 | |||
| Inmates | Review of 18 studies conducted in SA (EVISAT) | Mukinda and Mahomed, 2014 | Not specified | Various | 3900 | – | Prevalence: 0.04 Incidence: Not available |
Prevalence: 3900 from the EVISAT systematic review* Incidence: Since no incidence has been reported, the prevalence of 3900/100 000 was chosen† |
| Retrospective case-control at Mangaung correctional facility | Nyasulu et al, 2015 (2009–2010) |
1140 | Sputum microscopy | 8800 | – | |||
| Cross-sectional survey of inmates In the largest facility in Johannesburg | Telisinghe et al, 2014 (1966–2013) | 981 | Sputum microscopy and liquid culture | 7500 | – | |||
| Programme evaluation within four facilities in Gauteng | Zishiri et al, 2014 (2013) |
7426 | GeneXpert | 2700 | – | |||
| Cross-sectional survey of inmates In the largest facility in Johannesburg | Hanifa et al, 2015 (2009–2010) |
981 | Sputum microscopy and urine LAM | 3000 | – | |||
| Informal settlements | Cross-sectional survey of high TB and HIV burden community in the Western Cape | Wood et al, 2007 (2002–2004) |
762 | Sputum smear and culture | 3300 | – | Prevalence: 0.09 Incidence: 7.44 |
Prevalence: 3150 from systematic review* Incidence: 4500 from systematic review* |
| Review of 66 studies conducted in SA (EVISAT) | Claassens et al, 2014 (1966–2013) | 12 777 | Various | 3150 | 4500 | |||
| Cross-sectional study at a mobile clinic offering HIV testing in Cape Town | Kranzer et al, 2012 (2009–2011) |
6394 | Sputum smear and culture | 4960 | – | |||
| Routine chest screening in an ART service in a township in Cape Town | Dawson et al, 2010 | 235 | X-ray and sputum culture | 2468 | – | |||
| Cohort study of prospectively collected data on patients receiving ART in Cape Town | Gupta et al, 2012 (2002–2006) |
1544 | Symptom screening and sputum smear and culture | – | 7440 | |||
| Household Contacts | Retrospective programme analysis of TB household contact tracing programme in North West Province | Thind et al, 2012 (2009–2010) |
552 | Sputum smear and culture | 3100 | – | Prevalence: 0.04 Incidence: 1.33 |
Prevalence: 4000 from the meta-analysis‡ Incidence: 1300 from van Schalkwyk et al† |
| TB/HIV contact tracing study in North West Province | Shapiro et al, 2012 (2009) |
3033 | Sputum smear and culture | 6075 | – | |||
| A prospective cohort study on TB/HIV contact tracing study in North West Province | van Schalkwyk et al, 2014 (2009–2010) | 2377 | Sputum smear and culture | 9200 | 1300 | |||
| Pragmatic randomised trial in Matlosana District, North West province | Lebina et al, 2016 (2011–2012) |
1017 | Culture, microscopy and Gene Xpert | 5408 | – | |||
| Prospective study of index patients and household contacts in Johannesburg | Deery et al, 2014 (2012) |
1197 | Sputum microscopy | 749 | – | |||
| Children under 5 years | Prospective representative hospital surveillance data in Western Cape Province | Hesseling et al, 2009 (2004–2006) | 294 712 | Culture | – | 700 | Prevalence: 0.10 Incidence: 1.51 |
Prevalence: 664 from EVISAT systematic review* Incidence: 386 from EVISAT systematic review* |
| Review of 190 studies conducted in SA (EVISAT) | Garcia-Prats et al, 2014 (1947–2013) | Not specified | Various | 664 | 386 | |||
| Retrospective audit of neonates routinely screened for TB in a hospital setting in Cape Town | Bekker et al, 2012 (2009–2011) |
70 | Isolation of TB from gastric aspirate | 11 400 | – | |||
| Randomised control trial on children infected with HIV at two tertiary hospitals in Cape Town | Zar et al, 2007 (not specified) |
277 | Chest X-ray and culture | – | 1200 | |||
| Cohort analysis in children infected with HIV in Cape Town | Frigati et al, 2011 (2003–2007) |
298 | Chest X-ray and culture | 13 700 | 1200 | |||
| Cross-sectional study of children who are contacts of adult patients with MDR TB | Seddon et al, 2013 (2007–2009) |
228 | Chest X-ray and culture | 6578 | – | |||
| Elderly | Retrospective cohort study of patients newly diagnosed with TB in Soweto | Karstaedt and Bolhaar, 2014 (2003–2004) |
42 004 | Microbiological confirmation | – | 262 | Prevalence: Not available Incidence: 0.26 |
Case notification used as proxy for incidence (262)† |
| Migrants and refugees | Record review at an FBO in Johannesburg | McCarthy et al, 2009 (2004–2007) |
1297 | Not specified | 36 000 | 3300 | Prevalence: 0.36 Incidence: 3.29 |
Prevalence: 36 000 from McCarthy et al† Incidence: 3300 from McCarthy et al† |
| Women | Cross-sectional study at a primary healthcare facility in Cape Town | Oni et al, 2015 (2012–2013) |
10 234 | Not specified | 230 | – | Prevalence: 0.02 Incidence: Not available |
Prevalence: 230 from cross-sectional study† |
| HIV-infected | Prospective cohort of HIV positive HAART patients, KZN | Naidoo et al, 2014 (2007–2010) |
969 | Smear and culture | 17 850 | 4500 | Prevalence: 0.25 Incidence: 6.07 |
Prevalence: 3000 from EVISAT systematic review* Incidence: 4200 from EVISAT systematic review* |
| Review of 288 studies conducted in SA (EVISAT) | Wiysonge et al, 2014 (1947–2013) | Not specified | Not specified | 3000 | 4200 | |||
| Prospective cohort of patients referred to a clinic for ART initiation | Hanifa et al, 2012 (2007–2008) |
381 | Sputum smear and culture | 177 00 | – | |||
| Two clinical cohorts of HIV-positive patients | Golub et al, 2009 (2003–2007) |
2778 | Culture | – | 6228 | |||
| Observational community-based ART cohort in South Africa | Lawn et al, 2009 (2002–2006) |
770 | Sputum smear and culture | – | 7300 | |||
| Diabetes | Cross-sectional study on adults attending a diabetes clinic, Soweto | Majumder et al, 2016 (2014–2015) | 672 | Sputum smear | 1 240 | – | Prevalence: 0.01 Incidence: Not Available |
Prevalence: 1240 from Majumder et al* |
| Pregnant women | Prospective HIV-infected pregnant women in North West | Hoffman et al, 2013 (2010–2011) |
1451 | Sputum culture | 3 300 | – | Prevalence: 0.02 Incidence: 1.08 |
Prevalence: 2000 from the meta-analysis† Incidence: 1080 from Odayar et al† |
| A record review of pregnant women attending ANC in Northern Cape | Peters et al, 2015 (2011–2012) |
308 | Sputum culture | 13 600 | – | |||
| Cross-sectional integrating active TB case finding with ANC services in Soweto | Gounder et al, 2011 (2008–2009) |
3963 | Sputum smear and Xpert | 688 | – | |||
| Cohort of women visiting ANC in Cape Town | Odayar et al, 2016 (not specified) |
1507 | Symptom screening and bacteriologically | – | 1080 | |||
| Prospective cohort of pregnant and postpartum women routinely on anti-TB treatment in Cape Town | Bekker et al, 2016 (2011) |
8471 | Symptom screening and bacteriologically | 874 | – | |||
| Smokers | Prospective cohort of the Lung Health Survey from the electronic TB register, Cape Town | Ncayiyana 2010 (2003–2007) |
3971 | Smear and culture | – | 871 | Prevalence: Not Available Incidence: 0.87 |
Incidence: 817 from prospective Lung Health Survey† |
| Chronic alcohol users | Prospective cohort of the Lung Health Survey from the electronic TB register, Cape Town | Ncayiyana 2010 (2003–2007) |
3971 | Smear and culture | – | 949 | Prevalence: Not available Incidence: 0.95 |
Incidence: 949 from prospective Lung Health Survey† |
*Systematic reviews were considered representative.
†Only a single study was available to provide both estimates, and the available estimate was used as a proxy for the missing estimate.
‡Estimates derived after a meta-analysis of varying estimates from several studies.
ANC, antenatal clinic; ART, antiretroviral therapy; EVISAT, Evidence to Inform South African Tuberculosis Policies; FBO, faith-based organisation; HAART, highly active antiretroviral therapy; HAART, highly active antiretroviral therapy; HCW, healthcare worker; KZN, Kwa Zulu Natal; LAM, lipoarabinomannan; MDR, multidrug resistant; SA, South Africa; TB, tuberculosis.
Data collection
For every study, information on key characteristics was collected, which included the key population, study design, sample size, tuberculosis disease diagnosis, number of cases, follow-up period, study duration, prevalence and incidence rate.
Definition of key populations
Informal settlements were defined as either areas recently urbanised, overcrowded (plot size 250–800 m2) with informal dwellings for a population of low socioeconomic status,7 or townships, periurban or resource-limited settings.8–10 HCWs were individuals employed in healthcare facilities or providing healthcare in communities.11 12 Tuberculosis contacts lived in the same household as an index patient with tuberculosis.13 14 The estimated size of household tuberculosis contacts (1.6 million) was determined by the number of tuberculosis cases reported in 2016 (437 000)15 multiplied by the average household size (3.6)16 reported in the 2011 census. Chronic alcohol users’ consumption of alcohol resulted in physical or psychological adverse events.17 18 Mineworkers were those working in mining sites. Definition of the three categories of key populations was adopted from the Stop TB Partnership 2015 report.4
Selecting prevalence and incidence estimates
The overall prevalence and incidence estimates were derived from the WHO Global Tuberculosis Reports 201515 and 2018,19 respectively. For most of the key populations, previous estimates derived from the Evidence to Inform South African Tuberculosis Policies (EVISAT) conducted in 2014 on healthcare workers, inmates, mineworkers, children under 5 years and PLHIV were used, as they were considered most representative. The EVISAT reports were individual systematic reviews that contained 10–30 studies for each population. Of the remaining eight key populations discussed in this paper, estimates for diabetics, smokers, chronic alcohol users, migrants/refugees, women and the elderly were derived from a single article. The articles reported either an incidence or a prevalence rate and a case notification rate for the elderlies. However, prevalence and incidence rates for migrants/refugees were reported from a single paper. Prevalence estimates for last two populations, household tuberculosis contacts and pregnant women, were derived from a meta-analysis involving five and four studies, respectively. The incidence rates were derived from one article per key population.
The relative risk (RR) was extrapolated by dividing the incidence in each population by that of the general population. The number of cases in each population were calculated by multiplying the absolute population by the tuberculosis incidence per 100 000. The number needed to screen (NNS) was computed by inversing the tuberculosis prevalence in each population. The population attributable fraction (PAF), a function of RR and prevalence, was calculated by estimating the proportion of tuberculosis cases in the total population that would be averted if exposure to risk (key population) would be reduced using the formula , where p is the prevalence and RR is the relative risk of tuberculosis in a particular key population. All formulae used are summarised in online supplementary 1. Negative PAF values were not reported.
bmjgh-2020-002355supp001.pdf (108.6KB, pdf)
Quality assessment for observational studies
A modified Newcastle-Ottawa Scale for non-randomised studies was used in assessing studies quality.20 This tool assessed four study characteristics: study design, sample size, tuberculosis diagnosis method and duration of study. Each of these criteria was assigned a score of 1–4. The highest possible score was 16, and studies with scores above 9 were considered good quality. No studies were excluded from analysis based on their quality scores (online supplementary 2).
Quality assessment for randomised controlled trial studies
The Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist with 37 items was applied to evaluate the quality of the randomised controlled studies. A score of 1 was given if details required by the CONSORT were reported, and a score of 0 was reported where no item was reported. Compliance with the CONSORT was defined as the percentage of the items which was fulfilled by the paper, calculated by dividing the sum of the scores by 37. Papers that reported with randomisation, allocation concealment and blinding were considered high quality. No studies were excluded from analysis (online supplementary 3).
Meta-analysis
Meta-analyses were conducted to calculate an estimate of tuberculosis prevalence and incidence rate in selected key populations. For inclusion, studies reported on the number of tuberculosis cases, population size, incidence, prevalence and number of person years. Studies with survey, cross-sectional or evaluation designs contributed to prevalence, whereas cohort or trial designs provided incidence estimates. The associated SEs for incidence rates using the random effects model were calculated by estimating the inverse of the square root of the reported tuberculosis cases. These estimates were entered into Microsoft Excel V.2010, exported into STATA V.14 for analysis by using metaprop and metan commands for calculating pooled estimates for prevalence and incidence rates respectively. I2 estimates determined heterogeneity between studies in each population where >50% showed higher heterogeneity. Forest plots by the key population were drawn for tuberculosis prevalence and incidence rates.
Patient and public involvement
There has been no patient or public involvement during this review.
Results
Database searches yielded 120 citations and 20 reports (figure 1). A full-text review of 109 studies was conducted. Duplicate studies (n=13), those outside defined study period (n=9), not accessible (n=9), short communications (n=11), studies on tuberculosis infection (n=15) and tuberculosis resistance (n=19) were excluded. Overall, 51 studies were considered eligible for review, and from these, 32 (63 %) of acceptable quality (score ≥10) (see online supplementary 2 and 3) were selected for meta-analysis.
Figure 1.
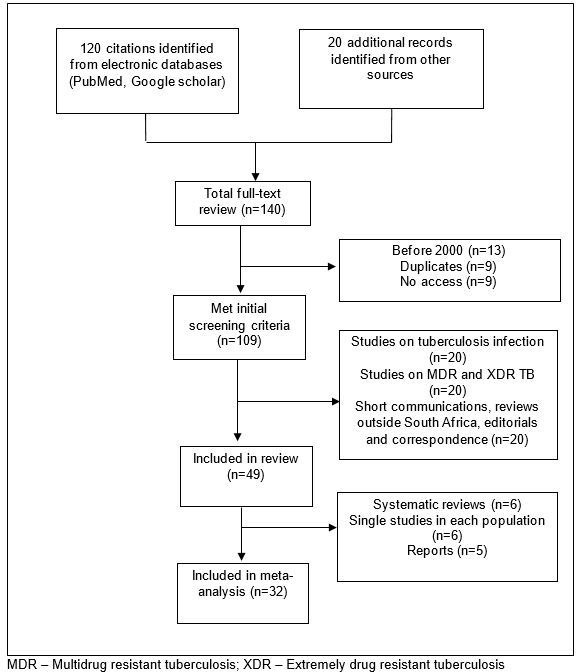
Flow diagram of article inclusion, 2000–2016. MDR, multidrug resistant; XDR, extremely drug resistant.
Study characteristics
The characteristics of the studies included in the review are summarised in table 2. The studies were published between 2000 and 2016. Observational study designs were commonly used to report prevalence and incidence rates, and these included cross-sectional (n=15), prospective and retrospective cohorts (n=16), and programmatic analysis (n=5). We also included randomised control trials (n=5) and systematic reviews (n=8).
Estimated population size
Several sources provided the estimated key population sizes as summarised in online supplementary 4. Women (24.6 million),21 those living in informal settlements (3.3 million)22 and smokers (9.5 million) made the largest population across the three categories.
Tuberculosis estimates for key populations
Approximately 281 000 tuberculosis cases were reported in South Africa, resulting in an estimated incidence of 520/100 000.15 The estimated tuberculosis prevalence was 696/100 000.23 Table 2 provides the different prevalence and incidence estimates and the rationale for selecting the final estimate. Prevalence and incidence estimates for mineworkers, inmates, people living in informal settlements, children under 5 years and PLHIV were derived from separate EVISAT systematic reviews. Estimates for migrants/refugees, smokers and chronic alcohol were derived from cohort studies. Two meta-analyses of several cohort studies provided prevalence and incidence estimates for household contacts and pregnant women. Prevalence estimates for diabetics and the elderly were derived from single cross-sectional studies.
Heterogeneity of tuberculosis prevalence and incidence in key populations
Heterogeneity was high in studies reporting on inmates, household TB contacts, HIV-infected and pregnant women (I2>90%, p=0.000) included in the meta-analysis to estimate tuberculosis prevalence (online supplementary 5). Studies on healthcare workers, informal settlements and children under 5 years were reportedly homogenous (I2=0%). Ten studies reporting on incidence rates were assessed for heterogeneity within the respective key populations. Heterogeneity within each key population was high (I2 >50%) in studies investigating tuberculosis incidence rate in healthcare workers (I2=99%, p=0.000), mineworkers (I2=98%, p=0.000) and HIV (I2=81%, p=0.006). There was less heterogeneity in studies within children under 5 years (I2=29%, p=0.236) (online supplementary 6).
bmjgh-2020-002355supp002.pdf (227.6KB, pdf)
bmjgh-2020-002355supp003.pdf (1MB, pdf)
Risk of tuberculosis in each key population
The risk of tuberculosis disease was highest in informal settlements (RR=5.8), HIV-infected (RR=5.4), inmate (RR=5.0) and refugee/migrant populations (RR=4.2).
Yield from tuberculosis screening
Populations with the lowest NNS and likely to produce a high yield from active tuberculosis screening were migrants/refugees (NNS=3), tuberculosis household contacts (NNS=25), inmates (NNS=26) and persons living in informal settlements (NNS=32) (table 3). Lowest yield of tuberculosis was observed in women (NNS=435), elderlies (NNS=382), children under 5 years (NNS=151) and smokers (NNS=115) (table 3).
Table 3.
Tuberculosis estimates for risk groups per 100 000, NNS, contribution to tuberculosis epidemic and PAF
| Risk group | Key population | Tuberculosis prevalence | Relative risk | NNS | Tuberculosis incidence | Estimated cases reported in 2016 | PAF | Studies reviewed (n) |
| People with increased risk of tuberculosis bacilli infection due to occupational or community exposure | Healthcare workers | 1400 | 1.9 | 71 | 1470 | 3397 | 3% | 7 |
| Mineworkers | 1300 | 3.8 | 77 | 3000 | 15 300 | 17% | 5 | |
| Inmates | 3900 | 5.0 | 26 | 3900 | 6318 | 31% | 5 | |
| Informal settlements | 3150 | 5.8 | 32 | 4500 | 148 500 | 43% | 5 | |
| Household tuberculosis contacts | 4000 | 1.7 | 25 | 1300 | 20 468 | 1.7% | 5 | |
| People with limited access to tuberculosis services | Children under 5 years | 664 | 0.5 | 151 | 386 | 22 002 | – | 6 |
| Elderly | 262 | 0.3 | 382 | 262 | 7860 | – | 1 | |
| Migrants and refugees | 36 000 | 4.2 | 3 | 3300 | 48 114 | 21% | 1 | |
| Women | 230 | 0.3 | 435 | 230 | 63 563 | – | 1 | |
| People at increased risk of tuberculosis due to biological or behavioural factors that compromise immune function | HIV-infected | 3000 | 5.4 | 33 | 4200 | 231 420 | 37% | 5 |
| Diabetics | 1240 | 1.6 | 81 | 1240 | 28 520 | 1.5% | 1 | |
| Pregnant women | 2000 | 1.4 | 50 | 1080 | 12 960 | 0.8% | 5 | |
| Smokers | 871 | 1.3 | 115 | 871 | 83 616 | 0.2% | 1 | |
| Chronic alcohol users | 94913 | 1.4 | 105 | 949 | 78 767 | 0.4% | 1 |
NNS, number needed to screen; PAF, population attributable fraction.
Contribution to the tuberculosis epidemic of South Africa
From approximately 291 000 tuberculosis cases reported in 2018, most contribution was from PLHIV (n=231 420) and informal settlements (n=148 500). The populations with a smaller contribution to the TB epidemic included healthcare workers (n=3397), inmates (6318) and the elderly (n=7860). The largest and smallest reduction in TB cases would occur in informal settlements (PAF=43%) and smokers (PAF=0.2%), respectively, if TB control interventions were put in place.
Discussion
Estimation of tuberculosis prevalence and incidence should include a measure of how much of its burden is attributed to key populations. Tuberculosis interventions including active case finding, passive case finding and improved access to primary healthcare facilities need to target populations where a large impact may be expected. From this review, the largest contribution is attributable to PLHIVs and those in informal settlements. If TB control efforts are intensified, it is possible to reduce TB cases in informal settlements and PLHIV by 43% and 37%, respectively. HIV is a well-documented risk factor for tuberculosis, with identified prevention and care strategies, including intensive case finding and treatment. Results from meta-analysis showed high tuberculosis prevalence in HIV-infected, children under 5 years, informal settlements and household contacts. Tuberculosis control activities in informal settlements are taking traction in South Africa, and our findings encourage enhancing active tuberculosis screening in this key population.
The review methods provide stakeholders with additional considerations needed to operationalise programmes for key populations. Extrapolated estimates, such as NNS and PAF, are useful in estimating the yield and the potential impact of interventions in key populations. These estimates can provide evidence necessary to inform tuberculosis case finding strategies and other interventions. The NNS identifies the populations most likely to generate higher yields from targeted screening activities, which informs priority setting and resource allocation while accounting for cost estimates.
This review provides estimates for cost-effectiveness of a screening intervention through identification of suitable diagnostic methods and feasibility of screening programmes in resource-strained settings. Findings from this research show that higher yields of tuberculosis disease in South Africa can be realised by screening in informal settlements, correctional facilities and in HIV-infected patients. This review suggests that informal settlements contribute largely to the tuberculosis epidemic in South Africa. As such, a targeted screening programme would yield significant tuberculosis cases. This finding is supported by similar findings in Brazil, where a transmission model of tuberculosis in Rio de Janeiro showed a high incidence in an area contributing to 36% of all reported tuberculosis cases.24 In Nigeria, a high tuberculosis yield was observed (NNS=16) after screening about 16 000 urban slum dwellers.25 In South Africa, there was a high likelihood of tuberculosis transmission in economically depressed areas where intimate and close contact occurred during social gatherings.26 These findings reveal the need to direct tuberculosis control efforts towards informal settlements as overcrowding and lower socioeconomic status are probably at play to explain high tuberculosis transmission.26 27
Another consideration is feasibility of implementing active case finding in accessible populations. Inmates may be relatively accessible compared with HCWs and mineworkers who are widely dispersed in small numbers across the country. Access to HCWs may be impeded by issues of confidentiality in the workplace.28 There may be additional considerations when investing in HCWs as they provide health services and are crucial to epidemic control. One study showed 60% of unreported tuberculosis cases among HCWs originated from unimplemented tuberculosis screening programmes.11 Mineworkers, employed in large numbers in specific locations, are easy to access, although issues regarding stigma, confidentiality and migration need consideration. For inmate populations, additional infection control strategies are necessary due to very crowded living conditions and reduced ventilation. Although not included in the South Africa NSP 2017–2022, accessing refugees/migrants for tuberculosis activities is essential. Access to this population may be complicated by legal issues, language barrier and lack of awareness of human rights.4 Some populations may prove less complicated to target, especially PLHIV accessing HIV care. The choice of screening tool in this population should be carefully considered as yield from symptom screening is generally overestimated and low sensitivity of GeneXpert MTB/RIF has been reported.29 Access to informal settlements may be difficult due to failure to locate addresses.13 Considerations for other populations include diagnosis difficulties (children and elderly), access to households (contacts) and absence of integrated services (diabetics).
Our review was not without limitations. First, there was scarcity of information on tuberculosis risk for all populations, explaining the high level of heterogeneity in estimates observed in studies across the populations. In addition, some populations had limited and outdated evidence limiting their generalisability outside of specific study settings. Information regarding diabetics, smokers, women, chronic alcohol users, the elderly and refugees/migrants was extracted from single studies, which may affect the external validity of the studies; interpretation should be done with caution. Data on pregnant women, mineworkers and migrant/refugees populations were limited to PLHIV and thus overestimated their contribution to tuberculosis burden. Our review therefore provides evidence that there are new gaps in the available literature, and these findings present the need for further research in these key population groups. Most studies in informal settlements were conducted in Western Cape and Gauteng provinces, reflecting a limited geographical representation. Despite these limitations, we have attempted to address the contribution of key populations to the tuberculosis epidemic in South Africa as comprehensively as possible. Where possible, we included both cross-sectional (prevalence) and longitudinal studies (incidence) on the same population to negate the obscurity that one summary statistic may have introduced. We believe that this review can serve as a vital tool for governmental national tuberculosis programmes and other relevant stakeholders active in tuberculosis control in assessing the expected yield of available case finding strategies given the screening and diagnostic capabilities available in South Africa.
Lastly, although not included in the South Africa NSP 2017–2022, findings suggest that populations such as refugees/migrants, the elderly, smokers, chronic alcohol users contribute to the overall tuberculosis epidemic. However, more studies in these populations are required to provide accurate estimates.
Conclusions
Using epidemiological evidence to estimate the yield and burden of tuberculosis in specific populations allows policy makers to focus the available case finding strategies where they are more likely to have an impact. Targeted interventions to informal settlements, in addition to PLHIV, mineworkers and inmates, are suggested as these populations are likely to have higher tuberculosis yields.
Acknowledgments
We thank Dr Evans Muchiri for providing guidance on the meta-analysis of study populations.
Footnotes
Handling editor: Seye Abimbola
Contributors: SC, NM and PS contributed to the study conceptualisation. LAC and NM conducted data collection. LAC, NM, SC and PS contributed to data analysis/interpretation and creation of the first draft of the manuscript. LAC, SC, GJC and CL contributed to interpretation and revisions of the manuscript. All authors gave the final approval of the version to be published.
Funding: The authors gratefully acknowledge the WHO for supporting this research through (award number 2017/703107-0).
Disclaimer: The Global Tuberculosis Programme of the World Health Organisation, Geneva, supported this review. The content is the sole responsibility of the authors and neither represents the view of The Aurum Institute nor Global Tuberculosis Programme of the WHO, Geneva.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request. All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information. Additional data are available on request.
References
- 1.World Health Organisation Global tuberculosis report 2018. Geneva, Switzerland: WHO, 2018. http://www.who.int/tb/publications/global_report/gtbr2018_main_text.pdf [Google Scholar]
- 2.Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol 2016;5:374–8. 10.1016/j.ijmyco.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Implementing the end TB strategy: the essentials. Geneva, Switzerland: WHO, 2015. http://www.who.int/tb/publications/2015/end_tb_essential.pdf?ua=1 [Google Scholar]
- 4.Stop TB Partnership The paradigm shift 2016-2020, 2011. Available: http://www.stoptb.org/assets/documents/global/plan/GlobalPlanToEndTB_TheParadigmShift_2016-2020_StopTBPartnership.pdf [Accessed 23 Nov 2017].
- 5.National Department of Health South Africa's national strategic plan for HIV, TB and STIs 2017-2022. Pretoria, South Africa: SANAC, 2017. http://sanac.org.za/wp-content/uploads/2017/05/NSP_FullDocument_FINAL.pdf [Google Scholar]
- 6.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 7.Wood R, Racow K, Bekker L-G, et al. Indoor social networks in a South African township: potential contribution of location to tuberculosis transmission. PLoS One 2012;7:e39246. 10.1371/journal.pone.0039246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kompala T, Shenoi SV, Friedland G. Transmission of tuberculosis in resource-limited settings. Curr HIV/AIDS Rep 2013;10:264–72. 10.1007/s11904-013-0164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middelkoop K, Bekker L-G, Morrow C, et al. Childhood tuberculosis infection and disease: a spatial and temporal transmission analysis in a South African township. S Afr Med J 2009;99:738–43. [PMC free article] [PubMed] [Google Scholar]
- 10.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010;14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Claassens MM, van Schalkwyk C, du Toit E, et al. Tuberculosis in healthcare workers and infection control measures at primary healthcare facilities in South Africa. PLoS One 2013;8:e76272. 10.1371/journal.pone.0076272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tudor C, Van der Walt M, Margot B, et al. Tuberculosis among health care workers in KwaZulu-Natal, South Africa: a retrospective cohort analysis. BMC Public Health 2014;14:891. 10.1186/1471-2458-14-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deery CB, Hanrahan CF, Selibas K, et al. A home tracing program for contacts of people with tuberculosis or HIV and patients lost to care. Int J Tuberc Lung Dis 2014;18:534–40. 10.5588/ijtld.13.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Schalkwyk C, Variava E, Shapiro AE, et al. Incidence of TB and HIV in prospectively followed household contacts of TB index patients in South Africa. PLoS One 2014;9:e95372. 10.1371/journal.pone.0095372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation Global tuberculosis report 2016. Geneva, Switzerland: World Health Organisation, 2016. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf [Google Scholar]
- 16.Statistics South Africa Census 2011, 2012. Available: https://www.statssa.gov.za/publications/P0302/P03022015.pdf [Accessed 04 Mar 2017].
- 17.Peltzer K. Conjoint alcohol and tobacco use among tuberculosis patients in public primary healthcare in South Africa. S Afr J Psych 2014;20:6. 10.4102/sajpsychiatry.v20i1.482 [DOI] [Google Scholar]
- 18.Peltzer K, Naidoo P, Louw J, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health 2013;13:699. 10.1186/1471-2458-13-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organisation WH Global tuberculosis report 2018, 2019. Available: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
- 20.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis 2011. Available: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf [Accessed 28 Apr 2018].
- 21.Statistics South Africa Mid year estimates 2015. Pretoria: Stats SA, 2015. https://www.statssa.gov.za/publications/P0302/P03022015.pdf [Google Scholar]
- 22.Housing Development Agency South Africa: informal settlements status (2013. Killarney, Johannesburg: HDA, 2013. http://www.thehda.co.za/uploads/files/HDA_South_Africa_Report_lr.pdf [Google Scholar]
- 23.World Health Organisation Tuberculosis fact sheet, 2015. Available: http://www.who.int/mediacentre/factsheets/fs104/en/ [Accessed 28 Oct 2015].
- 24.Dowdy DW, Golub JE, Chaisson RE, et al. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A 2012;109:9557–62. 10.1073/pnas.1203517109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogbudebe CL, Chukwu JN, Nwafor CC, et al. Reaching the underserved: active tuberculosis case finding in urban slums in southeastern Nigeria. Int J Mycobacteriol 2015;4:18–24. 10.1016/j.ijmyco.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Classen CN, Warren R, Richardson M, et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax 1999;54:136–40. 10.1136/thx.54.2.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopaul A, Kistnasamy EJ, Reddy P. Predictors of tuberculosis treatment defaulting in informal dwellers within the eThekwini Municipality, KwaZulu-Natal. South Afr J Infect Dis 2014;29:27–32. [Google Scholar]
- 28.Ayuk JN. A cross-sectional study of tuberculosis among workers in Tygerberg academic Hospital, Western Cape Province, South Africa. Stellenbosch: Stellenbosch University, 2013. [Google Scholar]
- 29.LaCourse SM, Cranmer LM, Matemo D, et al. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr 2016;71:219–27. 10.1097/QAI.0000000000000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-002355supp001.pdf (108.6KB, pdf)
bmjgh-2020-002355supp002.pdf (227.6KB, pdf)
bmjgh-2020-002355supp003.pdf (1MB, pdf)


