Abstract
Background:
As the number of older adults in the United States continues to grow, there will be increasing demands on health care providers to address the needs of this population. Cancer is of particular importance, with over half of all cancer survivors over age 65. In addition, depression, pain, and fatigue are concerns for older adults with cancer and have been linked to poorer physical outcomes.
Methods:
For this retrospective chart review, 1012 eligible participants were identified via a query of the Electronic Medical Record for all patients referred to one of four Survivorship Clinics at Memorial Sloan Kettering Cancer Center. All patients were between the ages of 30–55 (Younger Adults) or >65 (Older Adults). Depression was measured using the Patient Health Questionnaire-9 (PHQ-9).
Results:
The overall rate of depression in this sample of adult cancer survivors was 9.3%. There were no differences in the rates of clinically significant depression (defined as PHQ-9 score ≥10) between younger and older adult cohorts. However, there was a small trend toward higher mean PHQ-9 scores in the younger adult cohort (3.42 vs. 2.95; t=1.763, p=.10). Women reported greater rates of depression and higher pain and fatigue scores. Hispanic/Latino patients also reported significantly greater rates of depression.
Conclusion:
There were no observed differences in depression between older and younger adult cancer survivors. Gender and ethnic discrepancies in depression were observed. Future research should focus on understanding the nature of these differences and targeting interventions for the groups most vulnerable to depression after cancer treatment.
Keywords: cancer, depression, fatigue, gender, geriatrics, oncology, pain, PHQ-9, survivorship
INTRODUCTION
As the number of older adults in the United States and internationally continues to grow, there will be increasing demands on health care providers to address the unique needs of this population. This is of particular concern for cancer professionals as cancer is predominantly a disease of the elderly, with more than 50% of cancer survivors in the 65+ age group.1 This expansion of the older population, coupled with advancements in cancer screening and treatment, has led to an increase in the number of older-adult cancer survivors, with greater than 11 million survivors over age 65 in the United States expected by 2020.2 Yet the long-term effects of surviving this disease and the psychosocial needs of this growing cohort of older survivors are poorly understood.3
Depression is of particular clinical interest in this population, with rates of depression as high as 25% in older adults, well above the general population.4–11 Depression in older adults in community and primary care medical settings is associated with several important physical, psychosocial, and economic consequences: poor compliance, increased medical care utilization, and higher rates of mortality. 4–6, 10–15 Cancer-specific mortality has also been found to be substantially higher in older patients with a mental disorder, such as Major Depressive Disorder, compared to those without a mental disorder.16 Despite its prevalence and impact, depression is often unrecognized in elderly patients16 due to overlapping symptoms with comorbid medical illness, underreporting of depressive symptoms,7 the stigma of many physicians that depression is expected in older adults, and an atypical presentation of depressive symptoms (e.g., social withdrawal, irritability, somatic complaints, lack of motivation, or anhedonia).9 Furthermore, older adults seek mental health treatment at a rate lower than any other adult age group and are more likely to seek care from a primary care provider rather than a mental health specialist.16, 17
Emotional distress is also more common in people with cancer than in the general population, though some literature has found higher distress in younger as opposed to older cancer patients.12 This literature, however, has not teased apart the different aspects of distress (i.e., anxiety versus depression), and data in prostate cancer patients suggests that depression may actually increase with age.18 One meta-analysis of depression and anxiety in long-term cancer survivors found a prevalence of depression and anxiety of 11.6% and 17.9%, respectively, however they did not compare depression by age of survivors.19 Studies of depression in long-term cancer survivors have found that older survivors reported depression as much as 20 or even more than younger survivors.21 A better understanding of how depression affects this aging group of cancer survivors will be critical for improving cancer care in the 21st century.
In addition to depression, pain, and fatigue are important clinical outcomes in cancer survivors. There is literature to suggest that fatigue is common among cancer survivors and is linked to poorer physical and psychological outcomes, including depression and sleep disturbance.22 Pain is also common among cancer survivors, with studies suggesting a prevalence of up to 40% of survivors experiencing pain since diagnosis.23 Furthermore, older patients may somaticize depressive symptoms, suggesting pain and fatigue may be manifestations of emotional distress.9
The goal of the present study is to determine the prevalence of depression in older adult cancer survivors in relation to younger survivors, with the hypothesis that older survivors are a particularly vulnerable group to developing depressive symptoms. In addition, this study aims to compare the levels of pain and fatigue between younger and older adult cancer survivors. Finally, this study also aims to identify additional demographic (i.e., gender, ethnicity) predictors of depression, pain, and fatigue among cancer survivors.
METHODS
Participants and Procedures
This is a retrospective, cross-sectional review of EMR data collected from patients in one of four Survivorship Clinics at a cancer center in New York City between February 2008 and July 2015. The Survivorship Clinics provide cancer surveillance and health monitoring for patients who completed cancer treatment and are considered to have no evidence of disease (NED). Since this paper focuses on the broad spectrum of survivorship in different cancers, these patients will have been diagnosed in various stages of disease and have had various types of treatments. The purpose of this paper is not to examine variables such as stage of disease or impact of a specific type of treatment. The focus is on those patients who have been treated for cancer and who were NED at the time of their visit to the Survivorship Clinics.
As part of their regular Survivorship Clinic Visit, patients complete a (1) Survivor Self-Assessment Form and (2) Patient-Health Questionnaire-9 (PHQ-9), which are reviewed by a Nurse Practitioner and scanned into their EMR. We received IRB approval for a waiver of consent to abstract and analyze this archival data. Records eligible for inclusion had a Survivor Self-Assessment Form and PHQ-9 from the date of their initial Survivorship Clinic visit. Patients whose forms were incomplete or who only had forms from follow-up visits were excluded. Other eligibility criteria included: being either 30–55 (Younger Adult Cohort) or 65 and older (Older Adult Cohort) on the date of this initial Survivorship visit. We chose to define older adults as age 65 and older for several reasons, including convention in geriatrics health services research, recommendations from special reports,24 and Medicare definitions. We chose to define the younger-adult comparison group as beginning at age 30 in order to avoid the transition effects of “emerging adulthood” in the twenties, and capped the upper age limit at 55 years in order to keep the two age groups distinct and minimize any overlapping effects.
Measures
The Survivorship Self-Assessment form included information on demographic variables, exercise, past-month pain and fatigue, and health/cancer screening history. The PHQ-9 is the depression subscale of the full PHQ and includes the nine DSM-IV criteria that are used to diagnose major depressive disorder.25 Its psychometrics have been established in diverse general and medical populations.6, 26 Each item is rated 0 (not at all) to 3 (nearly every day) to indicate how much a person has been bothered by each symptom over a two-week period, and summed to produce total scores ranging from 0 to 27. A total score of ≥10 indicates clinically-significant depressive symptoms.27 Pain and fatigue were measured using the Visual Analogue Scale (VAS) in which participants circled a number between 0 and 10 indicating their self-reported level of pain and fatigue.28 In addition to these measures, we extracted additional demographic information including age, sex, race, and ethnicity from the EMR.
Data Analysis
There were not any relevant articles that reported effect sizes appropriate for this study. As this was a data abstraction from existing EMR, this study was limited by data availability rather than recruitment resources. However, recruitment of at least 500 participants in each cohort would be 80% powered to detect an effect size as small as d=0.18 based on a two tailed, independent samples t-test with 0.05 significance level.
All data were analyzed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA). We conducted descriptive analyses for demographic characteristics. Total PHQ-9 score was calculated as the sum of the 9 individual items, with up to three missing items included via substitution by the individual’s mean score. Older adults were compared to younger adults on the main study outcomes of depression, pain, and fatigue using t-tests. We examined the roles of sex and ethnic differences in these main dependent variables using χ2 tests. Differences in dependent variables across the four Survivorship Clinics (henceforth referred to as “Cancer Type”) were assessed by ANOVA. Finally, we fitted linear regression models to identify predictors of depression, pain, and fatigue, and to adjust for these effects in the assessment of a potential age-depression association. Predictor variables included: age, gender, race, ethnicity, cancer type, and marital/living status. These variables were chosen “a priori” based on the literature demonstrating a relationship between these variables and depressive symptoms.29–34 Case-wise deletion was used for missing values.
RESULTS
Sample Characteristics
This sample included 508 Older Adult and 504 Younger Adult Cancer Survivors (Table 1). The mean age of the Younger Adult cohort was 49.26 (SD=5.0) and 76.56 (SD=5.0) for the Older Adult cohort. The majority of patients (58.8%) came from the Breast Clinic, followed by Urologic (20.9%), Colorectal (12.6%), and Thoracic (7.8%) Clinics. Over half (59.7%) of patients were married and/or living with a partner. The majority of patients were White (72.9%), and 9.7% identified their ethnicity as Hispanic/Latino.
Table 1.
Demographics
| Younger Adults (N=504) | Older Adults (N=508) | All (N=1012) | Chi-square (df) | p-value | |
|---|---|---|---|---|---|
| DEMOGRAPHICS | |||||
| Age (M, SD) | 49.26 (5.0) | 76.56 (5.0) | 62.96 (14.5) | N/A | N/A |
| Females (N, %) | 400 (79%) | 379 (75%) | 779 (77%) | 3.23 (1) | 0.07 |
| Race | |||||
| White | 332 (66%) | 406 (80%) | 738 (73%) | 28.07 (3) | <0.001 |
| Black | 92 (18%) | 58 (11%) | 150 (15%) | ||
| Asian | 62 (12%) | 28 (6%) | 90 (9%) | ||
| Other/unknown | 18 (4%) | 16 (3%) | 34 (3%) | ||
| Ethnicity | |||||
| Non Hispanic/Latino | 384 (76%) | 283 (56%) | 667 (66%) | 43.56 (2) | <0.001 |
| Hispanic/Latino | 54 (11%) | 44 (9%) | 98 (10%) | ||
| Unknown | 66 (13%) | 181 (36%) | 247 (24%) | ||
| Cancer Type | |||||
| Breast | 351 (70%) | 244 (48%) | 595 (59%) | 95.28 (3) | <0.001 |
| Colorectal | 28 (6%) | 99 (19%) | 127 (13%) | ||
| Thoracic | 13 (3%) | 66 (13%) | 79 (8%) | ||
| Urologic | 112 (22%) | 99 (19%) | 211 (21%) | ||
| Marital/Living Status | |||||
| Married or living with partner | 352 (70%) | 252 (50%) | 604 (60%) | 43.18 (2) | <0.001 |
| Not married and living alone | 151 (30%) | 255 (50%) | 406 (40%) | ||
| Missing | 1 (<1%) | 1 (<1%) | 2 (<1%) | ||
Older versus Younger Adults
Depression
Using a cutoff of ≥10 on the PHQ-9 to indicate clinically-significant depression, 9.3% of the sample met criteria for depression in the past two weeks. Three patients, all in the older adult cohort, were missing more than three items on the PHQ-9 and could not be analyzed. There was no significant difference in the rates of depression between Older and Younger Adult cohorts (8.5% vs. 10.1%, χ2(1)=0.77, p=0.38). Mean PHQ-9 scores were compared across age cohorts, with younger adults having higher scores (M=3.42, SD=4.56) compared with older adults (M=2.95, SD =4.59), though this difference was not significant (t=1.63, p=0.103).
Each individual item on the PHQ-9 was then assessed independently to determine if older and younger patients experienced different components of depression at different rates. An independent samples t-test revealed that younger patients reported being bothered more frequently by one item, “Feeling bad about yourself/feeling like a failure” (t=2.53, p=0.012) when compared to older adults. This was the only significant difference found between older and younger adults when each PHQ-9 item was analyzed as a continuous variable. However, due to range and variability considerations, each item was also dichotomized into zero or non-zero values and Chi-square associations between each of the dichotomized outcomes to cohort were assessed. This investigation showed that older adults were more likely to report no impairment in feeling down (79% vs. 72%; p=0.012), sleep troubles (63% vs. 55%; p=0.014), feeling tired (55% vs. 47%; p=0.014), poor appetite (82% vs. 76%; p=0.038), feeling bad about themselves (90% vs. 82%; p<0.001), and trouble with concentration (87% vs. 81%; p=0.005) than their younger survivor counterparts. Mean PHQ-9 Total and item scores are reported by age group in Supplementary Table 1 (see online supplemental Tables).
Pain and Fatigue
Pain and fatigue scores both ranged from 0 to 10; mean pain for the sample was 1.71 (SD=2.63) and mean fatigue was 2.41 (SD=2.65). Two hundred forty-five (245) survivors (24%) were missing pain scores and 246 (24%) were missing fatigue scores. There was no significant difference in self-reported levels of pain between younger and older adults. Among the sample as a whole, people who were depressed reported significantly higher levels of both pain (4.03 vs. 1.48; t = −7.57, p < 0.001) and fatigue (5.74 vs. 2.09; F = −10.81, p < 0.001). Survivors in the younger cohort reported significantly more fatigue than older survivors (2.66 vs. 2.17; p=0.011); however this association was not sustained after adjustment for confounders.
Gender Differences
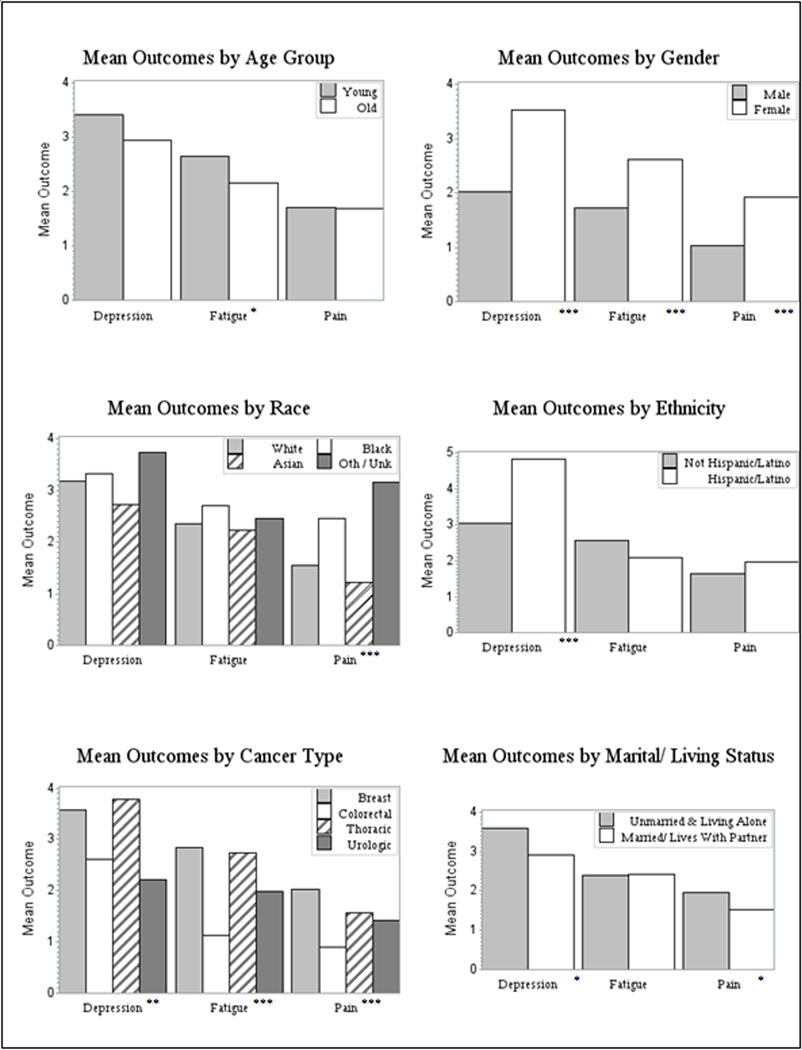
Women survivors had double the rate of clinically significant depression than men (10.5% vs. 5.2%; χ2 (1) = 6.152, p = 0.013). As depicted in Figure 1, mean PHQ-9 scores were significantly higher for women (M = 3.53, SD = 4.83) compared to men (M = 2.03, SD = 3.36; t=−4.43, p < 0.001). For individual PHQ item scores, women showed significantly higher scores (all p<0.05) for little interest or pleasure, feeling down, sleep problems, feeling tired, poor appetite, and trouble concentrating. Women also reported significantly higher levels of pain (t= 3.99, p <0.001) and fatigue (t = 4.01, p < 0.001) compared to male survivors.
Figure 1.
Mean Depression, Fatigue, and Pain Scores
Note: Significant group differences (i.e., ANOVA or t-test) on outcomes are indicated by asterisks (*). * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001.
Race and Ethnic Differences
There were no differences by race in mean PHQ-9 scores or rates of clinically significant depression in this sample. While there were no differences in fatigue scores across race, there were significant differences in mean pain scores (F(3,766) = 7.44, p < 0.001) (Figure 1). Pairwise tests indicated that mean self-reported pain scores for Black patients (M = 2.47, SD = 3.15) and Other/Unknown patients (M=3.15, SD = 3.34) were significantly different from those of White (M = 1.54, SD = 2.49) and Asian (M = 1.23, SD = 2.06) patients.
Patients who identified as ethnically Hispanic/Latino reported significantly higher rates of depression than Non-Hispanic/Latino patients (16.3% vs. 8.4%; χ2 (1, N = 765) = 6.303, p = 0.012), although ethnicity information was missing or refused for 24% of the study sample. Mean PHQ-9 scores were also significantly greater for Hispanic/Latino (M = 4.84, SD = 6.15) compared to Non-Hispanic/Latino patients (M = 3.03, SD = 4.24; t = −3.69, p < 0.001). There were no differences in pain or fatigue scores by ethnicity.
Other Correlates of Depression, Pain, and Fatigue
Cancer type was significantly associated with PHQ-9 score (F(3,1005) = 5.63, p < 0.01). Mean PHQ-9 scores for the breast cancer survivors (M=3.57, SD = 4.90) and thoracic cancer survivors (M = 3.77, SD = 5.70) were significantly different from the urologic cancer survivors (M = 2.22, SD = 3.32). There were also significant differences in pain scores (F(3,763) = 6.56, p < 0.001) and fatigue scores (F(3,762) = 14.83, p < 0.001), with survivors from the Breast clinic reporting the highest scores for pain and fatigue.
Being currently married and/or living with a partner/spouse was associated with lower rates of depression (7.1% vs. 12.6%, χ2(1) = 8.52, p = 0.004). Mean PHQ-9 scores were also lower for those who were married/living with a partner (M = 2.92, SD = 4.12) compared to those who were not (M = 3.58, SD = 5.19); t = 2.25, p = 0.03. Mean pain scores were higher for patients who were unmarried and/or living alone (M = 1.96, SD = 2.78) compared to those who were married/living with a partner (M = 1.53, SD = 2.50; t = 2.22, p = 0.03). There were no differences in fatigue scores. Tables with these data on the additional correlates of depression, pain, and fatigue are available on request.
Multivariable Regression
Given significant associations with one or more outcomes, we adjusted for the aforementioned potential covariates (age, gender, race/ethnicity, cancer type, and marital status) on our main outcome variables of depression, pain, and fatigue (Table 2). This resulted in a reduced effective sample size due to missingness in the ethnicity variable. However, an assessment of bivariate association of all predictors to each of the three outcomes showed no differential effects among this subset compared to the larger study sample.
Table 2.
Unadjusted and Adjusted Models of Depression, Pain, and Fatigue
| Covariate | Group | Depression | Pain | Fatigue | |||
|---|---|---|---|---|---|---|---|
| Unadjusted Beta | Adjusted Beta | Unadjusted Beta | Adjusted Beta | Unadjusted Beta | Adjusted Beta | ||
| Age1 | Older | −0.47 | −0.76* | −0.01 (0.97) | 0.03 | −0.49** | −0.14 |
| Gender | Female | 1.50*** | 0.69 | 0.87 *** | 0.44 | 0.90*** | 0.50 |
| Race2 | Black | 0.14 | 0.02 | 0.92*** | 0.81** | 0.35 | 0.26 |
| Asian | −0.47 | −0.11 | −0.32 | −0.18 | −0.13 | 0.03 | |
| Unk/ Oth | 0.55 | 0.41 | 1.61** | 1.65 | 0.10 | 0.56 | |
| Ethnicity | Hispanic | 1.81*** | 1.70*** | 0.34 | 0.13 | −0.49 | −0.52 |
| Cancer Type3 | Breast | 1.34*** | 0.48 | 0.61** | 0.23 | 0.85*** | 0.56 |
| Colorectal | 0.39 | −0.18 | −0.54 | −0.64 | −0.86** | −0.85* | |
| Thoracic | 1.55** | 1.81* | 0.13 | −0.10 | 0.75* | 0.70 | |
| Marital/Living Status | Married/With Partner | −0.66* | −0.88* | −0.43* | −0.42 | 0.05 | −0.08 |
| N4 | 763 – 1009 | 761 | 585 −767 | 583 | 577 – 766 | 575 | |
| R2 | − | 0.04 | − | 0.45 | − | 0.46 | |
Younger cohort (ages 30–55) used as reference group
White racial category used as reference group
Urologic clinic used as reference group
Sample size (N) ranges for unadjusted models indicate the minimum and maximum of the six models (e.g., age or gender) for the given outcome.
Note: Significant differences indicated by asterisks (*).
indicates p<0.05
indicates p<0.01
indicates p<0.001.
Depression
In fully adjusted models, a significant association was found between depression and age cohort; younger adults had a PHQ Total average of 0.76 points higher than older adults (p = 0.04) after controlling for confounders. Gender did not remain significant predictor of mean PHQ-9 Total score, with the average score for women 0.69 points higher than men (p = 0.23). With regard to cancer type, only the Thoracic cancer remained a significant predictor of depression, with a mean increase of 1.81 (p = 0.02) compared to Urologic. Breast cancer was no longer a significant predictor of depression once predictor variables were included. After adjustment, Hispanic/Latino patients had mean PHQ score 1.70 points higher than non Hispanic/Latino patients (p < 0.001). Finally, being unmarried and/or living alone also remained a significant predictor, with a score 0.88 points higher than those who were married and/or living with a partner (p = 0.02). Though the model was statistically significant, the r-square was only 0.05.
Pain
After adjustment, only race remained a significant predictor of pain scores, with Black patients having an adjusted mean of 0.81 points higher than Whites (p = 0.01). Gender, being a breast cancer survivor, and marital/living status were not significant predictors of pain after adjustment for race (Table 2).
Fatigue
In the adjusted model, age, gender, and being a breast cancer survivor all became non-significant predictors of fatigue (Table 2). Being a colorectal cancer survivor remained the only significant predictor of fatigue.
CONCLUSIONS
Clinical Implications
Our study revealed three important findings with significant clinical implications. First, we found no differences in either the prevalence or the severity of depression between younger and older adult cancer survivors; though a moderately statistically significant association emerged for depression after adjustment, the average effect was less than a one point difference between the groups. There are a few possible explanations for this finding. First, it may be possible that they PHQ-9 is an inadequate measure of depression among older adults, although previous studies on the sensitivity of brief self-report measures of depression in the elderly have been mixed. 35, 36 In addition, it is possible that our location in New York City, a busy urban setting, may preclude some of the social isolation seen in older adults who may live in less dense areas with fewer social services available. Finally, it is possible that because our sample was recruited from Survivorship Clinics, we investigated depression among a higher-functioning, more socially connected group of individuals who are still interested in attending clinic.
Interestingly, the overall rate of depression of 9.3% in this sample of cancer survivors was relatively similar to a sample of adults age 20 and older in New York City, which found a prevalence of major depression of 8% using the CIDI measure.37 It was also consistent with a previous meta analyses that found an overall prevalence of depression of 11.6% among over 50,000 pooled cancer survivors, and which also found no difference in depression rates among these cancer survivors and healthy controls.19
Secondly, we identified significant gender differences in both rates and levels of depression among cancer survivors, however this effect disappeared after adjustment for confounders. A review article on depression in cancer survivors by Massie (2004) found that the literature on gender differences in depression in cancer survivors is inconsistent, with some studies reporting no differences while others found higher rates in both men and women.31
Finally, our third important finding was the identification of higher rates of depression among Hispanic/Latino patients compared to Non-Hispanic/Latino patients. Of note, we did not have data on primary language spoken or socioeconomic status, so we are unable to determine if this observed difference is explained by other important demographic factors. Some prior literature has found rates of depression in Latina breast cancer survivors over three times that of the general population.38 However, this remains an understudied topic, especially when considering that the U.S. Hispanic population above age 65 is expected to increase over sixfold by 2050, compared to twofold for the Non-Hispanic population.39 Further investigation into the correlates of depression in this population with potential interventions designed specifically for Hispanic/Latino cancer survivors is warranted.
Collectively, these findings have important clinical implications for survivorship clinics. They suggest that routine depression screening is necessary in these clinics, and that age- and culture-appropriate (i.e., multiple languages, assessment of social isolation) screening tools are needed to pick up on depression in this population. Given the results indicating higher rates of depression in Hispanic/Latino patients, survivorship clinics should pay specific attention to this population both in screening and in the clinical encounter.
Study Limitations
The findings of this study should be considered in light of some limitations. First, the use of archival survey data from the EMR means that self-assessment forms were not completed in a controlled research environment by trained research staff, and were not always fully complete, leading to missing data. It is possible that survey responders and non-responders may differ on relevant measurements. While using existing data can be beneficial because it imposes no additional patient burden, it also means that many important correlate variables and/or predictors (e.g., social isolation, functional ability, medical comorbidities) may not be included in the dataset, limiting our ability to tease out some of the observed differences. To this end, though the adjusted models for pain and fatigue had r2 values within a normal range of other psychosocial research, the model for depression showed excessive variation, potentially indicating that another important predictor may have been omitted. Finally, our measures of pain and fatigue were only single-item measures, unlike our measure of depression.
In addition, this study relied on data gathered from patients exclusively in an urban setting who received treatment at a single institution and who were actively involved in a survivorship clinic. It is necessary replicate these findings in additional settings and include patients who are not followed in a survivorship clinic, as they may be more likely to experience social isolation. Furthermore, it is possible that the PHQ-9 was unable to pick up on the actual burden of depression experienced by the older adults, or the survivor population as a whole, where somatic complaints may be more prevalent than the affective symptoms of depression.40
This study also had some notable strengths. We had a large sample that included both older and younger cancer survivors. In addition, we conducted multivariable analyses in addition to bivariate in order to tease out the complex relationships between predictor variables and our three outcomes of interest. Finally, our use of a validated, multi-item measure of depression strengthens our findings and allows us to compare our results to other literature using this measure.
Future Directions
Overall, this study found relatively low rates of depression in cancer survivors, with no difference observed between younger and older adults. In addition, the findings of gender, racial, and ethnic differences in depression, pain, and fatigue levels among cancer survivors show possible opportunities for interventions to improve care for the survivorship population. Future work should focus on examining the burden of medical comorbidities experienced by the cancer survivor population and their relationship to depression, pain, and fatigue, which may provide useful information on the correlates of depression in this population.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by The Rosanne H. Silbermann Foundation. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA020449.
Ethical approval for this study was provided Memorial Sloan Kettering Cancer Center; approval number is WA0284-14.
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
Works Cited
- 1.Levit L, Balogh E, Nass S, Ganz PA. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington (DC)2013. [PubMed] [Google Scholar]
- 2.Medicine Io. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. In: Levit L, Balogh E, Nass S, Ganz PA, editors. Washington (DC): National Academies; 2013. [PubMed] [Google Scholar]
- 3.Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies; 2005. [Google Scholar]
- 4.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50(5):440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta psychiatrica Scandinavica. 2006;113(5):372–87. [DOI] [PubMed] [Google Scholar]
- 6.Ell K, Unutzer J, Aranda M, Sanchez K, Lee PJ. Routine PHQ-9 depression screening in home health care: depression, prevalence, clinical and treatment characteristics and screening implementation. Home health care services quarterly. 2005;24(4):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelan E, Williams B, Meeker K, Bonn K, Frederick J, Logerfo J, et al. A study of the diagnostic accuracy of the PHQ-9 in primary care elderly. BMC family practice. 2010;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. Journal of the National Cancer Institute Monographs. 2004(32):150–7. [DOI] [PubMed] [Google Scholar]
- 9.Seritan AL, McCloud MK, Hinton L. Geriatric Depression - Review for Primary Care. Current Psychiatry Reviews. 2009;5:137–42. [Google Scholar]
- 10.Sirey JA, Bruce ML, Carpenter M, Booker D, Reid MC, Newell KA, et al. Depressive symptoms and suicidal ideation among older adults receiving home delivered meals. Int J Geriatr Psychiatry. 2008;23(12):1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strine TW, Kroenke K, Dhingra S, Balluz LS, Gonzalez O, Berry JT, et al. The associations between depression, health-related quality of life, social support, life satisfaction, and disability in community-dwelling US adults. The Journal of nervous and mental disease. 2009;197(1):61–4. [DOI] [PubMed] [Google Scholar]
- 12.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113(12 Suppl):3519–29. [DOI] [PubMed] [Google Scholar]
- 13.Gantner AB, Schubert DS, Wolf SR, Creps P. Screening for depression in a geriatric rehabilitation sample. International journal of psychiatry in medicine. 2003;33(4):333–41. [DOI] [PubMed] [Google Scholar]
- 14.Glaesmer H, Riedel-Heller S, Braehler E, Spangenberg L, Luppa M. Age- and gender-specific prevalence and risk factors for depressive symptoms in the elderly: a population-based study. International psychogeriatrics / IPA. 2011;23(8):1294–300. [DOI] [PubMed] [Google Scholar]
- 15.Wittkampf K, van Ravesteijn H, Baas K, van de Hoogen H, Schene A, Bindels P, et al. The accuracy of Patient Health Questionnaire-9 in detecting depression and measuring depression severity in high-risk groups in primary care. Gen Hosp Psychiatry. 2009;31(5):451–9. [DOI] [PubMed] [Google Scholar]
- 16.Klapow J, Kroenke K, Horton T, Schmidt S, Spitzer R, Williams JB. Psychological disorders and distress in older primary care patients: a comparison of older and younger samples. Psychosom Med. 2002;64(4):635–43. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger MI, Mateo C, Sirey JA. Perceived barriers to mental health care and goal setting among depressed, community-dwelling older adults. Patient preference and adherence. 2009;3:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson CJ, Weinberger MI, Balk E, Holland J, Breitbart W, Roth AJ. The chronology of distress, anxiety, and depression in older prostate cancer patients. The oncologist. 2009;14(9):891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. The Lancet Oncology. 2013;14(8):721–32. [DOI] [PubMed] [Google Scholar]
- 20.Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. Journal of the American Geriatrics Society. 2009;57 Suppl 2:S289–92. [DOI] [PubMed] [Google Scholar]
- 21.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(12):M1119–24. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnt S, Ernst J, Singer S, Rüffer JU, Kortmann RD, Stolzenburg J, et al. Fatigue in Cancer Survivors – Prevalence and Correlates. Oncology Research and Treatment. 2009;32(6):312–7. [DOI] [PubMed] [Google Scholar]
- 23.Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, et al. Pain in cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(16):1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington DC: The National Academies; 2008. [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fann JR, Bombardier CH, Dikmen S, Esselman P, Warms CA, Pelzer E, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. The Journal of head trauma rehabilitation. 2005;20(6):501–11. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–59. [DOI] [PubMed] [Google Scholar]
- 28.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–19. [DOI] [PubMed] [Google Scholar]
- 29.Strine TW, Mokdad AH, Balluz LS, Gonzalez O, Crider R, Berry JT, et al. Depression and anxiety in the United States: findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr Serv. 2008;59(12):1383–90. [DOI] [PubMed] [Google Scholar]
- 30.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(31):3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massie MJ. Prevalence of Depression in Patients With Cancer. Journal of the National Cancer Institute Monographs. 2004;32:57–71. [DOI] [PubMed] [Google Scholar]
- 32.Apenteng BA, Hansen AR, Opoku ST, Mase WA. Racial Disparities in Emotional Distress Among Cancer Survivors: Insights from the Health Information National Trends Survey (HINTS). Journal of Cancer Education. 2016:1–10. [DOI] [PubMed] [Google Scholar]
- 33.Luckett T, Goldstein D, Butow PN, Gebski V, Aldridge LJ, McGrane J, et al. Psychological morbidity and quality of life of ethnic minority patients with cancer: a systematic review and meta-analysis. The Lancet Oncology. 2011;12(13):1240–8. [DOI] [PubMed] [Google Scholar]
- 34.Clark CJ, Fino NF, Liang JH, Hiller D, Bohl J. Depressive symptoms in older long-term colorectal cancer survivors: a population-based analysis using the SEER-Medicare healthcare outcomes survey. Supportive Care in Cancer. 2016;24(9):3907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saracino RM, Weinberger MI, Roth AJ, Hurria A, Nelson CJ. Assessing depression in a geriatric cancer population. Psycho-oncology. 2017;26(10):1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phelan E, Williams B, Meeker K, Bonn K, Frederick J, LoGerfo J, et al. A study of the diagnostic accuracy of the PHQ-9 in primary care elderly. BMC family practice. 2010;11:63-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gwynn RC, McQuistion HL, McVeigh KH, Garg RK, Frieden TR, Thorpe LE. Prevalence, diagnosis, and treatment of depression and generalized anxiety disorder in a diverse urban community. Psychiatr Serv. 2008;59(6):641–7. [DOI] [PubMed] [Google Scholar]
- 38.Holden AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2014;33(3):242–8. [DOI] [PubMed] [Google Scholar]
- 39.Vincent G, Velkoff V. The next four decades, the older population in the United States: 2010 to 2050 current population reports. . Washington DC: US Census Bureau; 2010. [Google Scholar]
- 40.Saracino RM, Rosenfeld B, Nelson CJ. Towards a new conceptualization of depression in older adult cancer patients: a review of the literature. Aging Ment Health. 2016;20(12):1230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.