Human cytomegalovirus (HCMV) is known for its broad cell tropism, as reflected by the different organs and tissues affected by HCMV infection. Hence, inhibition of HCMV entry into distinct cell types could be considered a promising therapeutic option to limit cell-free HCMV infection. Soluble forms of cellular entry receptor PDGFRα rather than those of entry receptor neuropilin-2 inhibit infection of multiple cell types. sPDGFRα specifically interacts with gO of the trimeric gH/gL/gO envelope glycoprotein complex. HCMV strains may differ with respect to the amounts of trimer in virions and the highly polymorphic gO sequence. In this study, we show that the major gO genotypes of HCMV that are also found in vivo are similarly well inhibited by sPDGFRα. Novel gO genotypic forms potentially emerging through recombination, however, may evade sPDGFRα inhibition on epithelial cells. These findings provide useful additional information for the future development of anti-HCMV therapeutic compounds based on sPDGFRα.
KEYWORDS: envelope glycoproteins, gO genotypes, genetic recombination, human cytomegalovirus, strain diversity, virus entry
ABSTRACT
Human cytomegalovirus (HCMV) envelope glycoprotein complexes, gH/gL/gO trimer and gH/gL/UL128-131 pentamer, are important for cell-free HCMV entry. While soluble NRP2-Fc (sNRP2-Fc) interferes with epithelial/endothelial cell entry through UL128, soluble platelet-derived growth factor receptor α-Fc (sPDGFRα-Fc) interacts with gO, thereby inhibiting infection of all cell types. Since gO is the most variable subunit, we investigated the influence of gO polymorphism on the inhibitory capacities of sPDGFRα-Fc and sNRP2-Fc. Accordingly, gO genotype 1c (GT1c) sequence was fully or partially replaced by gO GT2b, GT3, and GT5 sequences in the bacterial artificial chromosome (BAC) TB40-BAC4-luc background (where luc is luciferase). All mutants were tested for fibroblast and epithelial cell infectivity, for virion content of gB, gH, and gO, and for infection inhibition by sPDGFRα-Fc and sNRP2-Fc. Full-length and partial gO GT swapping may increase epithelial-to-fibroblast ratios due to subtle alterations in fibroblast and/or epithelial infectivity but without substantial changes in gB and gH levels in mutant virions. All gO GT mutants except recombinant gO GT1c/3 displayed a nearly complete inhibition at 1.25 μg/ml sPDGFRα-Fc on epithelial cells (98% versus 91%), and all experienced complete inhibition on fibroblasts (≥99%). While gO GT replacement did not influence sNRP2-Fc inhibition at 1.25 μg/ml on epithelial cells (97% to 99%), it rendered recombinant mutant GT1c/3 moderately accessible to fibroblast inhibition (40%). In contrast to the steep sPDGFRα-Fc inhibition curves (slope of >1.0), sNRP2-Fc dose-response curves on epithelial cells displayed slopes of ∼1.0, suggesting functional differences between these entry inhibitors. Our findings demonstrate that artificially generated gO recombinants rather than the major gO genotypic forms may affect the inhibitory capacities of sPDGFRα and sNRP2 in a cell type-dependent manner.
IMPORTANCE Human cytomegalovirus (HCMV) is known for its broad cell tropism, as reflected by the different organs and tissues affected by HCMV infection. Hence, inhibition of HCMV entry into distinct cell types could be considered a promising therapeutic option to limit cell-free HCMV infection. Soluble forms of cellular entry receptor PDGFRα rather than those of entry receptor neuropilin-2 inhibit infection of multiple cell types. sPDGFRα specifically interacts with gO of the trimeric gH/gL/gO envelope glycoprotein complex. HCMV strains may differ with respect to the amounts of trimer in virions and the highly polymorphic gO sequence. In this study, we show that the major gO genotypes of HCMV that are also found in vivo are similarly well inhibited by sPDGFRα. Novel gO genotypic forms potentially emerging through recombination, however, may evade sPDGFRα inhibition on epithelial cells. These findings provide useful additional information for the future development of anti-HCMV therapeutic compounds based on sPDGFRα.
INTRODUCTION
Human cytomegalovirus (HCMV) is a widely spread pathogen which may cause substantial harm in congenitally infected newborns and in patients undergoing severe immunosuppressive therapy (1). Natural HCMV transmission follows mainly through body fluids such as urine or saliva (2). Upon infection, HCMV is spread throughout the body, infecting many of the major somatic cell types such as fibroblasts, smooth muscle cells, endothelial cells, epithelial cells, neurons, and leukocytes (3).
It is suggested that several forms of the gH/gL-based glycoprotein (gp) complexes may be present in the virion envelope of HCMV, thereby playing important roles in host cell entry (4–7). The two best-characterized complexes are the trimer gH/gL/gO and the pentamer gH/gL/UL128-131. It has repeatedly been shown that cell-free virions, which are infectious for multiple cell types and thus demonstrate in vivo cell tropism, must harbor both gp complexes (8–11). HCMV strains show large differences in the relative levels of trimer and pentamer incorporated in their virions (12). It is suggested that the trimer-to-pentamer ratio influences the infection efficiency for the respective cell types (8, 13, 14) and that a number of HCMV genes have the capacity to impact the composition of the two gH/gL complexes (15).
Large sequence comparison analyses have shown that, among all subunits of the two gp complexes, glycoprotein O (gO) exhibits by far the highest sequence polymorphism, with up to 23% amino acid diversity among gO sequences (16–18). All known gO sequences cluster into five major groups which can further be divided into eight genotypes (GTs) (19, 20). A closer inspection of gO gene sequences in circulating HCMV strains revealed that recombination among distinct strains may have occurred at several positions along the gO gene (17, 18, 21–23), arguing that recombination may have also contributed to the observed gO polymorphism. Although it appears that all eight gO genotypes can form stable trimers (12), it is poorly understood what role gO polymorphism plays in cell tropism. As recently shown, gO genotypes may influence the efficiency of epithelial cell infection through specific sequence characteristics (24) or via affecting the relative levels of gH/gL complexes (12, 14). Moreover, it has recently been reported that the accessibility of certain gH or gH/gL epitopes for monoclonal antibodies (MAbs) differs among HCMV strains, probably due to the distinct gO genotype sequences of the respective strains (25).
Over the last few years, a number of cellular interaction partners for both the trimer and the pentamer have been identified (15, 26). One of these cellular receptors, platelet-derived growth factor receptor alpha (PDGFRα), was identified to directly and specifically interact with gO parts of the trimer (27–29). This interaction enables entry of cell-free virions into fibroblasts, the only cell type which shows high PDGFRα expression (30). Soluble forms of PDGFRα (sPDGFRα) can severely inhibit not only entry into fibroblasts but also entry into endothelial and epithelial cells (27–29), and first observations indicate that the inhibitory capacity of sPDGFRα is effective against several HCMV strains even when they harbor a different gO genotype sequence (28).
Neuropilin-2 (NRP2), another recently identified host cell receptor for HCMV, specifically interacts with the UL128 subunit of the pentamer (31). This interaction is required for entry into endothelial and epithelial cells, most likely through endocytosis, but seems to be dispensable for entry into fibroblasts. Accordingly, a soluble form of NRP2 (sNRP2) inhibits endothelial and epithelial infection but not infection of fibroblasts (31). Both PDGFRα and NRP2 likely function as primary entry receptors for the trimer and pentamer, respectively; however, the modes of entry downstream of receptor binding may substantially differ. In particular, it appears that the trimer functions at steps which are required for entry into all cell types (15), which makes sPDGFRα or derivatives thereof promising therapeutic tools against HCMV (32, 33).
In the present study, we now aimed to assess how gO polymorphism influences the inhibitory capacities of sPDGFRα and sNRP2. To this end, we generated a set of HCMV gO genotype (GT) mutant strains derived from the bacterial artificial chromosome (BAC) clone TB40-BAC4-luc (where luc is luciferase), with five strains harboring one of the major gO genotype sequences and two carrying a recombinant gO genotypic form. We showed that subtle to moderate differences in the inhibitory capacities of the two entry inhibitors, sPDGFRα and sNRP2, are attributed to a recombinant gO genotypic version rather than to the natural variation of gO.
RESULTS
Cell-free infectivity of HCMV strains upon swapping of gO genotype sequences.
In order to investigate the influence of gO polymorphism on the cell entry inhibitors sPDGFRα and sNRP2, we generated a panel of gO GT mutant viruses in which the parental gO sequence GT1c of TB40-BAC4-luc was fully or partially replaced by another gO GT sequence (Fig. 1A and B). Correctness of the whole unique long (UL) and unique short (US) regions of fibroblast-derived reconstituted viruses was validated by whole-genome sequencing. All experiments were done with two independently reconstituted mutant virus stocks without further passaging. For comparison analyses and comprehensiveness, both the parental strain, referred to as gO GT1c, and a previously generated gO GT mutant, gO GT4 (24), were included in all experiments.
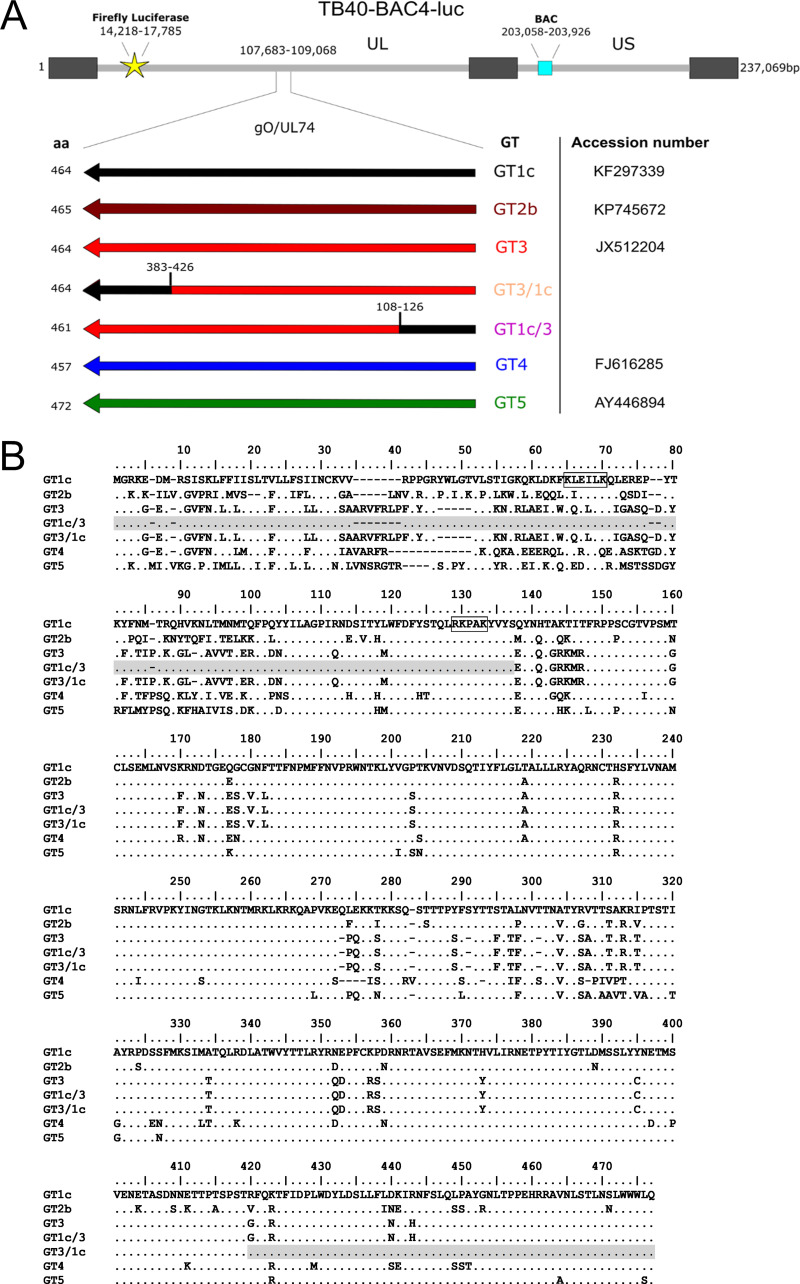
FIG 1.
Schematic illustration and gO sequences of BAC-derived gO genotype mutants. (A) The resident gO genotype (GT) 1c sequence of parental strain TB40-BAC4-luc was fully or partially replaced by the indicated gO GT sequences via en passant mutagenesis. Main genome characteristics are displayed. Arrows represent orientation and position of gO ORFs upon GT swapping. GTs and accession numbers of the HCMV strains from which the respective gO GT sequences are derived are shown on the right, and the length of gO amino acid (aa) sequence is shown on the left. The ranges of the recombination breakpoints of the chimeric mutants GT3/1c and GT1c/3 are depicted above the ORF. Cell-free bacterial artificial chromosome-derived mutant virus stocks were generated upon reconstitution in human foreskin fibroblasts. (B) Amino acid alignment of gO GT sequences in mutants. Putative PDGFRα binding sites as characterized recently (33) are boxed in black. The gray-shaded regions of recombinant GT1c/3 and GT3/1c mutants indicate the GT1c sequence part.
The use of HCMV strain TB40-BAC4-luc allowed quantitative determination of fibroblast and epithelial cell infection capacities by the monitoring of luciferase expression in cell lysates 2 days postinfection, as previously shown (34). In a first step, measuring relative light units (RLUs) as the readout for the extent of infection was validated for our experimental setup. Therefore, the same dilution series of the individual virus stocks was evaluated either by counting immediate early 1 (IE1)-positive cells or by luciferase assay. About 10 RLUs (7 to 20 in fibroblasts, 7 to 19 in epithelial cells) after subtraction of the background was measured for one IE1-infected cell independent of the cell type and of the gO GT mutant (Fig. 2A). The assay proved to be linear over the range of multiplicities of infection (MOIs) used for all experiments as determined by the number of plaque-forming units/cell in either human foreskin fibroblasts (HFFs) or ARPE-19 epithelial cells (Fig. 2B).
FIG 2.
Cell-free infectivity of gO genotype mutants for fibroblasts and epithelial cells using a luciferase reporter assay. (A and B) HFFs and in parallel ARPE-19 cells were infected on 96-well plates with a 2-fold serial dilution of HFF-reconstituted gO GT1c and the gO GT mutant virus stocks. Two days later cells were either stained for HCMV IE1 expression to count the number of infected cells or lysed and subjected to a luciferase assay to measure the relative light units (RLUs). Values shown in panel A are means ± standard deviations. In panel B the RLU values of the respective cell type are plotted against the MOI on HFFs (solid lines) and ARPE-19 cells (broken lines). (C and D) HFFs and ARPE-19 cells were infected with gO GT1c and gO GT mutants using a mean number of 7.4 log10 copies of encapsidated genome equivalents for HFFs and 7.8 log10 copies for ARPE-19 cells. At 2 days postinfection RLU values were assessed, and the ratio of the number of encapsidated genome equivalents to RLU counts was determined. All experiments were performed in triplicates, and data shown are means ± standard errors of the means of 2 to 5 independent experiments. (E and F) Results derived from individual 96-well luminometer plates are shown. Encapsidated genome equivalents were between 7.3 to 7.8 log10 copies for HFFs and 7.5 to 8.0 log10 copies for ARPE-19 cells. Two individual virus stocks from each mutant are plotted, indicated as follows: for HFFs, black (virus stock 1) and gray (virus stock 2) bars; for ARPE-19, and white (virus stock 1) and gray (virus stock 2) bars. (G) HFFs and ARPE-19 cells were simultaneously infected with gO GT1c and the gO GT mutants using the same 2-fold dilution series for both cell types. Undiluted virus stocks were adjusted to yield 3,000 to 8,000 RLUs in ARPE-19 cells. At 2 days postinfection the ratio of epithelial cell RLU values to fibroblast RLU values was calculated. Data shown are means of technical triplicates from two BAC-derived virus stocks of each strain. (H) Statistical significance was evaluated with data normalized to 1,000 to 2,000 RLUs in ARPE-19 cells by ANOVA with Dunnett’s test for multiple comparison (****, P < 0.0001). Data shown are means ± standard errors of the means of 3 to 4 independent experiments.
First, to assess the ability of the gO GT mutants to infect HFFs and ARPE-19 cells, cell-free virus stocks of gO GT1c and mutants were adjusted to a mean number of encapsidated genome equivalents of 8.4 log10 copies/ml (range, 8.0 to 9.2 log10 copies/ml) for HFFs and 8.8 log10 copies/ml (range, 8.3 to 9.3 log10 copies/ml) for ARPE-19 cells. From each adjusted virus stock, 100 μl for 10,000 cells was used for infection, and the corresponding mean MOI was 0.08 (range, 0.03 to 0.4) on HFFs and 0.009 (range, 0.003 to 0.02) on ARPE-19 cells. The number of RLUs in HFFs ranged from 1,600 to 16,000 and from 1,300 to 14,000 in ARPE-19 cells. The ratio of encapsidated genome equivalents to RLU values was calculated from two to five independent experiments. While GT3, GT3/1c, GT1c/3, and GT4 showed a trend toward lower fibroblast infectivity, it appears that GT5 displayed a slightly higher infection capacity for fibroblasts (Fig. 2C). In epithelial cells there was a trend toward a higher epithelial preference for all mutants (Fig. 2D). In Fig. 2E and F results from unique assays are presented in which distinct mutants along with the parental strain were investigated on the same plate using a narrow range of encapsidated genomes (7.3 to 7.8 log10 copies for HFFs and 7.5 to 8.0 log10 copies for ARPE-19) for infection. Results show that minor variations between individual plates and also between independently reconstituted virus stocks of the same mutant may occur.
Next, we determined the ratio of infection rates in ARPE-19/HFF cells by simultaneously infecting both epithelial cells and fibroblasts with 2-fold serial dilutions of the parental strain and mutant viruses. Undiluted virus stocks were adjusted to achieve a mean of 5,100 RLUs (range, 3,000 to 8,000) in ARPE-19-infected cells, which corresponds to a 0.005 to 0.016 MOI on this cell type. Infection efficiencies were determined by luciferase assay at 2 days postinfection, and the ratio of the number of RLUs for epithelial cells to the number of RLUs for fibroblasts was determined. Results from two clones from each mutant are shown in Fig. 2G. Both clones of the parental strain gO GT1c showed less than 10% epithelial cell infectivity relative to that of fibroblasts. Despite minor to moderate variations between different input virus titers and between the two clones of the same strain, all mutants showed an increase in the relative epithelial cell infectivity compared to that of GT1c. For statistical analyses, only data covering a narrow range of input RLU values (no more than 2-fold difference) were compared. The increase in the epithelial cell-to-fibroblast ratio proved to be statistically significant for the mutants GT1c/3 and GT4 independent of the input RLU values in ARPE-19 cell lysates (range, 350 to 6,000). In Fig. 2H results are shown for 1,000 to 2,000 input RLUs.
In summary, the data show that all mutants retained the preference for fibroblast over epithelial cell infectivity. However, it appears that subtle changes in infection efficiencies on both cell types may lead to significant changes in the epithelial cell-to-fibroblast tropism ratios.
Content of gB, gH, and gO in the envelope of gO GT mutant viruses.
We next sought to investigate whether changes of parental gO GT sequences in an otherwise unaltered TB40-BAC4-luc genomic background affected the overall gB, gH, and gO abundances in extracellular virions. To this end, parental and mutant virions were purified from fibroblast supernatant and subjected to semiquantitative Western blotting under reducing conditions. The total amount of virions was normalized to the level of the major capsid protein (MCP). One representative immunoblot for each mutant is given in Fig. 3, and the estimated virion content of gB, gH, and gO resulting from two to four independent experiments is shown in Table 1. Overall, the amounts of virion gB and gH were similar between gO mutants and the parental strain. The gO levels, on the other hand, displayed substantial variations. While the monoclonal anti-gO antibody (Ab), anti-gO.02, which is directed toward gO GT1c of TB40E (35), recognizes the gO genotypic forms GT3, GT1c/3, and GT3/1c, no gO bands were detected for gO GT2b virions (data not shown). When we included a previously generated gO GT1c_C343S mutant which is characterized by low gO and gH levels (24), we also found substantially lower gB levels in the mutant virions (Table 1), indicating that gH and gB similarly alter along with gO. This let us conclude that the various gO levels in the gO GT mutants are likely due to a reduced gO antibody recognition of the distinct gO sequences and may not necessarily reflect actual virion gO content. Since we found no detectable change in the gB and gH levels among the gO GT mutants, it appears that the overall composition of gB and the gH/gL-based complexes in virions is not affected upon full-length and partial gO GT replacement.
FIG 3.
Comparison of MCP, gB, gH, and gO content in cell-free virions between parental strain gO GT1c and gO mutants. Virions harvested from human foreskin fibroblast supernatant were subjected to reducing gel electrophoresis and analyzed by Western blotting using antibodies directed against major capsid protein (MCP) and the glycoproteins gB (anti-gB MAb 2F12), gO (anti-gO.02 MAb), and gH (AP86-SA4). The amounts of virions loaded on the gels were compared to the amount for MCP. Contents of gB, gH, and gO were compared between gO GT1c and the respective gO mutants. Band densities were determined relative to the GT1c reference band for each blot individually using Image Lab, version 6.0, software and are shown below the blots.
TABLE 1.
Glycoprotein content in fibroblast-derived cell-free virions
| gO GT mutant | Mean amount or protein (range [%])a
|
||
|---|---|---|---|
| gB | gH | gO | |
| GT1c | 121 (83–208) | 124 (63–200) | 72 (42–100) |
| GT2b | 136 (111–167) | 170 (91–205) | ND |
| GT3 | 142 (109–169) | 235 (179–303) | 59 (16–91) |
| GT3/1c | 116 (83–147) | 121 (100–143) | 28 (16–40) |
| GT1c/3 | 150 (111–189) | 84 (53–143) | 17 (6–27) |
| GT4 | 73 (67–80) | 119 (105–133)b | 98 (71–125)b |
| GT5 | 130 (74–172) | 102 (53–222) | 100 (45–200) |
| GT1c_C343S2 | 34 (22–67) | 40 (29–59)b | 29 (18–59)b |
Glycoprotein levels were normalized to the level of the major capsid protein. The data shown are the results of two to four independent experiments. ND, not detectable.
Data have been previously published (24).
Inhibition of cell-free fibroblast and epithelial cell infectivity by soluble PDGFRα-Fc.
Since all gO GT mutants retained the ability to infect fibroblasts and epithelial cells, we were able to directly compare the inhibitory capacity of sPDGFRα-Fc between the five major gO genotypes and the two recombinant forms. The inhibition experiments were performed with a fixed amount of infectious virus preincubated for 2 h with a 2-fold dilution series of sPDGFRα-Fc ranging from 0.0025 to 0.625 μg/ml. After another 2-h incubation on fibroblasts or epithelial cells, cells were washed and subsequently incubated with fresh medium for another 2 days. The RLU values were monitored by a luciferase assay and plotted against the concentration of sPDGFRα-Fc.
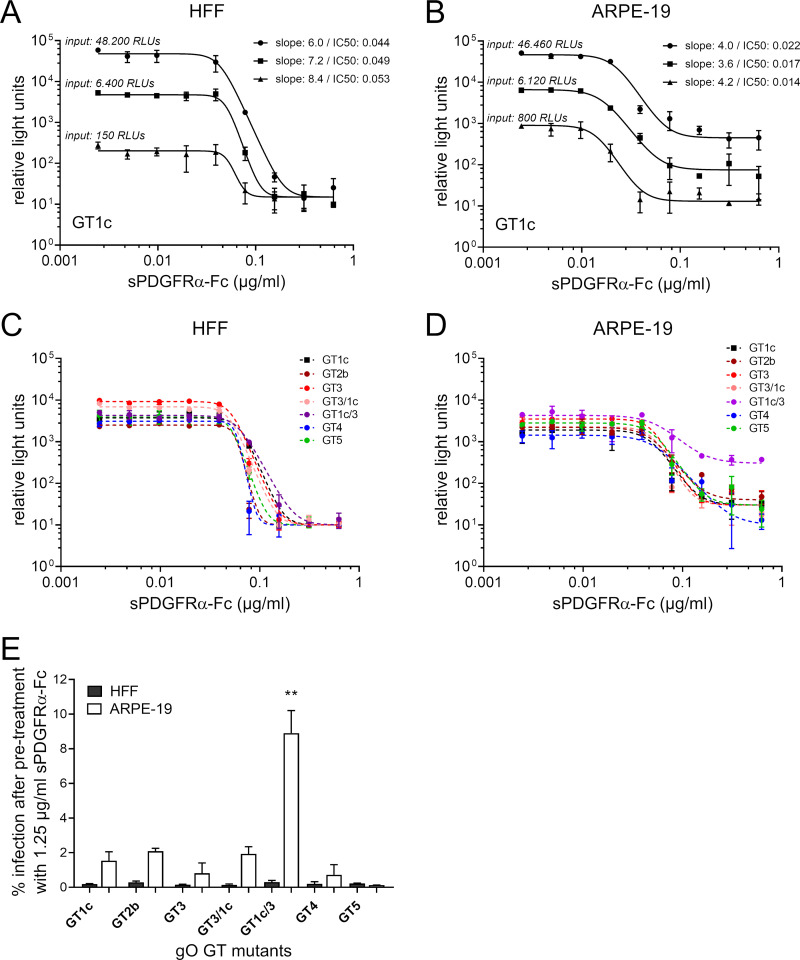
First, the appropriate amount of infectious input virus was determined using three different virus dilutions of parental strain gO GT1c, with amounts ranging from 150 to 48,200 RLUs (MOI of 0.01 to 0.5) on fibroblasts and from 800 to 46,460 RLUs (MOI of 0.002 to 0.04) on epithelial cells. As shown in Fig. 4A and B, there was no substantial change in the overall shape of the dose-dependent inhibition over a wide range of inputs. Thus, for all further inhibition experiments, the cell-free virus stocks were normalized to yield RLU values within this range, i.e., 2,500 to 13,700 RLUs of input virus in HFFs and 1,400 to 8,000 RLUs of input virus in ARPE-19 cells. In all mutants, cell-free infectivity was inhibited by sPDGFRα-Fc in a dose-dependent manner. Representative curves for gO GT1c and the gO GT mutants are shown in Fig. 4C and D. The half-maximal inhibition (50% inhibitory concentration [IC50]) as calculated by nonlinear regression ranged from 49 ng/ml to 73 ng/ml for fibroblasts and from 24 ng/ml to 56 ng/ml for epithelial cells (Table 2). None of the IC50 values for the mutants significantly differed from the IC50 of the parental strain. Moreover, there was no difference between parental strain and mutants in the overall steep shape of the dose-response curves (slopes of >1) in either fibroblasts or epithelial cells, except for one of the recombinant mutants, gO GT1c/3, in epithelial cells. This gO mutant showed a shallower dose-response curve, with a slope of 1.0 to 2.6 (Table 2). The slope parameter mathematically analogous to the Hill coefficient is a measure of cooperativity (36) in the binding of multiple ligands (e.g., sPDGFRα-Fc) to linked binding sites (e.g., gO). Dose-response curves with a slope of about 1.0 are indicative for noncooperativity, which means that the ligand binds at each site independently. In contrast, steep curves with slopes much larger than 1.0 are thought to result from a form of positive cooperative effects upon ligand binding (36). Hence, these findings suggest that the presumed positive cooperativity is weakened when sPDGFRα-Fc binds to gO GT1c/3.
FIG 4.
Inhibition of cell-free infectivity of gO genotype mutant viruses by soluble PDGFRα-Fc. The whole panel of gO genotype (GT) mutants along with the parental strain gO GT1c was preincubated with soluble PDGFRα-Fc (sPDGFRα-Fc) before infection of human foreskin fibroblasts (HFFs) or ARPE-19 (adult retinal pigment epithelial cell line 19) cells. Two days after infection, counts of relative light units (RLUs) were determined in cell lysates by a luciferase assay. (A and B) Three different virus stock concentrations of the parental strain gO GT1c (MOI of 0.01 to 0.5 on HFFs and MOI 0.002 to 0.04 on ARPE-19 cells), indicated as input RLU values, were used. (C and D) Mutant virus stocks were diluted to achieve RLU values ranging from 2,600 to 9,000 without treatment in HFFs (MOI of 0.05 to 0.1 on HFFs) and from 1,600 to 5,000 without treatment in ARPE-19 cells (MOI of 0.003 to 0.01 on ARPE cells). In the experiments shown in panels A to D, parental and mutant virus stocks were treated with serial 2-fold dilutions of sPDGFRα-Fc (range, 0.625 to 0.0244 μg/ml). Monitored RLU values were plotted against sPDGFRα-Fc concentrations. Four-parameter dose-response curves were generated, and the protein concentration causing 50% inhibition of infection (IC50) and the steepness of the curves were calculated (Table 2). In panels C and D, one representative curve from each mutant out of 2 to 4 independent experiments is shown. Data represent mean values ± standard deviations of triplicate determinations. (E) The percentage of infection of HFFs or ARPE-19 cells after pretreatment with 1.25 μg/ml sPDGFRα-Fc is shown. Input infectivity without treatment ranged from 2,500 to 13,700 RLUs in HFFs (MOI of 0.05 to 0.2) and from 1,400 to 8,000 RLUs in ARPE-19 cells (MOI of 0.003 to 0.015). Experiments were performed in triplicates, and data are means ± standard errors of the means of 3 to 4 independent experiments. Statistical significance was evaluated by ANOVA with Tukey’s test for multiple comparison (**, P < 0.01, for results in comparison to those with GT1c).
TABLE 2.
Dose-inhibition curve characteristics of gO genotype mutant strainsa
| gO GT mutant | sPDGFRα-Fc dose-response curve in: |
sNRP2-Fc dose-response curve in ARPE-19 cells |
||||
|---|---|---|---|---|---|---|
| HFFs |
ARPE-19 cells |
|||||
| Mean IC50 (range [ng/ml]) | Slope | Mean IC50 (range [ng/ml]) | Slope | Mean IC50 (range [ng/ml]) | Slope | |
| GT1c | 58 (52–63) | 6.0–8.0 | 39 (16–53) | 2.6–6.0 | 59 (40–79) | 1.4–1.6 |
| GT2b | 73 (54–92) | 6.0–8.0 | 36 (10–51) | 2.6–3.0 | 64 (39–89) | 1.4–1.7 |
| GT3 | 65 (53–82) | 4.8–10.0 | 47 (43–50) | 4.7–6.0 | 42 (28–56) | 1.5–1.6 |
| GT3/1c | 49 (46–54) | 6.0–9.5 | 50 (49–50) | 6.8–7.4 | 65 (41–100) | 0.9–1.8 |
| GT1c/3 | 69 (56–81) | 2.5–6.2 | 30 (13–62) | 1.0–2.6 | 88 (73–98) | 1.2–2.4 |
| GT4 | 52 (47–55) | 5.5–6.0 | 55 (52–56) | 5.3–6.0 | 54 (48–61) | 1.2 |
| GT5 | 68 (45–100) | 5.3–8.0 | 56 (54–58) | 5.3–6.0 | 63 (34–92) | 1.3–1.9 |
The data shown are the results of two to four independent experiments. Inhibitor concentration (IC50) and slope values were calculated from inhibitor concentration-versus-response four-parameter dose-response curves.
Next, we determined the maximal extent of inhibition at 1.25 μg/ml sPDGFRα-Fc, calculated as 1 – (RLU value after pretreatment/RLU value of untreated virus stocks). The inhibition of fibroblast infectivity was almost complete (>99%), and levels did not differ between gO GT1c and the mutants (Fig. 4E). In epithelial cells, in contrast, one of the recombinant mutants, gO GT1c/3, retained a significantly higher infectivity (mean, 9%) at this inhibitor concentration than gO GT1c. The infectivities of the other mutants did not differ from the infectivity of the parental strain. The reduced epithelial cell inhibition of gO GT1c/3 by sPDGFRα-Fc is accords well with the shallower shape of the dose-response curve (Fig. 4D). Notably, the inhibition efficiency was slightly less effective in epithelial cells (∼98% to 99%) than in fibroblasts for gO GT1c and the mutants GT2b, GT3, and GT3/1c (Fig. 4E).
In summary, these findings show that not only the five major genotypic forms of gO are recognized by sPDGFRα-Fc but also recombinant forms of gO. One recombinant version of gO seems to be less effectively inhibited by sPDGFRα-Fc on epithelial cells, however.
Inhibition of cell-free fibroblast and epithelial cell infectivity by soluble NRP2-Fc.
It has recently been reported that soluble forms of NRP2, which specifically bind to UL128, inhibit epithelial cell infection while fibroblast infection remains largely unaffected (31). We wanted to know whether virions with distinct gO sequences may indirectly affect the inhibitory capacity of sNRP2-Fc. To address this question, first we performed inhibition experiments on epithelial cells using a 2-fold dilution series of sNRP2-Fc (range, 0.0025 to 1.25 μg/ml) and RLU-normalized gO GT virions ranging from 700 to 9,500 RLUs, similar to the method described for sPDGFRα-Fc. Two to three independent experiments were performed for each mutant along with the parental strain, and one representative curve is shown in Fig. 5A. The dose-dependent inhibition was similar between the parental strain and gO GT mutants; the dose-response curves displayed slopes of about 1.0 (range, 0.9 to 2.4), and the IC50 values ranged from 42 to 88 ng/ml (Table 2). These findings indicate that changes in the gO genotypic forms do not influence the capacity of sNRP2-Fc for epithelial cell inhibition.
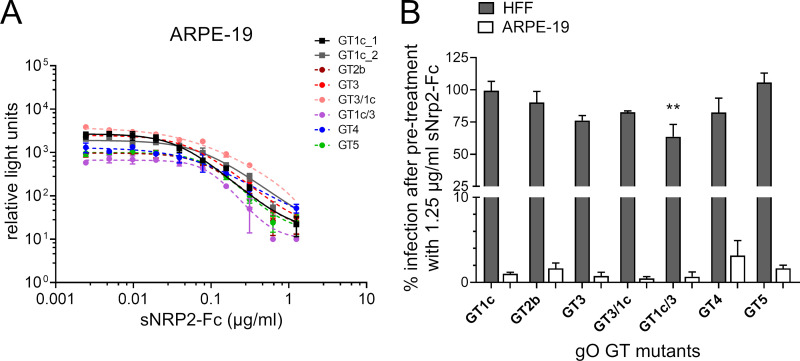
FIG 5.
Inhibition of cell-free infectivity of gO genotype mutant viruses by soluble NRP2-Fc. (A) Parental gO GT1c and gO GT mutant virus stocks were preincubated with serial 2-fold dilutions of soluble NRP2-Fc (sNRP2-Fc) (range, 1.25 to 0.0244 μg/ml) before infection of ARPE-19 cells (adult retinal pigment epithelial cell line 19). Two days after infection relative light unit (RLU) values were monitored and ranged from 700 to 9,500 RLUs (MOI of 0.001 to 0.02 on ARPE cells) in untreated controls. RLU values were plotted against sNRP2-Fc concentrations, and four-parameter dose-response curves were generated to calculate the protein concentration causing 50% inhibition of infection (IC50) and to determine the steepness of the curves. Two curves from gO GT1c and one representative curve from each mutant out of 2 to 3 independent experiments are shown. Data represent mean values ± standard deviations of triplicate determinations. (B) The percentage of infection of HFFs or ARPE-19 cells after pretreatment with 1.25 μg/ml sNRP2-Fc is shown. Input virus stock concentrations used for ARPE-19 cells were in the same range as those used for the experiment shown in panel A and ranged for HFFs from 900 to 13,700 RLUs without treatment (MOI of 0.02 to 0.2 on HFFs). Experiments were performed in triplicates, and data are means ± standard errors of the means of 3 to 5 independent experiments. Statistical significance was evaluated by ANOVA with Tukey’s test for multiple comparison (**, P < 0.01, for results in comparison to those with GT1c).
Finally, we wanted to assess whether the maximum inhibitory capacity of 1.25 μg/ml sNRP2-Fc on epithelial cells also has an effect on fibroblast infectivity. Input virus stock dilutions for ARPE-19 cells were in the same range as those used for the dose-inhibition curve analyses, and for HFFs input virus dilutions yielded RLU values from 900 to 13,700 without treatment. As shown in Fig. 5B, epithelial cell infectivity was reduced 97% to 99% in all mutant viruses along with the parental strain. Interestingly, although the fibroblast infectivity was almost unaffected in the parental strain and in two of the mutants, GT2b and GT5, a moderate reduction in fibroblast infectivity of 15% to 40% was observed for the other mutants, and this reached statistical significance for the recombinant mutant gO GT1c/3. From these data it appears that gO GT swapping may result in mutant virions which became partially accessible to sNRP2-Fc inhibition on fibroblasts.
DISCUSSION
The two envelope glycoprotein complexes, gH/gL/gO trimer and gH/gL/UL128-131 pentamer, which share the same gH/gL heterodimer, play major roles in HCMV cell entry. In the present study, we focused on gO, the critical subunit of the trimer. A special hallmark of gO is its high polymorphism, with an overall amino acid diversity of ∼20% (19, 20). To learn more about potential functional differences attributed to gO polymorphism, we fully or partially swapped gO gene sequences in the otherwise identical TB40-BAC4-luc background, tested the set of gO mutants for their capability to infect fibroblasts and epithelial cells and for their relative composition of envelope glycoproteins gB and gH in cell-free virions, and evaluated the inhibitory capacity of sPDGFRα-Fc in comparison to that of sNRP2 inhibition.
First, we demonstrated that BAC-derived viruses upon either partial or full-length gO GT swapping display only subtle variations in their infection efficiencies for fibroblasts and epithelial cells compared to those of gO GT1c. When we investigated the relative levels of gB and gH in cell-free virions, no substantial differences among the parental strain and mutants were observed, as similarly found for a gO GT4 mutant previously (24). Interestingly, in a recently published report, Zhang et al. showed that replacing gO in the two HCMV strain backgrounds, TR and Merlin, also had no effect on the virion amounts of gH/gL complexes and on fibroblast infectivity (14). Taking these findings together, it appears that the exclusive exchange of gO genotypic sequences within an otherwise unchanged genomic background do not substantially affect gH/gL-based complexes and cell-free fibroblast and epithelial cell infectivity. The epithelial-to-fibroblast tropism ratio, however, was substantially enhanced in gO GT1c/3 and the previously described gO GT4. This appears to be due to a combination of subtle changes in both fibroblast and epithelial cell infection efficiencies. Since gO sequence differences precluded relative quantitation of virion gO levels by the monoclonal anti-gO antibody gO.02, it cannot be excluded that subtle changes in the trimer-to-pentamer ratio may have occurred despite unchanged overall gH levels. However, when we included in the Western blot analyses a previously generated gO GT1c_C343S mutant in which a cysteine-to-serine mutation, C343S, affects trimer complex formation, we found that the gO levels altered along with those of gH and gB (24). Hence, it is more likely that gO GT sequence-specific characteristics rather than changes in the trimer-to-pentamer ratio may account for the observed alterations in epithelial-to-fibroblast tropism ratios, and this will be subject of further investigations.
Recombination among different HCMV strains may have contributed to HCMV evolution, as shown by numerous studies (37). Recently, the recombination density throughout the genome was deeply investigated by whole-genome sequence comparisons exploring past and recent recombination events as well (17, 18, 21, 22). A particularly interesting finding was the identification of pervasive genome-wide recombination generating diversity both within and between genes (17, 22), yet the intensity of recombination seems to be rare in highly variable regions (18, 22). So far, little is known about potential functional consequences for individual genes upon intragenic recombination. In the present study, we have now included two chimeric gO GT mutants, each of which carries a recombinant gO genotypic form composed of GT1c and GT3 sequences. One of them, gO GT3/1c, harbors the recombination breakpoint within the conserved C-terminal part of gO, and this mutant differs from full-length gO GT3 in only 4 amino acid residues. The recombination breakpoint of the other one, gO GT1c/3, is located in a small stretch of identical sequence between GT1c and GT3 in the otherwise highly polymorphic N-terminal part of gO. Recombination resulted in a severely altered gO sequence, with an amino acid diversity of 9% from GT1c and of 10% from GT3. Strikingly, these recombinant gO mutants not only retained the ability to infect fibroblasts and epithelial cells but also, as observed with GT1c/3, displayed a significant increase in the ARPE/HFF ratio compared to that with GT1c. Accordingly, these findings indicate that recombination within the gO gene may generate gene diversity either with or without modified functions and will allow novel combinations of neighboring loci when recombination occurs between genetically distinct strains. In fact, previously reported sequencing data have shown that recombination within gO may sporadically occur also in vivo despite a strong linkage between gO and the adjacent, partly overlapping gN gene (17, 18, 22, 23, 38).
Recent studies have shown that PDGFRα specifically interacts with the gO subunit of the trimer which is required for entry into fibroblasts (27–30). As soluble forms of PDGFRα or derivatives thereof can inhibit cell-free infection of several cell types (28), it appears that binding of sPDGFRα to gO interferes with trimer-mediated function(s) widely required for cell entry. We now demonstrate that representatives of the five major gO genotypic forms, GT1c, GT2b, GT3, GT4, and GT5, are similarly recognized by sPDGFRα and that upon pretreatment with sPDGFRα-Fc both fibroblast and epithelial cell infectivity levels were strongly inhibited. These data are well in line with previous reports showing the inhibitory capacity of sPDGFRα for several distinct HCMV strains (28). Notably, even at a concentration of 1.25 μg/ml sPDGFRα, we observed a residual infectivity of about 1% to 2% in epithelial cells, while in fibroblasts the inhibition was almost complete (≥99%), similar to previously shown results (29). Moreover, nearly complete inhibition of fibroblast infection seems to be present over a wide range of input virus whereas in epithelial cells the dynamic range of dose-dependent inhibition remains consistent between different input viruses, resulting always in about 1% to 2% residual infectivity. These data suggest that binding of sPDGFRα to gO trimer leads to a complete block of fibroblast receptor binding but may not completely abolish further entry functions of the gO trimer, for example, triggering of gB-mediated membrane fusion. This may well explain the residual infectivity seen in epithelial cells.
Remarkably, one of the two recombinant mutants, gO GT1c/3, displayed a significantly lower sensitivity for sPDGFRα inhibition on epithelial cells than the other mutants while fibroblast inhibition was similarly effective. As mentioned above, this mutant has its recombination site in the highly polymorphic N-terminal region of the protein, which only recently was suggested to contain the PDGFRα receptor binding domain (33). By mutational analysis, the authors identified a small stretch from amino acid 117 to 121 causing the strongest impairment of sPDGFRα binding to virus particles and consequently also reduced virus penetration into fibroblasts. Although this peptide site overlaps the recombination site of GT1c/3, the specific sequence remained unchanged upon recombination, suggesting that sPDGFRα binding to this recombinant form of gO is not impaired. This presumption is consistent with the finding that the gO GT1c/3 mutant retains its fibroblast infectivity while mutants with a mutation in this particular peptide sequence showed reduced penetration into fibroblasts (33). Hence, we assume that the impaired sPDGFRα inhibition for the gO GT1c/3 mutant on epithelial cells is not caused by a lower binding of sPDGFRα to this chimeric form of gO. Since the overall epithelial cell infectivity is also well preserved in this chimeric mutant, it is presumable that binding of sPDGFRα to this chimeric form of gO interferes less with the gO trimer functions that are necessary for epithelial cell entry. Whether a specific sequence motif generated upon recombining of these two gO GT sequences or an overall change in the structure and/or flexibility of this gO chimera accounts for this finding will be a subject of further studies.
The assumption that binding of sPDGFRα to gO trimer affects more entry properties than the unique block of the receptor binding site is further strengthened by our findings that inhibition with 2-fold serial dilutions of sPDGFRα led to steep dose-response curves in both fibroblasts and epithelial cells. Such steep inhibition curves with a slope much greater than 1 are thought to result from a form of positive cooperative effects upon ligand binding (36). Remarkably, the steepness of the sPDGFRα-Fc dose-inhibition curves was not affected by the amount of input virions. The underlying mechanisms are not yet clearly understood, but several scenarios exist that may explain why sPDGFRα-bound virions become rapidly inactive for cell entry. Binding of sPDGFRα to virions leads to (i) steric hindrance and/or conformational changes of the gO trimer which affects multiple sPDGFRα binding sites on the virion; (ii) cluster formation of trimers and/or other envelope complexes which causes multiple gO binding sites on the virion to be rapidly blocked; (iii) changes of gB prefusion into gB postfusion conformation (under the assumption that the trimer stabilizes the gB prefusion conformation) which renders virions inactive for entry; and/or (iv) cluster formation of multiple virions. Although these proposed scenarios await further clarification, from our data it becomes clear that a presumed cooperative effect triggered by sPDGFRα does not differ among the five major gO genotypes.
When we compared the dose-response curves of sPDGFRα with those of sNRP2, a recently identified entry inhibitor for epithelial cells (31), it became obvious that the mechanisms of action substantially differ between these two entry inhibitors. The dose-response curves of sNRP2 displayed a slope of about 1 to 2, meaning that binding of sNRP2 to its interaction partner UL128 of the pentamer causes no further effects beside the block of the binding site. There was also no difference seen among the gO GT mutants and parental strain, indicating that gO sequence replacement does not considerably influence the binding efficiency of sNRP2. Notably, all gO mutants along with the parental strain displayed residual epithelial cell infectivity of about 1% to 3% at a concentration of 1.25 μg/ml sNRP2. Whether NRP2-independent entry pathways and utilization of other cellular receptors such as the very recently identified pentamer-dependent host cell receptors CD147 (39) or OR14I1 (26) may circumvent complete inhibition or whether not all virions are neutralized by this concentration of sNRP2 awaits further investigation. As recently reported, fibroblast infection is largely unaffected by sNRP2 (31). Overall, this finding is in good concordance with our data. However, we observed a subtle inhibition of fibroblast infectivity by high concentrations of sNRP2 in the mutants GT3, GT3/1c, GT4, and, most notably, in the chimeric mutant GT1c/3. It is feasible that the lower fibroblast infectivity also observed in these four mutants partially contributes to this finding. Also, it is tempting to speculate that binding of large amounts of sNRP2 to virions sterically hinders the functional properties of the trimer when it is complexed with specific gO genotypic forms.
In conclusion, in this study we show that the major gO genotypic forms exert no substantial influence on the inhibitory capacities of sPDGFRα and sNRP2. But novel versions of gO potentially emerging through recombination may lead to moderate evasion of sPDGFRα inhibition on epithelial cells, a cell type which is important for interhost transmission. This might have implications when receptor inhibitors are considered as a therapeutic option for HCMV infection.
MATERIALS AND METHODS
Cells.
Human foreskin fibroblasts (HFFs) were cultured in minimum essential medium (modified Eagle’s medium) (MEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Capricorn Scientific, Ebsdorfergrund, Germany) and 0.5% neomycin (Sigma-Aldrich). Human adult retinal pigmented epithelial cells (ARPE-19; ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F12 (PAN-Biotech, Aidenbach, Germany) supplemented with 10% FBS and 1% penicillin-streptomycin (ThermoFisher) or in MEM supplemented with 10% FBS and 0.5% neomycin.
Generation of gO mutant BAC clones by en passant mutagenesis.
All HCMV gO mutant strains were derived from the bacterial artificial chromosome (BAC) clone TB40-BAC4-luc (34). By en passant mutagenesis in Escherichia coli GS1783 (40), the gO GT1c sequence of TB40-BAC4-luc was fully replaced by that of GT2b, GT3, and GT5 and partially replaced by that of gO GT3 at either the 5′ or 3′ end of the gO GT1c open reading frame (ORF). For generation of full-length gO BAC mutants, a gO deletion mutant was used in which the whole gO ORF sequence was deleted. This ensured recombination between transfer plasmid and BAC DNA solely upstream and downstream of the gO ORF sequence. For generation of recombinant BAC mutants GT3/1c and GT1c/3, original TB40-BAC4-luc BAC DNA was used, and both chimeric versions resulted from recombination within the gO GT1c ORF. First, a set of recombination cassettes was generated, and the primer pairs used are listed in Table 3. For this, inserts containing a kanamycin resistance gene, flanked on one side by an 18-bp I-SceI restriction sequence and a gO GT-specific 50-bp sequence and on both sides by a SacI or NdeI restriction site, were generated by PCR using pEP-Kan-S (kindly provided by Nikolaus Osterrieder). Second, each individual insert was cloned into the corresponding restriction site of gO GT sequence carried by pEX-A258, ordered from Eurofins Genomics (Luxembourg). The resulting transfer plasmids were used as templates to generate the PCR-derived recombination cassettes containing extensions of ∼50-bp sequences on each end for homologous recombination. The recombination cassettes were electroporated into recombination-competent E. coli GS1783 carrying full-length or gO-deleted TB40-BAC4-luc DNA. After electroporation, recombination-positive E. coli cells were subjected to kanamycin selection, and the introduced non-HCMV sequences were removed within E. coli by cleavage at the I-SceI site and a second red recombination. Positive kanamycin-sensitive, chloramphenicol-resistant bacteria colonies were selected. Finally, recombinant BAC DNAs were isolated from positive clones, and the correctness of the BAC DNA sequence was verified by whole-genome sequencing (see below). Further, overnight E. coli cultures of positive clones were stored at –80°C until further use.
TABLE 3.
Primer sequences for en passant mutagenesis
| TB40-BAC4-luc-derived gO GT mutant(s) | Template for PCR | PCR product | Forward primer (5′–3′) | Reverse primer (5′–3′) | BAC DNA for recombination in GS1783 |
|---|---|---|---|---|---|
| ΔgO | pEPKan-S | Recombination cassette | CAGAACTTTACTGCAACCACCACCAAAGGCTATTGAGGTTCCCCATGACAGAGGAGGAATAGGGATAACAGGGTAATCGATTT | GCAGACGGACGGTGCGGGGTTTCCTCCTCTGTCATGGGGAACCCTCAATAGCCTTTGGTGGCCAGTGTTACAACCAATTAACC | TB40-BAC4-luc |
| GT2b | pEPKan-S | Insert for cloning into pEX-A258-Pgt2b | TAAGGAGCTCATGTTGAGAGTACCGTAAATAGTGTACGGTGTTTCGTTACGGATCTAGGGATAACAGGGTAATCGATTT | GCCAGTGTTACAACCAATTAA | |
| Transfer plasmid | Recombination cassette | GATGGGAGCCTTTTGTATCGTA | GCCAAACCACAAGGCAGA | TB40-BAC4-lucΔgO | |
| GT5 | pEPKan-S | Insert for cloning into pEX-K4-Pgt5 | TAAGGAGCTCATGTCAAGAGTGCCATAAATAGTGTACGGCGTTTCGTTACGAATCTAGGGATAACAGGGTAATCGATTT | TAGCGAGCTCGCCAGTGTTACAACCAATTAACC | |
| Transfer plasmid | Recombination cassette | GGAGCCTTTTGTATCGTACTACGACATTGCTGCTTTCAGAACTTTACTGCGACCACCACCAAAGGCTATTG | AAACCACAAGGCAGACGGACGGTGCGGGGTTTCCTCCTCTGTCATGGGGAAAAAAGAGATGATAATGGTGAAAGGC | TB40-BAC4-lucΔgO | |
| GT3, GT3/1c, GT1c/3 | pEPKan-S | Insert for cloning into pEX-K4-Pgt3 | TAAGGAGCTCATGTCAAGAGTGCCGTAAATAGTGTACGGTGTTTCGTTGCGAATCTAGGGATAACAGGGTAATCGATTT | TAGCGAGCTCGCCAGTGTTACAACCAATTAACC | |
| Transfer plasmid | Recombination cassette | TTGCTGCTTTCAGAACTTTACTGCAACCACCACCAAAGGCTATTGAGGGTAGACAGATTTACAGCCCGGC | CAAGGCAGACGGACGGTGCGGGGTTTCCTCCTCTGTCATGGGGAGAAAAGGAGAGATGAGAGGTGTTTTTAACTTAT | TB40-BAC4-luc |
BAC-derived gO mutant HCMV strains.
Infectious viruses were generated by reconstitution as described previously (24). Briefly, mutant BAC DNAs were purified from E. coli using a NucleoBond BAC100 kit (Macherey-Nagel, Düren, Germany). The day before transfection, HFFs were seeded in six-well plates (3 × 105 cells/well), and then 2 μg of BAC DNA, 1 μg of pCMV71 DNA (plasmid kindly provided by Mark Stinski, University of Iowa), and 9 μl of ViaFect reagent (Promega, Madison, WI) were mixed with 100 μl of MEM without antibiotics, incubated for 15 min at room temperature (RT), and then added to the cells. At 24 h after transfection, cells were washed with phosphate-buffered saline (PBS), and fresh MEM with antibiotics was added. At 1 week after transfection, cells were trypsinized and transferred into 25-cm2 cell culture flasks. When cytopathic effect (CPE) was 90% to 100%, supernatants were cleared by centrifugation at 4°C for 20 min at 4,000 × g and stored as cell-free virus stocks in aliquots at –80°C. For infection and inhibition analyses, all aliquots were used only once to avoid multiple freeze-thaw cycles. Furthermore, one aliquot per reconstitution was subjected to (i) next-generation sequencing to confirm the correctness of the complete UL and US genomic regions, (ii) DNase treatment to assess the amount of encapsidated genomes, (iii) a plaque assay to determine the virus titers, and (iv) RLU measurements in order to normalize virus stocks in subsequent experiments. Two independent reconstitutions were performed for each mutant.
Whole-genome sequencing.
DNA from BAC purification (as described above) and extracted DNA from DNase-treated or untreated virus stocks from HFF cell culture supernatants upon reconstitution were quantified using a Qubit, version 2.0, fluorometer (ThermoFisher) according to the manufacturer’s instructions. One to two nanograms of DNA per sample was taken for library preparation using a Nextera XT DNA Library Preparation kit, and uniquely indexed samples using a Nextera XT Index kit were pooled and sequenced together (both from Illumina, San Diego, CA). Pooled libraries were sequenced with paired-end reads (2 by 150 to 250 bp) on a MiSeq system using version 2 or version 3 sequencing reaction chemistry (Illumina). Data were analyzed by CLC Genomics Workbench, version 12, software (Qiagen). Low-quality reads were trimmed, and on average 52 to 80% of reads mapped to the reference genome with an average coverage of at least 500.
Determination of encapsidated HCMV genomes in virus stocks.
In order to remove nonencapsidated viral DNA and free cellular DNA, fibroblast-derived virus stocks were treated with Turbo DNase (Thermo Fisher). For this, 100 μl of master mix (73 μl H2O, 20 μl of 10× DNase buffer, 5 μl of 10× PBS, 2 μl of Turbo DNase [2 U/μl]) was added to 100 μl of sample and incubated for 1 h at 37°C in a thermoshaker at 1,400 rpm. Immediately thereafter, the total reaction volume was added to 2 ml of lysis buffer, and DNA was extracted using a bead-based NucliSens EasyMag extractor (bioMérieux, Marcy-l’Étoile, France) according to the manufacturer’s protocol. DNA was eluted in 50 μl of nuclease-free H2O.
HCMV-specific quantitative PCR.
HCMV-DNA was quantitated using an in-house real-time quantitative PCR (qPCR), amplifying a conserved region within US17 (forward primer, GCGTGCTTTTTAGCCTCTGCA [10 pM]; reverse primer, AAAAGTTTGTGCCCCAACGGTA [10 pM]; TaqMan probe FAM-TGATCGGCGTTATCGCGTTCTTGATC-TAMRA [2 pM], where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine) as previously described (24).
Plaque assay and luciferase assay as readouts for the extent of infection.
The firefly luciferase gene of HCMV strain TB40-BAC4-luc allows monitoring of relative light units (RLUs) in infected cell lysates as a readout for infection efficiency (34). First, the number of RLUs per infected cell and the correlation of RLU counts with the multiplicities of infection (MOIs) were evaluated on both cell types. Thus, HFFs and ARPE-19 cells were seeded in 96-well Nunc plates for a plaque assay and in white, clear, flat-bottom 96-well plates (Corning, Corning, NY) for a luciferase assay at a density of 104 cells/well 1 day before infection. In parallel, 100 μl per well of the same 2-fold serial dilutions was used either to infect the cells to determine the number of PFU or to measure RLUs by a luciferase assay. Cells were infected for 2 h at 37°C, washed three times with PBS, supplied with 100 μl of medium, and incubated further at 37°C for 2 days. For plaque assay, infected cells were fixed for indirect immunofluorescence assay using a mouse monoclonal antibody against IE1 (clone 0841; Bio-Rad) and counted on a Leica DMi8 fluorescence microscope. For IE1 staining, technical duplicates were performed, and four microscopic fields per well were counted. RLU values were determined in technical triplicates by luciferase assay of cell lysates according to the manufacturer’s protocol (SteadyGlo Luciferase Assay System; Promega) and measured in a Victor Light 1420 plate reader (PerkinElmer, Waltham, MA).
Normalization of parental and mutant virus stocks.
For normalization of parental strain and mutant virus stocks to similar RLU values, HFFs and ARPE-19 cells were infected with serially 2-fold-diluted mutant virus stocks as described above. For determination of relative epithelial cell infectivity, virus stock generating 3,000 to 8,000 RLUs in ARPE-19 cells was used and 2-fold diluted an additional 3 to 4 times. For all inhibition assays virus stocks were diluted to yield RLU values within a range of 900 to 13,700 RLUs in HFFs (MOI of 0.02 to 0.2) and 700 to 9,500 RLUs in ARPE-19 cells (MOI of 0.001 to 0.02).
Fibroblast and epithelial cell infection efficiencies.
HFFs and ARPE-19 cells were seeded in white, clear, flat-bottom 96-well plates (Corning, Corning, NY) at a density of 104 cells/well. The following day, virus stocks were diluted to similar numbers of encapsidated genomes in cell culture medium to yield about 8.59 log10 encapsidated genome copies/ml in HFFs and ARPE-19 cells, which corresponds to an MOI of 0.1 in gO GT1c on HFFs. To determine the exact number of genomes, 5 μl of the freshly prepared virus dilution was again tested by qPCR after DNase I digestion as mentioned above, and 100 μl per well was used to infect the cells in triplicates for 2 h at 37°C. Cells were washed three times with PBS, supplied with 100 μl of medium, and incubated further at 37°C for 2 days before monitoring the mean RLU values of technical triplicates. The actual number of encapsidated genomes/milliliter ranged from 8.0 to 9.2 log10 copies/ml (mean, 8.4 log10 copies/ml) in HFFs and from 8.3 to 9.3 log10 copies/ml (mean, 8.8 log10 copies/ml) in ARPE-19 cells. The ratio of the number of encapsidated genome copies to the number of RLUs was calculated, and values for mutants were compared to those of gO GT1c. Two to five independent experiments per mutant were performed.
Relative epithelial cell infectivity.
Virus stocks were normalized to RLU values as described above, and the same virus stock dilution series was used for infection of both fibroblasts and epithelial cells, each seeded in white, clear, flat-bottom 96-well plates at a density of 1 × 104 cells/well 1 day before infection. Two days after infection, RLU values were determined by luciferase assay as described above, and the epithelial cell-to-fibroblast ratio of RLU values was calculated. All experiments were performed in technical triplicates, and three to four independent experiments were performed.
Production of purified virions for immunoblotting.
Supernatants from infected HFFs were harvested when cells displayed >90% CPE and then clarified by centrifugation at 4,000 × g for 30 min at 4°C. After filtration through a 0.45-μm-pore-size filter (Whatman; GE Healthcare Life Sciences/ThermoFisher), viruses were concentrated by centrifugation at 4°C using Vivaspin 20 concentrators with a molecular weight cutoff of 100,000 (Sartorius, Göttingen, Germany). Thereafter, virions were purified by ultracentrifugation through a 20% sucrose-TAN (0.05 M triethanolamine, 0.1 M NaCl, pH 8.0) cushion for 80 min at 70,000 × g at 4°C, and the pellets were gently resuspended in TAN buffer on ice and stored in aliquots at –80°C until further use.
Western blot analysis.
For sample preparation, virus stocks were mixed undiluted or diluted in TAN buffer with an equal volume of reducing 2× sample buffer (125 mM Tris-Cl, pH 6.8, 6% SDS, 10% glycerol, 10% 2-mercaptoethanol, 0.01% bromophenol blue) and incubated on ice for 10 min before being boiled at 95°C for 10 min. Samples were separated on 8 to 10% SDS-PAGE gels together with a high-range rainbow marker (Amersham ECL High-Range Rainbow molecular weight marker; GE Healthcare, UK). Separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immun-Blot; Bio-Rad, CA) in blotting buffer (40 mM Tris, 39 mM glycine, 1.3 mM SDS, 20% methanol) which were then incubated overnight in blocking buffer (PBS, 1% bovine serum albumin [BSA], 0.1% Tween 20) at 4°C. All antibodies (Abs) were diluted in blocking buffer. Primary mouse anti-gH (AP86-SA4) and anti-MCP monoclonal antibodies (MAbs), anti-gO.02 MAb, and gB antibody (2F12; Abcam, Cambridge, UK) were incubated for 2 h at RT. Sheep anti-mouse IgG-horseradish peroxidase (HRP) (Amersham, GE Healthcare, UK) was used as a secondary antibody and incubated for 1 h at RT. SuperSignal West Femto Maximum Sensitivity substrate (ThermoFisher) was applied for gO and gH detection according to the manufacturer’s instructions, and Pierce ECL Western blotting substrate (Thermo Fisher) was used for MCP and gB detection. Chemiluminescent signals were visualized and analyzed using a ChemiDoc Imager and Image Lab, version 6.0, software (both Bio-Rad).
Inhibition assays.
Cells were seeded in white, clear, flat-bottom 96-well plates at a density of 1 × 104 cells/well the day before infection. A fixed amount of virus as determined by RLU count normalization was preincubated with serial dilutions of soluble forms of PDGFRα-Fc or NRP2-Fc for 2 h before infection. Two hours after infection cells were washed twice with 1× PBS, supplied with 100 μl medium per well, and further incubated for 2 days before being subjected to a luciferase assay. Total RLU counts and the percentage relative to RLU counts of mock-preincubated controls were calculated. Two to five independent experiments per mutant virus were performed, and all experiments were carried out in technical triplicates. Another independently reconstituted BAC-derived virus per mutant was used to confirm the results.
Statistical analyses.
To compare relative epithelial cell infectivities (Fig. 2H) and percentages of infection between gO GT1c and gO GT mutants after pretreatment with the soluble entry inhibitors sPDGFRα and sNRP2 (Fig. 4 and 5), one-way analysis of variance (ANOVA) and Dunnett’s or Tukey’s tests for multiple comparison were used. Mean RLU values from three to five independently repeated experiments were used for statistical analyses. P values of <0.05 were considered significant. GraphPad Prism, version 7.01, was used for statistical analyses.
ACKNOWLEDGMENTS
We are very grateful to Michaela Binder, Sylvia Malik, Barbara Dalmatiner, and Andreas Rohorzka for excellent technical support. We thank Nikolaus Osterrieder for generously providing the plasmid pEP-Kan-S for BAC mutagenesis.
Funding for this research was provided by a grant from the Austrian Science Fund (FWF) to I.G. (project P26420-B13). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Boppana S, Britt WJ. 2013. Synopsis of clinical aspects of human cytomegalovirus disease, p 1–26. In Reddehase MJ. (ed), Cytomegaloviruses: from molecular pathogenesis to intervention. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 2.Cannon MJ, Schmid DS, Hyde TB. 2010. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 3.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 4.Huber MT, Compton T. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol 72:8191–8197. doi: 10.1128/JVI.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanarsdall AL, Howard PW, Wisner TW, Johnson DC. 2016. Human cytomegalovirus gH/gL forms a stable complex with the fusion protein gB in virions. PLoS Pathog 12:e1005564. doi: 10.1371/journal.ppat.1005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou M, Lanchy JM, Ryckman BJ. 2015. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J Virol 89:8999–9009. doi: 10.1128/JVI.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang XJ, Adler B, Sampaio KL, Digel M, Jahn G, Ettischer N, Stierhof YD, Scrivano L, Koszinowski U, Mach M, Sinzger C. 2008. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J Virol 82:2802–2812. doi: 10.1128/JVI.01550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wille PT, Knoche AJ, Nelson JA, Jarvis MA, Johnson DC. 2010. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J Virol 84:2585–2596. doi: 10.1128/JVI.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. 2015. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 112:1767–1772. doi: 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M, Yu Q, Wechsler A, Ryckman BJ. 2013. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope. J Virol 87:9680–9690. doi: 10.1128/JVI.01167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murrell I, Tomasec P, Wilkie GS, Dargan DJ, Davison AJ, Stanton RJ. 2013. Impact of sequence variation in the UL128 locus on production of human cytomegalovirus in fibroblast and epithelial cells. J Virol 87:10489–10500. doi: 10.1128/JVI.01546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Zhou M, Stanton R, Kamil J, Ryckman BJ. 2018. Expression levels of glycoprotein O (gO) vary between strains of human cytomegalovirus, influencing the assembly of gH/gL complexes and virion infectivity. J Virol 92:e00606-18. doi: 10.1128/JVI.00606-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen CC, Kamil JP. 2018. Pathogen at the gates: human cytomegalovirus entry and cell tropism. Viruses 10:704. doi: 10.3390/v10120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan A, Cunningham C, Hector RD, Hassan-Walker AF, Lee L, Addison C, Dargan DJ, McGeoch DJ, Gatherer D, Emery VC, Griffiths PD, Sinzger C, McSharry BP, Wilkinson GW, Davison AJ. 2004. Genetic content of wild-type human cytomegalovirus. J Gen Virol 85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 17.Sijmons S, Thys K, Mbong Ngwese M, Van Damme E, Dvorak J, Van Loock M, Li G, Tachezy R, Busson L, Aerssens J, Van Ranst M, Maes P. 2015. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J Virol 89:7673–7695. doi: 10.1128/JVI.00578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suarez NM, Wilkie GS, Hage E, Camiolo S, Holton M, Hughes J, Maabar M, Vattipally SB, Dhingra A, Gompels UA, Wilkinson GWG, Baldanti F, Furione M, Lilleri D, Arossa A, Ganzenmueller T, Gerna G, Hubacek P, Schulz TF, Wolf D, Zavattoni M, Davison AJ. 2019. Human cytomegalovirus genomes sequenced directly from clinical material: variation, multiple-strain infection, recombination, and gene loss. J Infect Dis 220:781–791. doi: 10.1093/infdis/jiz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattick C, Dewin D, Polley S, Sevilla-Reyes E, Pignatelli S, Rawlinson W, Wilkinson G, Dal Monte P, Gompels UA. 2004. Linkage of human cytomegalovirus glycoprotein gO variant groups identified from worldwide clinical isolates with gN genotypes, implications for disease associations and evidence for N-terminal sites of positive selection. Virology 318:582–597. doi: 10.1016/j.virol.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Stanton R, Westmoreland D, Fox JD, Davison AJ, Wilkinson GW. 2005. Stability of human cytomegalovirus genotypes in persistently infected renal transplant recipients. J Med Virol 75:42–46. doi: 10.1002/jmv.20235. [DOI] [PubMed] [Google Scholar]
- 21.Cudini J, Roy S, Houldcroft CJ, Bryant JM, Depledge DP, Tutill H, Veys P, Williams R, Worth AJJ, Tamuri AU, Goldstein RA, Breuer J. 2019. Human cytomegalovirus haplotype reconstruction reveals high diversity due to superinfection and evidence of within-host recombination. Proc Natl Acad Sci U S A 116:5693–5698. doi: 10.1073/pnas.1818130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassalle F, Depledge DP, Reeves MB, Brown AC, Christiansen MT, Tutill HJ, Williams RJ, Einer-Jensen K, Holdstock J, Atkinson C, Brown JR, van Loenen FB, Clark DA, Griffiths PD, Verjans G, Schutten M, Milne RSB, Balloux F, Breuer J. 2016. Islands of linkage in an ocean of pervasive recombination reveals two-speed evolution of human cytomegalovirus genomes. Virus Evol 2:vew017. doi: 10.1093/ve/vew017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan H, Koyano S, Inami Y, Yamamoto Y, Suzutani T, Mizuguchi M, Ushijima H, Kurane I, Inoue N. 2008. Genetic linkage among human cytomegalovirus glycoprotein N (gN) and gO genes, with evidence for recombination from congenitally and post-natally infected Japanese infants. J Gen Virol 89:2275–2279. doi: 10.1099/vir.0.83685-0. [DOI] [PubMed] [Google Scholar]
- 24.Kalser J, Adler B, Mach M, Kropff B, Puchhammer-Stöckl E, Görzer I. 2017. Differences in growth properties among two human cytomegalovirus glycoprotein O genotypes. Front Microbiol 8:1609. doi: 10.3389/fmicb.2017.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui X, Freed DC, Wang D, Qiu P, Li F, Fu TM, Kauvar LM, McVoy MA. 2017. Impact of antibodies and strain polymorphisms on cytomegalovirus entry and spread in fibroblasts and epithelial cells. J Virol 91:e01650-16. doi: 10.1128/JVI.01650-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.E X, Meraner P, Lu P, Perreira JM, Aker AM, McDougall WM, Zhuge R, Chan GC, Gerstein RM, Caposio P, Yurochko AD, Brass AL, Kowalik TF. 2019. OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc Natl Acad Sci U S A 116:7043–7052. doi: 10.1073/pnas.1814850116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L. 2016. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol 1:16082. doi: 10.1038/nmicrobiol.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegmann C, Hochdorfer D, Lieber D, Subramanian N, Stohr D, Laib Sampaio K, Sinzger C. 2017. A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells. PLoS Pathog 13:e1006273. doi: 10.1371/journal.ppat.1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M, Wildner S, Scrivano L, Adler B. 2017. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-alpha as a key for entry. PLoS Pathog 13:e1006281. doi: 10.1371/journal.ppat.1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu K, Oberstein A, Wang W, Shenk T. 2018. Role of PDGF receptor-alpha during human cytomegalovirus entry into fibroblasts. Proc Natl Acad Sci U S A 115:E9889–E9898. doi: 10.1073/pnas.1806305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho H, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C. 2018. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174:1158–1171.e19. doi: 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Stegmann C, Abdellatif ME, Laib Sampaio K, Walther P, Sinzger C. 2017. Importance of highly conserved peptide sites of HCMV gO for the formation of the gH/gL/gO complex. J Virol 91:e01339-16. doi: 10.1128/JVI.01339-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stegmann C, Rothemund F, Laib Sampaio K, Adler B, Sinzger C. 2019. The N terminus of human cytomegalovirus glycoprotein O Is Important for binding to the cellular receptor PDGFRα. J Virol 93:e00138-19. doi: 10.1128/JVI.00138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scrivano L, Sinzger C, Nitschko H, Koszinowski UH, Adler B. 2011. HCMV spread and cell tropism are determined by distinct virus populations. PLoS Pathog 7:e1001256. doi: 10.1371/journal.ppat.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laib Sampaio K, Stegmann C, Brizic I, Adler B, Stanton RJ, Sinzger C. 2016. The contribution of pUL74 to growth of human cytomegalovirus is masked in the presence of RL13 and UL128 expression. J Gen Virol 97:1917–1927. doi: 10.1099/jgv.0.000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill AV. 1910. A new mathematical treatment of changes of ionic concentration in muscle and nerve under the action of electric currents, with a theory as to their mode of excitation. J Physiol 40:190–224. doi: 10.1113/jphysiol.1910.sp001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puchhammer-Stöckl E, Görzer I. 2011. Human cytomegalovirus: an enormous variety of strains and their possible clinical significance in the human host. Future Virol 6:259–271. doi: 10.2217/fvl.10.87. [DOI] [Google Scholar]
- 38.Görzer I, Kerschner H, Redlberger-Fritz M, Puchhammer-Stöckl E. 2010. Human cytomegalovirus (HCMV) genotype populations in immunocompetent individuals during primary HCMV infection. J Clin Virol 48:100–103. doi: 10.1016/j.jcv.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Vanarsdall AL, Pritchard SR, Wisner TW, Liu J, Jardetzky TS, Johnson DC. 2018. CD147 promotes entry of pentamer-expressing human cytomegalovirus into epithelial and endothelial cells. mBio 9:e00781-18. doi: 10.1128/mBio.00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two-step markerless red recombination system. Methods Mol Biol 634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]