Abstract
Aspergillus tamarii grows abundantly in naturally composting waste fibers of the textile industry and has a great potential in biomass decomposition. Amongst the key (hemi)cellulose-active enzymes in the secretomes of biomass-degrading fungi are the lytic polysaccharide monooxygenases (LPMOs). By catalyzing oxidative cleavage of glycoside bonds, LPMOs promote the activity of other lignocellulose-degrading enzymes. Here, we analyzed the catalytic potential of two of the seven AA9-type LPMOs that were detected in recently published transcriptome data for A. tamarii, namely AtAA9A and AtAA9B. Analysis of products generated from cellulose revealed that AtAA9A is a C4-oxidizing enzyme, whereas AtAA9B yielded a mixture of C1- and C4-oxidized products. AtAA9A was also active on cellopentaose and cellohexaose. Both enzymes also cleaved the β-(1→4)-glucan backbone of tamarind xyloglucan, but with different cleavage patterns. AtAA9A cleaved the xyloglucan backbone only next to unsubstituted glucosyl units, whereas AtAA9B yielded product profiles indicating that it can cleave the xyloglucan backbone irrespective of substitutions. Building on these new results and on the expanding catalog of xyloglucan- and oligosaccharide-active AA9 LPMOs, we discuss possible structural properties that could underlie the observed functional differences. The results corroborate evidence that filamentous fungi have evolved AA9 LPMOs with distinct substrate specificities and regioselectivities, which likely have complementary functions during biomass degradation.
Introduction
For many years, enzymatic conversion of plant polysaccharides was thought to be achieved exclusively by a consortium of hydrolytic enzymes, i.e. glycoside hydrolases (GHs) such as cellulases. In 2010, however, oxidative enzymes, today referred to as lytic polysaccharide monooxygenases (LPMOs), were also reported to contribute to the depolymerization of recalcitrant polysaccharides [1]. Since then, it has been shown that the action of these copper-dependent LPMOs decreases the recalcitrance of plant cell walls and boosts GH activity, thus enabling more efficient hydrolysis of biomass [2–7]. LPMOs are classified in the CAZy database [8] among the Auxiliary Activities (AAs), where they are currently grouped into families AA9, AA10, AA11, AA13, AA14, AA15, and AA16. LPMOs, in particular those belonging to the AA9 family, differ in terms of substrate specificity and regioselectivity. Next to cellulose, members of the AA9 family may cleave a variety of β-glucans [9, 10] as well as xylan [11, 12]. Some specifically oxidize the C1 or the C4 carbon, whereas others can oxidize both C1 and C4 carbons of the targeted β-(1→4)-glycosidic bond [2, 13, 14]. Among the AA9 LPMOs, several [9, 10, 15, 16] have been reported to be active on tamarind xyloglucan. This xyloglucan, a soluble polysaccharide, has a β-(1→4)-linked D-glucan backbone with a cellotetraose repeating unit where three out of four D-glucosyl units are substituted [17]. To date, two cleavage patterns have been reported for LPMOs regarding xyloglucan activity, which can be easily distinguished by mass spectrometric analysis. Some LPMOs yield a clustered product profile because they only cleave next to unsubstituted glucosyl units (e.g. NcAA9C [9], here referred to as “substitution-intolerant” xyloglucan-active LPMOs), while others can cleave the xyloglucan backbone irrespective of substitutions and thus produce a myriad of products (e.g. GtAA9A-2 and FgAA9A [15, 16], here referred to as “substitution-tolerant” xyloglucan-active LPMOs).
Filamentous fungi are recognized for their great potential in biomass decomposition in nature. These characteristics are utilized within many fields of the biotechnological industry, e.g. in enzyme production, biodegradation of agro-industrial wastes, and a variety of industrial fermentation processes for upgrading low-value feedstocks [18–20]. Among the frequently employed species within the genus Aspergillus, Aspergillus tamarii, a saprophyte fungus, is recognized as an efficient protease [21, 22] and xylanase [23–26] producer. So far, the (putative) LPMOs of A. tamarii have not gained much attention. With regard to putative LPMOs from aspergilli, LPMO activity has been shown, to date, for five AA9s from A. fumigatus [27, 28], seven AA9s from A. terreus [27], two AA9s [29, 30] and one AA13 [31] from A. nidulans, an AA9 from A. niger [32], an AA11 [33] and AA13 [31] from A. oryzae, and an AA16 from A. aculeatus [34], whereas structural data are available for an AA9 (AfAA9B) from A. fumigatus [35] and an AA11 [33] and AA13 [31] from A. oryzae. So far, xyloglucan activity by these LPMOs has remained unexplored, except for one LPMO from A. nidulans (AN3046) for which xyloglucan activity has been proposed [29].
More than 90% of fungal genomes contain genes encoding LPMOs in families AA9-11, AA13-14 and/or AA16. Fungi tend to contain multiple genes encoding different members of the same LPMO family, and this is particularly true for AA9s, where gene numbers may reach more than 30 in some cases [36–38]. The functional characterization of sets of enzymes of a particular family from lignocellulolytic microorganisms is crucial for understanding their roles in the degradation of lignocellulosic biomass. In this context, this study was conducted to clone and characterize AA9 LPMOs from A. tamarii. We report here the properties of AtAA9A and AtAA9B, with different activities determined on cellulose and xyloglucan.
Materials and methods
Cloning and expression of Aspergillus tamarii LPMOs
Sequences were accessed from RNA-seq data for analysis of gene expression in Aspergillus tamarii strain BLU37 grown on steam-exploded sugarcane bagasse as carbon source [39]. In total, seven expressed AA9 genes were identified in the transcriptome datasets, with sequences of the predicted proteins listed in S1 Table in S1 Appendix. Sequences were analyzed for domains corresponding to carbohydrate-active enzymes or domains of unknown function using the dbCAN2 metaserver [40] and by multiple sequence alignment with similar domains in other proteins in the UniProt database using MUSCLE [41]. The genes encoding full-length AtAA9A, AtAA9B, and AtAA9G, excluding introns but including the native signal peptide, were codon optimized for Pichia pastoris (GenScript, Piscataway, NJ, USA). The synthetic genes were inserted into the pPink-GAP vector as previously described [42]. To generate truncated proteins containing the catalytic domain only, gene fragments encoding the AA9-domains of AtAA9A (AtAA9A-N; 702 nucleotides, encoding 234 residues) and AtAA9B (AtAA9B-N; 732 nucleotides, encoding 244 residues) were PCR amplified from the pPINK-GAP-AtAA9A and pPINK-GAP-AtAA9B constructs, respectively. PCR products were ligated into the pPINK_GAP_TaCel5A vector [42] using restriction enzymes EcoRI and Acc65I and the In-Fusion HD cloning kit (Clontech Laboratories, Mountain View, CA). The expression vectors were transformed into P. pastoris PichiaPink™ cells (Invitrogen, Carlsbad, CA, USA) and transformants screened for protein production in BMGY medium as previously described [42].
For the production of AtAA9A-N and AtAA9B-N, transformants with greatest expression for each protein were grown in 25 mL of BMGY medium (containing 1% (v/v) glycerol) in 250-mL Erlenmeyer flasks at 29°C and 200 rpm for 16 h. These pre-cultures were subsequently used to inoculate 500 mL BMGY medium (containing 1% (v/v) glycerol) in 2-L Erlenmeyer flasks, then incubated at 29°C and 200 rpm for 48 h. After 24 h of incubation, the media were supplemented with 1% (v/v) glycerol. Cells were removed by centrifugation at 8,000 g for 15 min at 4°C. The supernatants were dialyzed against 20 mM Tris-HCl pH 8.0 until they reached a conductivity of 4 mS.cm-1, then concentrated to 50 mL using a VivaFlow 200 tangential crossflow concentrator (MWCO 10 kDa, Sartorius Stedim Biotech Gmbh, Germany).
Purification and Cu(II) saturation
AtAA9A-N and AtAA9B-N were purified using a two-step purification protocol, starting with anion exchange chromatography followed by size exclusion chromatography. The concentrated broth after buffer exchange (see above) was loaded onto a 5-mL Q Sepharose FF column (GE Healthcare BioSciences AB, Sweden) equilibrated with 20 mM Tris-HCl pH 8.0. The bound proteins were eluted by applying a linear gradient from 0 to 0.5 M NaCl in the same buffer. Fractions were analyzed by SDS-PAGE and those containing AtAA9A-N or AtAA9B-N then pooled, dialyzed against 20 mM BisTris-HCl pH 6.0 and concentrated to 2 mL using Amicon Ultra centrifugal filters (MWCO 10 kDa, Merck Millipore, Carrigtwohill, Ireland). The concentrated samples were applied to a 120-mL Superdex 75 16/600 gel filtration column (GE Healthcare BioSciences AB) in 20 mM BisTris-HCl pH 6.0 supplemented with 150 mM NaCl. Protein purity was analyzed by SDS-PAGE and the fractions containing AtAA9A-N or AtAA9B-N were pooled and concentrated to 1 mL using Amicon Ultra centrifugal filters (MWCO 10 kDa, Merck Millipore), followed by sterilization by filtration through a 0.2-μm syringe filter. Protein concentrations were determined by measuring absorbance at 280 nm, using theoretical extinction coefficients calculated with the ExPASy server [43] (AtAA9A-N, 39545 M-1cm-1; AtAA9B-N, 41620 M-1cm-1). AtAA9A-N and AtAA9B-N were saturated with Cu(II) by incubating the enzymes with an excess of CuSO4 (3:1 molar ratio of copper to enzyme) for 30 min at room temperature, as described previously [44]. The solution was then loaded onto a PD MidiTrap G-25 desalting column (GE Healthcare, UK), equilibrated with 20 mM BisTris-HCl pH 6.0. Fractions containing AtAA9A-N and AtAA9B-N, eluted with 1 mL of the same buffer, were collected and stored at 4°C before further use.
In silico analysis of A. tamarii LPMOs
For phylogenetic analysis, a multiple sequence alignment of AA9 domains (without the C-terminal extension) was generated using MUSCLE [41] and the phylogenetic tree was generated using Interactive Tree of Life (iTOL) [45]. The sequence alignment of xyloglucan-active LPMOs was generated using T-Coffee’s Expresso tool [46]. Structural models of xyloglucan-active LPMOs were generated with SWISS-MODEL [47], using the templates with PDB IDs 3ZUD (for AtAA9B-N and FgAA9A-N), 4B5Q (for McAA9H), 4D7U (for MYCTH_79765 and PaAA9H-N), 4EIR (for MYCTH_100518), 4EIS (for GtAA9A-2-N, GtAA9B, and McAA9B), 4QI8 (for MYCTH_85556), 5N05 (for AtAA9A-N) and 6H1Z (for McAA9A-N and McAA9F). The images of protein structures were generated using PyMOL version 1.3 Schrödinger, LLC (New York, NY, USA).
Substrates
The following substrates were employed for exploring the activity of AtAA9A-N and AtAA9B-N: phosphoric acid swollen cellulose (PASC) prepared from Avicel PH-101 (Sigma Aldrich, St. Louis, MO, USA), as described by Wood [48]; tamarind xyloglucan, cellohexaose (Glc6), cellopentaose (Glc5), Icelandic moss lichenan, and ivory nut mannan, all obtained from Megazyme International Ireland (Wicklow, Ireland); birchwood xylan from Sigma-Aldrich.
Enzyme reactions
Reaction mixtures, in 200 μL total volumes, contained 0.2% (w/v) PASC or 1% (w/v) of the other substrates, and 1 μM of AtAA9A-N or AtAA9B-N in 50 mM of BisTris-HCl pH 6.0, supplied with 1 mM ascorbic acid as indicated. Purified recombinant cellobiose dehydrogenase (MtCDH) from Myriococcum thermophilum [49], at a concentration of 1 μM, was also used as an electron donor in reactions with PASC, instead of ascorbic acid. Samples were incubated at 37°C with shaking at 1000 rpm for 18 h. After incubation, soluble and insoluble fractions were separated using a 96-well filter plate (Merck Millipore) and a Merck Millipore vacuum manifold. C4-oxidized cello-oligosaccharide standards were produced by incubating PASC with 1 μM NcAA9C [50], using the same conditions as for AtAA9A-N and AtAA9B-N. C1-oxidized standards were generated in the same manner, using 1 μM NcAA9F [51]. Product formation was analyzed by high-performance anion-exchange chromatography (HPAEC) and MALDI-TOF mass spectrometry (MS), as described below.
Time course analysis and quantification of released oxidized products
Reaction mixtures with 0.2% (w/v) PASC, 1 μM of AtAA9 and 1 mM ascorbic acid were set up in 800 μL total volumes in 50 mM of BisTris-HCl pH 6.0 and incubated as specified above. Samples (150 μl) were collected after 20, 40, 60, 120, and 240 min of incubation and boiled at 97°C for 10 min to stop the reaction. Soluble and insoluble fractions were separated using a 96-well filter plate (Merck Millipore) and a Merck Millipore vacuum manifold. Next, 25 μl of the soluble fractions were supplemented with 1 μL TrCel7A in 150 mM Na-acetate pH 4.75 (to a final concentration of 1 μM), followed by incubation at 37°C for 18 h in order to convert the solubilized oxidized oligosaccharides to the corresponding oxidized dimers. After the incubation, the samples were incubated at 97°C for 10 min to stop the reaction. For product quantification, cellobionic acid (as C1-oxidized) and C4-oxidized dimer standards were prepared as described before [5, 52].
Analysis of enzyme products
Native and oxidized oligosaccharides were analyzed by HPAEC using a Dionex ICS-5000 system equipped with pulsed-amperometric detection (PAD) and a CarboPac PA1 analytical column with a CarboPac PA1 guard column (Dionex, Sunnyvale, CA, US). A 0.25 mL/min flow and 50-min gradient were employed as previously described [53]. Additional product analysis was performed by MALDI-TOF MS, using an Ultraflex MALDI-TOF/TOF instrument (Bruker Daltonics, Bremen, Germany) equipped with a nitrogen 337-nm laser beam, as described previously [1]. Prior to MALDI-TOF MS analysis, samples (1 μL) were spotted on an MTP 384 ground steel target plate TF (Bruker Daltonics) together with 1 μL of a saturated 2,5-dihydroxybenzoic acid solution and dried.
Results
Amino acid sequence analysis of A. tamarii AA9s
The genome of A. tamarii CBS 117626 has only recently been published, and it contains nine predicted proteins annotated as AA9 LPMOs [54]. Previous analysis of the transcriptome of A. tamarii BLU37 during cultivation on steam-exploded sugarcane bagasse as exclusive lignocellulosic carbon source [39] revealed seven expressed genes encoding putative AA9 enzymes, which we named AtAA9A, AtAA9B, AtAA9C, AtAA9D, AtAA9E, AtAA9F, and AtAA9G (see S1 Table for the predicted sequences, S2 Table for related LPMOs, including AA9s found in the A. tamarii CBS 117626 genome, as well as the closest related characterized LPMOs from aspergilli, and S3 Table for predicted properties in S1 Appendix). All seven AA9 LPMOs are secreted, as predicted using the SignalP program [55]. Of these, AtAA9D is a fragment only, AtAA9E is a single-domain LPMO, AtAA9A and AtAA9G carry a C-terminal CBM1, whereas AtAA9B, AtAA9C, and AtAA9F carry a 129-, 78- and 61-amino acid extension, respectively, at the C-terminal end, none of which are similar to any previously described domain (S1 and S3 Tables and S1 Fig in S1 Appendix). The C-terminal extension of AtAA9F seems to be a region of low complexity, while AtAA9B and AtAA9C are likely to carry small C-terminal domains of unknown function (S3 Table in S1 Appendix). Blasting the C-terminus of AtAA9B against the UniProt database (E = 0.001) resulted in 98 hits, all of which were LPMO sequences, with 96 originating from Aspergillus and Penicillium species (S1A Fig in S1 Appendix). This C-terminus potentially encodes a novel carbohydrate-binding module (CBM) that is characteristic to these species, with the sequence features are highlighted below in the Discussion below. Analysis of the C-terminus of AtAA9C against the UniProt database and the recently published A. tamarii genome [54] revealed that it may be a truncated version of a domain of unknown function. Comparison of the C-terminus of AtAA9C with the C-termini of proteins sharing >90% identity, which were all LPMOs from Aspergillus species, indicated that, in the AtAA9C sequence derived from the RNA-seq data, this domain lacks ca. 50 amino acids (S1B Fig in S1 Appendix).
Multiple sequence alignment of the AA9 domains with the AA9 LPMOs characterized to date revealed that the closest characterized relative of AtAA9A is LsAA9A, a C4-oxidizing LPMO from Lentinus similis (UniProt ID, A0A0S2GKZ1; 58% sequence identity) [56], while the closest relative of AtAA9B is TaAA9A, a well-studied C1/C4-oxidizing LPMO from Thermoascus aurantiacus (UniProt ID, G3XAP7; 71% and 69% sequence similarity, respectively) [2, 57] (S2 Fig and S2 Table in S1 Appendix). The closest characterized relatives of the other predicted LPMOs are listed in S2 Table in S1 Appendix. While sequence similarities may be indicative of regioselectivity and substrate specificity, they are not 100% predictive (S2 Fig in S1 Appendix and as discussed below).
Heterologous expression of A. tamarii AA9s
Of the seven AA9 LPMOs identified in the transcriptome of A. tamarii BLU37, five were upregulated after 48 hours when growing A. tamarii on sugarcane bagasse [39] (S2 Table in S1 Appendix), indicating a role in lignocellulosic biomass degradation. As a first step to understanding the LPMO potential of A. tamarii, we attempted to clone three of the five upregulated LPMOs, namely AtAA9A, AtAA9B and AtAA9G, with and without the C-terminal extension after the AA9 domains. AtAA9D was omitted because the sequence was incomplete, and AtAA9E was omitted because of sequence ambiguities. We successfully expressed in P. pastoris the catalytic domains of two of the three other LPMOs, namely AtAA9A-N and AtAA9B-N. The catalytic domain of AtAA9G was also expressed but in low quantities so we decided to focus on AtAA9A-N and AtAA9B-N. AtAA9A-N and AtAA9B-N were purified to homogeneity using two chromatographic steps (S3 Fig in S1 Appendix), and further characterized.
Electrophoretic analysis revealed that recombinant AtAA9A-N and AtAA9B-N had a slightly higher apparent molecular mass (27 kDa and 29 kDa, respectively) than the theoretical values (23 kDa and 24 kDa, respectively). This modest difference could be due to low levels of glycosylation. AtAA9A-N is predicted to have three potential O-glycosylation sites (Ser29, Thr37, and Thr42) and AtAA9B-N is predicted to have one potential N- (Asn135) and one potential O-glycosylation site (Ser34), as predicted by the NetNGlyc v1.0 [58] and NetOGlyc v4.0 [59] servers of the Technical University of Denmark. Based on the position of these amino acids in the predicted structure (models built with LsAA9A [PDB:5ACI] and TaAA9A [PDB:2YET], respectively; more details below), only Ser29 in AtAA9A is close to the catalytic surface, but still at a distance where an effect of a possible glycosylation on LPMO activity is unlikely.
Cellulolytic activity of AtAA9A-N and AtAA9B-N
The recombinant AtAA9A-N and AtAA9B-N were active on phosphoric acid-swollen cellulose (PASC), but with different regioselectivities (Fig 1 and S4 Fig in S1 Appendix). AtAA9A-N generated native and C4-oxidized cello-oligosaccharides only (Fig 1A), whilst AtAA9B-N generated both C1- and C4-oxidized (as well as native) cello-oligosaccharides (Fig 1B). No products were detected in control reactions without electron donor. When using MtCDH as an electron donor, MtCDH oxidized the reducing end of all solubilized cello-oligosaccharides, hence no native cello-oligosaccharides or C4-oxidized cello-oligosaccharides (or on-column degradation products thereof [60]) were detected, whereas small amounts of C1-oxidized cello-oligosaccharides were detected for both LPMOs (Fig 1A and 1B). These C1-oxidized cello-oligosaccharides, which were not observed in the control reaction without CDH and which must derive from native products generated by the LPMO, shows that CDH indeed was capable of driving the reactions with both LPMOs (Fig 1A and 1B).
Fig 1.
Oxidative cleavage of PASC by (A) AtAA9A-N and (B) AtAA9B-N. The graphs are HPAEC-PAD profiles of reaction mixtures containing 1 μM LPMO and 0.2% (w/v) PASC in 50 mM of BisTris-HCl pH 6.0, without electron donor (dotted black lines), with 1 mM ascorbic acid (AscA) as electron donor (solid black lines) or with 1 μM MtCDH as electron donor (dashed black lines), after incubation at 37°C for 18 h. For identification of C1- and C4-oxidized cello-oligosaccharides, product mixtures generated in reactions with strictly C1-oxidizing 1 μM NcAA9F (dashed grey lines) or strictly C4-oxidizing NcAA9C (solid grey lines), respectively, were used as a reference.
Incubation of AtAA9A-N and AtAA9B-N with cellohexaose (Glc6) and cellopentaose (Glc5) showed an absence of activity for AtAA9B-N (not shown), whereas AtAA9A-N displayed activity on both cello-oligosaccharides (Fig 2). AtAA9A-N generated mainly native Glc4 and Glc4gemGlc (C4-oxidized with degree of polymerization, DP, 2) and, to a smaller extent, Glc3 and Glc4gemGlc2 (C4-oxidized DP 3) from Glc6, and native Glc3 and Glc4gemGlc (C4-oxidized DP 2) from Glc5.
Fig 2.
Oxidative cleavage of cellohexaose (Glc6) (A) and cellopentaose (Glc5) (B) by AtAA9A-N. The graphs show HPAEC-PAD profiles of reaction mixtures containing 1 μM LPMO and 1% (w/v) oligomeric substrate in 50 mM of BisTris-HCl pH 6.0 with (solid black line) or without (dashed black line) ascorbic acid after incubation at 37°C for 18 h. The grey line shows native cello-oligosaccharide standards with DP 2–6.
Product formation by AtAA9A-N and AtAA9B-N over time was also assessed using PASC as a substrate. To facilitate quantification of product formation, the soluble products generated by the LPMOs were treated with a cellobiohydrolase, which converts both C1- and C4-oxidized cello-oligosaccharides to the corresponding oxidized dimers (for details, see the Materials and methods). As expected based on previous LPMO studies using the same reaction conditions (e.g. [61, 62]), both enzymes showed a linear phase of product formation followed by termination of the reaction. AtAA9A-N was faster than AtAA9B-N and reached a higher yield (AtAA9A-N, 199±9 μM in 120 min; AtAA9B-N, 38±2 μM in 60 min). In addition, product formation by AtAA9B-N leveled off sooner, after 60 min of incubation, while AtAA9A-N continued to release oxidized oligosaccharides for up to 120 min (Fig 3). The initial rates that can be estimated from the linear parts of the progress curves in Fig 3, 3.3 min-1, and 0.6 min-1 for AtAA9A-N and AtAA9B-N, respectively, are in the same range as the rates of other LPMOs working under the same conditions [63].
Fig 3. LPMO activity on cellulose over time.
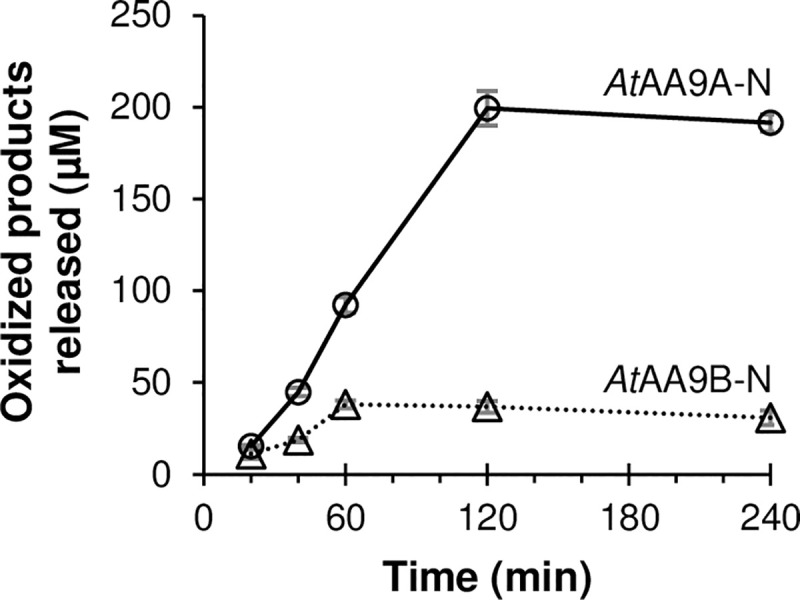
The graph shows the accumulation of soluble oxidized oligosaccharides generated from 1% (w/v) PASC by 1μM AtAA9A-N (solid line with circles) or AtAA9B-N (dotted line with triangles) in 50 mM of BisTris-HCl pH 6.0 in the presence of 1 mM ascorbic acid, over time. The reactions were incubated at 37°C and samples were collected after 20, 40, 60, 120, and 240 min of incubation.
Hemicellulolytic activity of AtAA9A-N and AtAA9B-N
AtAA9A-N and AtAA9B-N were both able to cleave tamarind xyloglucan but yielded different product mixtures (Figs 4 and 5). While the peaks in the chromatographic profiles could not be annotated due to unavailability of xyloglucan oligosaccharide standards, it is clear that these profiles are very different (Fig 4). The nature of this difference was revealed by MALDI-TOF MS analysis of the reaction products. The xyloglucan backbone contains an unsubstituted glucose (G) every four sugars, whereas the other glucoses are substituted with a pentose, xylose (X), which again may be substituted with another hexose, galactose (L) [17]. AtAA9A-N produced a clustered product profile typical for enzymes that can cleave xyloglucan only next to the unsubstituted glucose units, yielding for example a cluster of (oxidized) Hex4Pen3 (e.g. GXXX), Hex5Pen3 (e.g. GXXL) and Hex6Pen3 (e.g. GXLL) (Fig 5; [9]). From the current data, it is not possible to say whether, for example, the Hex4Pen3 product is oxGXXX or oxXXXG but previous detailed studies of NcAA9C, with a substrate-binding surface similar to that of AtAA9A-N (see below), have shown that this enzyme predominantly cleaves on the non-reducing side of a non-substituted glucose [9] and would thus, in this example, produce oxGXXX. On the other hand, AtAA9B-N produced a myriad of products indicating that xyloglucan was also cleaved in between substituted glucose units (Fig 5).
Fig 4. Oxidative cleavage of tamarind xyloglucan (TXG) by AtAA9A-N and AtAA9B-N.
The graph shows HPAEC-PAD profiles of reaction mixtures containing 1 μM AtAA9A-N (blue lines) or 1 μM AtAA9B-N (red lines) and 1% (w/v) TXG in 50 mM of BisTris-HCl pH 6.0, with (solid lines) or without (dashed lines) 1 mM ascorbic acid (AscA) after incubation at 37°C for 18 h.
Fig 5. Products generated from xyloglucan.
The picture shows MALDI-TOF MS spectra of xyloglucan-oligosaccharides released by AtAA9A-N (dark blue and light blue lines) and AtAA9B-N (red and orange lines) from tamarind xyloglucan (TXG). The indicated m/z values refer to sodium adducts of singly oxidized species (# indicates oxidation). The reactions contained 1% (w/v) TXG and 1 μM LPMO in 50 mM of BisTris-HCl pH 6.0, with (dark blue and red lines) or without (light blue and orange lines) 1 mM ascorbic acid (AscA). Reactions were incubated at 37°C for 18 h. The picture to the upper left shows xyloglucan and how it may be cleaved by AtAA9A and AtAA9B.
For both LPMOs, detected products were almost exclusively oxidized, as illustrated, e.g., by the product cluster for AtAA9A-N in Fig 5 showing oxidized GXXX (m/z 1083.5), GXXL (m/z 1245.6), and GXLL (m/z 1407.7). The relatively low signals for hydrated oxidized products (e.g. the signal at 1425.7 for GXLL) and the absence of signals representing the sodium salts of aldonic acids both suggest that oxidation of XG happened at C4 only for both LPMOs, although this cannot be concluded with certainty. Apart from activity on xyloglucan, we could not detect activity on the other hemicellulosic substrates tested (lichenan, ivory nut mannan, and birchwood xylan).
Discussion
Transcriptome analysis of A. tamarii growing on sugarcane bagasse as a carbon source revealed expression of seven AA9 LPMOs [39]. Of these, five were upregulated during the 48h growth period on this plant biomass, namely AtAA9A, AtAA9B, AtAA9D, AtAA9E and AtAA9G [39] (see also S2 Table in S1 Appendix). The differences in domain organization and variations in amino acid sequence indicate distinct roles of these LPMOs in biomass degradation. Here, we report characteristics of the catalytic domains of two of these LPMOs, AtAA9A, and AtAA9B.
AtAA9A and AtAA9B are both multi-modular enzymes. AtAA9A has a CBM1, which is commonly found attached to fungal AA9-type LPMOs. AtAA9B contains a short C-terminal domain of unknown function that seems specific to LPMOs of Aspergillus and Penicillium species. It is worth noting that the alignment of these small domains of approximately 40 residues (S1A Fig in S1 Appendix) shows a fully conserved Tyr/Trp, Trp, and His residue, i.e. residues that are often seen to contribute to the binding of carbohydrates. The differences in the C-terminal domains of the two proteins suggest that the two enzymes may target different parts of the plant cell wall.
Here, we have employed cleavage of PASC, soluble cello-oligosaccharides, and xyloglucan as a proxy for identifying functional differences. While AtAA9A-N cleaved cellulose with C4-oxidation and was active on cello-oligosaccharides (Glc6 and Glc5), AtAA9B-N cleaved cellulose with C1/C4-oxidation and was inactive on cello-oligosaccharides. Notably, AtAA9A-N produced only shorter C4-oxidized cello-oligosaccharides from PASC (Fig 1), which underpins its activity on cello-oligosaccharides, as observed before for NcAA9C [13, 50]. Initially it was postulated that LPMO activity on short cello-oligosaccharides may indicate activity on hemicelluloses that contain glucose in the polysaccharide backbone [50], which was later confirmed in multiple studies (e.g. [9, 10, 64, 65]). On the other hand, several LPMOs have been described that are inactive on soluble cello-oligosaccharides, but that are able to cleave polymeric hemicelluloses [15, 16, 57, 61, 62, 66] (Fig 6 and S4 Table in S1 Appendix). Correspondingly, despite the difference in activity towards cello-oligosaccharides, AtAA9A-N and AtAA9B-N both cleaved tamarind xyloglucan. The two AtAA9s characterized in this study show distinct cleavage patterns: AtAA9A-N exhibited substitution-intolerant cleavage, whereas AtAA9B-N exhibited substitution-tolerant cleavage. In general, the properties of AtAA9A-N resemble those of experimentally and structurally characterized NcAA9C [9, 67], LsAA9A [56] and CvAA9A [64]. The properties of the catalytic domain of AtAA9B resemble those of experimentally and structurally characterized TaAA9A [2, 57] and NcAA9M [68, 69].
Fig 6. Phylogenetic tree for AA9 LPMOs for which xyloglucan activity has been unequivocally demonstrated.
Red labels indicate LPMOs with a substitution-intolerant cleavage pattern, whereas blue labels indicate a less restricted cleavage pattern; black labels indicate that the cleavage pattern is unknown. Regioselectivity on cellulose (C1, C4, or C1/C4) and activity on soluble cello-oligosaccharides (full circle, active; cross, inactive; no sign, unknown) are indicated after the enzyme name. For more information on phylogeny, including less well characterized AA9 LPMOs, see S2 Fig in S1 Appendix.
LPMOs are prone to oxidative inactivation [70] and tend to be unstable under commonly used reaction conditions such as those employed here [71]. While in-depth assessment of LPMO stability is beyond the scope of this study, the progress curves with PASC revealed another difference between the two LPMOs: AtAA9A-N was more stable and gave higher product yields than AtAA9B-N. In both cases, the final yields (ca. 200 and 40 μM soluble oxidized products, respectively) stayed well below the theoretical maximum, which is defined by the presence of 1 mM ascorbic acid and the degree by which oxidized products become soluble [72]. It has recently been shown that efficient binding to substrate increases the redox stability of LPMOs and that removal of the CBM may lead to increased LPMO inactivation [72–75]. Thus, it is possible that the catalytic domains studied here are less stable than the full-length enzymes. Nevertheless, the difference in the progress curves of Fig 3 add to the notion that the catalytic domains of these two LPMOs have different properties.
Accumulating data for multiple LPMOs active on cello-oligosaccharides and xyloglucan, summarized in Fig 6 and S4 Table in S1 Appendix, now allow for meaningful speculation about the possible structural causes of the varying substrate specificities. In order to understand structural differences behind the distinct xyloglucan cleavage patterns and the ability to cleave soluble cello-oligosaccharides, we first aligned the sequences of xyloglucan-active AA9s with known cleavage types (S5 Fig in S1 Appendix), paying particular attention to the L2, L3, and LC loops that all contribute to shaping the substrate-binding surface [67, 69, 76] for which NMR and crystallographic studies have shown that they contain residues that are involved in binding of substrate [56, 64, 77]. Notably, existing data for LPMO–substrate interactions show that residues putatively involved in substrate binding not only occur in these three loops but also in a region between the LS and LC loops (residues from His147 to Tyr 166 for LsAA9A in S5 Fig in S1 Appendix). This was named “Seg4” in a recent study by Laurent et al. [78], based on the observation that this region is a meaningful discriminator for the phylogenetic grouping of AA9 LPMOs.
Multiple sequence alignment (S5 Fig in S1 Appendix) of xyloglucan-active AA9s showed that the substitution-tolerant XG-active AA9s have shorter L3 loop regions compared to their more restricted counterparts, with the structural effects of this difference illustrated in Fig 7 as well as in S6 and S7 Figs in S1 Appendix. Crystallographic and NMR studies have shown that the L3 loops of (substitution-intolerant) NcAA9C and LsAA9A carry multiple residues that interact with the substrate, namely His64 and Ala80 in NcAA9C [77] and His66, Asn67, Ala75 and Ser77 in LsAA9A [56]. The crystal structure of the LsAA9A–cellohexaose complex (PDB: 5ACI) reported by Frandsen et al. [56] revealed that the C6 hydroxyl of the glucose at subsite +1 is accommodated in a small pocket that is largely shaped by residues in the L3 loop, namely His1, His66, and Ala75, corresponding to His1, His64, and Ala80 in NcAA9C (Fig 7). This pocket is too small to accommodate a glucose with a xylosyl substitution at C6, as it would occur in xyloglucan [17]. In agreement with this, Agger et al. [9] concluded that NcAA9C converts the xyloglucan-oligosaccharide XG14 primarily to XXX and oxGXXXG, which implies that cleavage occurs when an unsubstituted glucose is bound to the +1 subsite. Recently, Sun et al. confirmed that NcAA9C cleaves polymeric XG predominantly by non-reducing side of a non-substituted glucose [69]. Notably, this pocket is lacking in substitution-tolerant XG-active AA9 LPMOs that have a shorter L3 loop and in which, with one exception (McAA9H), the Ala75/80 is replaced by a proline (Fig 7 and S5 Fig in S1 Appendix). This proline (or Tyr in McAA9H), which is part of a more open substrate-binding surface, may interact with a xylosyl moiety at subsite +1. Expectedly, the structural models of AtAA9A and AtAA9B (S7 Fig in S1 Appendix) correspond to their template structures LsAA9A (PDB ID, 5N05) and TaAA9A (PDB ID, 3ZUD), respectively, predicting a similar pocket formed by the corresponding residues in the L3 loop of AtAA9A and a surface-exposed proline in AtAA9B.
Fig 7.
The substrate-binding surface of (A) NcAA9C, (B) LsAA9A and (C) TaAA9A. Panels A and B show a cavity (black arrow) formed by the L3 loop of (A) NcAA9C (PDB: 4D7U) and (B) LsAA9A (PDB: 5ACI), both cleaving xyloglucan adjacent to an unsubstituted unit; panel C shows the lack of cavity and a conserved surface-exposed proline (white arrow) in TaAA9A (PDB: 2YET), being able to cleave xyloglucan between two substituted units. The cellohexaose was superposed from the LsAA9A–cellohexaose (PDB: 5ACI) structure. The same cavity is present in all other substitution-intolerant AA9 LPMOs with a known crystal structure, CvAA9A (PDB: 5NLT), NcAA9A (PDB: 5FOH) and NcAA9D (PDB: 4EIR). As to other substitution-tolerant AA9 LPMOs, to date structural data is available only for NcAA9M (PDB: 4EIS), which similarly shows the lack of cavity and a conserved surface-exposed proline. Note that panel C shows side chains of residues in the extended L2 region of substitution-tolerant TaAA9A, including aromatic Tyr24. More details of the structures are provided in S6 Fig in S1 Appendix, which shows a structural superposition.
Next to having a sterically less restrained +1 subsite, the substitution-tolerant xyloglucan-active AA9s contain insertions in their L2 loop that are absent in substitution-intolerant xyloglucan-active AA9s. Sun et al. recently suggested that this extension in the L2 loop (referred to as Seg1) combined with a shorter L3 loop (referred to as Seg2) may correlate with substitution-tolerant cleavage of XG [69]. While there is little experimental data in support of involvement of the L2 region in substrate binding, such involvement seems obvious from looking at available LPMO structures (Fig 7C and S8 Fig in S1 Appendix). The region with insertions (residues 16–40 for TaAA9A) carries one or two aromatic (Tyr or Phe) residues (S5 Fig in S1 Appendix). The structures of TaAA9A (Fig 7C) and NcAA9M, the only substitution-tolerant xyloglucan-active AA9s with a resolved crystal structure [2, 68], show that these aromatic residues may be surface exposed and thus contribute to binding of polymeric substrates. An extended substrate-binding surface could facilitate binding of a multitude of substrates, since the mere size of the interacting surface may compensate for sub-optimal interactions in one or a few subsites. Of note, while the correlations above seem quite general, and are supported by a recent comparative study by Sun et al. [69], PcAA9H is a notable exception, since its activity on XG is substitution-tolerant, while its sequence and predicted structure resemble that of substitution-intolerant XG-degrading LPMOs (Fig 6 and S5 and S8 Figs in S1 Appendix).
It is worthwhile noting that the substitution-intolerant xyloglucan-cleaving LPMOs cleave at C4, whereas the substitution-tolerant enzymes tend to show C1 and C4 oxidation (Fig 6 and S4 Table in S1 Appendix). Regioselectivity on xyloglucan has been confirmed unambiguously only for a handful of LPMOs, and the available data suggest that regioselectivity on xyloglucan corresponds to regioselectivity on cellulose [9, 15, 69, 79]. This adds to the notion that substitution-intolerant LPMOs have more restrained subsites that lead to tight and precise binding close to the catalytic copper, whereas substitution-tolerant LPMOs bind their substrate in a manner that is not disturbed by the substitutions present, leading to mixed oxidation patterns. In this respect, one might expect that the substitution-intolerant enzymes, with their smaller but potentially tighter binding substrate-binding surfaces, would be the only ones acting on soluble cello-oligosaccharides. However, while, indeed, compared to the substitution-tolerant LPMOs, a larger fraction of substitution-intolerant LPMOs cleaves soluble oligomers (Fig 6 and S4 Table in S1 Appendix), the correlation between the type of xyloglucan cleavage and the ability to cleave oligomers is far from absolute and may even not exist. As yet, it seems not possible to make meaningful predictions regarding the structural features that determine activity on soluble substrates. Such predictions await structural information for more LPMOs and more LPMO–substrate complexes.
In summary, we show that two of the AA9 LPMOs from A. tamarii have distinct substrate and product profiles, which corroborates that filamentous fungi have evolved LPMOs with diversified substrate specificities and oxidative regioselectivities and that these LPMOs likely complement each other in natural biomass degradation. Fungi are singular microorganisms that are adapted to multiple ecological niches and different conditions, thus displaying a potential for innumerable applications. Gaining a better knowledge of their enzymatic repertoire is crucial for exploiting and (re)designing their biotechnological applications.
Supporting information
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AMV was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível de Superior – Brasil (CAPES) – Finance Code 001. The authors acknowledge the receipt of financial support from the Brazilian National Council for Scientific and Technological Development (CNPq, project no. 303614/2017-0) and the Foundation for Research Support of the Federal District (FAPDF, project no. 0193.001195/2016) for EXFF. The authors thank the Research Council of Norway for financial support through the grants BioMim (project no. 243663) for DMP, VGHE and AV and Bio4Fuels (project no. 257622) for VGHE and AV. All funders provided support in the form of salaries for authors but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of the authors are articulated in the ‘Author contributions’ section.
References
- 1.Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sørlie M, et al. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science. 2010;330(6001):219–22. 10.1126/science.1192231 [DOI] [PubMed] [Google Scholar]
- 2.Quinlan RJ, Sweeney MD, Lo Leggio L, Otten H, Poulsen JC, Johansen KS, et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci USA. 2011;108(37):15079–84. 10.1073/pnas.1105776108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsberg Z, Vaaje-Kolstad G, Westereng B, Bunæs AC, Stenstrøm Y, MacKenzie A, et al. Cleavage of cellulose by a CBM33 protein. Protein Sci. 2011;20(9):1479–83. 10.1002/pro.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J, Arantes V, Pribowo A, Saddler JN. The synergistic action of accessory enzymes enhances the hydrolytic potential of a "cellulase mixture" but is highly substrate specific. Biotechnol Biofuels. 2013;6(1):112 10.1186/1754-6834-6-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller G, Várnai A, Johansen KS, Eijsink VGH, Horn SJ. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol Biofuels. 2015;8:187 10.1186/s13068-015-0376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KS. Discovery and industrial applications of lytic polysaccharide mono-oxygenases. Biochem Soc Trans. 2016;44(1):143–9. 10.1042/BST20150204 [DOI] [PubMed] [Google Scholar]
- 7.Harris PV, Xu F, Kreel NE, Kang C, Fukuyama S. New enzyme insights drive advances in commercial ethanol production. Curr Opin Chem Biol. 2014;19:162–70. 10.1016/j.cbpa.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490–D5. 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agger JW, Isaksen T, Várnai A, Vidal-Melgosa S, Willats WGT, Ludwig R, et al. Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc Natl Acad Sci USA. 2014;111(17):6287–92. 10.1073/pnas.1323629111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennati-Granier C, Garajova S, Champion C, Grisel S, Haon M, Zhou S, et al. Substrate specificity and regioselectivity of fungal AA9 lytic polysaccharide monooxygenases secreted by Podospora anserina. Biotechnol Biofuels. 2015;8:90 10.1186/s13068-015-0274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frommhagen M, Sforza S, Westphal AH, Visser J, Hinz SW, Koetsier MJ, et al. Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnol Biofuels. 2015;8:101 10.1186/s13068-015-0284-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hüttner S, Várnai A, Petrović DM, Bach CX, Kim Anh DT, Thanh VN, et al. Specific xylan activity revealed for AA9 lytic polysaccharide monooxygenases of the thermophilic fungus Malbranchea cinnamomea by functional characterization. Appl Environ Microbiol. 2019;85(23):pii: e01408–19. 10.1128/AEM.01408-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vu VV, Beeson WT, Phillips CM, Cate JHD, Marletta MA. Determinants of regioselective hydroxylation in the fungal polysaccharide monooxygenases. J Am Chem Soc. 2014;136(2):562–5. 10.1021/ja409384b [DOI] [PubMed] [Google Scholar]
- 14.Beeson WT, Phillips CM, Cate JH, Marletta MA. Oxidative cleavage of cellulose by fungal copper-dependent polysaccharide monooxygenases. J Am Chem Soc. 2012;134(2):890–2. 10.1021/ja210657t [DOI] [PubMed] [Google Scholar]
- 15.Nekiunaite L, Petrović DM, Westereng B, Vaaje-Kolstad G, Hachem MA, Várnai A, et al. FgLPMO9A from Fusarium graminearum cleaves xyloglucan independently of the backbone substitution pattern. FEBS Lett. 2016;590(19):3346–56. 10.1002/1873-3468.12385 [DOI] [PubMed] [Google Scholar]
- 16.Kojima Y, Varnai A, Ishida T, Sunagawa N, Petrović DM, Igarashi K, et al. Characterization of an LPMO from the brown-rot fungus Gloeophyllum trabeum with broad xyloglucan specificity, and its action on cellulose-xyloglucan complexes. Appl Environ Microbiol. 2016;82(22):6557–72. 10.1128/AEM.01768-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomivaara ST, Yaoi K, O'Neill MA, York WS. Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr Res. 2015;402:56–66. 10.1016/j.carres.2014.06.031 [DOI] [PubMed] [Google Scholar]
- 18.Conesa A, Punt PJ, van Luijk N, van den Hondel CA. The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol. 2001;33(3):155–71. 10.1006/fgbi.2001.1276 [DOI] [PubMed] [Google Scholar]
- 19.Nevalainen KM, Te'o VS, Bergquist PL. Heterologous protein expression in filamentous fungi. Trends Biotechnol. 2005;23(9):468–74. 10.1016/j.tibtech.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Sarrouh B, Santos TM, Miyoshi A, Dias R, Azevedo V. Up-to-date insight on industrial enzymes applications and global market. J Bioprocess Biotech. 2012;S4:002. [Google Scholar]
- 21.Anandan D, Marmer WN, Dudley RL. Isolation, characterization and optimization of culture parameters for production of an alkaline protease isolated from Aspergillus tamarii. J Ind Microbiol Biotechnol. 2007;34(5):339–47. 10.1007/s10295-006-0201-5 [DOI] [PubMed] [Google Scholar]
- 22.Boer CG, Peralta RM. Production of extracellular protease by Aspergillus tamarii. J Basic Microbiol. 2000;40(2):75–81. [Google Scholar]
- 23.El-Gindy AA, Saad RR, Fawzi EM. Purification of β-xylosidase from Aspergillus tamarii using ground oats and a possible application on the fermented hydrolysate by Pichia stipitis. Ann Microbiol. 2015;65(2):965–74. [Google Scholar]
- 24.Ferreira G, Boer CG, Peralta RM. Production of xylanolytic enzymes by Aspergillus tamarii in solid state fermentation. FEMS Microbiol Lett. 1999;173(2):335–9. [Google Scholar]
- 25.Monclaro AV, Aquino EN, Faria RF, Filho EXF, Ricart CAO, Freitas SM, et al. Characterization of multiple xylanase forms from Aspergillus tamarii resistant to phenolic compounds. Mycosphere. 2016;7(10):1554–67. [Google Scholar]
- 26.Gouda MK, Abdel-Naby MA. Catalytic properties of the immobilized Aspergillus tamarii xylanase. Microbiol Res. 2002;157(4):275–81. 10.1078/0944-5013-00165 [DOI] [PubMed] [Google Scholar]
- 27.Pierce BC, Agger JW, Zhang Z, Wichmann J, Meyer AS. A comparative study on the activity of fungal lytic polysaccharide monooxygenases for the depolymerization of cellulose in soybean spent flakes. Carbohydr Res. 2017;449:85–94. 10.1016/j.carres.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 28.de Gouvêa PF, Gerolamo LE, Bernardi AV, Pereira LMS, Uyemura SA, Dinamarco TM. Lytic polysaccharide monooxygenase from Aspergillus fumigatus can improve enzymatic cocktail activity during sugarcane bagasse hydrolysis. Protein Pept Lett. 2019;26(5):377–85. 10.2174/0929866526666190228163629 [DOI] [PubMed] [Google Scholar]
- 29.Jagadeeswaran G, Gainey L, Prade R, Mort AJ. A family of AA9 lytic polysaccharide monooxygenases in Aspergillus nidulans is differentially regulated by multiple substrates and at least one is active on cellulose and xyloglucan. Appl Microbiol Biotechnol. 2016;100(10):4535–47. 10.1007/s00253-016-7505-9 [DOI] [PubMed] [Google Scholar]
- 30.Jagadeeswaran G, Gainey L, Mort AJ. An AA9-LPMO containing a CBM1 domain in Aspergillus nidulans is active on cellulose and cleaves cello-oligosaccharides. AMB Express. 2018;8(1):171 10.1186/s13568-018-0701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo Leggio L, Simmons TJ, Poulsen JC, Frandsen KE, Hemsworth GR, Stringer MA, et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat Commun. 2015;6:5961 10.1038/ncomms6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du L, Ma L, Ma Q, Guo G, Han X, Xiao D. Hydrolytic boosting of lignocellulosic biomass by a fungal lytic polysaccharide monooxygenase, AnLPMO15g from Aspergillus niger. Ind Crops Prod. 2018;126:309–15. [Google Scholar]
- 33.Hemsworth GR, Henrissat B, Davies GJ, Walton PH. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nat Chem Biol. 2014;10(2):122–6. 10.1038/nchembio.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filiatrault-Chastel C, Navarro D, Haon M, Grisel S, Herpoël-Gimbert I, Chevret D, et al. AA16, a new lytic polysaccharide monooxygenase family identified in fungal secretomes. Biotechnol Biofuels. 2019;12:55 10.1186/s13068-019-1394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo Leggio L, Weihe CD, Poulsen JN, Sweeney M, Rasmussen F, Lin J, et al. Structure of a lytic polysaccharide monooxygenase from Aspergillus fumigatus and an engineered thermostable variant. Carbohydr Res. 2018;469:55–9. 10.1016/j.carres.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 36.Harris PV, Welner D, McFarland KC, Re E, Navarro Poulsen J-C, Brown K, et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry. 2010;49(15):3305–16. 10.1021/bi100009p [DOI] [PubMed] [Google Scholar]
- 37.Kracher D, Scheiblbrandner S, Felice AK, Breslmayr E, Preims M, Ludwicka K, et al. Extracellular electron transfer systems fuel cellulose oxidative degradation. Science. 2016;352(6289):1098–101. 10.1126/science.aaf3165 [DOI] [PubMed] [Google Scholar]
- 38.Lenfant N, Hainaut M, Terrapon N, Drula E, Lombard V, Henrissat B. A bioinformatics analysis of 3400 lytic polysaccharide oxidases from family AA9. Carbohydr Res. 2017;448:166–74. 10.1016/j.carres.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Midorikawa GEO, Correa CL, Noronha EF, Filho EXF, Togawa RC, Costa M, et al. Analysis of the transcriptome in Aspergillus tamarii during enzymatic degradation of sugarcane bagasse. Front Bioeng Biotechnol. 2018;6:123 10.3389/fbioe.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang Z, et al. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018;46(W1):W95–W101. 10.1093/nar/gky418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47(W1):W636–W41. 10.1093/nar/gkz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Várnai A, Tang C, Bengtsson O, Atterton A, Mathiesen G, Eijsink VGH. Expression of endoglucanases in Pichia pastoris under control of the GAP promoter. Microb Cell Fact. 2014;13(1):57 10.1186/1475-2859-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasteiger E, Hoogland C, Gattiker A, Duvaud Se, Wilkins MR, Appel RD, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005. p. 571–607. [Google Scholar]
- 44.Loose JS, Forsberg Z, Fraaije MW, Eijsink VGH, Vaaje-Kolstad G. A rapid quantitative activity assay shows that the Vibrio cholerae colonization factor GbpA is an active lytic polysaccharide monooxygenase. FEBS Lett. 2014;588(18):3435–40. 10.1016/j.febslet.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 45.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–W9. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang J-M, et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011;39(Web Server issue):W13–W7. 10.1093/nar/gkr245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–w303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood TM. Preparation of crystalline, amorphous, and dyed cellulase substrates. Methods Enzymol. 160: Academic Press; 1988. p. 19–25. 10.1016/0076-6879(88)59004-3 [DOI] [PubMed] [Google Scholar]
- 49.Zámocký M, Schumann C, Sygmund C, O'Callaghan J, Dobson AD, Ludwig R, et al. Cloning, sequence analysis and heterologous expression in Pichia pastoris of a gene encoding a thermostable cellobiose dehydrogenase from Myriococcum thermophilum. Protein Expr Purif. 2008;59(2):258–65. 10.1016/j.pep.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 50.Isaksen T, Westereng B, Aachmann FL, Agger JW, Kracher D, Kittl R, et al. A C4-oxidizing lytic polysaccharide monooxygenase cleaving both cellulose and cello-oligosaccharides. J Biol Chem. 2014;289(5):2632–42. 10.1074/jbc.M113.530196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kittl R, Kracher D, Burgstaller D, Haltrich D, Ludwig R. Production of four Neurospora crassa lytic polysaccharide monooxygenases in Pichia pastoris monitored by a fluorimetric assay. Biotechnol Biofuels. 2012;5(1):79 10.1186/1754-6834-5-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westereng B, Loose JSM, Vaaje-Kolstad G, Aachmann FL, Sørlie M, Eijsink VGH. Analytical tools for characterizing cellulose-active lytic polysaccharide monooxygenases (LPMOs). Methods Mol Biol. 2018;1796:219–46. 10.1007/978-1-4939-7877-9_16 [DOI] [PubMed] [Google Scholar]
- 53.Westereng B, Agger JW, Horn SJ, Vaaje-Kolstad G, Aachmann FL, Stenstrom YH, et al. Efficient separation of oxidized cello-oligosaccharides generated by cellulose degrading lytic polysaccharide monooxygenases. J Chromatogr A. 2013;1271(1):144–52. 10.1016/j.chroma.2012.11.048 [DOI] [PubMed] [Google Scholar]
- 54.Kjaerbølling I, Vesth T, Frisvad JC, Nybo JL, Theobald S, Kildgaard S, et al. A comparative genomics study of 23 Aspergillus species from section Flavi. Nat Commun. 2020;11(1):1106 10.1038/s41467-019-14051-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 56.Frandsen KE, Simmons TJ, Dupree P, Poulsen JC, Hemsworth GR, Ciano L, et al. The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat Chem Biol. 2016;12(4):298–303. 10.1038/nchembio.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrović DM, Bissaro B, Chylenski P, Skaugen M, Sørlie M, Jensen MS, et al. Methylation of the N-terminal histidine protects a lytic polysaccharide monooxygenase from auto-oxidative inactivation. Protein Sci. 2018;27(9):1636–50. 10.1002/pro.3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002;7:310–22. [PubMed] [Google Scholar]
- 59.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–88. 10.1038/emboj.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westereng B, Arntzen MØ, Aachmann FL, Várnai A, Eijsink VGH, Agger JW. Simultaneous analysis of C1 and C4 oxidized oligosaccharides, the products of lytic polysaccharide monooxygenases acting on cellulose. J Chromatogr A. 2016;1445:46–54. 10.1016/j.chroma.2016.03.064 [DOI] [PubMed] [Google Scholar]
- 61.Hegnar OA, Petrović DM, Bissaro B, Alfredsen G, Várnai A, Eijsink VGH. pH-dependent relationship between catalytic activity and hydrogen peroxide production shown via characterization of a lytic polysaccharide monooxygenase from Gloeophyllum trabeum. Appl Environ Microbiol. 2019;85(5):pii: e02612–18. 10.1128/AEM.02612-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrović DM, Várnai A, Dimarogona M, Mathiesen G, Sandgren M, Westereng B, et al. Comparison of three seemingly similar lytic polysaccharide monooxygenases from Neurospora crassa suggests different roles in plant biomass degradation. J Biol Chem. 2019;294(41):15068–81. 10.1074/jbc.RA119.008196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bissaro B, Várnai A, Røhr ÅK, Eijsink VGH. Oxidoreductases and reactive oxygen species in conversion of lignocellulosic biomass. Microbiol Mol Biol Rev. 2018;82(4):pii: e00029–18. 10.1128/MMBR.00029-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simmons TJ, Frandsen KEH, Ciano L, Tryfona T, Lenfant N, Poulsen JC, et al. Structural and electronic determinants of lytic polysaccharide monooxygenase reactivity on polysaccharide substrates. Nat Commun. 2017;8(1):1064 10.1038/s41467-017-01247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadowaki MAS, Várnai A, Jameson JK, AE TL, Costa-Filho AJ, Kumagai PS, et al. Functional characterization of a lytic polysaccharide monooxygenase from the thermophilic fungus Myceliophthora thermophila. PLoS ONE. 2018;13(8):e0202148 10.1371/journal.pone.0202148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frommhagen M, Koetsier MJ, Westphal AH, Visser J, Hinz SW, Vincken JP, et al. Lytic polysaccharide monooxygenases from Myceliophthora thermophila C1 differ in substrate preference and reducing agent specificity. Biotechnol Biofuels. 2016;9(1):186 10.1186/s13068-016-0594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borisova AS, Isaksen T, Dimarogona M, Kognole AA, Mathiesen G, Várnai A, et al. Structural and functional characterization of a lytic polysaccharide monooxygenase with broad substrate specificity. J Biol Chem. 2015;290(38):22955–69. 10.1074/jbc.M115.660183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Beeson WTt, Phillips CM, Marletta MA, Cate JH. Structural basis for substrate targeting and catalysis by fungal polysaccharide monooxygenases. Structure. 2012;20(6):1051–61. 10.1016/j.str.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun P, Laurent CVFP, Scheiblbrandner S, Frommhagen M, Kouzounis D, Sanders MG, et al. Configuration of active site segments in lytic polysaccharide monooxygenases steers oxidative xyloglucan degradation. Biotechnol Biofuels. 2020;13:95 10.1186/s13068-020-01731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bissaro B, Røhr ÅK, Müller G, Chylenski P, Skaugen M, Forsberg Z, et al. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat Chem Biol. 2017;13:1123–8. 10.1038/nchembio.2470 [DOI] [PubMed] [Google Scholar]
- 71.Eijsink VGH, Petrović D, Forsberg Z, Mekasha S, Røhr ÅK, Várnai A, et al. On the functional characterization of lytic polysaccharide monooxygenases (LPMOs). Biotechnol Biofuels. 2019;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Courtade G, Forsberg Z, Heggset EB, Eijsink VGH, Aachmann FL. The carbohydrate-binding module and linker of a modular lytic polysaccharide monooxygenase promote localized cellulose oxidation. J Biol Chem. 2018;293(34):13006–15. 10.1074/jbc.RA118.004269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forsberg Z, Bissaro B, Gullesen J, Dalhus B, Vaaje-Kolstad G, Eijsink VGH. Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J Biol Chem. 2018;293(4):1397–412. 10.1074/jbc.M117.817130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loose JSM, Arntzen MO, Bissaro B, Ludwig R, Eijsink VGH, Vaaje-Kolstad G. Multipoint precision binding of substrate protects lytic polysaccharide monooxygenases from self-destructive off-pathway processes. Biochemistry. 2018;57(28):4114–24. 10.1021/acs.biochem.8b00484 [DOI] [PubMed] [Google Scholar]
- 75.Chalak A, Villares A, Moreau C, Haon M, Grisel S, d'Orlando A, et al. Influence of the carbohydrate-binding module on the activity of a fungal AA9 lytic polysaccharide monooxygenase on cellulosic substrates. Biotechnol Biofuels. 2019;12:206 10.1186/s13068-019-1548-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu M, Beckham GT, Larsson AM, Ishida T, Kim S, Payne CM, et al. Crystal structure and computational characterization of the lytic polysaccharide monooxygenase GH61D from the Basidiomycota fungus Phanerochaete chrysosporium. J Biol Chem. 2013;288(18):12828–39. 10.1074/jbc.M113.459396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Courtade G, Wimmer R, Røhr ÅK, Preims M, Felice AK, Dimarogona M, et al. Interactions of a fungal lytic polysaccharide monooxygenase with beta-glucan substrates and cellobiose dehydrogenase. Proc Natl Acad Sci USA. 2016;113(21):5922–7. 10.1073/pnas.1602566113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laurent CVFP, Sun P, Scheiblbrandner S, Csarman F, Cannazza P, Frommhagen M, et al. Influence of lytic polysaccharide monooxygenase active site segments on activity and affinity. Int J Mol Sci. 2019;20(24):pii: E6219 10.3390/ijms20246219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fanuel M, Garajova S, Ropartz D, McGregor N, Brumer H, Rogniaux H, et al. The Podospora anserina lytic polysaccharide monooxygenase PaLPMO9H catalyzes oxidative cleavage of diverse plant cell wall matrix glycans. Biotechnol Biofuels. 2017;10:63 10.1186/s13068-017-0749-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.








