Abstract
目的
提高对人免疫缺陷病毒(HIV)阴性浆母细胞淋巴瘤的认识。
方法
回顾性分析北京协和医院1997年1月至2015年5月确诊的8例HIV阴性浆母细胞淋巴瘤患者的临床资料,分析其临床特征及转归。
结果
8例HIV阴性浆母细胞淋巴瘤中男3例,女5例,中位年龄60(43~80)岁,其中4例存在导致免疫功能低下的疾病或状态。8例患者均有结外受累,2例Ann Arbor分期为Ⅰ~Ⅱ期,6例为Ⅳ期,其中5例有骨髓受累。所有患者均弥漫表达CD38和CD138,B细胞标志包括PAX-5及Bcl-6少见。5例患者进行EBV-DNA检测,均为阴性。接受化疗并规律随访的7例患者中位随访36(11~57)个月,中位无进展生存时间为15(6~52)个月,中位总生存时间为36(2~52)个月;其中4例采用了硼替佐米联合化疗,3例有效,但疗效难以维持,分别于治疗后2、9、21个月疾病进展。2例Ⅰ~Ⅱ期患者均治疗有效,未出现疾病进展,持续存活;5例Ⅳ期患者化疗后虽然有效,但疗效难以维持,中位总生存时间仅12(6~52)个月,中位无进展生存时间仅10(2~21)个月。
结论
该组HIV阴性浆母细胞淋巴瘤患者以中老年为主,临床呈现高侵袭性,均出现结外(尤其是骨髓)受累,其免疫表型与浆细胞瘤较为接近,分期较晚的患者预后不良。
Keywords: 浆母细胞淋巴瘤, 临床特征, 治疗结果
Abstract
Objective
To deepen the knowledge of HIV-negative plasmablastic lymphoma (PBL).
Methods
Medical records from 8 HIV-negative PBL patients diagnosed in Peking Union Medical College Hospital from January 1997 to May 2015 were collected, and the clinical features and prognosis of these patients were analyzed.
Results
All of these 8 patients were diagnosed as HIV-negative PBL, 3 of 8 patients were males, and others were female. The median age was 60 (43–80) year. Among these patients, 4 cases had underlying immunosuppressive state. These patients all had extra-nodular involvement, and 6 cases of them were at stage Ⅳ according to Ann Arbor Staging, 5 patients had bone marrow involvement. CD38 and CD138 were diffusely positive for all patients, while the positive rate of B cell marker including PAX-5 and Bcl-6 were relative low. 5 of 8 patients had been detected for EBV-DNA, and all of them were negative. The median follow-up for the 7 patients receiving chemotherapy and regular follow-ups was 36 (11–57) months, the median progression-free survival (PFS) was 15 (6–52) months, and the median overall survival was 36 (2–52) months. Among these patients, 4 cases had received chemotherapy combined with Bortezomib, showing 3 cases of effective, but it seems to be difficult to keep the long term efficacy, and disease progression occurred in 2, 9, and 21 months after treatment. 2 patients at stageⅠ-Ⅱ were treated effectively, without disease progression and survival, 5 patients at stage Ⅳacquired the efficacy unsustainably, with a median PFS of 10 (2–21) months and a median overall survival of 12 (6–52) months.
Conclusion
HIV-negative PBL is relatively prevalent in elderly patients, and presenting with high invasiveness in clinical, extremely prone to extra-nodular involvement, especially the bone marrow. The immunophenotype of PBL is more resemble to that of plasmacytoma. Patients who were in late stage at diagnosis show poor prognosis.
Keywords: Plasmablastic lymphoma, Clinical characteristics, Treatment outcome
浆母细胞淋巴瘤(plasmablastic lymphoma,PBL)是一种罕见的后生发中心活化B细胞来源的弥漫大B细胞淋巴瘤,WHO 2016淋巴瘤分类标准将其定义为获得性免疫缺陷综合征相关的淋巴瘤(acquired immunodeficiency syndrome-related lymphoma,ARL)[1],与人免疫缺陷病毒(human immunodeficiency virus,HIV)和EBV的感染密切相关。近年来,HIV阴性PBL的报道也逐渐增多[2]–[5]。PBL侵袭性强,复发率和病死率较高,预后差,目前缺乏有效的治疗方法[6]–[7]。本文我们对我院确诊的8例HIV阴性PBL患者的临床特征、治疗及转归进行回顾性分析,以提高对该病的认识。
病例与方法
1.病例资料:以1997年1月至2015年5月我院诊断的共9例PBL患者为研究对象,诊断标准参照2008年WHO血液系统和淋巴组织肿瘤的分类标准,所有诊断均经过病理科医师二次审核,其中1例因最终病理复审诊断为浆细胞瘤而排除,其余8例符合PBL的诊断。收集患者的临床及随访资料,包括诊断时的年龄、性别、既往史、受累部位、Ann Arbor分期、血清HIV抗体检测、血清EBV DNA检测、治疗方案、疗效、随访时间、存活时间等。
2.疗效判断标准及生存定义:治疗后的疗效评估增强CT的判断标准根据2014年Cheson的修订标准[8],PET-CT的疗效评价依据2007年Cheson的修订标准[9],包括完全缓解(CR)、部分缓解(PR)、稳定(SD)和进展(PD)。总体生存(OS)期定义为确诊至死亡或末次随访的时间,无进展生存(PFS)期定义为确诊至疾病进展或末次随访的时间。
结果
1.临床特征:见表1。8例PBL患者中男3例,女5例,中位年龄60(43~80)岁,均无HIV感染。Ann Arbor分期:Ⅰ期、Ⅱ期各1例,Ⅳ期6例。国际预后指数(IPI)评分为0~2分3例,3~5分5例。8例患者中4例无导致免疫抑制的疾病或状态(如糖尿病、慢性感染、结缔组织病、服用激素或免疫抑制剂、器官移植术后、高龄等);4例存在导致免疫抑制的疾病或状态:例2为高龄(76岁),例5为高龄(80岁)、乳腺癌术后规律化疗、类风湿性关节炎服用免疫抑制剂,例7曾患结核性脑膜炎,例8为HBV携带者。所有患者均出现结外受累,其中5例为单纯结外受累而无结内器官受累,6例Ⅳ期的患者中5例出现骨髓受累;8例患者的受累部位包括骨髓(5例)、淋巴结(3例)、软组织(2例)、硬膜外(2例)、子宫(2例)、胸膜(2例)、鼻咽部(1例)、皮肤(1例)、口腔(1例)、直肠(1例)、横纹肌(1例)。8例患者中5例进行了EBV-DNA检测,均阴性。所有患者均未测定HHV-8。
表1. 8例人免疫缺陷病毒阴性浆母细胞淋巴瘤患者临床特征.
| 例号 | 年龄(岁) | 性别 | Ann Arbor分期 | IPI评分 | 结外受累 |
EBV DNA检测 | 导致免疫抑制的因素 | |
| 受累部位 | 是否骨髓受累 | |||||||
| 1 | 51 | 女 | ⅡA | 1 | 鼻咽部 | 否 | N/A | 无 |
| 2 | 76 | 男 | ⅣA | 4 | 右下颌软组织 | 是 | 阴性 | 高龄 |
| 3 | 50 | 男 | ⅣB | 4 | 皮肤、心膈角软组织、口腔 | 是 | 阴性 | 无 |
| 4 | 59 | 女 | ⅣA | 1 | 硬膜外 | 是 | 阴性 | 无 |
| 5 | 80 | 女 | ⅣA | 4 | 胸膜(胸腔积液) | 否 | 阴性 | 乳腺癌术后化疗、类风湿关节炎口服免疫抑制剂、高龄 |
| 6 | 61 | 女 | ⅠA | 1 | 子宫 | N/A | 阴性 | 无 |
| 7 | 60 | 男 | ⅣA | 5 | 硬膜外、横纹肌、胸膜(胸腔积液) | 是 | N/A | 结核性脑膜炎病史 |
| 8 | 43 | 女 | ⅣB | 3 | 子宫、直肠 | 是 | N/A | 携带HBV |
注:IPI:国际预后指数;N/A:不适用
2.组织病理及免疫组化特征:8例患者的病理标本均采用HE染色,并进行免疫组化染色分析,可见浆母细胞淋巴瘤的典型形态及免疫表型特点,见图1。具体免疫表型见表2。本组患者瘤细胞弥漫表达浆细胞标志物主要为CD38和CD138,其次为MUM-1;B细胞标志物CD20、CD79α、PAX-5及Bcl-6相对少见,3例患者表达CD3。Ki-67中位数为72%(40%~99%)。5例患者进行了EBV编码的小RNA原位杂交(EBER)检测,均为阴性。
图1. 人免疫缺陷病毒阴性浆母细胞淋巴瘤患者组织病理及免疫组化特征.
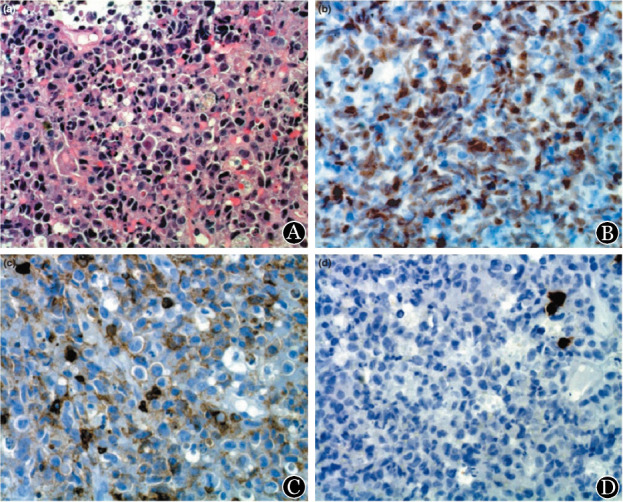
A:HE染色,低倍;细胞大、圆形或卵圆形、胞质丰富、偏心核、类似未成熟浆细胞。B:MUM-1免疫组化染色,低倍。C:抗CD45-RB免疫组化染色,低倍。D:抗CD79α免疫组化染色,低倍
表2. 8例人免疫缺陷病毒阴性浆母细胞淋巴瘤患者免疫表型.
| 例号 | CD38 | CD138 | MUM-1 | CD20 | CD79α | CD56 | Ki-67 | Bcl-6 | CD3 | PAX-5 | EBER |
| 1 | + | N/A | + | 散在+ | + | 散在+ | 40% | − | 部分+ | − | N/A |
| 2 | − | + | + | 部分+ | + | N/A | 60% | N/A | − | N/A | − |
| 3 | + | 部分+ | − | − | N/A | − | 50% | − | − | N/A | − |
| 4 | N/A | + | + | − | N/A | N/A | 84% | N/A | 弱+ | 弱+ | − |
| 5 | + | + | + | − | − | N/A | 90% | − | − | − | − |
| 6 | 散在+ | + | − | 散在+ | 散在+ | − | 99% | − | − | +/− | − |
| 7 | + | + | N/A | − | − | − | 85% | N/A | − | − | N/A |
| 8 | + | + | N/A | +/− | N/A | N/A | 60% | N/A | 散在+ | N/A | N/A |
注:EBER:EBV编码的小RNA原位杂交;N/A:不适用
3.遗传学特征:本组患者中有2例骨髓受累的患者采用FISH进行了骨髓染色体核型检测。例2同时存在del(1q21)、del(13q14)、del(17p13.1)和del(14q32);例7存在del(14q32)和1q21信号扩增。上述2例患者未见Myc基因的重排或扩增。
4.治疗及转归:除例7采用HyperCVAD(环磷酰胺、表柔比星、长春地辛、地塞米松)联合依托泊苷方案化疗1个疗程后失访,无法评价疗效及生存;其余7例均规律随访,中位随访时间36(11~57)个月。其中6例患者均以CHOP(环磷酰胺、表柔比星、长春地辛、泼尼松)方案或类CHOP方案联合或不联合其他药物作为一线化疗方案。2例(28.6%)患者接受预防性鞘注,例1为ⅡA期,累及鼻咽部,例4为ⅣA期,累及硬膜外,随访过程中所有患者均未出现中枢神经系统侵犯。
7例规律随访的患者中1例达到完全缓解(CR),5例达部分缓解(PR),1例疾病稳定(SD)。随访中5例患者疾病进展。中位PFS期为21(2~52)个月,中位OS期为36(6~52)个月。4例采用硼替佐米联合化疗的患者中3例PR,但是疗效均难以维持,分别于治疗后2、8、21个月疾病进展,于治疗后6、11、40个月死于原发病。规律随访的7例患者中Ⅰ~Ⅱ期2例,均治疗有效,1例CR,1例PR,未出现疾病进展,持续存活;其余5例患者均为Ⅳ期,化疗后虽然有效,但疗效难以维持,中位随访12(11~57)个月,中位OS时间仅12(6~52)个月,中位DFS时间仅10(2~21)个月(表3)。
表3. 8例人免疫缺陷病毒阴性浆母细胞淋巴瘤患者治疗及转归.
| 例号 | 治疗方案 | 疗效 | 随访时间(月) | 总生存时间(月) | 无进展生存时间(月) | 转归 |
| 1 | CHOP×4+TBI | 部分缓解 | 36 | 36 | 36 | 存活 |
| 2 | B+CHOP×1 | 部分缓解后进展 | 11 | 6 | 2 | 死亡 |
| 3 | BD+CHO×6 | 部分缓解后进展 | 11 | 11 | 8 | 死亡 |
| 4 | B+CHOP×2、CHP×4 | 部分缓解后进展 | 40 | 40 | 21 | 存活 |
| 5 | CHOP/CHP×9、MINE×5 | 部分缓解后进展 | 57 | 52 | 15 | 死亡 |
| 6 | 手术切除+化疗a | 完全缓解 | 52 | 52 | 52 | 存活 |
| 7 | 手术切除+E-hyperCVAD×1 | 失访 | 1b | N/A | N/A | N/A |
| 8 | CHOP×1+PAD×5 | 疾病稳定后进展 | 12 | 12 | 10 | 死亡 |
注:CHOP:环磷酰胺、表柔比星、长春地辛、泼尼松;B:硼替佐米;D:地塞米松;MINE:环磷酰胺、米托蒽醌、依托泊苷;E-hyperCVAD:依托泊苷、环磷酰胺、长春地辛、表柔比星、地塞米松;PAD:硼替佐米、表柔比星、地塞米松。a化疗方案不详;b为入院治疗时间;N/A:不适用
讨论
PBL是一种罕见的弥漫大B细胞淋巴瘤(DLBCL)亚型。Castillo等[6]对570例PBL患者进行回顾性分析,其中HIV阴性者占28%。大部分HIV阴性PBL患者存在免疫抑制状态,如老年、化疗后、骨髓移植、慢性感染或长期应用免疫抑制剂等[10]–[15],本组中4例患者存在导致免疫抑制的疾病或状态,另外4例无明确的免疫抑制状态,而免疫健全的PBL也有报道[2]–[3],[5]。PBL患者中男性约占75%,HIV阴性患者中女性比例更高,但仍以男性为主[6]。HIV阴性PBL患者诊断时的中位年龄为55岁,而HIV阳性PBL患者则为46岁[16]。
与HIV阳性PBL相比,HIV阴性PBL口外的结外器官受累更为多见,高达84%,骨髓受累比例约30%,且诊断时常为晚期[16],本组8例HIV阴性PBL均存在结外受累,且部位多样,包括硬膜外、子宫、胸膜、鼻咽部、皮肤等,5例骨髓受累。
PBL细胞常呈弥漫性增生,个大、圆或卵圆形、胞质丰富、核偏位、核仁单个或多个、呈“星空”样外观[17],免疫表型与浆细胞肿瘤相近[18],CD79a、IRF-4/MUM-1、CD38和CD138常阳性,而B细胞标志CD19、CD20、PAX-5常阴性,Ki-67常>90%[7]。本组7例PBL患者(1例未检测)的肿瘤细胞均弥漫表达浆细胞标志物CD38、CD138,其次为MUM-1,B细胞标志物PAX-5少见,与文献报道相似,而CD20表达率为50%,高于文献报道水平[6]。Ki-67中位数为72%(40%~99%),与既往报道相似[6]。EBER在HIV阴性PBL中阳性率较低,约为50%[6],本组患者中5例检测了EBER,均为阴性。Myc重排是PBL最常见的分子遗传学异常,约50%的患者存在Myc重排,且伴侣基因为IGH[19]。本组患者中仅2例进行了分子遗传学检测,均出现del(14q32),提示其可能有一定的诊断意义。
PBL重点需要与浆母细胞样浆细胞瘤、原发性渗出性淋巴瘤以及Burkitt淋巴瘤等鉴别。PBL的EBER常为阳性,Ki-67常较高,而浆母细胞样浆细胞瘤EBER为阴性,Ki-67常较低,常有单克隆M蛋白血症、高钙血症、溶骨性破坏等症状。比较基因组杂交技术,PBL在基因上与DLBCL更为接近[20],但DLBCL常CD20阳性,可鉴别。原发性渗出性淋巴瘤表型与PBL相似[7],但主要表现为胸腔或心包积液,而较少有淋巴结或脏器受累,且与HHV-8密切相关。典型Burkitt淋巴瘤CD20与Bcl-6均为阳性,也可与PBL鉴别[21]。
HIV阴性PBL患者预后差,总体生存期仅4个月[16]。免疫抑制状态为不良预后因素[12]。IPI评分可预测PBL患者预后[22]–[24],其中分期以及ECOG水平对预后的影响更为明确,而LDH水平、结外受累数目对预后影响较小,年龄是否影响预后尚存在争议[4],[6],[25]。EBV感染与预后是否相关尚不明确[16],[25]–[26]。其他预后不良因素还包括Myc基因重排和高Ki-67指数[22]。从本组患者来看,Ⅰ~Ⅱ期患者预后较好,OS时间分别为52和36个月,而Ⅳ期患者预后较差,中位OS时间仅为12(6~52)个月。
目前尚没有PBL的标准疗法,NCCN指南推荐强化疗,包括剂量调整的EPOCH(依托泊苷、泼尼松、长春新碱、环磷酰胺、多柔比星)或HyperCVAD等方案[1]。然而高强度化疗对患者的生存改善有限[22],[27]。自体造血干细胞移植(auto-HSCT)不论作为一线巩固还是挽救治疗,都明显改善PBL患者预后[28]。研究显示PBL患者诱导化疗CR后接受auto-HSCT,移植后2年复发率为30%,2年OS率为53%,中位随访30个月,11例患者至随访结束时仍无病存活[29]。本组患者均未行auto-HSCT,可能与其难以达到CR或患者状态较差有关。近年来,有硼替佐米治疗PBL的个案报道[30]–[32]。本组8例患者中4例接受硼替佐米联合化疗,3例有效,但疗效难以维持,分别于治疗后2、8、21个月疾病进展。此外,有报道利妥昔单抗在CD20阳性的PBL患者中有效[33],但多数PBL患者CD20阴性,因而限制了利妥昔单抗的应用。手术和放疗常作为姑息治疗[6]。
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J] Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JE, Kim YA, Kim WY, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in Korea[J] Leuk Lymphoma. 2009;50(4):582–587. doi: 10.1080/10428190902789173. [DOI] [PubMed] [Google Scholar]
- 3.Tani J, Miyoshi H, Nomura T, et al. A case of plasmablastic lymphoma of the liver without human immunodeficiency virus infection[J] World J Gastroenterol. 2013;19(37):6299–6303. doi: 10.3748/wjg.v19.i37.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases[J] Oncol Rep. 2015;33(4):1615–1620. doi: 10.3892/or.2015.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakral C, Thomas L, Gajra A, et al. Plasmablastic lymphoma in an immunocompetent patient[J] J Clin Oncol. 2009;27(25):e78–e81. doi: 10.1200/JCO.2009.22.2208. [DOI] [PubMed] [Google Scholar]
- 6.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma[J] Blood. 2015;125(15):2323–2330. doi: 10.1182/blood-2014-10-567479. [DOI] [PubMed] [Google Scholar]
- 7.Castillo JJ, Reagan JL. Plasmablastic lymphoma: a systematic review[J] Scientific World Journal. 2011;11:687–696. doi: 10.1100/tsw.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J] J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma[J] J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 10.Robak T, Urbańska-Ryś H, Strzelecka B, et al. Plasmablastic lymphoma in a patient with chronic lymphocytic leukemia heavily pretreated with cladribine (2-CdA): an unusual variant of Richter's syndrome[J] Eur J Haematol. 2001;67(5-6):322–327. doi: 10.1034/j.1600-0609.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi M, Ogawa F, Onishi T, et al. Plasmablastic lymphoma in an elderly immunocompetent patient[J] Pathol Int. 2012;62(5):347–350. doi: 10.1111/j.1440-1827.2012.02798.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Zhang L, Ayala E, et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review[J] Leuk Res. 2011;35(12):1571–1577. doi: 10.1016/j.leukres.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Van Vrancken MJ, Keglovits L, Krause J. Plasmablastic lymphoma following transplantation[J] Proc (Bayl Univ Med Cent) 2013;26(2):152–155. doi: 10.1080/08998280.2013.11928941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JZ, Min K, Fan L, et al. Plasmablastic lymphoma following combination treatment with fludarabine and rituximab for nongastric mucosa-associated lymphoid tissue lymphoma: a case report and review of literature[J] Int J Clin Exp Pathol. 2014;7(7):4400–4407. [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein J, Pezzella F, Gatter KC. Plasmablastic lymphomas may occur as post-transplant lymphoproliferative disorders[J] Histopathology. 2007;51(6):774–777. doi: 10.1111/j.1365-2559.2007.02870.x. [DOI] [PubMed] [Google Scholar]
- 16.Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma[J] Leuk Lymphoma. 2010;51(11):2047–2053. doi: 10.3109/10428194.2010.516040. [DOI] [PubMed] [Google Scholar]
- 17.Rafaniello Raviele P, Pruneri G, Maiorano E. Plasmablastic lymphoma: a review[J] Oral Dis. 2009;15(1):38–45. doi: 10.1111/j.1601-0825.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 18.Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles[J] Mod Pathol. 2005;18(6):806–815. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]
- 19.Valera A, Balagué O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas[J] Am J Surg Pathol. 2010;34(11):1686–1694. doi: 10.1097/PAS.0b013e3181f3e29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CC, Zhou X, Taylor JJ, et al. Genomic profiling of plasmablastic lymphoma using array comparative genomic hybridization (aCGH): revealing significant overlapping genomic lesions with diffuse large B-cell lymphoma[J] J Hematol Oncol. 2009;2:47. doi: 10.1186/1756-8722-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong HY, Scadden DT, de Leval L, et al. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm[J] Am J Surg Pathol. 2005;29(12):1633–1641. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 22.Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy[J] Cancer. 2012;118(21):5270–5277. doi: 10.1002/cncr.27551. [DOI] [PubMed] [Google Scholar]
- 23.Bibas M, Castillo JJ. Current knowledge on HIV-associated Plasmablastic Lymphoma[J] Mediterr J Hematol Infect Dis. 2014;6(1):e2014064. doi: 10.4084/MJHID.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center's experience[J] Leuk Lymphoma. 2015;56(1):267–269. doi: 10.3109/10428194.2014.911867. [DOI] [PubMed] [Google Scholar]
- 25.Loghavi S, Alayed K, Aladily TN, et al. Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: a clinicopathologic analysis of 61 patients[J] J Hematol Oncol. 2015;8:65. doi: 10.1186/s13045-015-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma[J] Oncologist. 2010;15(3):293–299. doi: 10.1634/theoncologist.2009-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo JJ, Winer ES, Stachurski D, et al. HIV-negative plasmablastic lymphoma: not in the mouth[J] Clin Lymphoma Myeloma Leuk. 2011;11(2):185–189. doi: 10.1016/j.clml.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Al-Malki MM, Castillo JJ, Sloan JM, et al. Hematopoietic cell transplantation for plasmablastic lymphoma: a review[J] Biol Blood Marrow Transplant. 2014;20(12):1877–1884. doi: 10.1016/j.bbmt.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Cattaneo C, Finel H, McQuaker G, et al. Autologous hematopoietic stem cell transplantation for plasmablastic lymphoma: the European Society for Blood and Marrow Transplantation experience[J] Biol Blood Marrow Transplant. 2015;21(6):1146–1147. doi: 10.1016/j.bbmt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Bose P, Thompson C, Gandhi D, et al. AIDS-related plasmablastic lymphoma with dramatic, early response to bortezomib[J] Eur J Haematol. 2009;82(6):490–492. doi: 10.1111/j.1600-0609.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 31.Saba NS, Dang D, Saba J, et al. Bortezomib in plasmablastic lymphoma: a case report and review of the literature[J] Onkologie. 2013;36(5):287–291. doi: 10.1159/000350325. [DOI] [PubMed] [Google Scholar]
- 32.Bibas M, Grisetti S, Alba L, et al. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone[J] J Clin Oncol. 2010;28(34):e704–708. doi: 10.1200/JCO.2010.30.0038. [DOI] [PubMed] [Google Scholar]
- 33.Yan M, Dong Z, Zhao F, et al. CD20-positive plasmablastic lymphoma with excellent response to bortezomib combined with rituximab[J] Eur J Haematol. 2014;93(1):77–80. doi: 10.1111/ejh.12286. [DOI] [PubMed] [Google Scholar]


