Abstract
Background and Purpose
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and abnormalities in insulin production. Apelin is associated with insulin resistance. According to the anti-diabetic properties of curcumin, the purpose of this study was to compare the effects of curcumin and nano-curcumin intake on insulin resistance and serum levels of fasting blood sugar (FBS), Apelin, and lipid profile (cholesterol, triglyceride, LDL, HDL and VLDL) in T2DM rats.
Materials and Methods
Forty-eight male Wistar rats were divided into six groups: Control, diabetic, diabetic treated with two doses of curcumin (100 and 200 mg/kg) and diabetic treated with two doses of nano-curcumin (100 and 200 mg/kg). Induction of T2DM was performed by intraperitoneal injection of Nicotinamide (110 mg/kg) and Streptozotocin (45 mg/kg) in the fasting state. Rats received different doses of nano-curcumin and curcumin by gavage (daily) for 28 days. At the end of the intervention period, insulin resistance and serum levels of FBS, apelin and lipid profiles were measured.
Results
Insulin resistance and serum levels of FBS, Apelin, cholesterol, triglycerides, LDL, and VLDL were significantly decreased in diabetic rats treated with curcumin and nano-curcumin (p<0.05) so that nano-curcumin in reducing lipid profile is more effective than curcumin (P<0.05). Serum level of HDL in nano-curcumin groups was significantly higher than diabetic and curcumin groups (p<0.05). Also, with increasing insulin resistance, serum level of apelin increased (P<0.05).
Conclusion
The therapeutic effects of curcumin and nano-curcumin were effective in decreasing insulin resistance, serum levels of FBS, apelin and lipid profile. The dose of 100 mg/kg nano-curcumin was more effective in reducing lipid profile.
Keywords: type 2 diabetes, nano-curcumin, apelin, insulin resistance, lipid profile, rat
Introduction
Diabetes is one of the most common chronic diseases in the world. It has many complications and its control imposes a huge financial burden on the society and new strategies for its treatment need to be explored.1 Type 2 diabetes mellitus (T2DM) is the most common type of diabetes, which is characterized by insulin resistance and dysfunction of pancreatic beta cells.1 Insulin resistance has been identified as the most important factor in the development of T2DM and its associated complications.2 Dyslipidemia is one of the complications of T2DM, caused by increased levels of LDL, triglyceride and total cholesterol and decreased level of HDL is common among diabetic patients.3
In recent years it has been reported that adipose tissue secretes an adipokine called apelin, which is involved in carbohydrate metabolism and insulin function4,5 it affects through a cell surface G protein-coupled receptor called APJ, which has structural similarity with angiotensin type I receptor.6 Apelin levels change with changes in insulin levels in the blood and appear to inhibit insulin secretion in the pancreas, circulating concentrations of apelin increase in obese humans and animals with insulin resistance.7,8 One study reported that treatment with apelin 13 for 10 weeks in type 2 diabetic rats prevented the destruction of pancreatic beta cells, thereby increasing serum insulin levels and decreasing hyperglycemia.9
Today, due to the lack of complete treatment of diabetes with synthetic drugs, the tendency to use natural compounds has increased; medicinal herbs have a significant role in the treatment of diabetic patients because of their low side effects, their availability and their effectiveness.10
Curcumin, dipropyl methane, is a hydrophobic polyphenol derived from the rhizome of Curcuma longa.11 This natural compound has a wide range of biological and pharmacological activities, including lowering blood cholesterol, lowering blood sugar, antioxidant and anti-inflammatory properties.11,12 Curcumin is an effective neutralizer of reactive oxygen species, the protective function of curcumin on the oxidative damage of biological membranes, DNA and protein in various diseases is mainly due to the removal of these radicals by curcumin.12
Despite these therapeutic effects, recent studies have shown that Cur is difficult to dissolve in water and is highly sensitive to physiological pH changes.13 In addition, its uptake through the gastrointestinal tract and in acidic environments is very poor. Therefore, it is useful to provide methods that can increase the solubility of functional herbal compounds with low solubility in water and protect them until they reach the target site in the body.13 In recent years, various strategies have been developed to increase the efficacy and effectiveness of curcumin. Liposomal curcumin, curcumin phospholipid complex, curcumin nanoparticles, and curcumin nanocapsules are examples of these methods.14,15 Animal models of diabetes are useful tools for research. Therefore, in the present study, Wistar rats were used. The purpose of the current study was to compare the effects of curcumin (CUR) and nano-curcumin (nCUR) on insulin resistance, serum levels of FBS, apelin and lipid profile in type 2 diabetic rats.
Materials and Methods
In this experimental study, forty-eight healthy male Wistar rats, aged 3 months, weighing 200–250 g, were purchased from the laboratory of animal research center of Zahedan University of Medical Sciences. All stages of the study were designed and implemented according to the guidelines of the laboratory animal ethics committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1397.357). The rats were housed in stainless steel cages, two rats per cage, in the animal research center of Zahedan University of Medical Sciences laboratory under standard conditions. The rats were exposed to dark/light cycle and allowed for free access to diet and water with suitable environmental conditions and good ventilation, and the environmental temperature was kept at 24 ± 2°C and 60 ± 5% humidity. The intervention was started after 2 weeks of acclimatization.16
Induction of T2DM
T2DM in the rats was induced by intraperitoneal injection of 110 mg/kg Nicotinamide (NA) (prepared by Merck, Germany) and 45 mg/kg Streptozotocin (STZ) (Sigma Aldrich, USA) in fasting state.17–19 0.1 M citrate buffer (pH = 4.5) was also used as Streptozotocin solvent (20). After 72 hrs, blood glucose levels were measured through the tail vein by the glucometer (Accu-Chek Performa, USA). All the animals with non-fasting blood glucose above 250 mg/dL at 72 hrs were considered diabetic and were included in the study.20
Experimental Design and Dietary Regimen
The rats were divided into six equal groups (eight rats per each group) including:
Group 1: Control group
Group 2: Diabetic control group
Group 3: Diabetic received CUR orally in a dose of 100 mg/kg/day
Group 4: Diabetic received CUR orally in a dose of 200 mg/kg/day
Group 5: Diabetic received nCUR orally in a dose of 100 mg/kg/day
Group 6: Diabetic received nCUR orally in a dose of 200 mg/kg/day
CUR is a lipophilic polyphenol and thus is insoluble in water, but it is readily soluble in organic solvents such as dimethyl sulfoxide (DMSO). A stock solution of CUR (Sigma Aldrich, USA, batch No: C7727) and Nano-micelle of curcumin (Exir Nano Sina, Iran, batch No: 1,228,225,765) was prepared at 100 mmol/L in DMSO. Therefore, concentrations of 100 and 200 mg/kg of body weight were selected as the treatment.21
The control and diabetic control groups received normal saline and the intervention groups received doses of 100 and 200 mg/kg CUR and nCUR (daily) by oral gavage (Using Needle Gavage) for 28 days.22
Collecting Samples
At the end of the experimental period, the rats were anesthetized with Ketamine (60 mg/kg, Alfasan, Woerden, Netherlands) and Xylazine (5 mg/kg, Alfasan, Woerden, Netherlands) after 12 hrs of overnight fasting.16 Blood samples were picked through cardiac puncture for the determination of biochemical parameters. Blood samples were centrifuged at 3000 rpm for 10 mins to obtain the serum. Serum samples were isolated in special microtubes and frozen at −75°C until biochemical assessments.16
Plasma Biochemical Assays
Serum levels of FBS, Triglycerides (TG), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and very low-density lipoproteins (VLDL) were assayed using an enzymatic photometric test, by Pars Azmoon test kits (Pars Azmoon Co., Iran).23 All tests were performed at Biochemistry Laboratory of Zahedan University of Medical Sciences, Zahedan, Iran.
The measurement method was based on the instructions in the kit manufacturer’s manual.24 Serum levels of insulin (Bioassay Technology Lab, China) and apelin-13 (Bioassay Technology Lab, China) were measured using rat-specific kits by ELISA using an ELISA reader (Stat Fax 2100, USA).25
Estimation of Insulin Resistance
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) formula was used to estimate insulin resistance. This index was calculated based on FBS and fasting insulin concentrations as follows. HOMA-IR= (insulin (µU/mL) × glucose (mg/dl)/405).26
Statistical Analysis
Statistical analysis was performed using PRISM GraphPad version 8. One-way ANOVA was used to compare multiple data sets and when the P. value obtained from ANOVA was significant (P<0.05), Tukey’s test was applied to test for differences among groups. P<0.05 was taken to indicate a significant difference between group means.
Results
Effects of Curcumin and Nanocurcumin on Insulin Resistance in Studied Rats
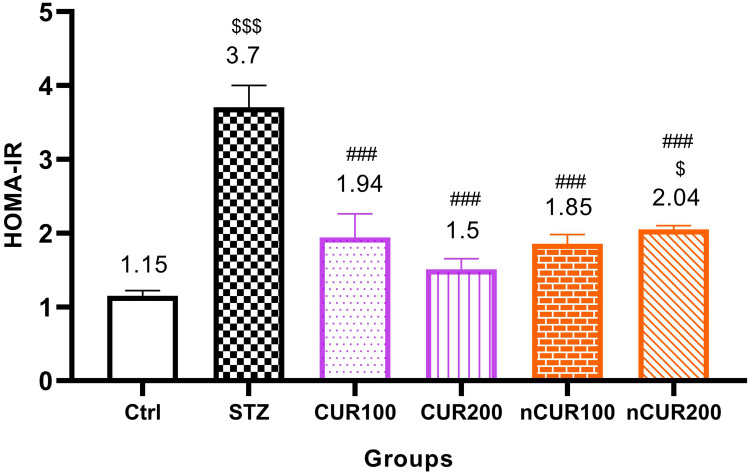
The effects of different doses of CUR and nCUR on insulin resistance were measured and the results are shown in Figure 1. Insulin resistance was significantly decreased in the CUR and nCUR groups compared to the diabetic control group (P <0.001). This decrease was more significant in the CUR 200 and nCUR 100 groups. There was no significant difference between CUR and nCUR groups (P>0.05).
Figure 1.
Effects of curcumin and nano-curcumin on insulin resistance in studied rats. $$$P <0.001 and $P <0.05 in comparision with control group. ###P <0.001 in comparision with STZ group. Data are expressed as Mean ± SD (n = 8), and analyzed by the One-way ANOVA and Tukey’s post hoc tests.
Abbreviations: Ctrl, control group; STZ, diabetic control group; CUR, curcumin; nCUR, nano-curcumin; HOMA-IR, homeostatic model assessment for insulin resistance.
Effects of Curcumin and Nano-Curcumin on FBS in Studied Rats
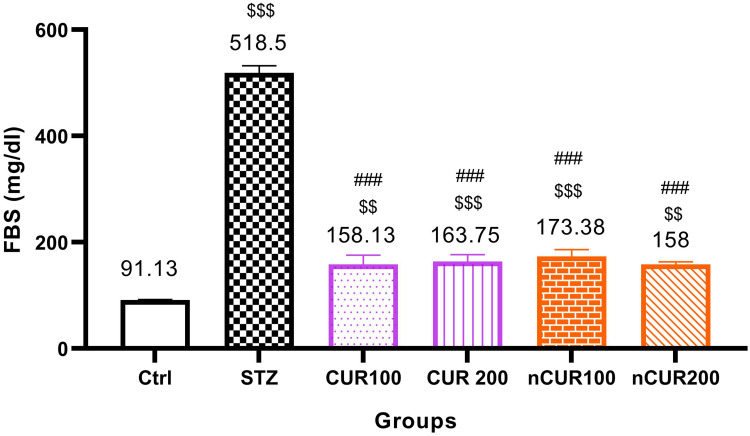
The effects of different doses of CUR and nCUR on FBS were measured and the results are shown in Figure 2. Serum level of FBS in the CUR and nCUR treated groups were significantly decreased compared to the diabetic control group (P <0.001). Also, there was no significant difference between the CUR and nCUR groups (P>0.05).
Figure 2.
Effects of curcumin and nano-curcumin on FBS in studied rats. $$$P <0.001 and $$P <0.01 in comparision with control group. ###P <0.001 in comparision with STZ group. Data are expressed as Mean ± SD (n = 8), and analyzed by the One-way ANOVA and Tukey’s post hoc tests.
Abbreviations: Ctrl, control group; STZ, diabetic control group; CUR, curcumin; nCUR, nano-curcumin; FBS, fasting blood sugar.
Effects of Curcumin and Nano-Curcumin on Serum Levels of Apelin in Studied Rats
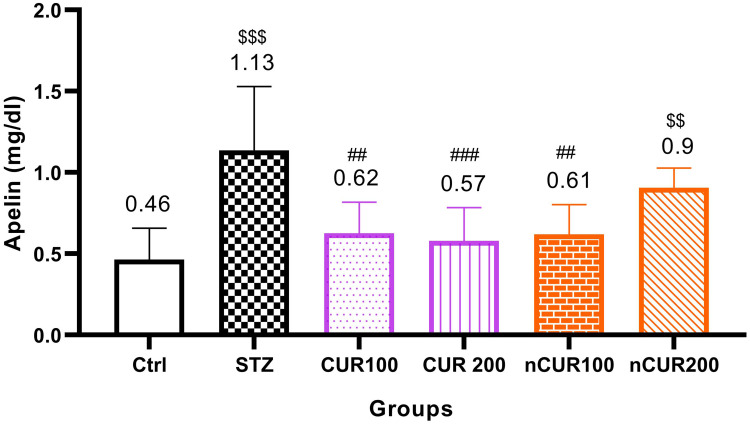
The effects of different doses of CUR and nCUR on serum levels of Apelin were measured and the results are shown in Figure 3. Serum level of apelin was significantly increased in the diabetic control group compared to the control, CUR 100, CUR 200 and nCUR 100 groups (P<0.05) but there was no significantly different with nCUR 200 group. There was no significant difference between CUR and nCUR groups (P>0.05).
Figure 3.
Effects of curcumin and nano-curcumin on serum levels of apelin in studied rats. $$$P <0.001 and $$P <0.01 in comparision with control group. ###P <0.001 and ##P <0.01 in comparision with STZ group. Data are expressed as Mean ± SD (n = 8), and analyzed by the One-way ANOVA and Tukey’s post hoc tests.
Abbreviations: Ctrl, control group; STZ, diabetic control group; CUR, curcumin; nCUR, nano-curcumin.
Effects of Curcumin and Nano-Curcumin on Serum Lipid Profile Levels in Studied Rats
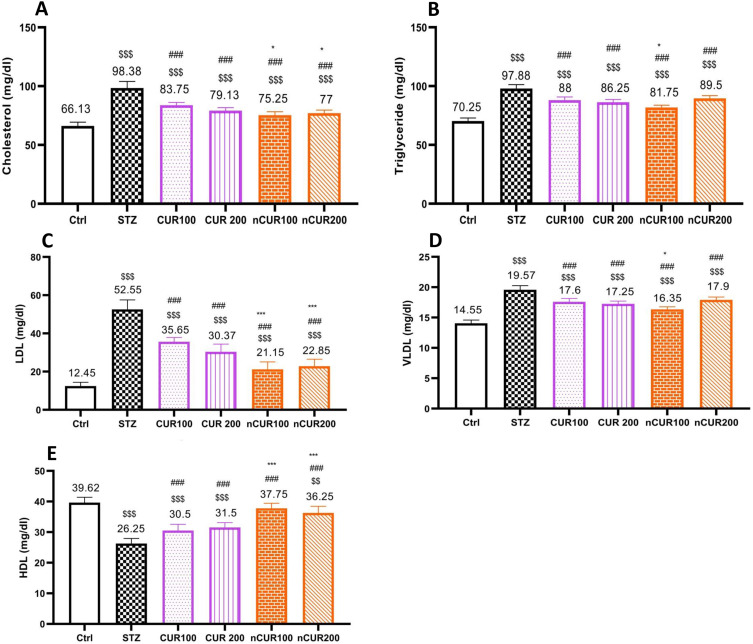
The effects of different doses of CUR and nCUR on serum levels of lipid profile were measured and the results are shown in Figure 4. Serum levels of cholesterol (Figure 4A), triglyceride (Figure 4B), LDL (Figure 4C) and VLDL (Figure 4D) were significantly decreased in the CUR and nCUR groups compared to the diabetic control group (P <0.05). Also, the mean of HDL serum (Figure 4E) was significantly increased in the nCUR and CUR groups compared to the diabetic control group (P <0.05). So that there was no significant difference between nCUR 100 and control group (P>0.05). The effects of nCUR groups on this variable were more effective than CUR groups (P <0.05).
Figure 4.
(A–E) Effects of curcumin and nano-curcumin on serum lipid profile levels in studied rats. Panel A, B, C, D and E show the average of serum levels of cholesterol, triglyceride, LDL, VLDL and HDL, respectively. $$$P <0.001 and $$P <0.01 in comparision with control group. ###P <0.00 in comparision with STZ group. ***P <0.001and *P <0.05 Nano-curcumin in comparision with curcumin group. Data are expressed as Mean ± SD (n = 8), and analyzed by the One-way ANOVA and Tukey’s post hoc tests.
Abbreviations: Ctrl, control group; STZ, diabetic control group; CUR, curcumin; nCUR, nano-curcumin; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein.
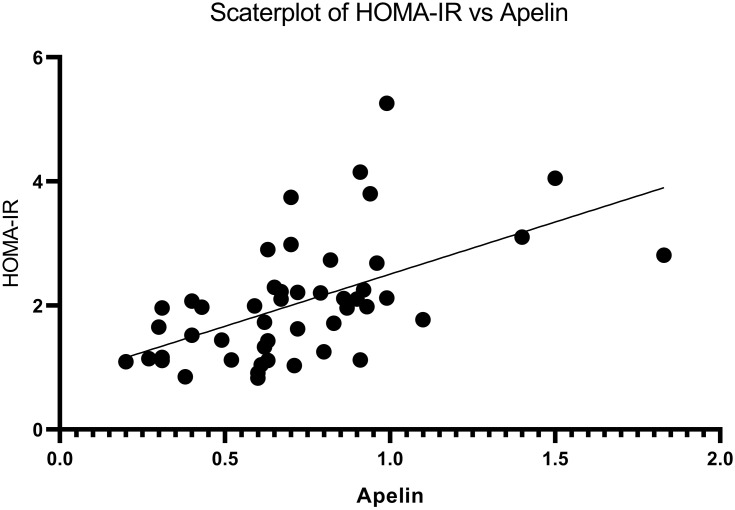
The Correlation Between Insulin Resistance and Serum Level of Apelin in Studied Rats
The significant positive correlation was found between Insulin resistance and serum level of apelin (p <0.05, r = 0.550). Serum level of apelin increased with increasing insulin resistance (Figure 5).
Figure 5.
Correlation between insulin resistance and serum level of apelin in studied rats. The results were analyzed by Pearson correlation coefficient. N = 48.
Abbreviation: HOMA-IR, homeostatic model assessment for insulin resistance.
Discussion
Numerous studies have shown that the pharmacological effects of curcumin on improving lipid profile and insulin resistance in diabetes mellitus are through increased PPAR-Y activation, NF-kB inhibition and modulate several transcription factors and cytokines.11,12 A great problem that should be considered is that several studies have shown that curcumin has poor bioavailability, low absorption, fast metabolism, and fast systemic elimination.13,16 To improve curcumin’s poor bioavailability, nano-micelle containing curcumin (SinaCurcumin®) was prepared for oral use, these nano-micelles are prepared from GRAS (generally recognized as safe) pharmaceutical excipients and C3-complex form of curcumin.27 The percentage of encapsulation of curcumin in this nano-micelle is close to 100% and their sizes are around 10 nm. Nano-curcumin has a significantly higher bioavailability after oral use as compared to simple powder of curcumin.27 After oral administration, nano-micelle of curcumin open in the stomach in less than 15 mins and are transferred to the small intestine. This product has a higher bioavailability than Ordinary curcumin.28
In the present study, the effects of Curcumin and Nano-curcumin on insulin resistance and serum levels of FBS, Apelin, cholesterol, triglyceride, HDL, LDL and VLDL were investigated in Streptozotocin-nicotinamide-induced diabetic rats. In this study, Nicotinamide and streptozotocin were used to induce T2DM by intraperitoneal injection.17,18 This study is the first study to compare the effects of CUR and nCUR on serum levels of Apelin, lipid profile and insulin resistance in T2DM rats.
In our study, insulin resistance and serum level of FBS were significantly decreased in the CUR and nCUR groups compared to the diabetic control group. These effects of CUR and nCUR are probably due to the activation of PPAR-Y, triggering glucokinase activity in the liver, inducing glucose transporter-4 (GLUT4) expression and attenuates tumor necrosis factor-alpha (TNFa).29 Numerous studies have shown that streptozotocin destroys pancreatic beta cells and induces diabetes in animal models by producing Reactive oxygen species (ROS).30,31 The mechanism of streptozotocin to destroy pancreatic beta cells is through GLUT2 receptors, which damage DNA and its alkylation. Alkylation of DNA helps to induce damage to pancreatic beta cells by producing too much nitric oxide (NO).31 Curcumin prevents the destruction of pancreatic beta cells by having anti-inflammatory (Inhibition of NF-κB), antioxidant and NO synthesis inhibition effects, thereby preventing high blood sugar and insulin resistance.29
Zhang et al reported that CUR has been shown to improve insulin sensitivity in muscle tissue of insulin-resistant rodents by an increase of the cellular glucose uptake as a result of the enhancement of GLUT4 translocation from intracellular compartments to the plasma membrane.32,33 In addition, increased glucose oxidation and glycogen synthesis have also been found in the muscle cell.32,33 In the study of Mantzorou et al CUR improved insulin resistance and decreased plasma glucose levels in diabetic rats.12 Also in the study of Rahimi et al, nCUR supplementation significantly reduced FBS compared to the placebo.27 In another study by Ganugula et al on rats with type 1 diabetes, nCUR (doses of 10 and 50 mg) reduced FBS by 32% and 37%, respectively.16 Best et al reported that CUR increased insulin secretion by affecting on pancreatic beta cells.34 A randomized, double-blind, placebo-controlled clinical trial to determine the effect of CUR on delayed development of T2DM in the pre-diabetes population was performed by Chuengsaman et al on 240 patients, in this study, all participants were randomly assigned to receive either 1500 mg/day CUR or placebo capsule for 9 months. The results showed that the CUR-treated group had lower insulin resistance and higher serum adiponectin levels.10 The results of these studies are consistent with the results of our study.
Several studies have shown that Curcumin analogues revealed a dose-dependent effect up to the dose of 30 mg/kg body weight, further increase in dose resulted in decreased anti-inflammatory activity.35,36 In another study Oral LD50 in mice was found to be more than 2.0 g/kg body weight.37 In our study, high doses of nCUR had the adverse effect on insulin resistance and serum levels of apelin and FBS, probably due to bioavailability and higher absorption of nCUR than CUR, high doses of this supplement have the adverse effects on improvement of insulin resistance and serum levels of apelin and FBS in diabetic rats.
In the present study, serum level of apelin in the CUR and nCUR treated groups (100 mg/kg dose) were significantly lower than the diabetic control group. The decrease in serum apelin in rats consuming CUR and nCUR is probably due to decreased insulin resistance and improved lipid profile. In clinical and experimental studies, serum level of apelin or its adipose tissue expression is increased in obese and insulin-resistant cases.38–40 Apelin has a role in energy metabolism, improves the sensitivity of insulin in insulin-resistant obese mice, and it is related to increase in glucose uptake in skeletal muscle.41
Studies have shown that CUR has a stronger antioxidant effect than vitamins E and C and can inhibit the activity of free radicals.42 On the other hand, CUR and nCUR may indirectly decrease serum apelin by decreasing blood glucose by improving insulin secretion from pancreatic beta cells and by decreasing diabetes complications.41 In the study of Ranjbar et al to investigate the effect of L-carnitine supplementation on serum apelin levels in diabetic rats, they were found that serum levels of apelin increase with the induction of diabetes and with the use of L-carnitine supplement, serum level of this factor is reduced.25
Bertrand et al described the effect of apelin on energy metabolism as follows, apelin activates AMPK (AMP-activated protein kinase) and eNOS (endothelial NO synthase) enzymes by effecting its Apj receptor on the cell surface, thereby enhancing intestinal absorption of glucose, mitochondrial biogenesis, fatty acid oxidation and lipolysis inhibition. It also inhibits insulin secretion by affecting on PDE3B (phosphodiesterase3B) enzyme.43
In our study, serum levels of cholesterol, triglyceride, LDL and VLDL were significantly decreased in the CUR and nCUR groups compared to the diabetic control group. This decrease was more significant in nCUR group compared to the CUR group. Also, serum levels of HDL in nCUR groups were significantly higher than diabetic control and CUR groups. Beneficial effects of CUR and nCUR on lipid profile are probably due to the activation of PPARα, improves insulin sensitivity and raising lipoprotein lipase activity of CUR.29 CUR is difficult to dissolve in water and is highly sensitive to physiological pH changes. In addition, its absorption through the gastrointestinal tract is very weak in acidic environments, instead nCUR has high bioavailability and uptake.44 It is probably for these reasons that nCUR is more effective in improving lipid profile than CUR.
In an RCT study, Neerati et al examined the potential beneficial effects of CUR capsules on blood lipids in T2DM treated with glyburide, the results showed that in the CUR-treated group, the serum levels of cholesterol, LDL, VLDL and triglycerides were significantly decreased and HDL levels were significantly increased.45 In the study of Rahimi et al, patients with T2DM were given nCUR supplementation (80 mg/day) in comparison with placebo for 3 months, and the results showed that nCUR supplementation compared to placebo significantly reduces FBS, triglyceride, cholesterol and LDL.27 In another study by Ding et al, that doses of 40 and 80 mg/kg CUR were administered to diabetic rats, results showed decrease in serum levels of FBS, insulin, cholesterol, triglyceride, LDL, and insulin resistance in the CUR-treated diabetic rats, the causes of these effects of CUR were reported as a decrease in lipid-regulating enzymes (HMG-COA reductase) in adipose tissue and hepatic gluconeogenic enzymes (glucose 6 phosphatase and Phosphoenolpyruvate carboxykinase) and SREBP (Sterol regulatory element-binding proteins) cycle regulation.46 The results of these studies are consistent with the results of our study.
In our study, it was observed that with increasing insulin resistance, serum level of apelin increased, so the correlation between these variables was positive and statistically significant.
Our findings in this content are consistent with recent studies.39,47,48 Nazari et al found that serum levels of apelin increased with insulin resistance in diabetic rats.38 Dray et al demonstrated that apelin increased glucose uptake in isolated soleus muscles. They postulated that apelin’s effects were mediated by the serine-threonine kinase AMPK in a pathway also involving eNOS. To support this, they determined that apelin-induced glucose uptake was impaired in both eNOS (‒/‒) mice and mice deficient in AMPK activity.49
Conclusion
According to the results of our study, it can be concluded that administration of Curcumin and Nano-curcumin in diabetic rats improved insulin resistance, serum levels of apelin, FBS and lipid profile. Also, the effects of Nano-curcumin on serum lipid profile are more effective than Curcumin, possibly due to increased solubility and bioavailability of Nano-curcumin. The effects of Nano-curcumin 100 are more effective than Nano-curcumin 200 in improving these variables, probably the effects of Nano-curcumin on these variables are dose-dependent, and with the increase in the supplement dose, the therapeutic effects are decreased. Therefore, it is recommended for future research, investigate the therapeutic effects of lower doses of Curcumin and Nano-curcumin in diabetic rats.
Limitation of the Study
Histopathological examination of the pancreas was not identified in this study. Less doses of CUR and nCUR (10, 25, and 50 mg/kg) were not investigated in this study.
Acknowledgments
The authors acknowledge the Zahedan University of Medical Science for providing this research facility and financial support. Data presented here were from an MSc thesis in Zahedan University of medical sciences.
Funding Statement
Funding was supported by the Zahedan University of Medical Sciences. The funding body did not have a role in the design of the study and collection, analysis, interpretation of data and in writing the manuscript.
Ethical Approval
The project and data collection procedure were approved by the Ethics Committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC. 1397.357).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work. The abstract of this paper was presented at the 5th International and 7th Iranian Congress of Endocrinology and Metabolism Updates as a poster presentation.
References
- 1.Rines AK, Sharabi K, Tavares CD, et al. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15(11):786. doi: 10.1038/nrd.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akter S, Rahman MM, Abe SK, et al. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: a nationwide survey. Bull World Health Organ. 2014;92(3):204–213A. doi: 10.2471/BLT.13.128371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367. doi: 10.1038/nrm2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Bo Y, Tao G-Z. Apelin protects against cardiomyocyte apoptosis induced by glucose deprivation. Chin Med J. 2009;122(19):2360–2365. [PubMed] [Google Scholar]
- 5.Kleinz MJ, Davenport A. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118(3):119–125. doi: 10.1016/j.regpep.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74(1):34–41. doi: 10.1046/j.1471-4159.2000.0740034.x [DOI] [PubMed] [Google Scholar]
- 7.Glassford AJ, Yue P, Sheikh AY, et al. HIF-1 regulates hypoxia-and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab. 2007;293(6):E1590–E6. doi: 10.1152/ajpendo.00490.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sörhede Winzell M, Magnusson C, Ahrén B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005;131(1):12–17. doi: 10.1016/j.regpep.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Zhao H, Du M, et al. The effect of apelin-13 on pancreatic islet beta cell mass and myocardial fatty acid and glucose metabolism of experimental type 2 diabetic rats. Peptides. 2019;114:1–7. doi: 10.1016/j.peptides.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 10.Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–2127. doi: 10.2337/dc12-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D-W, Fu M, Gao S-H, et al. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053. doi: 10.1155/2013/636053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantzorou M, Pavlidou E, Vasios G, et al. Effects of curcumin consumption on human chronic diseases: a narrative review of the most recent clinical data. Phytother Res. 2018;32(6):957–975. doi: 10.1002/ptr.6037 [DOI] [PubMed] [Google Scholar]
- 13.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17(1–2):71–80. doi: 10.1016/j.drudis.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Yu B, Zhao Y, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371(1–2):148–155. doi: 10.1016/j.ijpharm.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75x(1):R50–R57. doi: 10.1111/j.1750-3841.2009.01457.x [DOI] [PubMed] [Google Scholar]
- 16.Ganugula R, Arora M, Jaisamut P, et al. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of type 1 diabetes mellitus. Br J Pharmacol. 2017;174(13):2074–2084. doi: 10.1111/bph.13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananda PK, Kumarappan CT, Christudas S, et al. Effect of biophytum sensitivum on streptozotocin and nicotinamide-induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(1):31–35. doi: 10.1016/S2221-1691(11)60185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajarajeswari N, Pari L. Antioxidant role of coumarin on streptozotocin–nicotinamide‐induced type 2 diabetic rats. J Biochem Mol Toxicol. 2011;25(6):355–361. doi: 10.1002/jbt.20395 [DOI] [PubMed] [Google Scholar]
- 19.Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol Hung. 2014;101(4):408–420. doi: 10.1556/APhysiol.101.2014.4.2 [DOI] [PubMed] [Google Scholar]
- 20.Pari L, Srinivasan S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother. 2010;64(7):477–481. doi: 10.1016/j.biopha.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi S, Kayedpoor P, Karimzadeh-Bardei L, Nabiuni M. The effect of curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J Reprod Infertil. 2017;18(4):352. [PMC free article] [PubMed] [Google Scholar]
- 22.Faheem NM, El Askary A. Neuroprotective role of curcumin on the hippocampus against the structural and serological alterations of streptozotocin-induced diabetes in sprague Dawley rats. Iran J Basic Med Sci. 2017;20(6):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini M, Sedigheh A. Effects of dietary supplementation with ghee, hydrogenated oil, or olive oil on lipid profile and fatty streak formation in rabbits. ARYA Atheroscler. 2012;8(3):119. [PMC free article] [PubMed] [Google Scholar]
- 24.Soltanmohammadi E, Piran S, Mohammadi A, et al. Serum sdLDL-C and cellular SREBP2-dependent cholesterol levels; is there a challenge on targeting PCSK9. J Med Biochem. 2016;35(4):410–415. doi: 10.1515/jomb-2016-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranjbar Kohan N, Nazifi S, Tabandeh MR, et al. Effect of L-carnitine supplementation on apelin and apelin receptor (Apj) expression in cardiac muscle of obese diabetic rats. Cell J. 2018;20(3):427–434. doi: 10.22074/cellj.2018.5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oza MJ, Kulkarni YA. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front Pharmacol. 2018;9:739. doi: 10.3389/fphar.2018.00739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimi HR, Mohammadpour AH, Dastani M, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed. 2016;6(5):567–577. [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimi HR, Jaafari MR, Mohammadpour AH, et al. Curcumin: reintroduced therapeutic agent from traditional medicine for alcoholic liver disease. Asia Pac J Med Toxicol. 2015;4:25–30. [Google Scholar]
- 29.Poolsup N, Suksomboon N, Kurnianta PDM, Deawjaroen K, Atkin SL. Effects of curcumin on glycemic control and lipid profile in prediabetes and type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0215840. doi: 10.1371/journal.pone.0215840 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin–nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186(2):200–210. doi: 10.1016/j.cbi.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 31.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 32.Na LX, Zhang YL, Li Y, et al. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 2011;21(7):526–533. doi: 10.1016/j.numecd.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 33.Deng YT, Chang TW, Lee MS, et al. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J Agric Food Chem. 2012;60(4):1059–1066. doi: 10.1021/jf204496f [DOI] [PubMed] [Google Scholar]
- 34.Best L, Elliott AC, Brown PD. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem Pharmacol. 2007;73(11):1768–1775. doi: 10.1016/j.bcp.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Yang Y, Li X, Chen MF, Zheng SS. Study on the acute oral toxicity and genetic toxicity of curcumin. Chin J Health Lab Technol. 2011;21(7):1707–1709. [Google Scholar]
- 36.Abdel Rheim F, Ragab AA, Hammam FM, et al. Protective effects of curcumin for oxidative stress and histological alterations induced by pyrethroid insecticide in albino rats. Egypt J Hosp Med. 2015;58(1):63–73. doi: 10.12816/0009362 [DOI] [Google Scholar]
- 37.Kohli K, Ali J, Ansari MJ, et al. Curcumin: a natural antiinflammatory agent. Indian J Pharmacol. 2005;37(3):141. doi: 10.4103/0253-7613.16209 [DOI] [Google Scholar]
- 38.Nazari M, Moghimipour E, Tabandeh MR. Betaine down regulates apelin gene expression in cardiac and adipose tissues of insulin resistant diabetic rats fed by high-calorie diet. Int J Pept Res Ther. 2017;23(2):181–190. doi: 10.1007/s10989-016-9551-7 [DOI] [Google Scholar]
- 39.Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114(10):544–548. doi: 10.1055/s-2006-948309 [DOI] [PubMed] [Google Scholar]
- 40.Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19(11):1574–1580. doi: 10.1007/s11695-009-9955-y [DOI] [PubMed] [Google Scholar]
- 41.Yue P, Jin H, Aillaud M, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1):E59–E67. doi: 10.1152/ajpendo.00385.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 43.Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Front Physiol. 2015;6:115. doi: 10.3389/fphys.2015.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9289. doi: 10.1021/jf202135j [DOI] [PubMed] [Google Scholar]
- 45.Neerati P, Devde R, Gangi AK. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother Res. 2014;28(12):1796–1800. doi: 10.1002/ptr.5201 [DOI] [PubMed] [Google Scholar]
- 46.Ding L, Li J, Song B, et al. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol Appl Pharmacol. 2016;304:99–109. doi: 10.1016/j.taap.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 47.Kadoglou NP, Vrabas IS, Kapelouzou A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit. 2012;18(5):CR290. doi: 10.12659/MSM.882734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadoglou NP, Fotiadis G, Kapelouzou A, et al. The differential anti‐inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med. 2013;30(2):e41–e50. doi: 10.1111/dme.12055 [DOI] [PubMed] [Google Scholar]
- 49.Dray C, Knauf C, Daviaud D, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–445. doi: 10.1016/j.cmet.2008.10.003 [DOI] [PubMed] [Google Scholar]