Nipah virus (NiV) and Hendra virus (HeV), members of the Henipavirus genus, are recently emerged, highly lethal zoonotic pathogens that cause yearly outbreaks. NiV and HeV each encode a W protein that has roles in regulating host signaling pathways, including antagonism of the innate immune response. However, the mechanisms used by W to regulate these host responses are not clear. Here, characterization of the interaction of NiV and HeV W with 14-3-3 identifies modulation of 14-3-3-regulated host signaling pathways not previously associated with W, suggesting new avenues of research. The cocrystal structure of the NiV W:14-3-3 complex, as only the second structure of a 14-3-3 mode III interactor, provides further insight into this less-well-understood 14-3-3 binding motif.
KEYWORDS: 14-3-3, Hendra virus, Henipavirus, Nipah virus, W protein
ABSTRACT
Nipah virus (NiV) and Hendra virus (HeV), members of the Henipavirus genus in the Paramyxoviridae family, are recently emerged, highly lethal zoonotic pathogens. The NiV and HeV nonsegmented, negative-sense RNA genomes encode nine proteins, including the W protein. Expressed from the P gene through mRNA editing, W shares a common N-terminus with P and V but has a unique C-terminus. Expressed alone, W modulates innate immune responses by several mechanisms, and elimination of W from NiV alters the course of infection in experimentally infected ferrets. However, the specific host interactions that allow W to modulate innate immunity are incompletely understood. This study demonstrates that the NiV and HeV W proteins interact with all seven isoforms of the 14-3-3 family, regulatory molecules that preferentially bind phosphorylated target proteins to regulate a wide range of cellular functions. The interaction is dependent on the penultimate amino acid residue in the W sequence, a conserved, phosphorylated serine. The cocrystal structure of the W C-terminal binding motif with 14-3-3 provides only the second structure of a complex containing a mode III interactor, which is defined as a 14-3-3 interaction with a phosphoserine/phosphothreonine at the C-termini of the target protein. Transcriptomic analysis of inducible cell lines infected with an RNA virus and expressing either wild-type W or W lacking 14-3-3 binding, identifies new functions for W. These include the regulation of cellular metabolic processes, extracellular matrix organization, and apoptosis.
IMPORTANCE Nipah virus (NiV) and Hendra virus (HeV), members of the Henipavirus genus, are recently emerged, highly lethal zoonotic pathogens that cause yearly outbreaks. NiV and HeV each encode a W protein that has roles in regulating host signaling pathways, including antagonism of the innate immune response. However, the mechanisms used by W to regulate these host responses are not clear. Here, characterization of the interaction of NiV and HeV W with 14-3-3 identifies modulation of 14-3-3-regulated host signaling pathways not previously associated with W, suggesting new avenues of research. The cocrystal structure of the NiV W:14-3-3 complex, as only the second structure of a 14-3-3 mode III interactor, provides further insight into this less-well-understood 14-3-3 binding motif.
INTRODUCTION
Nipah virus (NiV) and Hendra virus (HeV), members of the Henipavirus genus within the Paramyxoviridae family, are recently emerged, highly pathogenic, zoonotic viruses that can cause fatal encephalitis or severe respiratory disease in humans, with case fatality rates between 40 and 75% (1, 2). HeV first appeared in 1994 in Australia, causing fatal disease in both horses and humans, while NiV emerged in Malaysia in 1998, causing lethal disease in humans (1, 3). Two strains of NiV have been identified, Malaysia (NiVM) and Bangladesh (NiVB), with NiVB being the more pathogenic strain (4, 5). Concerningly, human-to-human transmission of NiV has been reported and, despite the continued outbreaks of NiV and HeV, therapeutics and antivirals are currently lacking, and the determinants of virulence are poorly understood (1, 6).
The nonsegmented, negative-sense single-stranded RNA (ssRNA) genome of NiV and HeV encodes nine proteins from six genes, including the innate immune antagonist W (1). Expressed from the P gene through mRNA editing, W shares a common N-terminus with the P and V proteins but has a unique C-terminus. Through the shared N-terminus, P, V, and W interact with STAT1, inhibiting STAT1 phosphorylation and the interferon (IFN) response (7–10). In a ferret model of infection, recombinant NiV lacking STAT1 binding resulted in a reduction of IFN inhibition and an altered disease course but remained lethal, indicating the importance of additional functional roles, independent of Jak-STAT signaling inhibition, by the NiV P gene products (8).
The unique C-terminus of W contains a classical nuclear localization signal that interacts specifically with the Qip-1 subfamily of importin alpha (IMPA) nuclear transporters, importin α3 (IMPA3) and importin α4 (IMPA4) (11, 12). Through this interaction, W localizes to the nucleus, whereas P and V are predominantly cytoplasmic (9). W nuclear localization is required for its inhibition of Toll-like receptor 3 (TLR3) signaling (11). W also has described roles in regulating inflammatory responses to NiV in ferret models of infection and inhibiting signaling through IKKε and TBK1 (11, 13, 14). Despite this previous work, the specific host proteins and pathways engaged by W to regulate host responses to viral infection are not yet clear.
The W-host protein interactome has been examined through several mass spectrometry screens (15, 16). Among the identified host interactors of HeV and NiV W were several members of the 14-3-3 family of regulatory molecules (16). The 14-3-3 family of proteins consists of seven isoforms in humans; ε, β, ζ, γ, η, σ, and θ (17). Preferentially binding phosphorylated substrates, 14-3-3 proteins regulate a variety of cellular processes through their interactions with target proteins, affecting protein-protein interactions, trafficking, and enzymatic activities (17). Notably, roles for 14-3-3 in innate immune signaling have been described (17–19).
Given the functional impact of 14-3-3 on cellular signaling, including innate immune signaling, the potential interaction between NiV and HeV W and 14-3-3 was of interest. In this study, the capacity of W to interact with each of the 14-3-3 family members is demonstrated, and a high-resolution X-ray crystal structure of the W C-terminus in complex with 14-3-3σ is described. The results define the binding interface, demonstrate that the interaction requires phosphorylation of the penultimate amino acid residue in W, S449, and indicate that the interaction of IMPA3 with the W NLS can disrupt its interaction with 14-3-3. Functional assays comparing wild-type and mutant W indicate that innate immune regulatory functions are not substantially affected by 14-3-3 interaction. However, loss of interaction of W with 14-3-3 is demonstrated to modulate a range of cellular signaling pathways in inducible W-expressing cell lines. The data suggest novel functions for NiV and HeV W proteins and their modulation by 14-3-3 proteins.
RESULTS
NiV and HeV W interact with 14-3-3 family members.
Through mRNA editing, the P gene of NiV and HeV expresses the P, V, and W proteins that share a common N-terminus (NT) but have unique C-termini (CT) (Fig. 1A). A coimmunoprecipitation (co-IP) assay demonstrated that 14-3-3ε interacts specifically with NiV W and not NiV P, V, or the common NT (Fig. 1B). That 14-3-3ε interacts with NiV W but not V indicates that the binding site is located within the unique CT region of W. NiV W shares ∼59% identity at the amino acid level with HeV W (data not shown). Using a co-IP assay, HeV W, along with NiV W, coprecipitated 14-3-3ε, indicating conservation of the interaction between NiV and HeV W proteins (Fig. 1C).
FIG 1.

NiV and HeV W interacts with 14-3-3 family members. (A) Schematic of P gene products, P, V, and W, using NiV amino acid numbering to indicate the final amino acid in each gene (P, 709 amino acids; V, 456 amino acids; and W, 450 amino acids). The dashed line at amino acid 407 indicates the site of mRNA editing. NT, N-terminus; CT, C-terminus. (B) A co-IP assay with anti-HA antibody was performed on HEK293T lysates transfected with plasmids encoding Flag-tagged 14-3-3ε (2 μg) and HA-tagged NiV P, V, W, or the common N-terminus (NT) of the P gene products (2 μg), as indicated. Western blotting was performed for HA and Flag. “pCAGGS” denotes the empty vector control. (C) A co-IP assay was performed on HEK293T lysates transfected with plasmids encoding Flag-tagged NiV W or HeV W (2 μg) and HA-tagged 14-3-3ε (2 μg), as indicated. IP was performed with anti-Flag antibody. Western blotting was performed as in panel C. (D) A co-IP assay was performed on HEK293T lysates transfected with plasmids encoding Flag-tagged NiV W or empty vector (pCAGGS) (5 μg). Western blotting was performed for Flag and endogenous 14-3-3β, ε, γ, η, θ, ζ, and σ. (E) Co-IP assays and Western blotting were performed as in panel C on HEK293T lysates transfected with HA-tagged NiV W (2 μg) and Flag-tagged-14-3-3ε, ζ, σ, θ, and η (2 μg), as indicated. For all assays, “Input” indicates Western blotting performed on cell lysates used for the co-IP. Western blots for β-actin served as a loading control.
The 14-3-3 family of regulatory proteins consists of seven isoforms in humans—ε, β, ζ, γ, η, σ, and θ (20). To determine the specificity of NiV W interaction across the 14-3-3 family, co-IP assays with endogenous 14-3-3 were performed (Fig. 1D). As a control, IMPA3 was included as a known NiV W interactor (11, 12) (Fig. 1D). NiV W coprecipitated endogenous 14-3-3ε, β, γ, η, and θ. Endogenous 14-3-3ζ and σ were weakly visible in the input samples and were not detectable in the IP (Fig. 1D). Therefore, to further assess the breadth of interaction, co-IP assays with transfected Flag-tagged 14-3-3ζ and σ, as well as 14-3-3ε, θ, and η were performed. NiV W coprecipitated Flag-tagged 14-3-3 ζ, σ, ε, θ, and η (Fig. 1E). Together, these co-IP assays indicate that NiV W interacts with all seven isoforms of human 14-3-3.
NiV W S449 is required for interaction with 14-3-3.
Given that the interaction with 14-3-3 is shared between NiV and HeV W and is not mediated by the common N-terminus of the P gene products, it is likely facilitated by the unique W C-terminus (residues 408 to 450 of NiV W or residues 406 to 448 of HeV W) (Fig. 1A and B and 2A). As 14-3-3 proteins preferentially recognize targets containing phosphorylated threonine (T) or serine (S) residues, alignment of the NiV and HeV W C-termini identified three “T” and one “S” residue (T410, T420, T438, and S449 in NiV W) conserved between both strains of NiV and HeV W that could mediate the interaction with 14-3-3 (Fig. 2A) (20). To assess which residue(s) were required, the four “T” and “S” residues were individually mutated to alanine (A) in the context of a NiV W C-terminus construct fused to glutathione S-transferase (GST). A co-IP assay demonstrated that the C-terminus (CT) of NiV W was sufficient to mediate interaction with 14-3-3ε (Fig. 2B). NiV W mutants T410A, T420A, and T438A maintained interaction with 14-3-3ε, while NiV W S449A demonstrated a loss of interaction (Fig. 2B). In further support of the specificity of the NiV W interaction with 14-3-3, the unique CT of NiV V fused to GST was unable to coprecipitate 14-3-3ε. Expression of GST alone also did not interact with 14-3-3ε (Fig. 2B). Similar results were demonstrated in the context of full-length NiV W, as mutants T410A, T420A, and T438A interacted with 14-3-3ε, while mutant S449A did not (Fig. 2C). Co-IP assays with the seven isoforms of 14-3-3 further demonstrated the requirement of NiV W S449 for interaction across the family (Fig. 2D and E). In addition, the NiV W S449A mutant maintained interaction with IMPA3 (Fig. 2E). Together, these data indicate that the NiV W interaction with 14-3-3 is mediated through NiV W S449 in the unique C-terminus.
FIG 2.
NiV W S449 is required for interaction with 14-3-3. (A) Alignment of amino acid residues in unique C-termini of NiV and HeV W. Conserved serine (S) and threonine (T) residues are indicated in bold and underlined. Numbering indicates amino acid residue location in full-length W. (B) A co-IP assay was performed with anti-GST antibody on lysates from HEK293T cells transfected with plasmids encoding Flag-tagged 14-3-3ε (2 μg) and GST fused NiV W C-terminus (CT) wild-type or mutant constructs, GST fused NiV V CT, or GST alone (2 μg), as indicated. Western blotting was performed for GST and Flag. pCAGGS denotes the empty vector control. (C) A co-IP assay was performed with anti-Flag antibody on lysates of HEK293T cells transfected with plasmids encoding HA-tagged 14-3-3ε (2 μg) and Flag-tagged full-length NiV W wild-type or mutant constructs (2 μg), as indicated. Western blotting was performed for Flag and HA. (D) A co-IP assay was performed on lysates from HEK293T cells transfected with plasmids encoding HA-tagged NiV W, NiV W S449A, or pCAGGS empty vector (5 μg). Western blotting was performed for Flag and endogenous 14-3-3β, ε, γ, η, θ, ζ, and σ. (E) co-IP assays and Western blotting were performed as in panel C on lysates from HEK293T cells transfected with plasmids encoding HA-tagged NiV W or NiV W S449A (2 μg) and Flag-tagged 14-3-3ε, ζ, σ, θ, and η (2 μg), as indicated. For all co-IP assays, “Input” indicates Western blots performed on cell lysates used for the co-IP. Western blots for β-actin served as a loading control.
Cocrystal structure of NiV W in complex with 14-3-3.
X-ray crystallography was used to solve a cocrystal structure of 14-3-3σ in complex with a peptide containing NiV W amino acids 441 to 450 (ARVSMRRMSpN), with a phosphoserine at position 449, to 2.3 Å (Fig. 3A and Table 1). The final five amino acids of the NiV W peptide (amino acids 446 to 450, RRMSpN) were resolved in the structure. In the absence of a phosphoserine at position 449, the NiV W peptide did not form crystals in the presence of 14-3-3σ, supporting the necessity of the phosphoserine to mediate the interaction with 14-3-3. The 14-3-3σ protein structure contained nine antiparallel α-helices (Fig. 3B), with α3, α5, α7, and α9 forming a deep groove and binding site for the NiV W peptide. The Sp449 forms a series of specific interactions with 14-3-3, mediated through R56, R129, and Y130 (Fig. 3C). Other interactions outside of the Sp449 binding region that also participate in the interface include 14-3-3 N175 binding to NiV W N450, 14-3-3 E182 binding to NiV W R447, and 14-3-3 N226 binding to NiV W M448 (Fig. 3B). The strong bonding at the phosphate provides a structural basis for the importance of phosphorylation, consistent with our observation that phosphomimetics were insufficient for binding in both co-IP assays and during attempts to purify protein complexes for crystallization (data not shown). This is consistent with literature describing that aspartate and glutamate are poor phosphomimetic residues for 14-3-3 interactions (21, 22).
FIG 3.
A cocrystal structure demonstrates that NiV W interacts with 14-3-3 through a mode III binding motif. (A) Structure of 14-3-3σ bound to the C-terminus of NiV W protein containing a phosphorylated serine 449 (S449) (PDB: 6W0L). 14-3-3 is shown in surface (left) and cartoon (right) in gray, bound to NiV W in ball and stick mode in orange. 14-3-3σ comprises nine α-helices, and NiV W is bound in a pocket created by α3/α5/α7/α9. (B) Topology diagram highlighting the binding of NiV W to 14-3-3 α-helices. (C) Detailed bonding between 14-3-3σ and NiV W. (D) A co-IP assay was performed with anti-HA antibody on lysates from HEK293T cells transfected with plasmids encoding HA-tagged 14-3-3σ wild-type or mutants (3 μg) and Flag-tagged NiV W (2 μg), as indicated. Western blotting was performed for Flag and HA. “pCAGGS” denotes the empty vector control. (E) A co-IP assay was performed with anti-Flag antibody on lysates from HEK293T cells transfected with plasmids encoding Flag-tagged NiV W wild type or mutants (2 μg) and HA-tagged 14-3-3σ (3 μg), as indicated. Western blotting was performed as in panel D. For all co-IP assays, “Input” indicates Western blots performed on cell lysates used for the co-IP. Western blots for β-actin served as a loading control.
TABLE 1.
Diffraction data collection and refinement statistics for 14-3-3 bound with NiV W C-terminus
| Data collection and refinement parameters | Valuea |
|---|---|
| Resolution range (Å) | 24.69 to 2.3 (2.382 to 2.3) |
| Space group | C 2 2 21 |
| Unit cell dimensions | |
| a, b, c (Å) | 83.04, 112.00, 62.50 |
| α, β , γ (°) | 90, 90, 90 |
| Total no. of reflections | 63,050 (5,697) |
| No. of unique reflections | 13,246 (1,277) |
| Multiplicity | 4.8 (4.5) |
| Completeness (%) | 99.7 (99.9) |
| Mean I/σ(I) | 5.2 (2.1) |
| Wilson B-factor (Å2) | 24.4 |
| R-merge | 0.174 (0.615) |
| R-pim | 0.096 (0.370) |
| CC1/2 | 0.978 (0.344) |
| CC* | 0.988 (0.511) |
| No. of reflections used in refinement | 13,233 |
| No. of reflections used for R-free | 644 |
| R-work | 0.2102 |
| R-free | 0.2647 |
| No. of nonhydrogen atoms | 1,963 |
| Macromolecules | 1,930 |
| Solvent | 32 |
| No. of protein residues | 239 |
| RMS (bonds) | 0.005 |
| RMS (angles) | 0.64 |
| Ramachandran favored (%) | 98.28 |
| Ramachandran allowed (%) | 1.72 |
| Ramachandra outliers (%) | 0.00 |
| Rotamer outliers (%) | 0.96 |
| Clashscore | 1.81 |
| Average B-factor (Å2) | 36.16 |
| Macromolecules (Å2) | 36.23 |
| Solvent (Å2) | 31.65 |
Values in brackets refer to the highest-resolution shell.
The 14-3-3 proteins preferentially interact with phosphorylated substrates through one of three modes, the well characterized mode I and II and the less-well-defined mode III (20). Mode I and II consist of two canonical internal binding motifs that contain a phosphorylated threonine or serine residue, often followed by a proline residue at the +2 position to accommodate binding. Mode III, in contrast, occurs between 14-3-3 and a phosphoserine/phosphothreonine in the C-terminus of the target protein (20). NiV W S449 is the penultimate amino acid residue in the NiV W sequence (Fig. 2A); therefore, the 14-3-3 interaction with NiV W is a mode III binding interaction (20).
Using the cocrystal structure, mutations were designed in 14-3-3σ and NiV W to disrupt the identified interface. Amino acids R56 and R129 in 14-3-3σ coordinate the interaction with the phosphoserine at NiV W S449 (Fig. 3C). A co-IP assay demonstrated that individual mutation of R56 or R129 to “A” leads to the loss of interaction with NiV W, highlighting the importance of the contacts between 14-3-3 and the NiV W phosphoserine (Fig. 3D). Within the NiV W interface, mutations to “A” were made for R446, R447, M448, and N450. Used in a co-IP assay along with NiV W S449A, a loss of interaction between NiV W and 14-3-3σ was identified for the mutant NiV W R446A and NiV W S449A (Fig. 3E). NiV W R447A, M448A, and N450A maintained interaction with 14-3-3σ (Fig. 3E). These results validate the determined structure, confirm that NiV W interacts with 14-3-3 through a mode III binding motif, and demonstrate that phosphorylation of S449 is essential to the interaction.
IMPA3 can outcompete 14-3-3 interaction with NiV W.
The cocrystal structure of the C-terminal peptide of NiV W with 14-3-3σ supports the direct interaction of these two proteins (Fig. 3A). In cells, NiV W is localized to the nucleus through the interaction of a nuclear localization signal (NLS) located near the C-terminus with IMPA3 and IMPA4, while the loss of interaction with the IMPA proteins results in NiV W cytoplasmic localization (11, 12). To assess whether subcellular localization of NiV W influences interaction with 14-3-3, a co-IP assay was performed with 14-3-3ε and wild-type NiV W or the mutant NiV W K439A/K440A (KKA), which contains mutations to the W NLS residues critical for interaction with the major NLS binding sites on IMPA3 and IMPA4 (Fig. 4A) (12). Both NiV W and NiV W KKA coprecipitated 14-3-3ε, suggesting that nuclear localization of NiV W is not necessary to mediate 14-3-3 interaction (Fig. 4B).
FIG 4.
IMPA3 can outcompete the 14-3-3 interaction with NiV W. (A) Amino acid residues in the unique C terminus of NiV W. S449 is indicated with a double underline, while the classical bipartite nuclear localization signal (NLS) is indicated by the solid and dashed single underline. Amino acids K439 and K440 that interact with the major NLS binding site of IMPA are indicated in bold. Numbering indicates the amino acid residue location in full-length W. (B) A co-IP assay performed with anti-Flag antibody on lysates from HEK293T cells transfected with plasmids encoding Flag-tagged NiV W or the IMPA3 binding mutant, NiV W RKA, and HA-tagged 14-3-3σ, as indicated. Western blotting was performed with Flag and HA. “pCAGGS” denotes the empty vector control. (C and D) Competition co-IP assays were performed with anti-Flag antibody on lysates from HEK293T cells transfected with plasmids encoding Flag-tagged NiV W (2 μg) and (C) HA-tagged 14-3-3ε (3 μg) and increasing concentrations of HA-tagged IMPA3 (1, 2, and 3 μg) or (D) HA-tagged IMPA3 (2 μg) and increasing concentrations of HA-tagged 14-3-3ε (1, 2, and 4 μg), as indicated. Western blotting was performed as in panel B. For all co-IP assays, “Input” indicates Western blots performed on cell lysates used for the co-IP. Western blots for β-actin served as a loading control.
The 14-3-3 binding motif in NiV W (amino acid residues 446 to 450) is adjacent to the NiV W NLS (amino acid residues 421 to 446) (Fig. 4A) (12). To determine whether NiV W can interact concurrently with 14-3-3 and IMPA3, competition co-IP assays were performed (Fig. 4C and D). NiV W coprecipitated 14-3-3ε in the absence of overexpressed IMPA3; however, upon coexpression of increasing concentrations of IMPA3, the interaction of NiV W with 14-3-3ε rapidly decreased, while the interaction of NiV W with IMPA3 increased (Fig. 4C). In contrast, the interaction of NiV W with IMPA3 was not disrupted by the expression of increasing concentrations of 14-3-3ε (Fig. 4D). Together, these data suggest that NiV W does not simultaneously interact with 14-3-3 and IMPA3 and that IMPA3 outcompetes 14-3-3 for interaction with NiV W.
NiV W interaction with 14-3-3 does not affect inhibition of interferon responses.
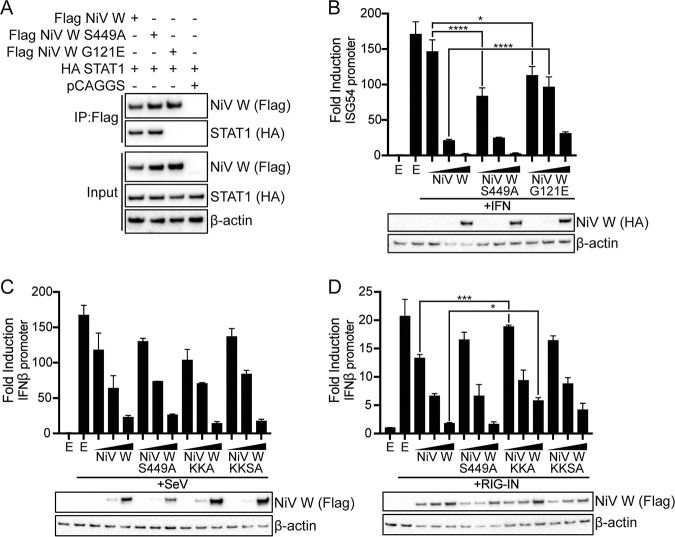
NiV W is a known antagonist of IFN responses, and several 14-3-3 isoforms have been demonstrated to contribute to innate immune signaling pathways (7–9, 11, 18, 19, 23, 24). Antagonism of the IFN response by NiV W is partially exerted through an N-terminal interaction with STAT1 (7–9). A co-IP assay demonstrated that both NiV W and the mutant NiV W S449A coprecipitate STAT1 (Fig. 5A). As a control for loss of interaction, NiV W G121E, a mutant known to have a disrupted interaction with STAT1, was included in the co-IP (7, 8). As expected, NiV W G121E did not interact with STAT1 (Fig. 5A). To further assess the functional outcome of NiV W S449A on antagonism of the IFN response, an ISG54 promoter luciferase assay was used. Treatment of cells with IFN-β induced ISG54 luciferase activity that was inhibited in a concentration-dependent manner by NiV W (Fig. 5B). Similar inhibition was demonstrated by the mutant NiV W S449A, while NiV W G121E showed a loss of inhibitory activity consistent with its lack of STAT1 binding and previous studies (Fig. 5B) (7, 8). Together, these data indicate that the interaction of NiV W with 14-3-3 does not affect its interaction with STAT1 or contribute to its inhibition of IFN-induced ISG54 promoter gene expression.
FIG 5.
NiV W interaction with 14-3-3 does not affect inhibition of interferon responses. (A) A co-IP assay was performed with anti-HA antibody on lysates from HEK293T cells transfected with plasmids encoding HA-tagged NiV W, NiV W S449A and NiV W G121E (2 μg), and HA-tagged STAT1 (2 μg), as indicated. Western blotting was performed for HA and Flag. “pCAGGS” denotes the empty vector control. “Input” indicates Western blots performed on cell lysates used for the co-IP. Western blots for β-actin served as a loading control. (B) HEK293T cells were transfected with the ISG54 promoter luciferase reporter, a constitutively expressed Renilla luciferase plasmid, and increasing concentrations of plasmids encoding HA-tagged NiV W, NiV W S449A, or NiV W G121E (1, 10, and 100 ng), as indicated. At 24 h posttransfection, cells were treated with 1,000 units (U) interferon (IFN) for an additional 24 h. The firefly luciferase signal was normalized to the Renilla luciferase signal, and error bars represent the standard deviation (SD) for triplicate experiments. NiV W expression was assessed by Western blotting for the HA tag. “E” denotes the empty vector control. (C) HEK293T cells were transfected with the IFN-β promoter luciferase reporter, a constitutively expressed Renilla luciferase plasmid, and increasing concentrations of plasmids encoding Flag-tagged NiV W, NiV W S449A, NiV W KKA, or NiV W KKSA (2.5, 25, and 250 ng), as indicated. To induce reporter activity, cells were infected with SeV (200 HAU) 24 h posttransfection for an additional 18 h. Luciferase activity and protein expression were assessed and analyzed as in panel B. “E” denotes the empty vector control. (D) HEK293T cells were transfected with the IFN-β promoter luciferase reporter, a constitutively expressed Renilla luciferase plasmid, Flag-tagged RIG-IN (5 ng), and increasing concentrations of plasmids encoding Flag-tagged NiV W, NiV W S449A, NiV W KKA, or NiV W KKSA (62.5, 125, and 250 ng), as indicated. Eighteen hours posttransfection, luciferase activity and protein expression were assessed and analyzed as in panel B. “E” denotes the empty vector control. (B to D) Statistical significance was assessed by a one-way ANOVA, followed by Tukey’s test; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
While NiV W is known to inhibit type I IFN production, the mechanistic basis is not yet clear (11). Interestingly, 14-3-3ε is required for the stable interaction of RIG-I and TRIM25, facilitating efficient RIG-I signaling (19). To assess the contribution of NiV W interaction with 14-3-3 on inhibition of IFN production, we used an IFN-β promoter luciferase assay, inducing activity with infection by the known RIG-I activator Sendai virus (SeV) or by coexpression with a constitutively active RIG-I (RIG-IN) (25). As 14-3-3 exerts its function on RIG-I signaling in the cytoplasm, we determined the inhibitory activity of wild-type NiV W and NiV W S449A, as well as that of the cytoplasmic localizing IMPA binding mutant NiV W KKA and a dual mutant, NiV W KKSA, which contained both the IMPA binding mutations at K439A/K440A and the 14-3-3 binding mutation at S449A (Fig. 5C and D). SeV-induced IFN-β promoter activity was inhibited in a concentration dependent manner by NiV W, and the mutant NiV Ws S449A, KKA, and KKSA maintained this inhibition of luciferase activity (Fig. 5C). Similarly, RIG-IN-induced IFN-β promoter reporter activity was inhibited to a comparable extent by both NiV W and NiV W S449A (Fig. 5D). The IMPA binding mutant, NiV W KKA, demonstrated a modest but statistically significant loss of inhibitory activity at the lowest and highest concentrations used, while no statistically significant changes in activity were detected for the dual mutant, NiV W KKSA (Fig. 5D). Together, these results indicate that the interaction of NiV W with 14-3-3 is not required for its antagonistic activity on the IFN production pathway under the conditions tested.
NiV W interaction with 14-3-3 modulates host gene expression in the context of RNA virus infection.
To gain insight into the functional impact of the NiV W interaction with 14-3-3, RNAseq was performed on Tet-inducible, monoclonal, stable A549 cell lines that express a Flag-tag, Flag-tagged NiV W, or Flag-tagged NiV W S449A. Cells were induced by doxycycline treatment for 24 hours and then mock- or SeV-infected for an additional 24 hours to activate an antiviral response. RNAseq was then performed on duplicate samples to assess the impact of wild-type and mutant W on gene expression. Alignment of RNAseq reads to the SeV genome demonstrated that 12% of the reads in the SeV-infected control aligned to SeV (Fig. 6A). This slightly decreased in the NiV W-expressing cells (8.8% and 9.5%) while remaining similar in those expressing NiV W S449A. Expression of NiV W S449A, as measured by percentage aligned reads, was slightly decreased relative to NiV W (Fig. 6A). Principal-component analysis (PCA) demonstrated distinct profiles for each set of samples (Fig. 6B).
FIG 6.
NiV W interaction with 14-3-3 modulates host gene expression in the context of virus infection. (A) The percentage of RNAseq reads that align to the SeV genome or NiV W sequence for each set of samples was determined. (B) Principal-component analysis (PCA) of RNAseq samples using DESeq2. (C to D) Functional enrichment using Metascape of DEGs where (C) expression is downregulated in SeV-infected, wild-type W-expressing cells compared to S449A-expressing cells or (D) expression is upregulated in SeV-infected, wild-type W-expressing cells compared to S449A-expressing cells. Significant terms were defined as those with a P value of <0.01. The number of DEGs that correspond with each term is indicated in white on each bar of the graph. (E to F) Transcription factor binding site (TFBS) enrichment analysis in the promoters of genes downregulated in SeV-infected, wild-type NiV W-expressing cells compared to NiV W S449A-expressing cells (E) and upregulated in SeV-infected, wild-type NiV W-expressing cells compared to NiV W S449A-expressing cells (F), using oPOSSUM 3.0. Significantly enriched transcription factor binding sites (TFBS) using a Z-score cutoff of 20 (E, red circles) or 10 (F, blue circles) are indicated. (G) Transcription factor (TF) functional association network generated by STRING for TFs that bind the enriched TFBS in the downregulated gene subset (from panel E; Z-score ≥ 10). (H) Functional enrichment of TFs in panel G using STRING. The number of TFs that correspond with each term is indicated in white on each bar of the graph.
Differentially expressed gene (DEG) analysis using edgeR identified 630 DEGs between SeV-infected cells expressing NiV W and those expressing NiV W S449A, with 449 DEGs downregulated (71%) in NiV W versus NiV W S449A and 181 DEGs upregulated (29%). Gene ontology (GO) enrichment analysis was performed on the down- and upregulated DEGs using Metascape (26). DEGs downregulated in NiV W-expressing cells relative to those expressing NiV W S449A enriched to GO terms associated with metabolism (e.g., hormone and organic hydroxy compound metabolic processes), cellular signaling (e.g., regulation of MAPK cascade), and extracellular matrix organization (Fig. 6C). Analysis of the 181 DEGs upregulated in the NiV W-expressing cells compared to those expressing NiV W S449A enriched to GO processes involved in signaling (e.g., cytokine-mediated signaling pathway, positive regulation of protein kinase activity, and cation homeostasis) and metabolism (e.g., regulation of nucleotide and phosphate metabolic processes) (Fig. 6D). These results indicate that the loss of NiV W interaction with 14-3-3 alters a wide range of cellular signaling pathways.
To further understand which types of genes may be regulated by NiV W and its interaction with 14-3-3, oPOSSUM 3.0 single-site analysis (SSA) was used to analyze the enrichment of transcription factor binding sites (TFBS) in the up- and downregulated DEGs. Using a threshold Z-score of >10, 47 TFBS were significantly enriched in the downregulated DEGs, while only six were significantly enriched in the upregulated DEGs (Fig. 6E and F). Notably, interferon response-related transcription factors STAT1, STAT3, RELA, and AP1 TFBS were enriched in the DEGs downregulated in NiV W-expressing cells relative to the NiV W S449A mutant (Fig. 6E).
Forty-seven transcription factors (TFs) that interact with the significant TFBS identified in the downregulated DEG subset were further analyzed by STRING to generate a functional association network that demonstrated connections between 34 of the TFs (Fig. 6G) (27). Functional enrichment found GO terms consistent with the downregulated DEG analysis, including processes involved in regulation of cellular metabolism and immune system processes (Fig. 6C and H). Cell differentiation, fate commitment, and processes involved in RNA metabolism and regulation of RNA polymerase II transcription were also highly enriched for (Fig. 6H).
DISCUSSION
NiV and HeV W proteins are virulence factors. A recombinant NiV (rNiV) lacking W expression maintained lethality in ferret models of infection but resulted in an increased cytokine response, delayed time to death, and altered disease course such that the W mutant caused encephalitis rather than the pneumonia caused by the wild-type NiV, although the mechanistic basis for these differences are not yet understood (13, 14). The NiV and HeV W proteins are also noteworthy among those expressed by most members of the Paramyxoviridae family, including other henipaviruses, as they possess a relatively long, unique C-terminus, rather than terminating in a stop codon shortly after the mRNA editing site as seen in most paramyxovirus W proteins (28). This C-terminal domain is important for several NiV and HeV W functions, including facilitating the nuclear localization of NiV and HeV W, through interaction with the IMPA3 and IMPA4 nuclear transport adaptor proteins (11, 12). The nuclear localization of W is required for some of its identified innate immune antagonism roles, such as inhibition of TLR3 signaling (11). NiV W must also be present in the nucleus to carry out recently identified functions in modulating p53 signaling and affecting the activity of the PRP19 complex, although the impact of these functions on virus have yet to be determined (15).
In this study, the interaction between NiV W and the seven isoforms of 14-3-3 was mapped to the unique C-terminus, although nuclear localization of W is not necessary to facilitate interaction. Instead, a conserved phosphorylated serine residue in NiV and HeV W (S449 in NiV W, S447 in HeV W) is required to mediate interaction with 14-3-3. This serine is the penultimate amino acid residue in the NiV and HeV W sequence, suggesting a mode III interaction interface. The cocrystal structure of NiV W in complex with 14-3-3σ confirmed a mode III binding motif in NiV W, containing residues 446RRMSPN450. The consensus sequence for mode III consists of (pS/pT)X1-2-COOH (20, 29). Selectivity for amino acids upstream of the phosphorylated serine or threonine are absent from the consensus, as there is great variability in the binding motifs currently described for mode III interactors (20). Mutational analysis of the NiV W binding motif identified a loss of interaction with mutation to alanine of R446, in addition to S449. While not absolutely required, upstream arginine residues are often found in the binding motifs of mode I to III interactors and have been shown to be important for kinase recognition and phosphorylation-dependent 14-3-3 binding (30, 31). Therefore, it is possible that NiV W R446A lost interaction with 14-3-3 due to disruption of phosphorylation at S449. Mode III interactors are less well characterized than mode I and II, and only one cocrystal structure of a mode III interactor, TWIK-related acid-sensitive K-channels (TASK) in complex with 14-3-3σ, has previously been reported (32). Therefore, the interaction interface mapping and structure of NiV W with 14-3-3σ provides additional insight into 14-3-3 interactions with mode III binding motif-containing target proteins. The mode III interaction interface also provides a tool to further understand the role of both 14-3-3 and W in NiV and HeV infection, as a specific stabilizer of mode III interactors has been developed (32). It will be of interest to determine the effect stabilization of this interaction may have on infection.
14-3-3 proteins are known to function in the regulation of intracellular protein localization, sequestering or trafficking target proteins throughout the cell (17). The subcellular localization of W in NiV-infected cell lines can vary, with NiV W localized in the nucleus of neuronal cells but cytoplasmic in endothelial cell lines (33). Therefore, it is of interest to determine how NiV W nuclear localization is regulated. While the NiV W interaction with 14-3-3 does not require interaction with IMPA, competition co-IPs indicate that IMPA can outcompete 14-3-3 for interaction with NiV W. The reciprocal co-IP demonstrated that 14-3-3 does not disrupt IMPA interaction with NiV W. The results of the competition co-IPs suggest that the interaction of NiV W with 14-3-3 does not disrupt IMPA-driven NiV W nuclear localization and that 14-3-3 is not a regulator of W nuclear import. Rather, because NiV W does not simultaneously interact with both IMPA and 14-3-3, the interaction with IMPA may have a regulatory role on the NiV W:14-3-3 interaction. Further studies will be required to assess this possibility.
The NS3 proteins of Zika virus and dengue virus are described interactors of 14-3-3 proteins, which bind through phosphomimetic binding motifs rather than phospho-serine or -threonine (23, 24). Via their interaction with 14-3-3, these NS3 proteins inhibit innate immune signaling, as efficient RIG-I and MDA5 signaling have been demonstrated to require 14-3-3ε and 14-3-3η, respectively (18, 19). This suggested a potential mechanistic role for the NiV W interaction with 14-3-3. Inhibition of SeV-induced IFNβ-promoter activity by NiV W has been previously demonstrated (11). While the inhibition is attributed to a decrease in IFN regulatory transcription factor 3 (IRF3) phosphorylation, the mechanism of action is not fully understood (11). Using IFNβ promoter luciferase assays, NiV W inhibited RIG-I signaling, induced by either SeV infection or expression of RIG-IN, and inhibition was not lost following disruption of the 14-3-3 interaction. As the activity of 14-3-3ε on RIG-I signaling occurs in the cytoplasm, it is possible that the nuclear localized NiV W was unable to affect this function of 14-3-3. To address this, NiV W expression was shifted to the cytoplasm by mutating the major IMPA NLS binding site in the C-terminus of W and combining it with the 14-3-3 binding mutant (NiV W KKSA). Notably, these mutants demonstrated inhibition similar to that of wild-type NiV W. Together, these data indicate that the interaction of NiV W with 14-3-3 does not facilitate its inhibition of RIG-I signaling, as measured through the IFNβ promoter luciferase assay. Attempts were made to determine if the NiV W interaction with 14-3-3η affects MDA5 signaling; however, NiV W-mediated inhibition of MDA5 signaling was minimal and inconsistent (data not shown). Therefore, assessment of the activity of the 14-3-3 binding mutant of NiV W on MDA5 signaling was not pursued.
Due to the multifunctionality of the 14-3-3 family of proteins, RNAseq was used to gain an understanding of what processes may be affected by the NiV W:14-3-3 interaction in the presence of an RNA virus infection. Inducible cell lines were used to ensure that all cells would express the transgene, but only after induction. Given the previously described innate immune inhibitory functions of W, we performed the study in the presence of SeV infection, because SeV is a strong activator of innate antiviral signaling. Several of the enriched terms were related to processes 14-3-3s are involved in, including cellular metabolism, regulation of apoptosis, cell adhesion, and cellular signaling (34–36). This suggests that NiV W exerts its effect on these signaling pathways through its interaction with 14-3-3 (13). Given that lack of W expression during NiV infection results in an altered course of disease, it is possible that the interaction of W with 14-3-3 may contribute to this. Further studies will be required to understand and clarify the impact of these newly identified functions.
Enrichment of genes involved in innate immune antagonism and responses to viral infection were identified in the pathway analysis, and transcription factor binding sites (TFBS) for innate immune-related transcription factors STAT1, STAT3, RELA, and AP1 were enriched for in the downregulated DEG subset. This suggests that the interaction of NiV W with 14-3-3 did modulate innate immune responsive gene expression in this more sensitive experimental system, relative to the IFNβ promoter luciferase assays.
The roles of NiV and HeV W in infection remain unclear. Previous studies mostly focused on its functions in the antagonism of innate immune signaling (7–9, 11, 13, 14). However, the modulation of diverse signaling pathways and processes regulated by 14-3-3 suggests potential new functions for W that are worthy of further exploration. The contribution of the W interactions to pathogenesis is of interest. Because the W open reading frame overlaps with those of P and V, one cannot readily mutate NiV W without also mutating either P or V. One possibility would be to build a recombinant NiV with a W knockout and then introduce wild-type or mutant Ws as an extra gene. However, adding an extra gene would likely be somewhat attenuating. Therefore, examining the contribution of 14-3-3 binding to virulence will be complicated.
MATERIALS AND METHODS
Plasmids and lentiviruses for cell culture.
The pCAGGS HA and Flag-tagged NiV P, NiV V, NiV W, HeV W, pCAGGS HA-tagged NiV W G121E, pCAGGS Flag-tagged NiV W K439A/K440A (KKA), pCAGGS TRIF, pCAGGS hemagglutinin (HA)-tagged IMPA3, pCAGGS Flag-tagged RIG-IN, pCAGGS HA-tagged STAT1, and ISG54 and IFNβ promoter firefly luciferase reporters have been previously described (7, 11, 12, 37). pRL-TK was purchased from Promega. pcDNA3.1 Myc-tagged 14-3-3ζ, pcDNA3.1 HA-tagged 14-3-3ε, and pcDNA3 HA-tagged 14-3-3σ were purchased from Addgene (Addgene no. 48798, 48787, and 11946) and were cloned by PCR and inserted into pCAGGS Flag and HA-tagged vectors. 14-3-3θ and η were cloned by PCR from A549 cDNA and inserted into pCAGGS Flag and HA-tagged vectors. pCAGGS Flag-tagged NiV W T410A, T420A, T438A, S449A, R446A, R447A, M448A, and N450A and pCAGGS HA-tagged 14-3-3σ R56A and R129A were generated by primer-inserted mutations or overlapping PCR. The NiV W CT (amino acids 408 to 450) was cloned into pCAGGS as a GST fusion, and mutants T410A, T420A, T438A, and S449A were generated by primer-inserted mutations or overlapping PCR. NiV V CT (amino acids 408 to 457) was cloned into pCAGGS as a GST fusion. Flag-tagged NiV W and NiV W S449A, or a Flag-tag alone, were cloned into pLVX-TetOne-Puro lentivirus vector (Clontech).
Lentiviruses were generated by transfecting 2 × 106 HEK293T cells with 3 μg of the pLVX-TetOne-Puro lentiviral plasmid expressing Flag-tagged NiV W, Flag-tagged NiV S449A, or Flag-tag, 2.25 μg of psPAX2, and 0.75 μg of pMD2.G (38). Supernatants were collected at 48 and 72 h posttransfection, combined, and clarified by centrifugation at 4,000 rpm for 15 min and stored at –80°C.
Plasmids for recombinant protein expression and purification.
The gene encoding full-length 14-3-3σ was codon optimized and synthesized by GenScript. A Tobacco etch virus (TEV)-protease was incorporated at the N-terminus and cloned into the pET-30a expression vector at the BamHI site. The plasmid was transformed into BL21(DE3) pLyS, and expression of recombinant protein was performed using the auto-induction method (39). Cultures were incubated for 30 h on an orbital platform with shaking at 90 rpm. Pellets were resuspended in His buffer (50 mM phosphate buffer [pH 8.0], 300 mM NaCl, and 20 mM imidazole). Cells were lysed by three freeze-thaw cycles and treatment with 1 ml of lysozyme (20 mg/ml) and 10 μl of DNase (50 mg/ml) per 50 ml of resuspended culture. Cell debris was removed by centrifugation at 12,000 rpm, and the supernatant was filtered through a 0.45-μm low protein affinity filter (Millipore). The soluble extract was injected onto a 5-ml Ni-Sepharose HisTrap HP (GE Healthcare). The column was washed with 10 to 15 column volumes of His buffer and eluted using a gradient of 20 mM to 500 mM of imidazole over five column volumes. The elution fractions were pooled, treated with TEV protease overnight, and purified by size exclusion chromatography on a Superdex 200 26/60 size exclusion column (GE Healthcare), equilibrated in 50 mM Tris (pH 8.0) and 125 mM NaCl. Peaks were pooled and concentrated using a 10-kDa Amicon filtration device to 30 mg/ml, aliquoted, and stored at –80°C.
Crystallization, data collection, and processing.
All crystallization was performed using the hanging drop vapor diffusion technique in 48-well plates (Hampton Research). Each well contained 300 μl of reservoir solution, covered by a siliconized glass cover slip with 1.5 μl of reservoir solution mixed with 1.5 μl of protein:peptide mixed at a 1:2 molar ratio. The final crystallization solution contained 31% PEG 400, 0.1 M HEPES (pH 7.5), 0.2 M CaCl2, and 2 mM dithiothreitol (DTT). Crystals were flash-cooled in liquid nitrogen and diffracted at the Australian synchrotron MX1 and MX2 beamlines (40, 41). Diffraction data were integrated using iMosfilm and merged and scaled using Aimless, and Phaser was used for initial phase determination and electron density maps (42–44). Model building and refinement were performed using Coot and Phenix, respectively (45, 46).
Cells.
HEK293T cells (CRL-3216) and A549 cells (CCL-185) were obtained from ATCC and were maintained in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and cultured at 37°C and 5% CO2. A549 cells expressing Tet-inducible Flag-tag, Flag-tagged NiV W, or NiV W S449A were generated by transducing 1 × 105 A549 cells with 1 ml of lentivirus and 8 μg of Polybrene. Forty-eight hours postransduction, cells were treated with 1 μg/ml puromycin to select for cells that integrated the lentiviral vector, and monoclonal cell lines were generated. Expression of Flag-tagged NiV W or NiV W S449A was assessed by treating monoclonal cell lines with 1 μg/ml doxycycline for 24 h, followed by Western blot analysis for the Flag-tag. A single clonal population of each cell line was selected for use and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and cultured at 37°C and 5% CO2.
Luciferase assays.
For interferon (IFN)-induced ISG54 promoter luciferase assay, HEK293T cells (7.5 × 104/well) were transfected with 30 ng of the ISG54 promoter firefly luciferase reporter, 30 ng of a constitutively expressed Renilla luciferase reporter (pRLTK), and increasing concentrations of HA-tagged NiV W, NiV W S449A, and NiV W G121E (1, 10, and 100 ng), using Lipofectamine 2000 (Thermo Fisher Scientific). Twenty-four hours posttransfection, cells were treated with 1,000 U IFN-β as indicated for a further 24 h. Luciferase activity was assessed using a dual-luciferase assay (Promega) and read on an EnVision plate reader. Firefly luciferase values were normalized to Renilla luciferase values. The assay was performed in triplicate; error bars indicate the standard deviation for the triplicate. For Sendai virus (SeV)-induced IFN-β promoter luciferase assay, HEK293T cells (7.5 × 104/well) were transfected with 30 ng of the IFN-β promoter firefly luciferase reporter, 30 ng of a constitutively expressed Renilla luciferase reporter (pRLTK), and increasing concentrations of the indicated Flag-tagged NiV W and NiV W mutants (2.5, 25, and 250 ng), using Lipofectamine 2000 (Thermo Fisher Scientific). Twenty-four hours posttransfection, cells were infected with 200 HA units (HAU) of SeV for 18 h. Luciferase activity was assessed and analyzed as described above. For RIG-IN-induced IFN-β promoter luciferase assay, HEK293T cells (7.5 × 104/well) were transfected with 30 ng of the IFN-β promoter firefly luciferase reporter, 30 ng of a constitutively expressed Renilla luciferase reporter (pRLTK), 5 ng of RIG-IN, and increasing concentrations of the indicated Flag-tagged NiV W and NiV W mutants (62.5, 125, and 250 ng) as indicated, using Lipofectamine 2000 (Thermo Fisher Scientific). Eighteen-hour posttransfection luciferase activity was assessed and analyzed as described above.
Coimmunoprecipitation assays.
HEK293T cells (1 × 106) were transfected with the indicated plasmids using Lipofectamine 2000 (Thermo Fisher Scientific). At 24 h posttransfection, cells were lysed in NP-40 lysis buffer (50 mM Tris [pH 7.5], 280 mM NaCl, 0.5% NP-40, 0.2 mM EDTA, 2 mM EGTA, 10% glycerol, protease inhibitor [complete; Roche, Indianapolis, IN]). Anti-FLAG M2 magnetic beads, EZview Red anti-HA agarose affinity gel (Sigma-Aldrich), or Pierce glutathione magnetic agarose beads (Thermo Fisher Scientific) were incubated as indicated with lysates for 1 h at 4°C, washed five times in NP-40 lysis buffer, and eluted using 3× FLAG or HA peptide (Sigma-Aldrich) at 4°C for 30 min or by boiling in sample buffer for 5 min. Whole-cell lysates (4% of sample) and coprecipitation samples (25% of sample) were analyzed by Western blotting. For coimmunoprecipitation of endogenous 14-3-3, 7 × 106 HEK293T cells were transfected with the indicated plasmids using Lipofectamine 2000. At 24 h posttransfection, cells were lysed in NP-40 lysis buffer, and anti-FLAG M2 or EZview Red anti-HA agarose affinity gel beads were incubated as indicated with lysates for 8 h or 16 h, respectively. Beads were washed, eluted, and analyzed as above.
Western blots.
Lysates were run on 10% Bis-Tris Plus polyacrylamide gels (Thermo Fisher) and transferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Membranes were probed with the indicated antibodies and were developed by Western Lightning Plus ECL (Perkin Elmer) and imaged on a ChemiDoc MP imaging system (Bio-Rad).
Antibodies.
Rabbit and mouse anti-Flag and rabbit anti-HA antibodies were purchased from Sigma-Aldrich. Rabbit anti-β-actin, rabbit anti-GST, and anti-14-3-3 antibodies were purchased from Cell Signaling.
Library generation and sequencing.
To generate the RNA, each stable cell line (5 × 105 cells) was plated in duplicate in a 6-well plate and allowed to rest for 24 h, after which they were treated with 1 μg/ml doxycycline for an additional 24 h. Cells were then infected with Sendai virus (SeV) (200 HAU) in the presence of 1 μg/ml doxycycline to induce an antiviral response. Twenty-four hours postinfection, cells were harvested in TRIzol. RNA was isolated using a Zymo Research Direct-zol RNA mini-prep kit (Zymo Research), and RNA quality and concentration were determined using a 2100 bioanalyzer (Agilent Genomics). The NEB poly(A) mRNA magnetic isolation kit was used in conjunction with the NEB Ultra II RNA directional kit to prepare the libraries. Multiplexed libraries were subjected to single-end 75-bp sequencing using the Illumina NextSeq 500 platform (Illumina).
RNA-Seq analysis.
Data analysis was performed using the Georgia State University (GSU) high-performance computing resources (48). RNA reads were trimmed using Trim Galore with an average phred score cutoff of 30 and minimum length of 50 bp. FastQC function was used to generate quality reports. Reads that passed quality control (2.5 × 107 to 3.6 × 107 reads/sample) were aligned to a custom reference genome that contained the human genome (Homo_sapiens.GRCh38.dna.primary_assembly.fa) and NiV W sequences and a custom annotation file (Homo_sapiens.GRCh38.94.gtf) containing NiV W using HiSat2, with percent alignment between 79 and 97% per sample (47). To determine raw expression values, gene-level read counts were generated using the htseq-count function in HTseq, counting reads overlapping exonic regions of genes and discarding reads that map to ambiguous regions (54). The edgeR package was used for normalization and statistical analysis of differentially expressed genes (DEGs). For further analysis, DEGs were defined as those with a fold change of ≥2 and a false-discovery rate (FDR)-corrected P value of ≤0.05, and only protein-coding genes with an average of one read per kilobase of transcript per million mapped reads (RPKM) were included. The principal component diagram was generated using DESeq2.
Functional enrichment of DEGs by Metascape identified genes mapping to specific biological pathways, under gene ontology (GO) terms (26). Enrichment of transcription factor binding sites (TFBS) was analyzed by Opussom-3.0 human single site analysis, using the default settings (49–51). Transcription factors that interact with the enriched TFBS were analyzed by STRING using a high confidence interaction score to generate a functional association network and identify the enriched GO terms (27). The resulting network was imported into Cytoscape for generation of the figure (52). The percentage of read alignment to SeV and NiV W was determined by alignment of trimmed RNA-seq reads to the sequence of SeV (GenBank accession number AB855653.1) or NiV W, using GSNAP in Sequencher v5.4.6 (GeneCodes). Alignments were imported into Tablet v1.16.09.06, and the percentage of reads aligned to unaligned was calculated (53).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 8 with significance determined by a one-way analysis of variance (ANOVA), followed by Tukey’s correction for multiple comparisons. Data points were considered significantly different if the P value was <0.05. Statistical details can be found in the figure legends.
Data availability.
Models are available in the Protein Data Bank (PDB) under PDB code 6W0L. The GenBank BioProject number for the RNA-sequencing data reported in this paper is PRJNA625125.
ACKNOWLEDGMENTS
We thank Kate M. Smith for assistance with preliminary studies and Sampriti De for technical support. We acknowledge the High Throughput DNA Sequencing and Molecular Evolution core at the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology for the use of their shared equipment, services, and expertise. We also acknowledge the use of Georgia State’s research computing resources, which are supported by Georgia State’s Research Solutions. This research was undertaken in part using the MX2 beamline at the Australian Synchrotron, part of ANSTO, and using the Australian Cancer Research Foundation (ACRF) detector.
This work was supported by grants R21AI144880, U19AI109945, and P01AI120943 from the NIH to C.F.B.
Author contributions were as follows. Megan R. Edwards: conceptualization, investigation, formal analysis, writing—original draft. Mikayla Hoad: investigation, formal analysis. Sofiya Tsimbalyuk: investigation, formal analysis. Andrea R. Menicucci: formal analysis, writing—review and editing. Ilhem Messaoudi: supervision, writing—review and editing. Jade K. Forwood: conceptualization, funding acquisition, supervision, writing—original draft. Christopher F. Basler: conceptualization, funding acquisition, supervision, writing—original draft.
REFERENCES
- 1.Ksiazek TG, Rota PA, Rollin PE. 2011. A review of Nipah and Hendra viruses with an historical aside. Virus Res 162:173–183. doi: 10.1016/j.virusres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Broder CC. 2012. Henipavirus outbreaks to antivirals: the current status of potential therapeutics. Curr Opin Virol 2:176–187. doi: 10.1016/j.coviro.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvey LA, Wells RM, McCormack JG, Ansford AJ, Murray K, Rogers RJ, Lavercombe PS, Selleck P, Sheridan JW. 1995. Infection of humans and horses by a newly described morbillivirus. Med J Aust 162:642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 4.Mire CE, Satterfield BA, Geisbert JB, Agans KN, Borisevich V, Yan L, Chan Y-P, Cross RW, Fenton KA, Broder CC, Geisbert TW. 2016. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: implications for antibody therapy. Sci Rep 6:30916. doi: 10.1038/srep30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brian HH, Luis L, Azaibi T, Zhenhua Y, Bettina B, Nadine B, Pierre ER, James AC, Thomas GK, Mohammed Jahangir H, Emily SG, Robert FB, William JB, Paul AR. 2005. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerging Infectious Dis J 11:1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weatherman S, Feldmann H, de Wit E. 2018. Transmission of henipaviruses. Curr Opin Virol 28:7–11. doi: 10.1016/j.coviro.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciancanelli MJ, Volchkova VA, Shaw ML, Volchkov VE, Basler CF. 2009. Nipah virus sequesters inactive STAT1 in the nucleus via a P gene-encoded mechanism. J Virol 83:7828–7841. doi: 10.1128/JVI.02610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satterfield BA, Borisevich V, Foster SL, Rodriguez SE, Cross RW, Fenton KA, Agans KN, Basler CF, Geisbert TW, Mire CE. 2019. Antagonism of STAT1 by Nipah virus P gene products modulates disease course but not lethal outcome in the ferret model. Sci Rep 9:16710. doi: 10.1038/s41598-019-53037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw ML, Garcia-Sastre A, Palese P, Basler CF. 2004. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J Virol 78:5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez JJ, Cruz CD, Horvath CM. 2004. Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J Virol 78:5358–5367. doi: 10.1128/jvi.78.10.5358-5367.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw ML, Cardenas WB, Zamarin D, Palese P, Basler CF. 2005. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3-triggered signaling pathways. J Virol 79:6078–6088. doi: 10.1128/JVI.79.10.6078-6088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KM, Tsimbalyuk S, Edwards MR, Cross EM, Batra J, Soares da Costa TP, Aragao D, Basler CF, Forwood JK. 2018. Structural basis for importin alpha 3 specificity of W proteins in Hendra and Nipah viruses. Nat Commun 9:3703. doi: 10.1038/s41467-018-05928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterfield BA, Cross RW, Fenton KA, Agans KN, Basler CF, Geisbert TW, Mire CE. 2015. The immunomodulating V and W proteins of Nipah virus determine disease course. Nat Commun 6:7483. doi: 10.1038/ncomms8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satterfield BA, Cross RW, Fenton KA, Borisevich V, Agans KN, Deer DJ, Graber J, Basler CF, Geisbert TW, Mire CE. 2016. Nipah virus C and W proteins contribute to respiratory disease in ferrets. J Virol 90:6326–6343. doi: 10.1128/JVI.00215-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Gil L, Vera-Velasco NM, Mingarro I. 2017. Exploring the human-Nipah virus protein-protein interactome. J Virol 91:e01461-17. doi: 10.1128/JVI.01461-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichlmair A, Kandasamy K, Alvisi G, Mulhern O, Sacco R, Habjan M, Binder M, Stefanovic A, Eberle CA, Goncalves A, Burckstummer T, Muller AC, Fauster A, Holze C, Lindsten K, Goodbourn S, Kochs G, Weber F, Bartenschlager R, Bowie AG, Bennett KL, Colinge J, Superti-Furga G. 2012. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature 487:486–490. doi: 10.1038/nature11289. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty MK, Morrison DK. 2004. Unlocking the code of 14-3-3. J Cell Sci 117:1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- 18.Lin JP, Fan YK, Liu HM. 2019. The 14-3-3eta chaperone protein promotes antiviral innate immunity via facilitating MDA5 oligomerization and intracellular redistribution. PLoS Pathog 15:e1007582. doi: 10.1371/journal.ppat.1007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu HM, Loo YM, Horner SM, Zornetzer GA, Katze MG, Gale M Jr. 2012. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coblitz B, Wu M, Shikano S, Li M. 2006. C-terminal binding: an expanded repertoire and function of 14-3-3 proteins. FEBS Lett 580:1531–1535. doi: 10.1016/j.febslet.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Johnson C, Crowther S, Stafford MJ, Campbell DG, Toth R, MacKintosh C. 2010. Bioinformatic and experimental survey of 14-3-3-binding sites. Biochem J 427:69–78. doi: 10.1042/BJ20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killoran RC, Fan J, Yang D, Shilton BH, Choy W-Y. 2015. Structural analysis of the 14-3-3ζ/Chibby interaction involved in Wnt/β-catenin signaling. PLoS One 10:e0123934. doi: 10.1371/journal.pone.0123934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YK, Gack MU. 2016. A phosphomimetic-based mechanism of dengue virus to antagonize innate immunity. Nat Immunol 17:523–530. doi: 10.1038/ni.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedl W, Acharya D, Lee JH, Liu G, Serman T, Chiang C, Chan YK, Diamond MS, Gack MU. 2019. Zika virus NS3 mimics a cellular 14-3-3-binding motif to antagonize RIG-I- and MDA5-mediated innate immunity. Cell Host Microbe 26:493–503.e6. doi: 10.1016/j.chom.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. 2019. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni S, Volchkova V, Basler CF, Palese P, Volchkov VE, Shaw ML. 2009. Nipah virus edits its P gene at high frequency to express the V and W proteins. J Virol 83:3982–3987. doi: 10.1128/JVI.02599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC. 2005. Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc Natl Acad Sci U S A 102:1222–1227. doi: 10.1073/pnas.0406871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobe B, Kampmann T, Forwood JK, Listwan P, Brinkworth RI. 2005. Substrate specificity of protein kinases and computational prediction of substrates. Biochim Biophys Acta 1754:200–209. doi: 10.1016/j.bbapap.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Shikano S, Coblitz B, Sun H, Li M. 2005. Genetic isolation of transport signals directing cell surface expression. Nat Cell Biol 7:985–992. doi: 10.1038/ncb1297. [DOI] [PubMed] [Google Scholar]
- 32.Anders C, Higuchi Y, Koschinsky K, Bartel M, Schumacher B, Thiel P, Nitta H, Preisig-Muller R, Schlichthorl G, Renigunta V, Ohkanda J, Daut J, Kato N, Ottmann C. 2013. A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chem Biol 20:583–593. doi: 10.1016/j.chembiol.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Lo MK, Miller D, Aljofan M, Mungall BA, Rollin PE, Bellini WJ, Rota PA. 2010. Characterization of the antiviral and inflammatory responses against Nipah virus in endothelial cells and neurons. Virology 404:78–88. doi: 10.1016/j.virol.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Morrison DK. 2009. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennington KL, Chan TY, Torres MP, Andersen JL. 2018. The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 37:5587–5604. doi: 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleppe R, Martinez A, Doskeland SO, Haavik J. 2011. The 14-3-3 proteins in regulation of cellular metabolism. Semin Cell Dev Biol 22:713–719. doi: 10.1016/j.semcdb.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Edwards MR, Liu G, Mire CE, Sureshchandra S, Luthra P, Yen B, Shabman RS, Leung DW, Messaoudi I, Geisbert TW, Amarasinghe GK, Basler CF. 2016. Differential regulation of interferon responses by Ebola and Marburg virus VP35 proteins. Cell Rep 14:1632–1640. doi: 10.1016/j.celrep.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen B, Mulder LC, Martinez O, Basler CF. 2014. Molecular basis for ebolavirus VP35 suppression of human dendritic cell maturation. J Virol 88:12500–12510. doi: 10.1128/JVI.02163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Studier FW. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Cowieson NP, Aragao D, Clift M, Ericsson DJ, Gee C, Harrop SJ, Mudie N, Panjikar S, Price JR, Riboldi-Tunnicliffe A, Williamson R, Caradoc-Davies T. 2015. MX1: a bending-magnet crystallography beamline serving both chemical and macromolecular crystallography communities at the Australian Synchrotron. J Synchrotron Radiat 22:187–190. doi: 10.1107/S1600577514021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPhillips TM, McPhillips SE, Chiu HJ, Cohen AE, Deacon AM, Ellis PJ, Garman E, Gonzalez A, Sauter NK, Phizackerley RP, Soltis SM, Kuhn P. 2002. Blu-Ice and the Distributed Control System: software for data acquisition and instrument control at macromolecular crystallography beamlines. J Synchrotron Radiat 9:401–406. doi: 10.1107/s0909049502015170. [DOI] [PubMed] [Google Scholar]
- 42.Evans PR, Murshudov GN. 2013. How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG. 2011. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 67:271–281. doi: 10.1107/S0907444910048675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCoy AJ. 2007. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr 63:32–41. doi: 10.1107/S0907444906045975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. 2012. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarajlic S, Edirisinghe N, Wu Y, Jiang Y, Faroux G. 2017. Training-based workforce development in Advanced Computing for Research and Education (ACoRE), p 1–4. Proceedings of the Practice and Experience in Advanced Research Computing 2017 on Sustainability, Success and Impact; New Orleans, LA: Association for Computing Machinery. doi: 10.1145/3093338.3104178. [DOI] [Google Scholar]
- 49.Ho Sui SJ, Fulton DL, Arenillas DJ, Kwon AT, Wasserman WW. 2007. oPOSSUM: integrated tools for analysis of regulatory motif over-representation. Nucleic Acids Res 35:W245–W252. doi: 10.1093/nar/gkm427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho Sui SJ, Mortimer JR, Arenillas DJ, Brumm J, Walsh CJ, Kennedy BP, Wasserman WW. 2005. oPOSSUM: identification of over-represented transcription factor binding sites in co-expressed genes. Nucleic Acids Res 33:3154–3164. doi: 10.1093/nar/gki624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon AT, Arenillas DJ, Worsley Hunt R, Wasserman WW. 2012. oPOSSUM-3: advanced analysis of regulatory motif over-representation across genes or ChIP-Seq datasets. G3 (Bethesda) 2:987–1002. doi: 10.1534/g3.112.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milne I, Stephen G, Bayer M, Cock PJ, Pritchard L, Cardle L, Shaw PD, Marshall D. 2013. Using Tablet for visual exploration of second-generation sequencing data. Brief Bioinform 14:193–202. doi: 10.1093/bib/bbs012. [DOI] [PubMed] [Google Scholar]
- 54.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Models are available in the Protein Data Bank (PDB) under PDB code 6W0L. The GenBank BioProject number for the RNA-sequencing data reported in this paper is PRJNA625125.