Abstract
It is often said that substantial retinal ganglion cells (RGCs) are lost before glaucomatous damage is detected by standard automated perimetry (SAP). There are 4 key articles referenced to support this belief. To test the hypothesis that the 4 key articles are incorrectly cited, the publications in the first 6 months of 2019 that reference one or more of these 4 articles were examined. In particular, the degree to which the quotes from these 2019 publications accurately reflected the evidence in the 4 key articles was assessed. These quotes are inadequately supported by the data, and in some cases even by the conclusions found in the abstracts of the key articles. This is in spite of several review articles that have questioned the evidence in these key articles. Further, a case can be made that the evidence in the key articles better supports the opposite conclusion. That is, the data suggest that sensitivity loss can be seen on SAP before RGCs are missing.
Keywords: glaucoma, retinal ganglion cell, perimetry, visual fields, optical coherence tomography, OCT
It is often said that substantial retinal ganglion cells are lost/missing before glaucomatous damage is detected by standard automated perimetry (SAP). This belief has important clinical implications, as typically glaucoma is diagnosed based upon both structural (anatomical) and functional (perimetric) evidence. This perspective considers the 4 most commonly referenced articles in support of this belief.1–4 First, in spite of review articles questioning the evidence for this belief,5–7 it was my impression from talks given at annual meetings that this belief was still commonly held and that the 4 key articles mentioned above are still misinterpreted. To test this hypothesis, the journal articles published in the first 6 months of 2019 that referenced one or more of these 4 articles were examined. The quotes from these 2019 articles were then assessed to determine the degree to which they accurately reflected the evidence in the 4 key articles. Finally, the implications for the clinical diagnosis of glaucoma, in particular, and the issue of structural vs functional damage, in general, are considered.
FOUR WIDELY CITED ARTICLES IN SUPPORT OF THE COMMON BELIEF
The first column of Table 1 contains the authors and year of publication of the 4 key articles1–4 that are often referenced in support of the general belief that retinal ganglion cell (RGC) loss precedes loss seen with perimetry. According to Google Scholar, as of June 30, 2019, these 4 articles have been cited from almost 500 times in the case of ref. D4 to almost 1400 times in the case of ref A1. Further, in the first half of this year (2019), there were 61 citations to these 4 articles in 47 journal articles (next to the last column in Table 1). In 25 of these 47 publications,8–32 one or more of the 4 key articles were cited in the context of structural and functional damage in glaucoma, for a total of 33 citations (last column in Table 1). (The other 22 publications mentioned other aspects of the 4 articles that do not bear on structure vs. function.) All relevant quotes from the 25 publications8–32 can be found in Table S1. It is safe to say that these statements support the assertion that it is generally believed that RGC or RGC axon loss precedes visual field (VF) loss. Further, not a single quote questioned this common belief, in spite of published work that has.5–7 Let’s take a closer look at the 4 commonly cited articles to evaluate the evidence for these quotes. (See also refs 5–7.)
Table 1.
Number of citations of 4 widely referenced articles
Quigley, Addicks, Green (1982)1
This ground-breaking study counted axons in post-mortem optic nerve head tissue from 5 eyes of 4 patients and 5 eyes of 5 “normal” individuals. The abstract concluded (Table 2, second column): “Definite loss of axons occurs prior to reproducible visual field defects in some patients suspected of having glaucoma.” In fact, this conclusion is based upon the results from two eyes. As the authors say: “The two of the three nerves from eyes suspected of having glaucoma with normal visual field test results had fewer than normal number of axons. In the most obvious case, only 60% of the normal number of fibers remained.”1
Table 2.
Relevant quotes from publications citing the 4 commonly referenced articles
| Publication | Article | Quote from publication citing one or more of the 4 articles | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 8 | X | - | - | - | “Quigley et al. found that VF sensitivity began to decline soon after the initial loss of RGCs in a sample of 6 cadaver eyes.” |
| 9 | X | - | - | - | “Indeed, it is recognized that approximately 30–50% of retinal ganglion cells are lost before visual field defects are detected with automated perimetry” |
| 10 | X | - | - | - | “At least 40% of RGCs are lost before the VFs show a defect, hence the emphasis is on early diagnosis.” |
| 11 | X | - | - | - | “From 30% to 50% of the retinal ganglion cells from an area of the VF can be lost prior to detecting a VF defect by SAP.” |
| 12 | X | X | - | X | “Previous studies indicate that VF defect may not be clinically detectable until 25 to 35% of all RGCs are lost.” |
| 13 | X | - | - | - | “Approximately 40% of the optical fibers may not be accompanied by the slightest anomaly in Goldmann’s field of vision.” |
| - | X | - | - | “This is percentage is lower, of the order of 10 to 20% when the visual field is measured in automated perimetry.” | |
| 14 | - | X | - | - | “. . . substantial RGC loss can occur before perimetric loss becomes manifest” |
| 15 | - | X | X | - | “Clinically speaking, at least 25% to 50% of RGCs must be lost before significant visual field (VF) defects appear on automated perimetry testing” |
| 16 | - | X | X | X | “. . . studies have found that, by the time visual field abnormalities are detectable on static perimetry, 20–50% of the RGC is already damaged” |
| 17 | - | X | - | - | “Glaucomatous axonal loss and associated structural changes have long been known to precede the manifestation of VF defects, as measured with standard automated perimetry (SAP).” |
| 18 | - | X | - | - | “It has been shown that 35% to 50% of RGCs may die before changes in visual field tests are detected.” |
| 19 | - | X | - | - | “. . . about 40% of RGCs will be lost before signs of damage can be perceived through perimetry.“ |
| 20 | - | X | X | X | “Indeed, many studies reported that a high percentage of retinal ganglion cells (from 25 to 50%) are already dead when a visual field defect is detectable.” |
| 21 | - | - | X | - | “In many eyes, as many as 30%–50% of GCs may be lost before detection by standard visual field testing.” |
| 22 | - | - | X | - | “A reduction of at least 25% of the RGC complex is required to produce a corresponding statistical abnormality on automated perimetry.” |
| 23 | - | - | X | - | “Glaucoma usually remains asymptomatic until late-stage disease, when about 30% of the RGCs are lost. Then, abnormalities in automated visual field testing can be noted.” |
| 24 | - | - | X | - | “The subtle onset of the disease usually leads to a late diagnosis, since visual field defects are often first detectable by visual field testing when 25–35% of RGCs are already lost.” |
| 25 | - | - | X | - | “this disease leads to a progressive degeneration of the RGCs and subsequent visual field loss” |
| 26 | - | - | X | - | “At present, perimetry can only be used to identify a visual field defect when 40–50% of the RGC population has been lost.” |
| 27 | - | - | X | - | “. . . discordance between the loci of structural and functional loss has been widely reported, where observable structural loss largely preceded measurable functional loss.” |
| 28 | - | - | X | - | “25–30% of ganglion cell loss happens before it can be detected on field test.” |
| 29 | - | - | X | - | Moreover, the visual field defect cannot be detected until 30%–50% of RGCs are lost. |
| 30 | - | - | X | - | Previous studies have shown that depletion of RGCs can occur before clinical detection of VF defects. |
| 31 | - | - | X | “. . . 30–40% of retinal nerve fiber layer (RNFL) death takes place before it can manifest a visual field defect.” | |
| 32 | - | - | - | X | Even in mild glaucoma without any visual field defect, approximately 50% of ganglion cells are already lost. |
Red: this quote cited the wrong article and/or there was no evidence to support this quote in abstract of article they cited.
Orange: this quote incorrectly summarizes, and/or overstates, the conclusion in the abstract of the cited article.
Yellow: this quote agrees with the abstract of the article cited, but the results of the cited article provide weak, or no, support for the quote.
Green: this quote is an accurate, or a reasonably accurate, statement of results
Contrast the authors’ quotes with the citations in Table S1, article A, references 8–13. Of the 6 publications citing article A, only one (a) is shown with a ‘green X’, indicating that ‘this quote is an accurate, or a reasonably accurate, statement of results’ (see key to Table S1), although even this publication could have noted that the conclusion in article A was based upon 2 eyes, not all 6. More important, 2 articles (10 and 13) are coded with an “orange X”, indicating that ‘this quote incorrectly summarizes, and/or overstates, the conclusion in the cited article.’ For example, reference 10 stated, “At least 40% of RGCs are lost before the VFs show a defect, hence the emphasis is on early diagnosis.” However, only one eye showed 40% loss, the other 2 with normal Goldmann fields showed a far less loss. Finally, even more remarkable, the remaining 3 of 6 publications (9, 11, 12) are coded with a ‘red X’, indicating that ‘this quote cited the wrong article and/or there was no evidence to support this quote in the article they cited.’ For example, ref. 9 states: “Indeed, it is recognized that approximately 30–50% of retinal ganglion cells are lost before visual field defects are detected with automated perimetry”. The 30–50% must come from someplace else as article A did not use automated perimetry and did not conclude “30–50%”.
Quigley, Dunkelberger, Green (1989)2
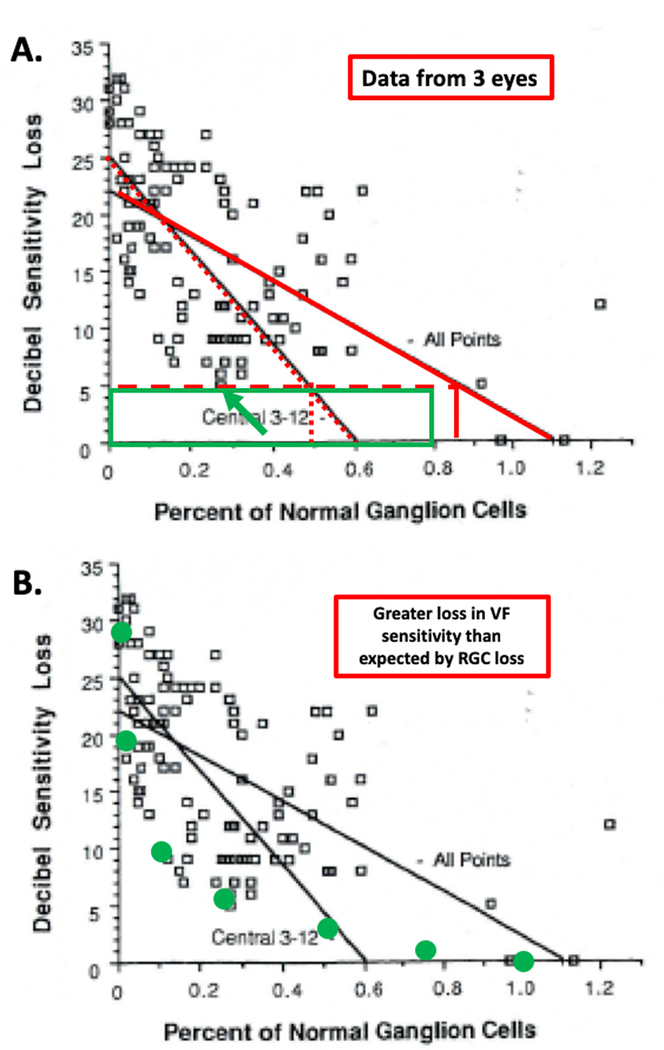
This study improved on the earlier study by counting RGCs in post-mortem histology and comparing local RGC loss to SAP VF loss in corresponding locations. The study involved 5 normal eyes and 6 eyes with glaucoma, although only 3 of the latter had data from SAP. The abstract concludes: “Throughout the central 30 degrees of the retina, 20% of the normal number of cells were gone in locations with 5-dB sensitivity loss, …” This conclusion refers to their Fig. 13, reproduced as Fig. 1 below, with the red lines and green rectangle added. These data are from the 3 eyes with SAP data and show a plot of sensitivity loss vs. RGC loss for 24, 36 or 48 VF locations, depending upon the eye. The conclusion that “20% of the normal number of cells were gone in locations with 5-dB sensitivity loss” comes from the solid red regression line. The import of a 5-dB loss is made clear in the Discussion that states a “uniform decrease of 5-dB across the entire field would be statistically abnormal.”2 The authors conclude that a 5-dB loss corresponds to a loss of 20%, see solid vertical red line. [Note: a regression line through only the points within 3 to 12° (red dotted slanting line) suggests a loss of about 50% for a 5-dB sensitivity loss (dotted vertical line).]
Figure 1.
Figure is modified from Fig. 13 of Quigley et al.2 For 3 eyes with glaucoma, local visual field (VF) sensitivity loss measured with static automated perimetry (SAP) is shown vs. the proportion of normal retinal ganglion cells (RGC) remaining, measured at the same locations from post-mortem tissue. A. the green rectangle indicates the region of visual field sensitivity within 5 dB of normal and with RGCs ranging from 0 to 80% of normal. The green arrow indicates the point falling within this region. The vertical dotted and solid red lines indicate the proportion of RGC associated with the two regression lines and a loss of 5 dB. B. The green symbols are the prediction of a linear model relating sensitivity loss to loss in RGCs.6 The points falling above the green symbols indicate a greater loss in VF sensitivity than expected by RGC loss based upon the linear model.
Of the 9 publications citing this article with relevant quotes, 4 did not accurately represent the conclusion (red X in the column under B in Table S1). In another 2 (orange X), the range (e.g., 25 to 50%) of RGC loss is misleading. Further, the remaining 3 publications are coded with a yellow X indicating that ‘this quote agrees with the abstract of the article cited, but the results of the cited article provide weak, or no, support for the quote’ that “20% of the normal number of cells were gone in locations with 5-dB sensitivity loss”.
There are two reasons for this assertion. First, there is at most one data point consistent with the conclusion that 20% of the RGCs are gone in locations with 5-dB or less sensitivity loss (i.e. ‘normal VF’). In particular, the data points consistent with this conclusion would fall within the green rectangle in Fig. 1A. At most one data point (green arrow) shows more than a 20% RGC loss, and a ‘normal’ VF (i.e. VF better than −5 dB). Second, there is a need for a null model. That is, what is the expected loss in VF for a given loss in RGCs. A number of studies33–36 have argued for a linear model relating VF sensitivity to structural loss. By linear is meant that a proportional loss in RGCs will result in the same proportional loss in sensitivity, where sensitivity is expressed on a linear scale, not in dB, which is a log scale. That is, according to the linear model, a loss of 50% of the RGCs corresponds to a 50% loss in VF sensitivity, which is a 3 dB loss. The green circles in Fig. 1B show the prediction of a linear model. Notice that all the points fall above a curve through these green circles. (The data for the central points would deviate even further from the nonlinear model of Swanson and colleagues.37–38) Based upon either model there is a greater loss in VF sensitivity than expected by RGC loss. This is the opposite of what is commonly believed.
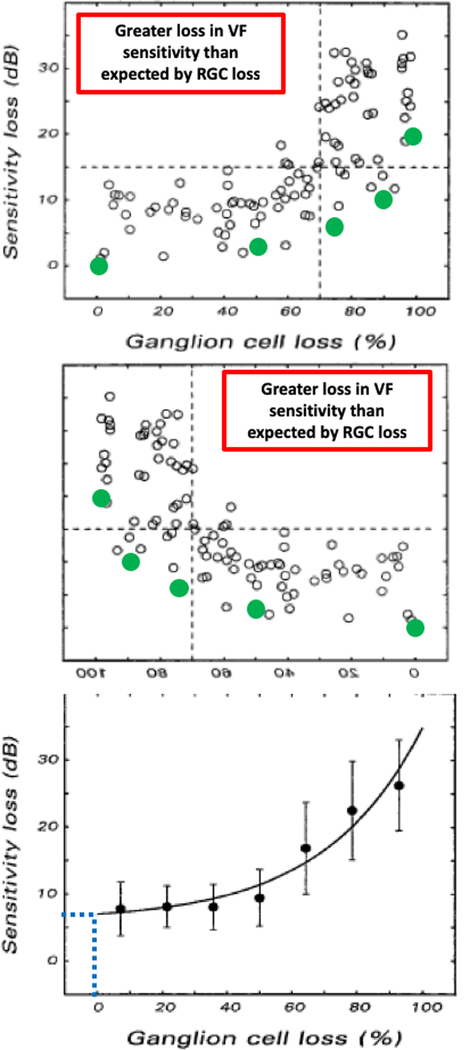
Kerrigan-Baumrind, Quigley, Pease (2000)3
Technically, this is the most complete and most impressive examination of post-mortem human data. As the authors point out, prior to their study, RGC counts had only been compared to SAP in 5 eyes with glaucoma. They included 17 eyes from 13 patients and 17 eyes from age-matched controls. The number of RGCs were measured in a number of histological sections at 28 locations in the VF. The relative RGC loss at these 28 locations was compared to the loss of SAP. They did not show a plot as in Fig. 1; note the R2 was only 0.03. They did, however, show the average data for the 17 eyes and their Fig. 3 is reproduced in Fig. 2. Figure 2B shows this figure rotated along the horizontal axis so that the y-axis indicates better VF (less loss) at the bottom of the figure as in Fig. 1. Notice that as in Fig. 1, the points fall above the prediction of the linear model, again consistent with greater loss in VF sensitivity than expected by RGC loss. Also notice that according to the regression line, 100% of normal RGC is associated with a 6 dB loss in Fig. 2A, suggesting VF loss before RGC loss - the reverse of the common belief.
Figure 3.
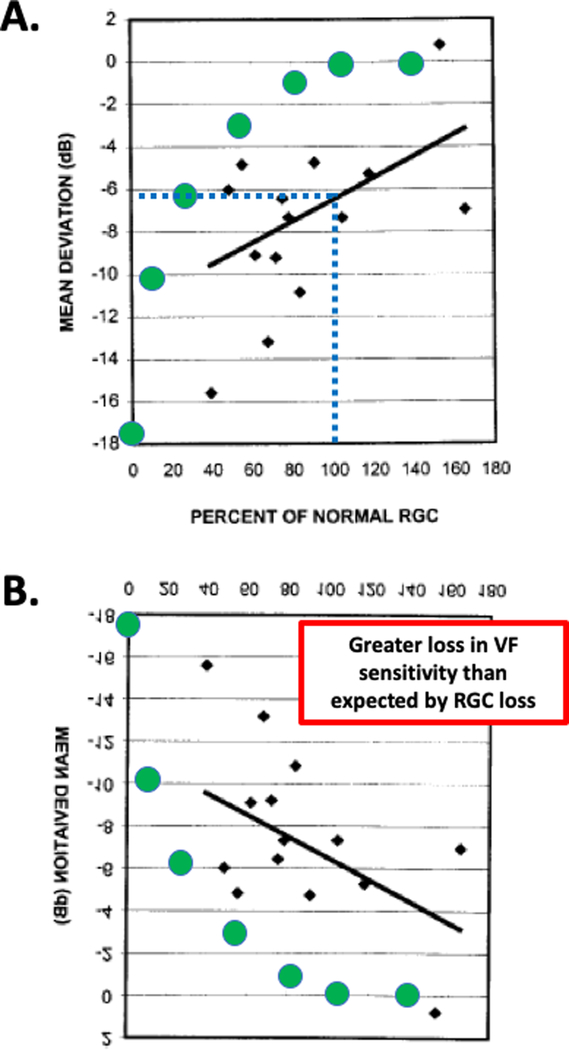
Figure is modified from Fig. 3 of Harwerth et al.4 For 10 monkey eyes with unilateral experimental glaucoma, mean deviation loss in sensitivity (dB) on SAP is shown versus the average percent loss of normal retinal ganglion cells (RGC) remaining as compared to the unaffected eye. A. The green symbols are the prediction of a linear model relating sensitivity loss to loss in RGCs.6 The points falling above the green symbols indicate a greater loss in VF sensitivity than expected by RGC loss. B. Same as in panel A, but rotated to agree with the orientation of Fig. 1. C. The mean ± SD for the data in panel A. The dotted blue lines indicate that a normal (100%) complement of RGCs is associated with about a 7 dB loss in MD.
Figure 2.
Figure is modified from Fig. 3 of Kerrigan-Baumrind et al.3 For 17 post-mortem eyes, mean deviation loss in sensitivity on SAP is shown versus the average percentage of normal retinal ganglion cells (RGC) remaining for each eye. A. The dotted blue line indicates that according to the regression line, a 6 dB loss in MD is associated with a normal (100%) complement of RGCs. The green symbols are the prediction of a linear model relating sensitivity loss to loss in RGCs.6 The points falling below the green symbols indicate a greater loss in VF sensitivity than expected by RGC loss. B. Same as in panel A, but rotated to agree with the orientation of Fig. 1.
However, their conclusion states, “At least 25% to 35% RGC loss is associated with statistical abnormalities in automated visual field testing.” This conclusion is relatively accurately reflected in 12 (yellow X) of the 14 relevant quotes in 2019 (Table S1). However, none of these are marked “green”, because the quote from the Kerrigan-Baumrind et al. abstract is not consistent with their data in Fig. 2.6,7
Harwerth, Carter-Dawson, Shen (1999)4
In this study, monkeys with unilateral experimental glaucoma, were trained to perform SAP. The post-mortem analysis compared histology (RGC counts) in the eye with experimentally induced glaucoma to the untreated control eye. Harwerth et al.4 concluded: “Current perimetry regimens with either white or monochromatic stimuli do not provide a useful estimate of ganglion cell loss until a substantial proportion have died.” Unlike the studies above, the emphasis here is on the variability of the RGC estimate, rather than the variability of the SAP. They do not say that RGCs are lost before SAP sensitivity is abnormal. In any case, all 5 of the quotes in Table S1 do not accurately reflect their conclusion (red Xs). Instead, the quotes reflect the commonly held belief.
On the other hand, the Harwerth et al. data support our working hypothesis that there is a greater loss in VF sensitivity than expected by RGC loss. Figure 3A shows their Fig. 34 and Fig. 3B the same figure flipped vertically so the x-axis agrees with Fig. 1. Notice that as in Fig. 1B and 2B, the points, in general, fall above the predictions for the linear model (green circles). Further, the experimental eye showed on average a loss of about 7 dB at 100% RGC (blue dotted line in Fig 3C), in agreement with the human data in Fig. 2A (blue dotted lines), as previously noted.6,7
A testable hypothesis
To summarize, the data in Figs 1–3 actually support an alternative hypothesis, that is, Before significantly detectable RGC loss occurs, there is a detectable loss in visual field loss as measured with perimetry. With the recent advances in imaging,39 it should soon be possible to test this hypothesis by visualizing individual RGCs in vivo.
WHAT DOES IT MEAN TO ASK WHICH COMES FIRST STRUCTURAL OR FUNCTIONAL DAMAGE IN GLAUCOMA?
“Which comes first structural or functional damage in glaucoma?” is a metaphysical question, like the classic chicken-or-egg causality dilemma in philosophy. As a metaphysical question, it only has metaphysical answers, unless it is turned into a scientifically testable hypothesis/question. To do so, the particular functional and structural test, as well as the particular measure of each, must be specified.
Thus, I am not making a general statement about structural vs functional loss. Instead, I am asserting that if by structural loss one means the number of RGCs missing in histological tissue and by functional loss one means SAP VF total deviation loss, then the evidence so far argues for functional loss before structural loss. On the other hand, the existing RGCs could be sick, but not missing, and if there were a structural correlate that measured this dysfunction, then perhaps these structural RGC changes would precede the 24–2 VF loss.
WHAT IS THE CLINICAL RELEVANCE?
Because we cannot yet see individual RGCs per se in the clinic, it is of little direct clinical significance whether or not a loss in SAP sensitivity occurs before, or after, a loss in RGCs. However, the clinical diagnosis of glaucoma does depend upon the extent to which a clinical measure of structure agrees with SAP, the commonly used functional measure in the clinic. Thus, it is critical to know the extent to which particular clinical structural and functional measures use in the clinic agree.
Recently, Hood and DeMoraes40 argued that clinically abnormal structure (aS) and abnormal functional (aF) will agree even in eyes with early glaucoma, but only if abnormal regions on OCT RGC and RNFL probability/deviation maps are compared to deviation maps for 10–2 and 24–2 VF. In fact, we recently developed an automated method to make this comparison.41 Using this method, we42 found aS-aF agreement in almost 90% of the eyes with early glaucoma. While, in general, there is aS-aF agreement, individual eyes can show damage first on OCT, and others will show it first on VF. Which is seen first will depend upon test variability and, most importantly, on the individual’s level of sensitivity and OCT thickness when healthy.6
Interestingly, our data also indicated that if one uses the typical measures of structure (i.e., OCT RNFL thickness) and function (PSD and GHT of 24–2VF), then aS occurs first more often than does aF.41
CONCLUDING THOUGHTS
There are a few lessons to be learned from the analyses here. First, in general, statements about which comes first, structural or functional damage in glaucoma, have NO MEANING, unless one specifies the question/hypothesis in terms of the test and test measures employed. Second, the common belief concerning the loss of RGC before the detection of VF damage with SAP is poorly supported by the data reported in the four most commonly cited studies. Third, the histological data appear to be better aligned with the alternative hypothesis that VF damage is seen before loss of RGCs. Finally, while it is beyond the scope here to analyze the causes of the errors in citation seen in Table S1, the psychological concept of “confirmation bias” is relevant. This term refers to “… the seeking or interpreting of evidence in ways that are partial to existing beliefs, expectations, or a hypothesis in hand.”43
Supplementary Material
Table 3.
Conclusions in Abstract of the 4 commonly cited articles.
| Ref # | From Abstract Conclusions | Comment on Results |
|---|---|---|
| A | “Definite loss of axons occurs prior to reproducible visual field defects in some patients suspected of having glaucoma.” | Two eyes showed results consistent with this conclusion. The more extreme had a normal Goldmann VF, but 40% loss of axons |
| B | “Throughout the central 30 degrees of the retina, 20% of the normal number of cells were gone in locations with 5-dB sensitivity loss,…” | First, only 3 post-mortem eyes were involved. Second, the data supply weak support for the conclusion. Third, based upon a linear model, the data suggest VF sensitivity is lost before RGCs are missing. |
| C | “At least 25% to 35% RGC loss is associated with statistical abnormalities in automated visual field testing.” | First, the data supply weak support for the conclusion. Second, based upon a linear model, the data suggest VF sensitivity is lost before RGCs are missing. |
| D | “Current perimetry regimens with either white or monochromatic stimuli do not provide a useful estimate of ganglion cell loss until a substantial proportion have died.” | First, the data support the conclusion. Second, based upon a linear model, the data suggest VF sensitivity is lost before RGCs are missing. |
Acknowledgments
Dongwon Lee helped with the literature search and he, Gus DeMoraes and Manos Tsamis provided important feedback on earlier drafts.
Financial disclosures: DCH receives lecture fees, research support, and equipment from Topcon, Inc. and Heidelberg Engineering.
Supported by: NIH/ NEI grant: EY002115
REFERENCES
- 1.Quigley HA, Addicks EM, Green WR. Optic Nerve Damage in Human Glaucoma: III. Quantitative Correlation of Nerve Fiber Loss and Visual Field Defect in Glaucoma, Ischemic Neuropathy, Papilledema, and Toxic Neuropathy. JAMA Ophthalmol. 1982;100(1):135–146. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–464. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan–Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741–748. [PubMed] [Google Scholar]
- 4.Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(10):2242–2250. [PubMed] [Google Scholar]
- 5.Garway-Heath DF, 2004. Comparison of structural and functional methods In: Weinreb RN, Greve EL (Eds.), Glaucoma Diagnosis. Structure and Function. Kugler Publications, The Hague, pp. 135–143. [Google Scholar]
- 6.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik R, Swanson WH, Garway-Heath DH. The ‘structure-function’ relationship in glaucoma: past thinking and current concepts. Clin Experiment Ophthalmol. 2012;40: 369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouri-Mahdavi K, Fatehi N, Caprioli J. Longitudinal Macular Structure-Function Relationships in Glaucoma and Their Sources of Variability. Am J Ophthalmol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr Opin Neurol. 2019;32(1):115–123. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande GA, Bawankule PK, Raje DV, et al. Linear discriminant score for differentiating early primary open angle glaucoma from glaucoma suspects. Indian J Ophthalmol. 2019;67(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morejon A, Mayo-Iscar A, Martin R, et al. Development of a new algorithm based on FDT Matrix perimetry and SD-OCT to improve early glaucoma detection in primary care. Clin Ophthalmol. 2019;13:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen MJ, Yang HY, Chang YF, et al. Diagnostic ability of macular ganglion cell asymmetry in Preperimetric Glaucoma. BMC Ophthalmol. 2019;19(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahnoux-Zabsonre A, Keita C, Safede K, et al. Prevalence of primary chronic open-angle glaucoma in Ivory Coast. J Fr Ophtalmol. 1998;21(9):643–647. [PubMed] [Google Scholar]
- 14.Nguyen AT, Greenfield DS, Bhakta AS, et al. Detecting Glaucoma Progression Using Guided Progression Analysis with OCT and Visual Field Assessment in Eyes Classified by International Classification of Disease Severity Codes. Ophthalmol Glaucoma. 2019;2(1):36–46. [DOI] [PubMed] [Google Scholar]
- 15.Gupta L, Rahmatnejad K, Gogte P, et al. Reproducibility of minimum rim width and retinal nerve fibre layer thickness using the Anatomic Positioning System in glaucoma patients. Can J of Ophthalmol. 2019;54(3):335–341. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Tanabe Y, Itoh Y, et al. Association between Combined Structure Function Index and Glaucoma Severity. J Ophthalmol. 2019;2019:9414675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouchi J, Kunikata H, Omodaka K, et al. Color visual acuity in preperimetric glaucoma and open-angle glaucoma. PLoS One. 2019;14(4):e0215290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brin TA, Tarita-Nistor L, González EG, et al. Vection Responses in Patients With Early Glaucoma. J Glaucoma. 2019;28(1):68–74. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Huang W, Li F, et al. Subcortical visual pathway may be a new way for early diagnosis of glaucoma. Med Hypotheses. 2019;123:47–49. [DOI] [PubMed] [Google Scholar]
- 20.Rolle T, Dallorto L, Cafasso R, et al. Reading Ability in Primary Open-angle Glaucoma: Evaluation with Radner Reading Charts. Optom Vis Sci. 2019;96(1):55–61. [DOI] [PubMed] [Google Scholar]
- 21.Kurokawa K, Zhang F, Crowell JA, et al. Method to track and measure loss of inner retinal neurons in the living human eye. SPIE. 2019;Vol 10858. [Google Scholar]
- 22.Chan JW, Hills NK, Bakall B, et al. Indirect Traumatic Optic Neuropathy in Mild Chronic Traumatic Brain InjuryIndirect Traumatic Optic Neuropathy (ITON). Invest Ophthalmol & Vis Sci. 2019;60(6):2005–2011. [DOI] [PubMed] [Google Scholar]
- 23.Reinehr S, Koch D, Weiss M, et al. Loss of retinal ganglion cells in a new genetic mouse model for primary open-angle glaucoma. J Cell Mol Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutgen VM, Perumal N, Pfeiffer N, et al. Autoantibody Biomarker Discovery in Primary Open Angle Glaucoma Using Serological Proteome Analysis (SERPA). Front Immunol. 2019;10(381). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miltner AM, La Torre A. Retinal Ganglion Cell Replacement: Current Status and Challenges Ahead. Dev Dyn. 2019;248(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamsher E, Davis BM, Yap TE, et al. Neuroprotection in glaucoma: old concepts, new ideas. Expert Rev Ophthalmol. 2019;14(2):101–113. [Google Scholar]
- 27.Phu J, Kalloniatis M, Wang H, et al. Optimising the Structure-Function Relationship at the Locus of Deficit in Retinal Disease. Front Neurosci. 2019;13:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George R, Patel T, Ariga M, et al. Interpretation of optical coherence tomography. TNOA J Ophthalmic Sci Res. 2019;57(1):34–48. [Google Scholar]
- 29.Xu X-Y, Xiao H, Luo J-Y, et al. Evaluation of spectral domain optical coherence tomography parameters in discriminating preperimetric glaucoma from high myopia. Int J Ophthalmol. 2019;12(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ustaoglu M, Solmaz N, Onder F. Discriminating performance of macular ganglion cell-inner plexiform layer thicknesses at different stages of glaucoma. Int J Ophthalmol. 2019;12(3):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verzosa C, Ongkeko-Perez J, Aquino M Sr. With recent advances in optic nerve imaging, are baseline optic nerve stereoscopic photos still necessary? Philipp J Ophthalmol. 2019;44:37–40. [Google Scholar]
- 32.Lee CS, Larson EB, Gibbons LE, et al. Ophthalmology-Based Neuropathology Risk Factors: Diabetic Retinopathy is Associated with Deep Microinfarcts in a Community-Based Autopsy Study. J Alzheimers Dis. 2019;68(2):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garway–Heath DF, Caprioli J, Fitzke FW, et al. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41(7):1774–1782. [PubMed] [Google Scholar]
- 34.Garway-Heath DF, Holder GE, Fitzke FW, et al. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43(7):2213–2220. [PubMed] [Google Scholar]
- 35.Hood DC, Greenstein VC, Odel JG, et al. Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol. 2002;120(12):1672–1681. [DOI] [PubMed] [Google Scholar]
- 36.Schlottmann PG, De Cilla S, Greenfield DS, et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45(6):1823–1829. [DOI] [PubMed] [Google Scholar]
- 37.Pan F, Swanson WH, Dul MW. Evaluation of a two-stage neural model of glaucomatous defect: an approach to reduce test-retest variability. Optom Vis Sci. 2006;83(7):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45(2):466–472. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Kurokawa K, Zhang F, Lee JJ, Miller DT. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc Natl Acad Sci USA. 2017;114(48):12803–12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hood DC, De Moraes CG. Four questions for every clinician diagnosing and monitoring glaucoma. J Glaucoma. 2018;27(8):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsamis E, Bommakanti N, Sun A et al. An automated method for assessing topographical structure-function agreement in abnormal glaucomatous regions. [Under Review] [DOI] [PMC free article] [PubMed]
- 42.Hood DC, Tsamis E, Bommakanti N et al. Structure-function agreement is better than commonly thought in eyes with early glaucoma. Invest Ophthalmol Vis Sci. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nickerson RS. Confirmation bias: A ubiquitous phenomenon in many guises. Rev Gen Psychol. 1998;2(2):175–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.