Abstract
Complete gonadal dysgenesis (CGD) or Swyer syndrome is characterised by sexual infantilism in a phenotypic female with 46, XY karyotype. Patients with gonadal dysgenesis and Y-chromosome material are at a high risk of developing gonadoblastoma and dysgerminoma. A 16-year-old girl presented with progressive virilisation, poor breast development and primary amenorrhea. On evaluation, she was found to have male-range serum testosterone, large abdominopelvic mass lesion, elevated germ cell tumour markers and 46, XY karyotype. She underwent surgical excision of left gonadal mass and right streak gonad, histopathology of which revealed dysgerminoma and gonadoblastoma, respectively. A diagnosis of virilising germ cell tumour arising in the setting of 46, XY CGD was, therefore, made. This case highlights a rare presentation of 46, XY CGD and the need to consider early prophylactic gonadectomy in patients affected with this rare condition. The presence of dysgerminoma/gonadoblastoma should be suspected if a hitherto phenotypic female with CGD undergoes virilisation.
Keywords: reproductive medicine, cancer - see oncology
Background
Disorders of sex development (DSDs) are congenital conditions characterised by atypical chromosomal, gonadal or anatomical sex. These disorders could be broadly classified into: (a) sex chromosomal DSD, (b) 46, XY DSD and (c) 46, XX DSD, depending on the karyotype. 46, XY DSD results from either defect in gonadal (testicular) development or defect in androgen biosynthesis and action. 46, XY complete gonadal dysgenesis (CGD) or Swyer syndrome is characterised by the presence of dysgenetic abdominal gonads, which fail to secrete both anti-Müllerian hormone (AMH) and testosterone, during the antenatal period. As a result, subjects with this disorder are born with normal female external and internal genitalia and are often undetected until adolescence when they present with delayed puberty and primary amenorrhea.1 Hormonal evaluation reveals hypergonadotropic hypogonadism and imaging studies show the presence of hypoplastic uterus with bilateral streak gonads.2
It is known that individuals with gonadal dysgenesis and Y-chromosome material have a high risk of gonadoblastoma development with the potential for malignant transformation to dysgerminoma. The onset seems to be greatest at or after the time of puberty. The risk of gonadal malignancy in 46, XY CGD ranges from 37% to 45%.3 Radiological features are not well established; however, most tumours appear solid and may demonstrate heterogeneity and mottled calcification.4
Here, we present the case of a 16-year-old subject, reared as a girl, who presented with progressive virilisation, lack of development of secondary sexual characteristics and primary amenorrhea. Based on a detailed biochemical, radiological and cytogenetic evaluation, a diagnosis of 46, XY CGD with virilising germ cell tumour was considered. The diagnosis was confirmed on surgical exploration, and subsequent histopathology. Our case highlights a rare presentation of Swyer syndrome and the need to consider early prophylactic gonadectomy in patients affected with this rare condition.
Case presentation
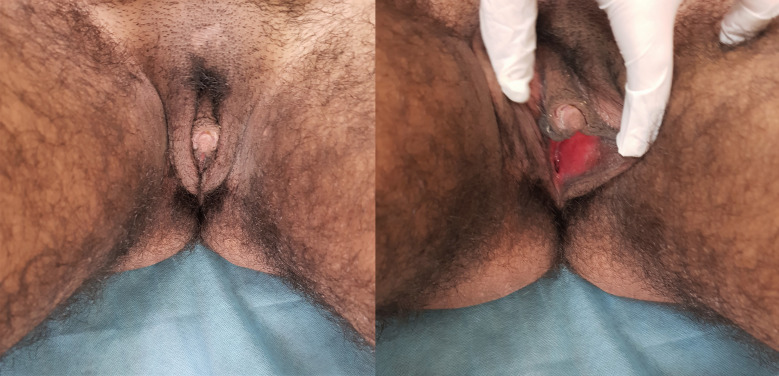
A 16-year-old subject, reared as a girl, presented with a history of progressive virilisation since the age of 12 years. She noticed increased growth of dark, terminal hair initially over her face and subsequently over the rest of her body: chest, arms, legs, abdomen and back. She also noted increased clitoral size and heaviness of her voice. She had poor breast development, and did not attain menarche. The patient was borne of a non-consanguineous marriage and there was no history of genital ambiguity at birth or antenatal maternal virilisation. Her medical history and family history were unremarkable. She denied exposure to exogenous androgen supplements. Examination revealed a young girl with a height of 166 cm, weight of 54 kg and body mass index of 19.6 kg/m2. She had severe generalised hirsutism with modified Ferriman-Gallwey score of 36/36, acneiform eruptions, increased muscle bulk and low pitch voice (figure 1). Breast development was absent (Tanner stage B1), while pubic hair development corresponded to Tanner stage 5. Genital examination revealed clitoromegaly with clitoral length of 25 mm and width of 12 mm (figure 2). Posterior labial fusion was absent and three separate openings could be visualised in the perineum: vaginal, urethral and anal. Rest of her general and systemic examination was unremarkable.
Figure 1.
Clinical photograph showing generalised hirsutism involving the face, chest and abdomen and poor breast development.
Figure 2.
Clinical photographs showing clitoromegaly with the absence of posterior labial fusion and separate urethral and vaginal openings.
Investigations
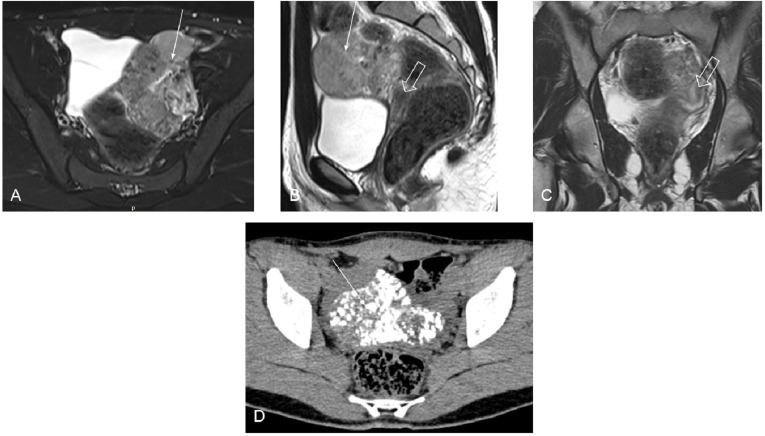
Her complete blood count, liver and kidney function tests were unremarkable. She had an elevated male-range serum testosterone of 24.9 nmol/L (N: 0.28–1.67 nmol/L) with low level of serum gonadotropins: luteinising hormone (LH) of 1.0 IU/L (N: 2.4–12.6 IU/L) and follicle stimulating hormone (FSH) of 0.9 IU /L (N: 3.5–12.5 IU/L). Serum 17β-estradiol was also low at 21.3 pmol/L. Her thyroid function tests, serum 8 am cortisol, serum prolactin, serum dehydroepiandrosterone sulfate (DHEAS) and serum insulin-like growth factor-1 (IGF-1) were normal. We proceeded with abdominal and pelvic imaging to exclude a neoplastic virilising mass lesion. Ultrasonography revealed the presence of hypoplastic uterus and a large mass lesion with variable echogenicity and internal vascularity in the left iliac region. No tract or communication was seen between rectum and uterus or cervix. Vagina was normal in calibre and signal intensity; ovaries could not be visualised. MRI and CT scan of the abdomen and pelvic region were performed next to confirm the findings. MRI revealed the presence of a large multilobulated heterogeneously T2 hyperintense mass (with multiple hypointense areas) of size 8.3×7.5×4.3 cm in the left iliac fossa (figure 3). The mass showed heterogeneous enhancement on postcontrast images and diffusion restriction on diffusion-weighted sequences. Additionally, chunky calcification was noted throughout the mass on non-contrast CT images (figure 3). Considering the possibility of a virilising germ cell tumour, tumour markers were obtained: serum beta-human chorionic gonadotropin (β-hCG) 29.95 IU/L (N: <5 IU/L), serum alpha-fetoprotein (AFP) 1.72 ng/mL (N: 0.89–8.78 ng/mL) and serum lactate dehydrogenase (LDH) 939 IU/L (N: 114–240 U/L). Since germ cell tumours are known to arise from immature gonocytes in the setting of a dysgenetic gonad, chromosomal analysis (GTG banding) was also performed, revealing a 46, XY karyotype. A diagnosis of 46, XY CGD with a virilising germ cell tumour was, therefore, considered.
Figure 3.
MRIs (A–C) showing a heterogeneous mass lesion (thin arrows) in the left adnexal region and hypoplastic uterus (thick arrows). Extensive calcification within the mass lesion can be seen on CT scan (D).
Differential diagnosis
Our patient presented with pubertal virilisation, lack of development of secondary sexual characteristics and primary amenorrhea. She had elevated male-range serum testosterone, low serum estradiol and low gonadotropins, while the rest of her hormone profile was normal. The following differentials were considered: virilising ovarian or adrenal neoplasm, ovarian hyperthecosis, congenital adrenal hyperplasia, exogenous androgen use, aromatase deficiency and complete androgen insensitivity syndrome.5–10 The finding of a normal serum DHEAS level, the onset of hyperandrogenism at gonadarche rather than pubarche and normal adrenal imaging findings excluded the possibility of congenital adrenal hyperplasia. Similarly, the absence of adrenal mass lesion on imaging and normal adrenocortical hormone profile excluded virilising adrenal mass, while the presence of suppressed gonadotropins and imaging evidence of a localised mass lesion excluded ovarian hyperthecosis. A history of exogenous androgen use was firmly denied by the patient. Patients with aromatase deficiency are known to have a similar clinical presentation; however, they have biochemical evidence of hypergonadotropic hypogonadism (elevated LH, FSH and low estradiol). Besides, genital ambiguity at birth and antenatal maternal virilisation is often seen in aromatase deficiency, which was not the case here. Finally, although girls with complete androgen insensitivity syndrome present with primary amenorrhea and male-range serum testosterone, they have normal breast development, lack features of pubertal virilisation and demonstrate evidence of elevated gonadotropins (LH>FSH) on biochemical evaluation. Our patient had imaging evidence of a large abdominopelvic mass lesion on the left side. Since germ cell tumours (gonadoblastoma/dysgerminoma) arising from immature germ cells in dysgenetic testis are also known to result in virilisation, we performed chromosomal study, revealing a 46, XY karyotype. Therefore, we made a diagnosis of 46, XY CGD (Swyer syndrome) with virilising germ cell tumour and proceeded for surgery after taking informed consent from the patient’s family.
Treatment
Patient underwent exploratory laparotomy under general anesthesia—the surgeon identified a streak gonad on the right side and a large mass lesion on the left side, both of which were excised. Her postoperative course was uneventful. On postoperative day 2, serum testosterone reduced to 0.76 nmol/L, while serum LH and FSH increased to 9.97 IU/L and 35.44 IU/L, respectively. On pathological examination, the left gonadal tumour measured 9.5×7.0×4.0 cm. It was encapsulated, greyish white in colour, and showed multiple foci of calcification. The examined sections showed features of dysgerminoma and the tumour cells were immunopositive for CD117 and SALL4. Additionally, foci of gonadoblastoma with hyalinisation and calcification were also noted. The examined sections from the right streak gonad also showed features of gonadoblastoma. These findings confirmed our preoperative diagnosis.
Outcome and follow-up
At a follow-up visit 4 months later, the patient reported no recurrence of symptoms, and her serum testosterone declined to 0.55 nmol/L, while serum FSH increased to 137 IU/L. She was started on pubertal induction with estradiol valerate 0.5 mg/day, with plan for gradual dose uptitration and eventual addition of progesterone for withdrawal bleeding.
Discussion
46, XY CGD was first described in 1955 by Swyer in two phenotypic women with 46, XY karyotype and unambiguously female external genitalia, tall stature, primary amenorrhea and normal Müllerian structures.1 The incidence of this rare condition has been estimated at around 1 in 80 000 live births.2 Unlike partial gonadal dysgenesis, genital ambiguity at birth is not seen in Swyer syndrome due to lack of androgen production by dysgenetic gonads in the fetal life. The diagnosis is often delayed till adolescence, when affected patients present with delayed puberty and primary amenorrhea.11 The median age of onset of thelarche and menarche in apparently healthy Indian girls are 10.8 years and 12.4 years, respectively.12 Considering these data, the presentation in our case was delayed by at least 4–5 years. An earlier presentation and diagnosis could have guided prophylactic gonadectomy before the actual tumour development.
The clinical presentation and hormonal profile in the index case were not typical of Swyer syndrome. Elevated levels of gonadotropins are typically seen in such patients; however, secretion of high level of androgens by gonadoblastoma could have resulted in suppression of gonadotropins in this case. It is known that individuals with gonadal dysgenesis and Y-chromosome material have a high risk of developing gonadoblastoma. Bilateral gonadectomy is recommended in such cases; however, its appropriate timing remains controversial. In a study by Jiang et al, 67 patients with CGD were evaluated. Tumours were reported in 15 (22.4%) cases and included gonadoblastoma, seminoma, dysgerminoma and choriocarcinoma.13 A timely diagnosis of CGD is desirable not only to decrease the obvious risk of malignancy, but also for initiation of appropriate pubertal development and maintenance of bone health.14 Although gonadoblastoma is a benign tumour, it has the potential to transform into malignant dysgerminoma.3 This was seen in our patient who was found to harbour bilateral gonadoblastoma and left dysgerminoma.
To conclude, patients with Swyer syndrome remain at a high risk of developing gonadoblastoma and dysgerminoma from immature germ cells in the dysgenetic gonad. Our case illustrates a rare presentation of Swyer syndrome and highlights the need for early prophylactic gonadectomy in patients affected with this condition.
Patient’s perspective.
I thank my team of doctors for their efforts. While they have made me understand that this is a congenital condition, I am happy that the tumours causing masculine changes have been removed surgically. I hope my story helps clinicians better understand and manage those affected with this rare condition.
Learning points.
Swyer syndrome or 46, XY complete gonadal dysgenesis is a disorder of sex development characterised by sexual infantilism in a phenotypic female with 46, XY karyotype.
Individuals with gonadal dysgenesis and Y-chromosome material are at a high risk of developing gonadoblastoma and dysgerminoma.
The presence of dysgerminoma/gonadoblastoma should be suspected if a hitherto phenotypic female with Swyer syndrome undergoes virilisation.
Early prophylactic gonadectomy should be offered to patients affected with Swyer syndrome.
Acknowledgments
The authors thank Dr Vatsala Dadhwal (Department of Obstetrics and Gynecology, All India Institute of Medical Sciences) and her team for performing surgical intervention on the patient. We are also grateful to Dr Devasenathipathy Kandasamy (Department of Radiodiagnosis, All India Institute of Medical Sciences) and Dr Yashdeep Gupta (Department of Endocrinology and Metabolism, All India Institute of Medical Sciences) for their help in managing the patient.
Footnotes
Twitter: @hiya21288
Contributors: SA, HB, AG and RK diagnosed and managed the patient, did the literature search and drafted the manuscript. All authors reviewed and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Swyer GI. Male pseudohermaphroditism: a hitherto undescribed form. Br Med J 1955;2:709–12. 10.1136/bmj.2.4941.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michala L, Goswami D, Creighton SM, et al. Swyer syndrome: presentation and outcomes. BJOG 2008;115:737–41. 10.1111/j.1471-0528.2008.01703.x [DOI] [PubMed] [Google Scholar]
- 3.McCann-Crosby B, Mansouri R, Dietrich JE, et al. State of the art review in gonadal dysgenesis: challenges in diagnosis and management. Int J Pediatr Endocrinol 2014;2014:4. 10.1186/1687-9856-2014-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaioannou G, Sebire NJ, McHugh K. Imaging of the unusual pediatric 'blastomas'. Cancer Imaging 2009;9:1–11. 10.1102/1470-7330.2009.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolthers OD, Cameron FJ, Scheimberg I, et al. Androgen secreting adrenocortical tumours. Arch Dis Child 1999;80:46–50. 10.1136/adc.80.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bús D, Buzogány M, Nagy G, et al. Rare virilizing granulosa cell tumor in an adolescent. Mol Clin Oncol 2017;6:88–90. 10.3892/mco.2016.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forest MG, Nicolino M, David M, et al. The virilized female: endocrine background. BJU Int 2004;93(Suppl 3):35–43. 10.1111/j.1464-410X.2004.04707.x [DOI] [PubMed] [Google Scholar]
- 8.Goyal A, Malhotra R, Kulshrestha V, et al. Severe hyperandrogenism due to ovarian hyperthecosis in a young woman. BMJ Case Rep 2019;12:e232783. 10.1136/bcr-2019-232783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulun SE. Aromatase and estrogen receptor α deficiency. Fertil Steril 2014;101:323–9. 10.1016/j.fertnstert.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakes MB, Eyvazzadeh AD, Quint E, et al. Complete androgen insensitivity syndrome--a review. J Pediatr Adolesc Gynecol 2008;21:305–10. 10.1016/j.jpag.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 11.Mota BC, Oliveira LMB, Lago R, et al. Clinical profile of 93 cases of 46, XY disorders of sexual development in a referral center. Int Braz J Urol 2015;41:975–81. 10.1590/S1677-5538.IBJU.2014.0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khadgawat R, Marwaha RK, Mehan N, et al. Age of onset of puberty in apparently healthy school girls from Northern India. Indian Pediatr 2016;53:383–7. 10.1007/s13312-016-0857-5 [DOI] [PubMed] [Google Scholar]
- 13.Jiang J-F, Xue W, Deng Y, et al. Gonadal malignancy in 202 female patients with disorders of sex development containing Y-chromosome material. Gynecol Endocrinol 2016;32:338–41. 10.3109/09513590.2015.1116509 [DOI] [PubMed] [Google Scholar]
- 14.Berglund A, Johannsen TH, Stochholm K, et al. Incidence, prevalence, diagnostic delay, and clinical presentation of female 46, XY disorders of sex development. J Clin Endocrinol Metab 2016;101:4532–40. 10.1210/jc.2016-2248 [DOI] [PubMed] [Google Scholar]